Introduction
Bone metabolism is regulated strictly by two types
of functional cells, osteoblasts and osteoclasts, which are
responsible for bone formation and bone resorption, respectively
(1). Bone remodeling is known to
be the outcome of the coupling and fine-tuning process of
osteoblastic bone formation and osteoclastic bone resorption
(1). Numerous humoral factors,
including cytokines and prostaglandins, have been demonstrated to
participate in bone remodeling (2). Osteoprotegerin, which belongs to the
tumor necrosis factor receptor family, along with receptor
activator of nuclear factor-κB (RANK), is synthesized by
osteoblasts and plays an inhibitory role in osteoclastic
differentiation and activation (3). Osteoprotegerin, secreted by
osteoblasts, binds to RANK ligand (RANKL) as a decoy receptor, and
prevents RANKL from binding to RANK, resulting in the inhibition of
bone resorption (3). It has been
reported in previous research that RANKL knock-out mice and
osteoprotegerin knock-out mice suffered from severe osteopetrosis
and osteoporosis, respectively (4,5).
Therefore, it has been firmly established that the
RANK/RANKL/osteoprotegerin axis is a major regulatory aspect of
bone remodeling (6).
It has previously been noted that prostaglandins act
as autacoids in osteoblasts (7).
Prostaglandins, which have previously been recognized as potent
bone-resorptive agents (8), are
known to play important roles also in the process of bone formation
(8,9). Of the prostaglandins, prostaglandin
F2α (PGF2α) has been shown to act as a bone
remodeling mediator (9). In
relation to osteoblasts, we have previously demonstrated that
PGF2α induces activation of p38 mitogen-activated
protein (MAP) kinase, p44/p42 MAP kinase and stress-activated
protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in
osteoblast-like MC3T3-E1 cells, and that PGF2α-induced
osteoprotegerin synthesis is mediated through these MAP kinases
(10). We have also shown that
PGF2α stimulates the synthesis of interleukin-6 (IL-6),
a multifunctional cytokine modulating bone metabolism (11,12), via the activation of p38 MAP
kinase and p44/p42 MAP kinase but not SAPK/JNK in osteoblast-like
MC3T3-E1 cells (13,14). However, the details of the effects
of PGF2α on osteoblasts still remain unclear.
Mimosine, a plant amino acid, is well known to
chelate iron and inhibit mammalian DNA replication (15). Mimosine is additionally recognized
as a normoxic inducer of hypoxia-inducible factor (HIF) (16). HIF is a DNA-binding transcription
factor that interacts with specific nuclear cofactors under low
oxygen conditions, and activates a series of hypoxia-associated
genes to facilitate responses to hypoxic environments (17). A large number of target genes of
HIF have been identified, e.g., erythropoietin, glucose transporter
protein 1 and vascular endothelial growth factor (VEGF) (16). HIF is known to play an important
role in angiogenesis, erythropoiesis and metabolism (16). Regarding HIF and bone metabolism,
it has been previously demonstrated that hypoxia regulates
osteoclast-mediated bone resorption (17). In osteoblasts, HIF-1α reportedly
promotes bone formation by direct stimulation of osteoblast
proliferation (18).
Additionally, HIF-1α promotes angiogenesis and stimulates bone
regeneration (19). However, the
exact role of HIF in bone metabolism has not yet been
clarified.
In the present study, we investigated the effect of
mimosine, a normoxic inducer of HIF, on the
PGF2α-induced synthesis of osteoprotegerin and IL-6, and
the exact mechanism in osteoblast-like MC3T3-E1 cells. Herein, we
show that that mimosine suppresses the PGF2α-induced
osteoprotegerin synthesis without affecting IL-6 synthesis in these
cells.
Materials and methods
Materials
Mimosine and deferoxamine were purchased from
Sigma-Aldrich Co. (St. Louis, MO, USA). A mouse osteoprotegerin
enzyme-linked immunosorbent assay (ELISA) kit, mouse IL-6 ELISA
kit, mouse VEGF ELISA kit and PGF2α were obtained from
R&D Systems, Inc. (Minneapolis, MN, USA). HIF-1α antibodies
(ab16066) were obtained from Abcam (Cambridge, UK).
Phospho-specific p38 MAP kinase antibodies (#4511), p38 MAP kinase
antibodies (#9212), phospho-specific p44/p42 MAP kinase antibodies
(#9101), p44/p42 MAP kinase antibodies (#9102), phospho-specific
SAPK/JNK antibodies (#4671) and SAPK/JNK antibodies (#9252) were
all obtained from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Actin antibodies were obtained from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). An ECL western blot detection system
was obtained from GE Healthcare (Buckinghamshire, UK). Other
materials and chemicals were obtained from commercial sources.
PGF2α was dissolved in ethanol. Mimosine was disolved in
phosphate-buffered saline (PBS) supplemented with 0.01% bovine
serum albumin (BSA) containing 7.5% NaHCO3. Deferoxamine
was disolved in PBS supplemented with 0.01% BSA. The maximum
concentration of ethanol was 0.1%, which did not affect the assay
for osteoprotegerin release, IL-6 release, osteoprotegerin mRNA
expression, or western blot analysis.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from
newborn mouse calvaria (20) were
maintained as previously described (21). Briefly, the cells were cultured in
α-minimum essential medium (α-MEM) containing 10% fetal bovine
serum (FBS) at 37°C in a humidified atmosphere of 5%
CO2/95% air. The cells were seeded into 35-mm diameter
dishes (5×104 cells/dish) or 90-mm diameter dishes
(2×105 cells/dish) in α-MEM containing 10% FBS. After 5
days, the medium was exchanged for α-MEM containing 0.3% FBS. The
cells were then used for experiments after 48 h.
Assay for osteoprotegerin or IL-6
The cultured cells were pretreated with various
doses of mimosine or deferoxamine for 60 min, and then stimulated
with 10 µM PGF2α or the vehicle (PBS supplemented
with 0.01% BSA containing 0.1% ethanol) in 1 ml of α-MEM containing
0.3% FBS for the indicated periods of time. The conditioned medium
was collected at the end of incubation, and the concentrations of
osteoprotegerin or IL-6 were subsequently measured using an
osteoprotegerin ELISA kit or IL-6 ELISA kit according to the
manufacturer's instructions.
Assay for VEGF
The cultured cells were treated with various doses
of mimosine or deferoxamine in 1 ml of α-MEM containing 0.3% FBS
for 48 h. The conditioned medium was collected at the end of
incubation, and the VEGF concentrations were then measured using a
VEGF ELISA kit according to the manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
Cultured cells were pretreated with 700 µM
mimosine, 500 µM deferoxamine or vehicle for 60 min, and
then stimulated with 10 µM PGF2α or vehicle in
α-MEM containing 0.3% FBS for 3 h. Total RNA was isolated and
transcribed into cDNA using TRIzol reagent (Invitrogen Co.,
Carlsbad, CA, USA) and an Omniscript Reverse Transcriptase kit
(Qiagen, Inc., Valencia, CA, USA), respectively. RT-qPCR was
performed using a LightCycler system with capillaries and the
FastStart DNA Master SYBR-Green I provided with the kit (Roche
Diagnostics, Basel, Switzerland). Sense and antisense primers for
mouse osteoprotegerin mRNA were purchased from Takara Bio, Inc.
(Tokyo, Japan) (primer set ID: OPG; MA026526) (osteoprotegerin
primer sequences (5′→3′): forward, CAATGGCTGGCTTGGTTTCATAG and
reverse, CTGAACCAGACATGACAGCTGGA), whereas mouse VEGF mRNA or
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA primers were
synthesized based on the study of Simpson et al (22) (VEGF primer sequences (5′→3′):
forward, TTACTGCTGTACCTCCACC and reverse, ACAGGACGGCTTGAAGATG; and
GAPDH primer sequences (5′→3′) forward, AACGACCCCTTCATTGAC and
reverse, TCCACGACATACTCAGCAC. The amplified products were
determined using a melting curve analysis and agarose gel
electrophoresis. The mRNA levels of osteoprotegerin or VEGF were
normalized to those of GAPDH mRNA, respectively.
Western blot analysis
The cultured cells were pretreated with various
doses of mimosine or deferoxamine for 60 min, and then stimulated
with 10 µM PGF2α or vehicle in α-MEM containing
0.3% FBS for the indicated periods of time. The cells were washed
twice with phosphate-buffered saline, and then lysed, homogenized
and sonicated in a lysis buffer containing 62.5 mM Tris/HCl, pH
6.8, 2% sodium dodecyl sulfate (SDS), 50 mM dithiothreitol and 10%
glycerol. SDS-polyacrylamide gel electrophoresis (PAGE) was
performed according to the method of Laemmli (23) in 10% polyacrylamide gels. The
protein was fractionated and transferred onto Immun-Blot PVDF
membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked
with 5% fat-free dry milk in Tris-buffered saline-Tween-20 (TBS-T;
20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.1% Tween-20) for 1 h before
incubation with primary antibodies. Western blot analysis was
performed as described previously (24) using HIF-1α antibodies, actin
antibodies, phospho-specific p38 MAP kinase antibodies, p38 MAP
kinase antibodies, phospho-specific p44/p42 MAP kinase antibodies,
p44/p42 MAP kinase antibodies, phospho-specific SAPK/JNK antibodies
or SAPK/JNK antibodies as primary antibodies, and
peroxidase-labeled antibodies raised in goat anti-rabbit IgG were
used as secondary antibodies (074-1506, KPL, Inc., Gaithersburg,
MD, USA). The primary and secondary antibodies were diluted at
1:1,000 with 5% fat-free dried milk in TBS-T. The peroxidase
activity on the PVDF membranes was visualized on X-ray film by
means of the ECL western blot detection system.
Densitometric analysis
Densitometric analysis was performed using scanner
and image analysis software (ImageJ version 1.48; National
Institutes of Health, Bethesda, MD, USA). The phosphorylated
protein levels were calculated as follows: the
background-subtracted signal intensity of each phosphorylation
signal was normalized to the respective total protein signal and
plotted as the fold increase in comparison to control cells which
had not been stimulated by PGF2α nor treated with
mimosine or deferoxamine.
Statistical analysis
The data were analyzed by ANOVA followed by the
Bonferroni method for multiple comparisons between pairs, and a
p-value <0.05 was considered to indicate a statistically
significant difference. All data are presented as the means ± SEM
of triplicate determinations from three independent cell
preparations.
Results
Effects of mimosine and deferoxamine on
HIF-1α protein levels in MC3T3-E1 cells
Mimosine, which is an inhibitor of the prolyl
hydroxylase domain proteins responsible for degrading HIF-1α, is
known to act a normoxic inducer of HIF-1α (25). In the present study, we first
examined the effect of mimosine on HIF-1α protein levels in
osteoblast-like MC3T3-E1 cells. We noted that mimosine markedly
increased the HIF-1α protein levels (Fig. 1A).
It has previously been noted that deferoxamine, an
iron chelator, exerts its angiogenic effects through stimulation of
the HIF-1α pathway (26), and
deferoxamine is known to be another inducer of HIF-1α (26). We also found that HIF-1α protein
expression levels were markedly upregulated by deferoxamine
(Fig. 1B).
Effects of mimosine and deferoxamine on
PGF2α-induced osteoprotegerin release in MC3T3-E1
cells
We have recently shown that PGF2α
stimulates osteoprotegerin synthesis in osteoblast-like MC3T3-E1
cells (10). Thus, in the present
study we examined the effect of mimosine and deferoxamine on
PGF2α-induced osteoprotegerin release in MC3T3-E1 cells.
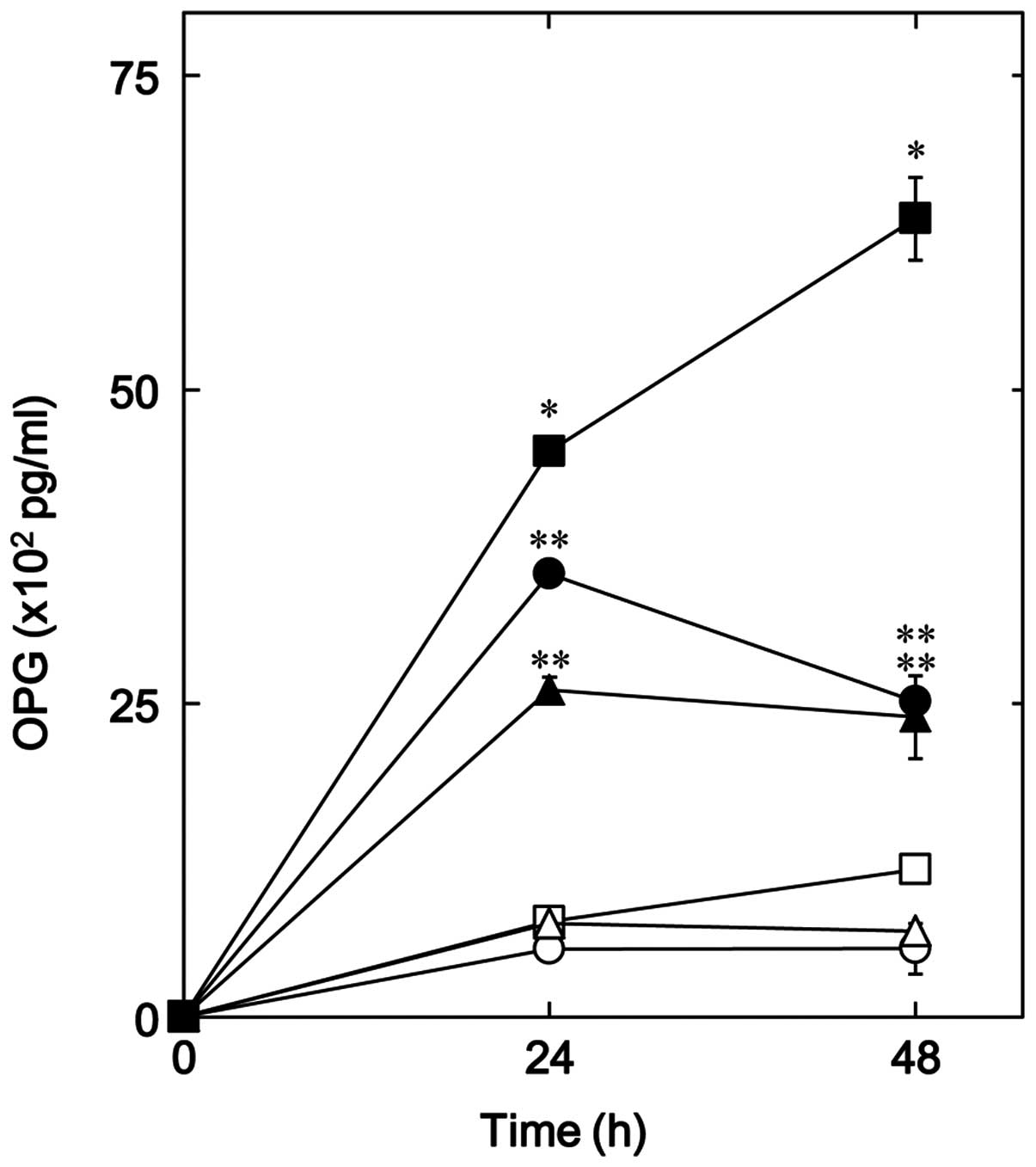
Mimosine was noted to significantly reduce osteoprotegerin release
induced by PGF2α up to 48 h (Fig. 2). The suppressive effect of
mimosine on osteoprotegerin release is clearly dose-dependent in
the range between 300 and 700 µM (Fig. 3A); mimosine at 500 µM
caused a 65% decrease in PGF2α-induced OPG release. In
addition, we also noted that deferoxamine significantly decreased
the release of osteoprotegerin induced by PGF2α
(Fig. 2), and the inhibitory
effect was dose-dependent in the range between 100 and 500
µM (Fig. 3B). The maximum
inhibitory effect of deferoxamine in relation to OPG release was
observed at 500 µM, which caused approximately an 80%
decrease in PGF2α-induced OPG release.
Effects of mimosine or deferoxamine on
the PGF2α-induced release of IL-6 in MC3T3-E1 cells
We have previously reported that PGF2α
stimulates IL-6 synthesis in osteoblast-like MC3T3-E1 cells
(13,14). Thus, in the present study we
examined the effect of mimosine or deferoxamine on the
PGF2α-induced release of IL-6. It was clear that
mimosine up to 700 µM failed to markedly affect the IL-6
release induced by 10 µM PGF2α (Fig. 4A). In addition, we noted that
deferoxamine up to 500 µM did not markedly effect
PGF2α (10 µM)-induced IL-6 release (Fig. 4B).
Effects of mimosine or deferoxamine on
PGF2α-induced osteoprotegerin mRNA expression in
MC3T3-E1 cells
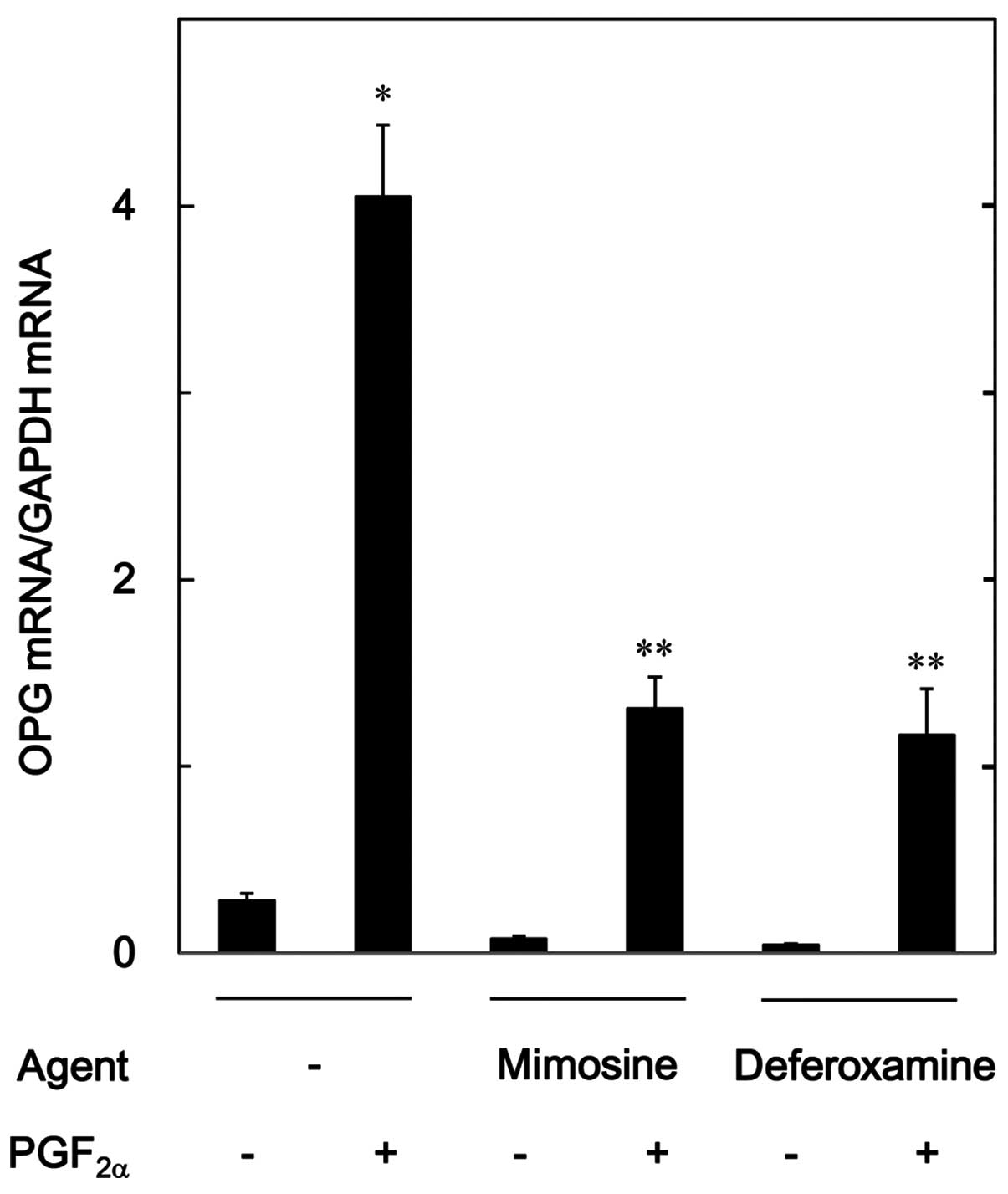
To investigate whether the inhibitory effect of
mimosine or deferoxamine on PGF2α-induced
osteoprotegerin release is mediated by transcriptional events in
osteoblast-like MC3T3-E1 cells, we examined the effect of mimosine
and deferoxamine on PGF2α-induced osteoprotegerin mRNA
expression. Mimosine (700 µM) and deferoxamine (500
µM), which alone hardly affected the osteoprotegerin mRNA
level, significantly attenuated the increase in the mRNA expression
level of osteoprotegerin induced by 10 µM of
PGF2α (Fig. 5).
Effects of mimosine or deferoxamine on
the release of VEGF and the expression of mRNA in MC3T3-E1
cells
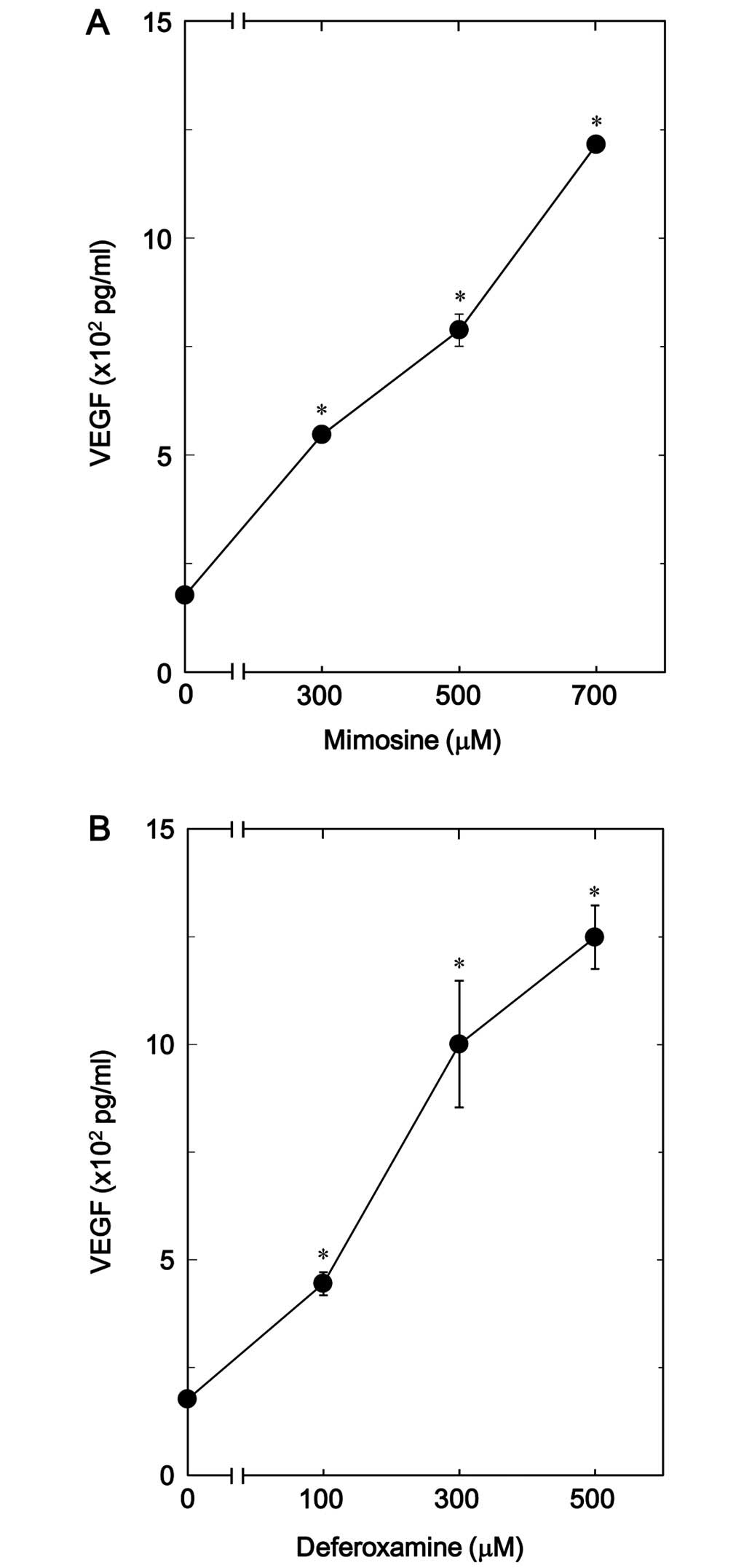
It has been noted previously that HIF increases
oxygen-regulated gene expression, including VEGF, and promotes
angiogenesis and osteogenesis (27). Therefore, we examined whether
mimosine or deferoxamine upregulates VEGF release in
osteoblast-like MC3T3-E1 cells. The release of VEGF was
significantly upregulated by mimosine in a dose-dependent manner in
the range between 300 and 700 µM (Fig. 6A). Additionally, we noted that
deferoxamine dose-dependently increased VEGF release in the range
between 100 and 500 µM (Fig.
6B).
We further investigated the effects of mimosine or
deferoxamine on VEGF mRNA expression in MC3T3-E1 cells. Both
mimosine and deferoxamine significantly upregulated VEGF mRNA
expression levels (Fig. 7).
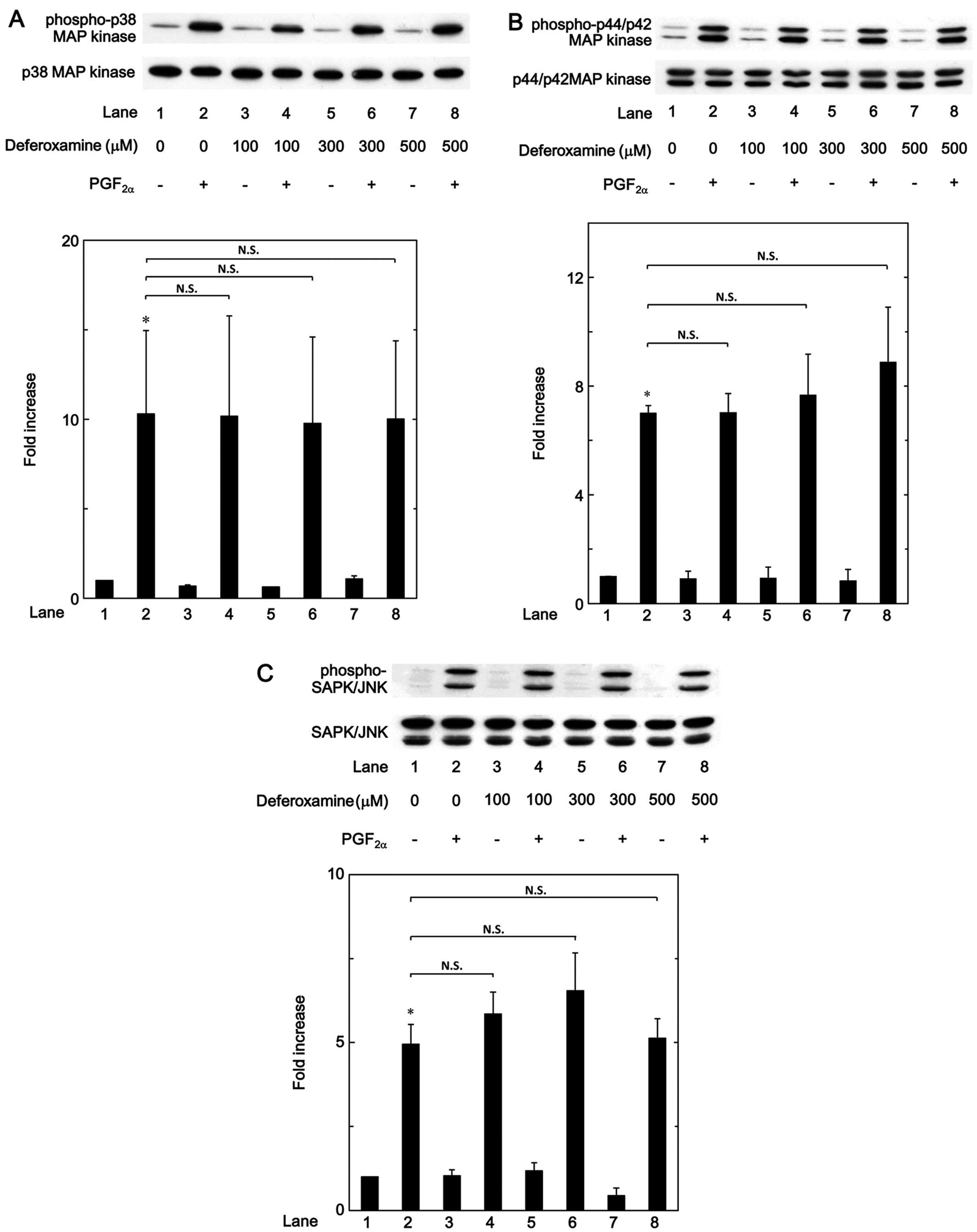
Effects of mimosine or deferoxamine on
PGF2α-induced phosphorylation of p38 MAP kinase, p44/p42
MAP kinase or SAPK/JNK in MC3T3-E1 cells
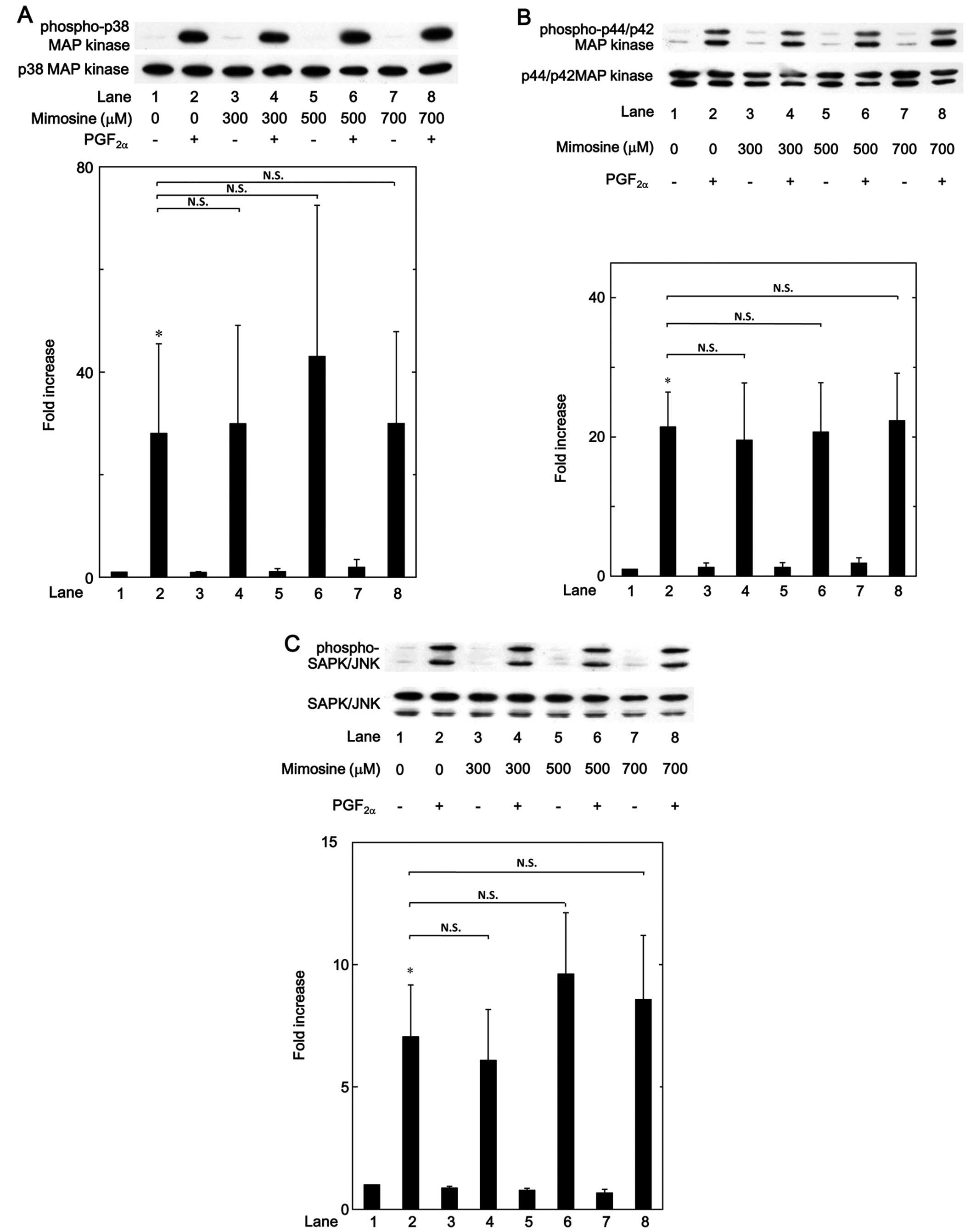
In a previous study, we have demonstrated that
PGF2α-induced osteoprotegerin synthesis is mediated
through activation of p38 MAP kinase, p44/p42 MAP kinase and
SAPK/JNK in osteoblast-like MC3T3-E1 cells (10). Therefore, in the present study we
examined whether mimosine and deferoxamine affected the
PGF2α-induced phosphorylation of p38 MAP kinase, p44/p42
MAP kinase or SAPK/JNK in these cells. We noted that the
phosphorylation of p38 MAP kinase, p44/p42 MAP kinase and SAPK/JNK
induced by PGF2α was not markedly affected by mimosine,
up to 700 µM (Fig. 8). It
also became evident that deferoxamine exerted little effect on the
PGF2α-induced phosphorylation of p38 MAP kinase, p44/p42
MAP kinase and SAPK/JNK, up to 500 µM (Fig. 9).
Discussion
In the present study, we demonstrated that mimosine
significantly reduced the PGF2α-induced release of
osteoprotegerin in osteoblast-like MC3T3-E1 cells. It is well known
that mimosine is an inhibitor of the prolyl hydroxylase domain
proteins responsible for degrading HIF-1α and also that it is an
inhibitor of DNA replication (16,25). Additionally, we showed in the
present study that the release of osteoprotegerin caused by
PGF2α was significantly suppressed by deferoxamine,
which is another inhibitor of the prolyl hydroxylase domain
proteins responsible for degrading HIF-1α, as has also been
previously stated (26). We found
that the protein levels of HIF-1α were considerably upregulated by
both mimosine and deferoxamine in osteoblast-like MC3T3-E1 cells.
Thus, it seems likely that the inhibitory effect of mimosine or
deferoxamine on osteoprotegerin release is mediated through the
HIF-1α-dependent pathway in MC3T3-E1 cells. In addition, we
demonstrated that both mimosine and deferoxamine suppressed the
PGF2α-induced osteoprotegerin mRNA expression.
Therefore, our findings suggest that the suppressive effect of
mimosine and deferoxamine on PGF2α-induced
osteoprotegerin release is exerted at a point upstream of the
transcriptional level in these cells. It is important to note that
HIF-1 is a transcription factor which plays a pivotal role in the
cellular response to hypoxia, and that VEGF is a target gene of
HIF-1 (16). In this study, we
showed that mimosine and deferoxamine by themselves induced the
release and the expression of VEGF mRNA. It has been noted that the
HIF-1 consists of two subunits, HIF-1α and HIF-1β, and the
expressed HIF-1α is degraded immediately in normoxic cells by the
ubiquitin-proteasome system, and that chemical hydroxylase
inhibitors including mimosine and deferoxamine suppress the
degradation of HIF-1α, resulting in its stabilization (16,25,26). Therefore, it is probable that
mimosine and deferoxamine, as normoxic inducers of HIF-1α, actually
stimulate VEGF synthesis in MC3T3-E1 cells. Taken together, our
findings suggest that mimosine and deferoxamine suppress the
PGF2α-induced synthesis of osteoprotegerin via
stabilization of HIF-1α expression in osteoblast-like MC3T3-E1
cells.
In our previous studies (13,14), we reported that PGF2α
induces the synthesis of IL-6 and also osteoprotegerin in
osteoblast-like MC3T3-E1 cells. IL-6 is known as a bone-resorptive
cytokine, and it plays an important role in bone metabolism
(6). Thus, we examined the effect
of mimosine or deferoxamine on PGF2α-induced IL-6
release, and demonstrated that neither mimosine nor deferoxamine
affected the release of IL-6 induced by PGF2α. Thus, it
is possible that the suppressive effects of mimosine and
deferoxamine on PGF2α induction are specific to
osteoprotegerin synthesis in osteoblast-like MC3T3-E1 cells.
The MAP kinase superfamily is known to play a
central role in a variety of cellular functions such as
proliferation, differentiation and survival (28). Three major MAP kinases, p38 MAP
kinase, p44/p42 MAP kinase and SAPK/JNK, are the main elements used
by cells to transfer diverse messages (29). Regarding the regulatory mechanism
of PGF2α-induced osteoprotegerin synthesis in
osteoblasts, we have previously reported that the activation of p38
MAP kinase, p44/p42 MAP kinase and SAPK/JNK are involved in the
PGF2α-induced osteoprotegerin synthesis in
osteoblast-like MC3T3-E1 cells (10). Thus, in the present study we
further examined the effect of mimosine or deferoxamine on the
PGF2α-induced phosphorylation of these MAP kinases in
MC3T3-E1 cells. We noted that the PGF2α-induced
phosphorylation of p38 MAP kinase, p44/p42 MAP kinase or SAPK/JNK
was not markedly affected by mimosine or deferoxamine. Thus, it
seems unlikely that the modulations of these MAP kinase activities
are involved in the suppressive effect which both mimosine and
deferoxamine exert on PGF2α-induced osteoprotegerin
synthesis in osteoblast-like MC3T3-E1 cells. Further investigations
are required in order to clarify the exact mechanism underlying the
effects of mimosine and deferoxamine on osteoprotegerin synthesis
in osteoblast-like MC3T3-E1 cells.
It is well known that RANKL-mediated osteoclastic
bone resorption constitutes the initial step of bone remodeling
(1). Osteoprotegerin produced by
osteoblasts plays a crucial role in the regulation of bone
remodeling as a decoy receptor of RANKL (3). However, it is also well known that
osteoblasts, osteoclasts and capillary endothelial cells are
closely coordinated during bone remodeling (30). VEGF, a specific growth factor of
vascular endothelial cells, produced by osteoblasts, is considered
to promote bone formation by supplying micro-vasculature (31). To maintain the quality of bone,
proper bone remodeling is essential to ensure the removal of old,
fragile bone and the renewal of the skeleton. Therefore, our
present findings demonstrating the inhibitory effects of mimosine
and deferoxamine, which act as normoxic inducers of HIF-1α in the
PGF2α-induced osteoprotegerin synthesis in osteoblasts,
provide new insights regarding hypoxic conditions in bone
metabolism. Further investigation is now necessary to elucidate in
more detail the mechanisms of HIF in bone metabolism.
In conclusion, our findings strongly suggest that
mimosine, a normoxic inducer of HIF, inhibits
PGF2α-induced osteoprotegerin synthesis without
affecting IL-6 synthesis in osteoblasts.
Acknowledgments
We are very grateful to Yumiko Kurokawa for her
skillful technical assistance. This research was supported in part
by a Grant-in-Aid for Scientific Research (19591042) from the
Ministry of Education, Science and the Research Funding for
Longevity Sciences (25-4, 26-12) from the National Center for
Geriatrics and Gerontology (NCGG), Japan.
References
|
1
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parfitt AM: Targeted and nontargeted bone
remodeling: relationship to basic multicellular unit origination
and progression. Bone. 30:5–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: a novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kong YY, Yoshida H, Sarosi I, Tan HL,
Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G,
Itie A, et al: OPGL is a key regulator of osteoclastogenesis,
lymphocyte development and lymph-node organogenesis. Nature.
397:315–323. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizuno A, Amizuka N, Irie K, Murakami A,
Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, et
al: Severe osteoporosis in mice lacking osteoclastogenesis
inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun.
247:610–615. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hikiji H, Takato T, Shimizu T and Ishii S:
The roles of prostanoids, leukotrienes, and platelet-activating
factor in bone metabolism and disease. Prog Lipid Res. 47:107–126.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blackwell KA, Raisz LG and Pilbeam CC:
Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab.
21:294–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agas D, Marchetti L, Hurley MM and
Sabbieti MG: Prostaglandin F2α: a bone remodeling
mediator. J Cell Physiol. 228:25–29. 2013. View Article : Google Scholar
|
|
10
|
Kuroyanagi G, Tokuda H,
Matsushima-Nishiwaki R, Kondo A, Mizutani J, Kozawa O and Otsuka T:
Resveratrol suppresses prostaglandin F2α-induced
osteoprotegerin synthesis in osteoblasts: inhibition of the MAP
kinase signaling. Arch Biochem Biophys. 542:39–45. 2014. View Article : Google Scholar
|
|
11
|
Hirano T: Revisiting the 1986 molecular
cloning of interleukin 6. Front Immunol. 5:4562014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Franchimont N, Wertz S and Malaise M:
Interleukin-6: an osteotropic factor influencing bone formation?
Bone. 37:601–606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tokuda H, Kozawa O, Harada A and Uematsu
T: p42/p44 mitogen-activated protein kinase activation is involved
in prostaglandin F2α-induced interleukin-6 synthesis in
osteoblasts. Cell Signal. 11:325–330. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minamitani C, Otsuka T, Takai S,
Matsushima-Nishiwaki R, Adachi S, Hanai Y, Mizutani J, Tokuda H and
Kozawa O: Involvement of Rho-kinase in prostaglandin
F2α-stimulated interleukin-6 synthesis via p38
mitogen-activated protein kinase in osteoblasts. Mol Cell
Endocrinol. 291:27–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Warnecke C, Griethe W, Weidemann A,
Jürgensen JS, Willam C, Bachmann S, Ivashchenko Y, Wagner I, Frei
U, Wiesener M and Eckardt KU: Activation of the hypoxia-inducible
factor-pathway and stimulation of angiogenesis by application of
prolyl hydroxylase inhibitors. FASEB J. 17:1186–1188.
2003.PubMed/NCBI
|
|
16
|
Schofield CJ and Ratcliffe PJ: Oxygen
sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 5:343–354.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Knowles HJ, Cleton-Jansen AM, Korsching E
and Athanasou NA: Hypoxia-inducible factor regulates
osteoclast-mediated bone resorption: role of angiopoietin-like 4.
FASEB J. 24:4648–4659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Wan C, Deng L, Liu X, Cao X,
Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC,
et al: The hypoxia-inducible factor alpha pathway couples
angiogenesis to osteogenesis during skeletal development. J Clin
Invest. 117:1616–1626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan C, Gilbert SR, Wang Y, Cao X, Shen X,
Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC,
et al: Activation of the hypoxia-inducible factor-1α pathway
accelerates bone regeneration. Proc Natl Acad Sci USA. 105:686–691.
2008. View Article : Google Scholar
|
|
20
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2
in osteoblast-like cells. Exp Cell Res. 198:130–134. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simpson DA, Feeney S, Boyle C and Stitt
AW: Retinal VEGF mRNA measured by SYBR green I fluorescence: a
versatile approach to quantitative PCR. Mol Vis. 6:178–183.
2000.PubMed/NCBI
|
|
23
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells.
J Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen X, Wan C, Ramaswamy G, Mavalli M,
Wang Y, Duvall CL, Deng LF, Guldberg RE, Eberhart A, Clemens TL and
Gilbert SR: Prolyl hydroxylase inhibitors increase neoangiogenesis
and callus formation following femur fracture in mice. J Orthop
Res. 27:1298–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donneys A, Deshpande SS, Tchanque-Fossuo
CN, Johnson KL, Blough JT, Perosky JE, Kozloff KM, Felice PA,
Nelson NS, Farberg AS, et al: Deferoxamine expedites consolidation
during mandibular distraction osteogenesis. Bone. 55:384–390. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan L, Li J, Yu Z, Dang X and Wang K: The
hypoxia-inducible factor pathway, prolyl hydroxylase domain protein
inhibitors, and their roles in bone repair and regeneration. BioMed
Res Int. 2014:2393562014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
29
|
Widmann C, Gibson S, Jarpe MB and Johnson
GL: Mitogen-activated protein kinase: conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999.PubMed/NCBI
|
|
30
|
Erlebacher A, Filvaroff EH, Gitelman SE
and Derynck R: Toward a molecular understanding of skeletal
development. Cell. 80:371–378. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zelzer E and Olsen BR: Multiple roles of
vascular endothelial growth factor (VEGF) in skeletal development,
growth, and repair. Curr Top Dev Biol. 65:169–187. 2005. View Article : Google Scholar : PubMed/NCBI
|