Introduction
Paraquat (PQ) is widely used as a herbicide;
however, it is strongly toxic to humans (1). Multiple organ damage is induced by
PQ, which is absorbed through the skin, the respiratory tract and
the digestive tract (1), and it
mainly accumulates in lung cells. The PQ concentration in lung
tissue is 6–10-fold higher than that in plasma, and it causes acute
pulmonary edema, progressive dyspnea and pulmonary fibrosis
(2). PQ poisoning can lead to the
production of reactive oxygen species (ROS) and PQ radicals through
cyclic reduction/oxidation. Redox cycling is believed to play an
essential role in the initiation of lung damage and fibrosis
resulting from PQ poisoning (3).
Silent information regulator 2-related enzyme 1
(SIRT1) is a NAD+-dependent protein deacetylase
(4) and is involved in various
cellular processes, including cell proliferation, apoptosis and
inflammation (5). Previous
studies have confirmed that SIRT1 plays a significant role in lung
injury caused by smoking, bronchial asthma and oxidative
stress-related lung diseases (6–8).
Nuclear factor E2-related factor 2 (NRF2) is an
important transcription factor, which regulates genes associated
with the scavenging of oxygen free radicals, and is regarded as an
effective target for antioxidant therapy (9,10).
Under conditions of oxidative stress, NRF2 translocates to the
nucleus, and then induces the gene expression of downstream
detoxification enzymes and antioxidant enzymes, such as superoxide
dismutase (SOD) and heme oxygenase-1 (HO-1), in order to produce an
antioxidant effect (11–13). Previous studies have revealed that
SIRT1 promotes the activity of NRF2 and upregulates the expression
of NRF2 downstream genes, such as SOD (14,15), and that the knockdown of SIRT1
results in the downregulation of NRF2 expression (16). In our previous study, we confirmed
that NRF2 is activated and plays a role in protecting A549 lung
cells against oxidative damage following exposure to PQ (17). However, the association between
SIRT1 and NRF2, as well as their effects on PQ-induced oxidative
stress remain to be elucidated.
Therefore, in the present study, we aimed to
determine whether the SIRT1/NRF2/ARE signaling pathway plays an
important role in lung injury induced by PQ. For this purpose,
mouse type II alveolar epithelial cells (AECs-II) were exposed to
various concentrations of PQ and the effects of PQ on the levels of
antioxidant enzymes were determined. In addition, to examine the
association between SIRT1 and NRF2, SIRT1 expression was either
induced or silenced by transfection of the cells with a SIRT1
overexpression vector or shRNA targeting SIRT1, respectively.
Materials and methods
Animals and reagents
ICR mice (n=20, weighing 18–30 g) of clean grade
were provided by the Animal Experimental Center of Wenzhou Medical
University (Wenzhou, China). All experimental procedures involving
animals were pre-approved in accordance with the Institutional
Animal Care and Use Committee guidelines of Wenzhou Medical
University. PQ used in this study was purchased from Sigma-Aldrich
(St. Louis, MO, USA) and compounded into a liquid of corresponding
concentrations. The antibodies, including anti-SIRT1 (#3931),
anti-NRF2 (#12721), anti-glyceraldehyde-3-phosphate dehydrogenase
(GAPDH; #5174) and anti-acetyl lysine (#9441) antibodies were
obtained from Cell Signaling Technology (Danvers, MA, USA).
Isolation and culture of mouse
AECs-II
The mice were sacrificed by cervical dislocation,
and both lungs were quickly removed and placed into 75% ethanol for
5 min. Subsequently, both lungs were moved to a superclean bench
and residual tracheal tissue and connective tissue were cleared to
the maximum extent possible. Finally, the cleared lung tissues were
placed in sterile pre-cold PBS and washed 2 to 3 times. The lung
tissues were washed twice in phosphate-buffered saline (PBS) and
cut into small sections (approximately 1 mm3) and then
digested with 0.25% trypsinase (Gibco, Carlsbad, CA, USA) under
gentle agitation at 37°C. After 5 min, digestion was terminated
using RPMI-1640 medium with 10% fetal calf serum (both from Gibco,
Carlsbad, CA, USA). The above process was repeated once.
Subsequently, 0.5% collagenase type I (Sigma-Aldrich) was added at
37°C for 15 min, after which the tissues were centrifuged at 1,000
× g for 5 min and washed twice with RPMI-1640 medium containing 10%
fetal calf serum. The cells were then transferred to a 25
cm2 culture flask and cultured in a CO2
incubator (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C.
Transfection and transfection efficiency
analysis
For the upregulation or silencing of SIRT1,
lentiviral particles to induce the overexpression of SIRT1 and
shRNA specific to SIRT1 to silence SIRT1 were synthesized by
Shanghai GeneChem Co., Ltd. (Shanghai, China). A lentiviral
overexpression vector (LV-SIRT1) and shRNA targeting SIRT1
(shRNA-SIRT1) were used to induce the overexpression of the
silencing of SIRT1, respectively. The shRNA-SIRT1 sequence was
5′-GAUGAAGUUGACCUCCUCATT-3′. The cells were seeded in 6-well plates
until they grew to approximately 80% coverage, and solutions of the
viral particles equal to a multiplicity of infection (MOI) of 80
were then pre-mixed with RPMI-1640 medium and added to the 6-well
plates. After 12 h, the medium was replaced with fresh medium.
After a further 48 h, the cells were observed under a fluorescence
microscope (Olympus CKX41SF; Olympus Corp., Tokyo, Japan), in order
to determine the percentage of cells synthesizing green fluorescent
protein (GFP). The ratio of the number of cells that emit green
fluorescence to the same field of view is the transfection
efficiency. We then detected the protein expression of SIRT1 in the
transfected mouse AECs-II by western blot analysis.
Exposure of cells to PQ
The AECs-II were either exposed to various
concentrations of PQ (0, 200, 400, 800 and 1000 μM) for 24 h
or to 800 μM PQ for various periods of time (6, 12, 24 and
48 h). The control cells were untreated cells.
Western blot analysis
After harvesting, the mouse AECs-II were lysed on
ice using lysis buffer (Beyotime Biotechnology, Jiangsu, China) for
30 min. Subsequently, the cell lysates were centrifuged at 16,000 ×
g for 15 min at 4°C. The supernatants were collected and the
protein concentration was detected using the bicinchoninic acid
(BCA) method. Equal amounts of protein (40 μg/lane) were
loaded onto a polyacrymide gel and run at 80 V for 1 h, followed by
a 120 V run for 30 min, and then transferred to polyvinylidene
fluoride (PVDF) membranes (0.45 μm; Millipore, Billerica,
MA, USA) by electroblotting. After blocking with 5% skimmed
powdered milk for 2 h, the membranes were correspondingly incubated
with anti-SIRT1 (1:1,000), anti-NRF2 (1:1,000) or anti-GAPDH
(1:5,000) antibodies overnight at 4°C. The membranes were then
washed in Tris-buffered saline-Tween-20 (TBST) 3 times for 10 min
each and further incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibodies (Biogot
Technology, Co., Ltd., Shanghai, China) for 2 h at room temperature
and detected using an ECL detection system (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Determination of SOD and catalase (CAT)
activities, as well as glutathione (GSH) and malondialdehyde (MDA)
levels
Following exposure to PQ, either supernatant or cell
samples were collected. The SOD and CAT activities were detected
according to the manufacturer's instructions (provided with the SOD
and CAT assay kits; Jiancheng Bioengineering Institute, Nanjing,
China). We also measured the MDA and GSH levels following the
instructions provided by the manufacturer (Jiancheng Bioengineering
Institute). The samples were analyzed using a spectrophotometer.
The SOD and CAT activities were expressed in U/mgprot. The GSH
levels were expressed in μmol/gprot and the MDA levels were
expressed in nmol/mgprot.
Determination of HO-1 activity by
ELISA
The cell supernatants were harvested following
exposure of the cells to PQ. HO-1 activity was measured using
commercially available enzyme-linked immunosorbent assay (ELISA)
kits (Westang Biotechnology, Shanghai, China). ELISA was performed
strictly according to the manufacturer's instructions. The samples
were analyzed using a spectrophotometer. HO-1 activity was
expressed in μg/gprot.
Analysis of cell apoptosis
The cell apoptotic rate was detected using a FACScan
flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA) with an
Annexin V-FITC/propidium iodide (PI) apoptosis detection kit
(Nanjing Keygen Biotechnology Co. Ltd., Nanjing, China). Following
exposure to PQ, the cells were harvested, washed with ice-cold PBS
and then re-suspended in 500 μl binding buffer at a
concentration of 1×106 cells/ml. Subsequently, the cells
were stained with 5 μl of Annexin V-FITC and 5 μl of
PI, followed by incubation for 15 min at room temperature in the
dark. Finally, apoptosis was assessed by flow cytometry.
Analysis of protein stability
The cells were seeded in 6-well plates and exposed
to 800 μM PQ for 24 h. Subsequently, the culture solution
was changed to cycloheximide solution (CHX Sigma-Aldrich) at a
concentration of 100 g/ml and treated for 0, 6, 12 and 24 h. The
treated cells were collected and processed for western blot
analysis following the removal of cycloheximide.
Detection of acetylated NRF2 expression
in cells
After harvesting, the whole cell lysates were
prepared using non-denaturing lysis buffer (Thermo Fisher
Scientific) on ice and immunoprecipitation was performed with the
whole cell lysates. Briefly, the lysates were incubated with 1
μg each of either normal rabbit IgG (Cell Signaling
Technology) or anti-NRF2 at 4°C with gentle rotation overnight and
collected by incubating with Protein A/G Plus Agarose (Thermo
Fisher Scientific). The agarose beads were washed and then boiled
for 10 min with 1X SDS loading buffer followed by centrifugation at
1,000 × g for 1 min. The immunocomplexes were then detected by
western blot analysis using anti-acetyl-lysine antibody (#9441;
Cell Signaling Technology).
Statistical analysis
All data are presented as the means ± SD and were
analyzed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA) was
used to prepare the figures. The comparisons between groups were
analyzed using one-way ANOVA. A p-value <0.05 was considered to
indicate a statistically significant difference.
Results
Transfection efficiency analysis
As shown in Fig.
1A, the cells in the negative control group cells did not
express green fluorescence. Under the condition of a multiplicity
of infection (MOI) of 20, the transfection efficiency of the
lentivirus in the mouse AECs-II is very low. When the MOI was
increased to 80, the transfection efficiency of the lentivirus in
the mouse AECs-II increased by >80%. Thus, in all the
experiments, the lentivirus was transfected at an MOI of 80. As
shown in Fig. 1B, compared with
the control group, SIRT1 protein expression in the shRNA-SIRT1
group decreased significantly, while that in the LV-SIRT1 group
significantly increased (all p<0.05).
PQ induces the apoptosis of mouse
AECs-II
The proportion of early apoptotic and late apoptotic
cells was counted. As shown in Fig.
2, following exposure to increasing concentrations of PQ, the
proportion of early apoptotic cells increased; however, when the
concentration reached 1000 μM, the percentage of late
apoptotic cells increased significantly more than the proportion of
early apoptotic cells at the same time. Similarly, in the groups of
cells exposed to a PQ concentration of 800 μM for different
periods of time, the proportion of apoptotic cells also increased
in a time-dependent manner.
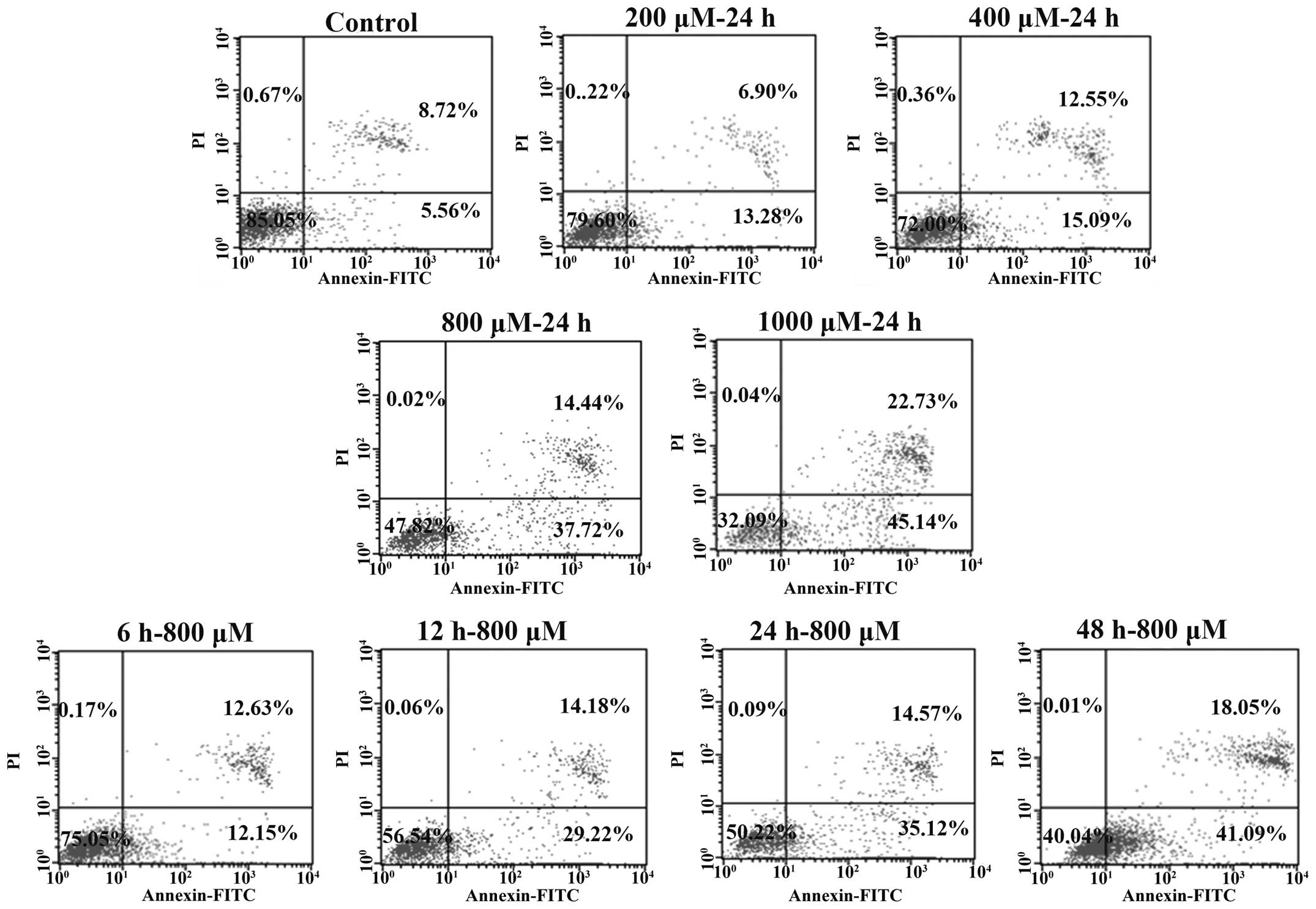 | Figure 2Paraquat (PQ)-induced apoptosis of
mouse type II alveolar epithelial cells (AECs-II). The mouse
AECs-II were treated with various concentrations of PQ (0, 200,
400, 800 and 1000 μM; top and middle panels) for 24 h, and
also treated with 800 μM PQ for 6, 12, 24 and 48 h (bottom
panels). The apoptosis of the cells was detected using a
FACSCalibur flow cytometer. The 4 quadrants indicate the following:
left upper quadrant, mechanically broken cells; left lower
quadrant, surviving cells; upper right quadrant, late apoptotic
cells; lower right quadrant, early apoptotic cells. |
Protein expression of SIRT1 and NRF2 in
mouse AECs-II following exposure to PQ
To assess the potential roles of SIRT1 and NRF2 in
PQ-induced lung injury, the protein levels of SIRT1 and NRF2 were
measured by western blot analysis in the mouse AECs-II following
exposure to various concentrations of PQ for 24 h (Fig. 3A) and exposure to 800 μM PQ
for different periods of time (Fig.
3B). The results revealed that exposure of the cells to low
concentations (200 and 400 μM) of PQ upregulated the protein
expression of SIRT1 and NRF2. With the increasing concentations
(800 and 1000 μM) of PQ, the protein expression of SIRT1 and
NRF2 gradually decreased. Furthermore, during the short periods of
exposure to PQ, the protein expression of SIRT1 and NRF2 was
significantly elevated and peaked at 12 h. As the duration of
exposure to PQ was extended, the protein expression of SIRT1 and
NRF2 gradually decreased.
Effects of PQ on SOD, CAT and HO-1
activities, as well as on GSH and MDA levels in mouse AECs-II
Following exposure to low concentrations of PQ (200
and 400 μM) for 24 h (Fig.
4A–D), the SOD, CAT and HO-1 activities, as well as the GSH
levels significantly increased compared with the control groups. As
the PQ concentration increased (800 and 1000 μM), the
activities of SOD, CAT and HO-1, as well as the levels of GSH
gradually decreased. The levels of MDA (Fig 4E) in the cells increased in a PQ
concentration-dependent manner.
Following a 12-h challenge with 800 μM PQ
(Fig. 4F–H), the GSH levels and
CAT activity significantly increased compared with the untreated
control cells, reaching peak levels. SOD activity reached a peak at
6 h. With the extension of the duration of exposure to PQ, the
activities of SOD and CAT, as well as the GSH levels were markedly
decreased compared with the control groups. The levels of MDA and
HO-1 (Fig. 4I and J) in the cells
increased in a time-dependent manner.
Overexpression of SIRT1 inhibits the
apoptosis of AECs-II
Following the knockdown or upregulation of SIRT1
expression, the mouse AECs-II were challenged with 800 μM PQ
for 24 h. The overexpression of SIRT1 significantly decreased the
cell apoptotic rate. On the contrary, the downregulation of SIRT1
enhanced the apoptotic rate of the cells (Fig. 5A–D).
SIRT1 protein suppresses PQ-induced
oxidative stress in mouse AECs-II
Compared with normal conditions, SOD and CAT
activity, as well as the GSH levels were markedly inhibited in the
mouse AECs-II following exposure to 800 μM PQ (Fig. 5E–G). The silencing of SIRT1 by
shRNA targeting SIRT1 (shRNA-SIRT1) suppressed the activity of SOD
and CAT, as well as the GSH levels to an even greater extent
compared with the cells epxosed to PQ and not transfected with
shRNA, whereas the overexpression of SIRT1 using a SIRT1
overexpression vector (LV-SIRT1) markedly increased the
activity/levels of these enzymes (Fig. 5E–G). Conversely, the MDA levels
and HO-1 activity (Fig. 5H and I)
were notably elevated in the mouse AECs-II following exposure to
PQ. The silencing of SIRT1 increased the expression of MDA, whereas
the upregulation of SIRT1 had the opssosite effect. However, the
overexpression of SIRT1 promoted HO-1 activation and upregulated
its expression.
SIRT1 protein expression upregulates NRF2
protein expression
The mouse AECs-II in which SIRT1 was either
overexpressed or knocked down were exposed to PQ and harvested at
24 h. As shown in Fig. 6A and B,
the overexpression of SIRT1 promoted the expression of NRF2,
whereas the knockdown of SIRT1 blocked NRF2 activation. NRF2
expression correlated with the levels of SIRT1.
 | Figure 6Silent information regulator
2-related enzyme 1 (SIRT1) protein upregulates the expression and
stability of nuclear factor E2-related factor 2 (NRF2) protein by
deacetylating NRF2. Mouse type II alveolar epithelial cells
(AECs-II) were treated with 800 μM paraquat (PQ) for 24 h
after successful transfection. (A and B) The protein expression
levels of SIRT1 and NRF2 were then measured by western blot
analysis. (E–I) Transfected mouse AECs-II were treated with 100
μg/ml of cycloheximide (CHX) for 0, 6, 12 and 24 h following
exposure to PQ. The protein stability of NRF2 was measured by
western blot analysis. (E) Control group; (F) PQ group; (G) PQ +
shRNA-SIRT1 group; (H) PQ + LV-SIRT1 group. (C and D) Following
successful transfection, the NRF2 protein was immunoprecipitated
with anti-NRF2 antibody. The acetylated NRF2 protein was detected
by western blot analysis using anti-acetyl-lysine antibody. Lane 1,
Control group; lane 5, shRNA-SIRT1, group; lane 6, LV-SIRT1 group.
The values are presented as the means ± SD (n=4 experiments).
*p<0.05, statistically significant difference when
compared with the control group; #p<0.05,
statistically significant difference when compared with the PQ
group. Lane or bar 1, control group; lane or bar 2, PQ group; lane
or bar 3, PQ + shRNA-SIRT1 group; lane or bar 4, PQ + LV-SIRT1
group; lane or bar 5, shRNA-SIRT1 group; lane or bar 6, LV-SIRT1
group. |
SIRT1 protein enhances the stability of
NRF2 protein
The stability of NRF2 protein expression gradually
decreased following exposure of the cells to PQ for 6 h. In the
control group (Fig. 6E and I),
the expression of NRF2 decreased by 24.7 and 42.51% at 12 and 24 h,
respectively. In the PQ group (Fig.
6F and I), NRF2 expression decreased by 53.77 and 71.6%,
following treatment with CHX for 12 and 24 h, respectively. In the
PQ + shRNA-SIRT1 group (Fig. 6G and
I), the downregulation of SIRT1 significantly damaged the
stability of NRF2, with the decrease in expression reaching 71.7
and 74.8% at 12 and 24 h, respectively. In the PQ + LV-SIRT1 group
(Fig. 6H and I), the upregulation
of SIRT1 protein expression played a negative role in the
degradation of NRF2 protein, with the decline only reaching 41.0
and 47.9% at the corresponding time points.
SIRT1 protein deacetylates NRF2 protein
in mouse AECs-II
To determine whether SIRT1 plays a vital role in the
acetylation of NRF2 protein, the levels of acetylated NRF2 in the
cells were detected. Our data (Fig.
6C and D) revealed that the NRF2 protein acetylation level was
markedly upregulated by the depletion of SIRT1, whereas the
overexpression of SIRT1 reduced the NRF2 acetylation level.
Discussion
Previous studies have reported that PQ-induced
pulmonary toxicity directly results in bronchial toxicity,
inflammation and oxidative stress (18,19,20). It has been reported that excessive
amounts of ROS-mediated oxidative stress play an important role in
the process of PQ-induced pulmonary injury (21,22). PQ is an influential inducer of ROS
and may prompt the generation of ROS, such as superoxide anions, as
well as hydroxyl and peroxyl radicals (1,23).
After cellular uptake, PQ has been found to target the mitochondria
and through cyclic reduction-oxidation reactions, causes an
increase in intracellular ROS generation, which then leads to the
apoptosis of cells (10,24). The findings of the present study
revealed that exposure of the cells to high concentrations of PQ
induced a significant increase in the MDA content, whereas the
activities of SOD and CAT were decreased, which was consistent with
the findings of a previous study (25).
In a previous study, NRF2 was considered as an
effective target for the antioxidant therapy of PQ poisoning
(15). The levels of oxidative
stress were assessed by measuring the levels/activity of MDA, SOD,
GSH and CAT, which were also associated with its anti-apoptotic
properties (26). It has been
proven that NRF2 expression maintains the balance of the
oxidant-antioxidant system and inhibits inflammation, as well as
apoptotic factors, which are associated with an anti-apoptotic
effect on cell injury induced by PQ (27). As NRF2 may activate cellular
responses through enhanced transcription by antioxidant response
element (ARE), this leads to increases in the expression levels of
antioxidant enzymes. including CAT, SOD, GSH and glutathione
peroxidase (GPx) (15,28). It has been demonstrated that the
ability to eliminate electrophiles and free radicals is enhanced by
these defensive proteins, which collectively promotes the
detoxification of ROS, and increases the capacity of cells to
resist apoptosis (12,29). Previous studies have confirmed
that the depletion of NRF2 by siRNA down-regulates the expression
of SOD and upregulates the levels of MDA and apoptotic factors,
including phosphorylated p53, Bax and the active form of caspase-3,
which significantly enhances PQ-induced oxidative damage in cells
in vitro (18,30). Moreover, NRF2 enhances the
inhibitory effects of HO-1 on the intracellular production of ROS
(31). Additionally, it has been
demonstrated that the overexpression of NRF2 effectively alleviates
PQ-induced lung injury and cell apoptosis (32). In the present study, we verified
that NRF2 overexpression induced by transfection with LV-SIRT1
played an important protective role by reducing the cell apoptosis
induced by PQ. This study provides evidence of the involvement of
the NRF2 signaling pathway in the antioxidant effects exerted by
SIRT1, thereby improving the endogenous cellular anti-apoptotic
system.
In unstressed cells, NRF2 has been found to be
sequestered by its inhibitor, Keap1, thereby promoting rapid
proteasome-mediated degradation (33). However, in response to oxidative
stress, NRF2 dissociates from Keap1, through modifications to the
Keap1-NRF2 complex, such as ubiquitination, proteasomal degradation
or phosphorylation (34). NRF2
has been found to be regulated by acetylation; the results of a
previous study indicated that NRF2 acetylation at lysine residues
in the Neh1 DNA-binding domain was responsible for NRF2
transactivation rather than for stability (35). However, a previous study also
demonstrated that the downregulation of histone deacetylase (HDAC)2
may cause the impaired functioning of NRF2, which leads to the
reduced expression of the antioxidant responsive genes and thus, it
was concluded that HDAC2 may regulate NRF2 activity through
deacetylation (36). In this
study, we found that SIRT1 deacetylated NRF2 in cells, thus
increasing its stability, resulting in the enhanced expression of
antioxidant genes and decreased sensitivity to oxidative
stress.
It is well known that sirtuins are involved in
various aspects of biological processes through deacetylation, such
as the regulation of gene expression, cellular stress response and
DNA repair (37). There is a
close association between SIRT1 expression and oxidative stress.
Cigarette smoke-mediated oxidative stress has been reported to
decrease SIRT1 protein levels and activity in the lungs of rats and
human beings (4,38). In the present study, SIRT1
overexpression was associated with a high level of GSH, as well as
SOD and CAT activities, the downstream genes of NRF2-anti-ARE,
whereas the depletion of SIRT1 markedly increased the activity of
MDA and HO-1, which suggested that SIRT1 protein may play an
important role in mediating the oxidant-antioxidant balance in
PQ-induced pulmonary injury (39)
and enhanced cell resistance to oxidative stress. In a previous
study of ours (40), resveratrol,
an activator of SIRT1, increased the SIRT1 and NRF2 levels, and
through the activation of the NRF2-ARE pathway led to the
suppression of ROS production.. In that study, we demonstrated that
PQ stimulated the compensatory overexpression of SIRT1 and NRF2,
whereas exposure to PQ for a longer period of time significantly
decreased the expression of SIRT1, as well as NRF2 in the lungs of
mice. Additionally, treatment with resveratrol not only upregulated
the expression of SIRT1 and NRF2, but also increased the activities
of HO-1, SOD and CAT. The depletion of SIRT1 has also been shown to
block NRF2-ARE pathway activation and reverse the antioxidative
capacity (15). In this study,
our results revealed that the upregulation of SIRT1 expression
markedly increased the expression of NRF2, whereas the knockdown of
SIRT1 had a negative influence on the levels of NRF2 expression.
Therefore, we hypothesized that SIRT1 protein would alleviate
PQ-induced oxidative stress through the NRF2 pathway. It has
previously been confirmed that SIRT1 promotes nuclear accumulation,
DNA binding and the transcriptional activities of NRF2 and promotes
the expression of the NRF2 downstream genes, HO-1, and SOD,
resulting in the reduction of ROS levels in a deacetylase-dependent
manner (14). Importantly, SIRT1
is a redox-sensitive deacetylase that is post-translationally
modified by oxidants and carbonyl stress (4). We found that the upregulation of
SIRT1 caused NRF2 deacetylation, resulting in the increased
stability and activity of NRF2, thus causing the suppression of
apoptosis and resistance to oxidative stress.
Collectively, our results demonstrated that SIRT1
enhanced and stabilized NRF2 protein expression. We demonstrated
that SIRT1 was associated with NRF2 and prevents its degradation
possibly through the deacetylation of various residues. As the
reduced SIRT1 activity is observed in PQ poisoning due to oxidative
stress, this may consequently reduce the activity of NRF2, thus
inhibiting an increase in the expression of antioxidant enzymes;
this in turn increases oxidative stress, which then further reduces
SIRT1 activity; this process is an ongoing vicious circle. SIRT1
overexpression in cells may upregulate Nrf2 expression by
increasing Nrf2 deacetylation, resulting in the enhancement of
normal antioxidant defences.
Acknowledgments
This study was supported by the State Key Program of
Natural Science Foundation of Zhejiang province (no. LZ12H26001),
and the Medical and Health Research Program of Zhejiang province
(no. 2012ZDA034).
References
|
1
|
Gawarammana IB and Buckley NA: Medical
management of paraquat ingestion. Br J Clin Pharmacol. 72:745–757.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shao X and Chen JH: Progress on
pathogenesis and treatment of paraquat-induced pulmonary fibrosis.
Zhejiang Da Xue Xue Bao Yi Xue Ban. 43:717–727. 2014.In
Chinese.
|
|
3
|
He X, Wang L, Szklarz G, Bi Y and Ma Q:
Resveratrol inhibits paraquat-induced oxidative stress and
fibrogenic response by activating the nuclear factor erythroid
2-related factor 2 pathway. J Pharmacol Exp Ther. 342:81–90. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caito S, Rajendrasozhan S, Cook S, Chung
S, Yao H, Friedman AE, Brookes PS and Rahman I: SIRT1 is a
redox-sensitive deacetylase that is post-translationally modified
by oxidants and carbonyl stress. FASEB J. 24:3145–3159. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salminen A, Kaarniranta K and Kauppinen A:
Crosstalk between oxidative stress and SIRT1: impact on the aging
process. Int J Mol Sci. 14:3834–3859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao H, Sundar IK, Huang Y, Gerloff J,
Sellix MT, Sime PJ and Rahman I: Disruption of sirtuin 1-mediated
control of circadian molecular clock and inflammation in chronic
obstructive pulmonary disease. Am J Respir Cell Mol Biol.
53:782–792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chun P: Role of sirtuins in chronic
obstructive pulmonary disease. Arch Pharm Res. 38:1–10. 2015.
View Article : Google Scholar
|
|
8
|
Yao H, Hwang JW, Sundar IK, Friedman AE,
McBurney MW, Guarente L, Gu W, Kinnula VL and Rahman I: SIRT1
redresses the imbalance of tissue inhibitor of matrix
metalloproteinase-1 and matrix metalloproteinase-9 in the
development of mouse emphysema and human COPD. Am J Physiol Lung
Cell Mol Physiol. 305:L615–L624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong GL, Cai QQ, Tan JP, Jiang XZ, Zhao
GJ, Wu B, Li MF, Qiu QM and Lu ZQ: Mifepristone-inducible
recombinant adenovirus attenuates paraquat-induced lung injury in
rats. Hum Exp Toxicol. 34:32–43. 2015. View Article : Google Scholar
|
|
10
|
Hong GL, Liu JM, Zhao GJ, Wang L, Liang G,
Wu B, Li MF, Qiu QM and Lu ZQ: The reversal of paraquat-induced
mitochondria-mediated apoptosis by cycloartenyl ferulate, the
important role of Nrf2 pathway. Exp Cell Res. 319:2845–2855. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dinkova-Kostova AT: The role of sulfhydryl
reactivity of small molecules for the activation of the KEAP1/NRF2
pathway and the heat shock response. Scientifica (Cairo).
2012:6061042012.
|
|
12
|
Giudice A, Arra C and Turco MC: Review of
molecular mechanisms involved in the activation of the Nrf2-ARE
signaling pathway by chemopreventive agents. Methods Mol Biol.
647:37–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Motohashi H and Yamamoto M: Nrf2-Keap1
defines a physiologically important stress response mechanism.
Trends Mol Med. 10:549–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang K, Huang J, Xie X, Wang S, Chen C,
Shen X, Liu P and Huang H: Sirt1 resists advanced glycation end
products-induced expressions of fibronectin and TGF-β1 by
activating the Nrf2/ARE pathway in glomerular mesangial cells. Free
Radic Biol Med. 65:528–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang K, Chen C, Hao J, Huang J, Wang S,
Liu P and Huang H: Polydatin promotes Nrf2-ARE anti-oxidative
pathway through activating Sirt1 to resist AGEs-induced
upregulation of fibronetin and transforming growth factor-β1 in rat
glomerular messangial cells. Mol Cell Endocrinol. 399:178–189.
2015. View Article : Google Scholar
|
|
16
|
Potteti HR, Rajasekaran S, Rajamohan SB,
Tamatam CR, Machireddy N and Reddy SP: Sirtuin 1 promotes
hyperoxia-induced lung epithelial death independent of NRF2
activation. Am J Respir Cell Mol Biol. Oct 14–2015.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai Q, Lu Z, Hong G, Jiang X, Wu Z, Zheng
J, Song Q and Chang Z: Recombinant adenovirus Ad-RUNrf2 reduces
paraquat-induced A549 injury. Hum Exp Toxicol. 31:1102–1112. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu H, Chang Z, Han W, Wang L and Hong G:
Curcumin reduces paraquat-induced oxidative injury in A549 cells by
activation of the Nrf2-ARE pathway. Zhonghua Lao Dong Wei Sheng Zhi
Ye Bing Za Zhi. 32:44–49. 2014.PubMed/NCBI
|
|
19
|
Dinis-Oliveira RJ, Pontes H, Bastos ML,
Remião F, Duarte JA and Carvalho F: An effective antidote for
paraquat poisonings: the treatment with lysine acetylsalicylate.
Toxicology. 255:187–193. 2009. View Article : Google Scholar
|
|
20
|
Kim YS, Zerin T and Song HY: Antioxidant
action of ellagic acid ameliorates paraquat-induced A549
cytotoxicity. Biol Pharm Bull. 36:609–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang SR, Park JR and Kang KS: Reactive
oxygen species in mesenchymal stem cell aging: implication to lung
diseases. Oxid Med Cell Longev. 2015:p4862632015. View Article : Google Scholar
|
|
22
|
Toygar M, Aydin I, Agilli M, Aydin FN,
Oztosun M, Gul H, Macit E, Karslioglu Y, Topal T, Uysal B and Honca
M: The relation between oxidative stress, inflammation, and
neopterin in the paraquat-induced lung toxicity. Hum Exp Toxicol.
34:198–204. 2015. View Article : Google Scholar
|
|
23
|
Wang X, Luo F and Zhao H: Paraquat-induced
reactive oxygen species inhibit neutrophil apoptosis via a p38
MAPK/NF-κB-IL-6/TNF-α positive-feedback circuit. PLoS One.
9:e938372014. View Article : Google Scholar
|
|
24
|
Dinis-Oliveira RJ, Duarte JA,
Sánchez-Navarro A, Remião F, Bastos ML and Carvalho F: Paraquat
poisonings: mechanisms of lung toxicity, clinical features, and
treatment. Crit Rev Toxicol. 38:13–71. 2008. View Article : Google Scholar
|
|
25
|
Senator A, Rachidi W, Lehmann S, Favier A
and Benboubetra M: Prion protein protects against DNA damage
induced by paraquat in cultured cells. Free Radic Biol Med.
37:1224–1230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Zhang Y, Li H, Xu W, Chu K, Chen
L and Chen X: Antioxidant and anti-excitotoxicity effect of Gualou
Guizhi decoction on cerebral ischemia/reperfusion injury in rats.
Exp Ther Med. 9:2121–2126. 2015.PubMed/NCBI
|
|
27
|
Jiang XZ, Song Q, Xu XP, Cai QQ, Hong GL,
Liang H and Lu ZQ: The effects of Nrf2 gene expression induced by
RU486 at different doses on A549 cell damage induced by paraquat.
Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 30:268–272. 2012.In
Chinese. PubMed/NCBI
|
|
28
|
Lee HJ, Han J, Jang Y, Kim SJ, Park JH,
Seo KS, Jeong S, Shin S, Lim K, Heo JY and Kweon GR:
Docosahexaenoic acid prevents paraquat-induced reactive oxygen
species production in dopaminergic neurons via enhancement of
glutathione homeostasis. Biochem Biophys Res Commun. 457:95–100.
2015. View Article : Google Scholar
|
|
29
|
Giudice A and Montella M: Activation of
the Nrf2-ARE signaling pathway: a promising strategy in cancer
prevention. Bioessays. 28:169–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Varì R, Scazzocchio B, Santangelo C,
Filesi C, Galvano F, D'Archivio M, Masella R and Giovannini C:
Protocatechuic acid prevents oxLDL-induced apoptosis by activating
JNK/Nrf2 survival signals in macrophages. Oxid Med Cell Longev.
3518272015.
|
|
31
|
Ci X, Lv H, Wang L, Wang X, Peng L, Qin FX
and Cheng G: The antioxidative potential of farrerol occurs via the
activation of Nrf2 mediated HO-1 signaling in RAW 264.7 cells. Chem
Biol Interact. 239:192–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He XY, Zhao GJ, Lu ZQ, Hong GL, He F,
Liang H, Qiu QM and Li JR: Oxidative stress of acute paraquat
poisoned rats and sodium dimercaptopropane sulfonate intervention.
Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 27:476–479. 2009.In
Chinese.
|
|
33
|
Kwak MK, Wakabayashi N, Itoh K, Motohashi
H, Yamamoto M and Kensler TW: Modulation of gene expression by
cancer chemopreventive dithiolethiones through the Keap1-Nrf2
pathway. Identification of novel gene clusters for cell survival. J
Biol Chem. 278:8135–8145. 2003. View Article : Google Scholar
|
|
34
|
Baird L, Llères D, Swift S and
Dinkova-Kostova AT: Regulatory flexibility in the Nrf2-mediated
stress response is conferred by conformational cycling of the
Keap1-Nrf2 protein complex. Proc Natl Acad Sci USA.
110:15259–15264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Z, Chin YE and Zhang DD: Acetylation
of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2
during the antioxidant response. Mol Cell Biol. 29:2658–2672. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mercado N, Thimmulappa R, Thomas CM,
Fenwick PS, Chana KK, Donnelly LE, Biswal S, Ito K and Barnes PJ:
Decreased histone deacetylase 2 impairs Nrf2 activation by
oxidative stress. Biochem Biophys Res Commun. 406:292–298. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang WJ, Wang DL and Zhu WG: Mechanism of
regulating deacetylase SIRT1 expression and activity. Yi Chuan.
32:1003–1008. 2010.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao H, Sundar IK, Ahmad T, Lerner C,
Gerloff J, Friedman AE, Phipps RP, Sime PJ, McBurney MW, Guarente L
and Rahman I: SIRT1 protects against cigarette smoke-induced lung
oxidative stress via a FOXO3-dependent mechanism. Am J Physiol Lung
Cell Mol Physiol. 306:L816–L828. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tyagi N, Kumari A, Dash D and Singh R:
Protective effects of intranasal curcumin on paraquot-induced acute
lung injury (ALI) in mice. Environ Toxicol Pharmacol. 38:913–921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li S, Zhao G, Chen L, Ding Y, Lian J, Hong
G and Lu Z: Resveratrol protects mice from paraquat-induced lung
injury: The important role of SIRT1 and NRF2 antioxidant pathways.
Mol Med Rep. 13:1833–1838. 2016.
|




















