Introduction
Epidural fibrosis (EF), which causes spinal epidural
adhesion and scarring, is frequently found in the epidural space
and contributes greatly to postoperative pain and recurrent lumbar
disc herniation after a laminectomy (1,2).
Various agents or mechanical barriers for preventing EF have been
studied both in animal models and humans, including autologous fat
grafts, Adcon-L, polytetrafluoroethylene membranes, and
fibrinolytic agents (3). However,
it is still difficult to treat patients with established spinal
EF.
EF formation is known to be a complicated process,
involving hypernomic proliferation of fibroblasts, the increased
expression of inflammatory cytokines, the exorbitant production of
collagens and other changes in cellular and molecular components
(4,5). After laminectomy, fibrous connective
tissues adjacent to the laminar traumas are activated to repair the
wound; fibroblast proliferation and inflammatory cytokine release
are greatly increased during the repair process (6). As a major cell component involved in
this repair, fibroblasts are driven to excessively proliferate and
produce collagens (mainly Col I) by the increased growth factors
and inflammatory mediators (7).
Transforming growth factor β (TGF-β) is a secreted
protein that controls proliferation, differentiation, metastasis
and other functions in the majority of types of cells (8). TGF-β has been shown to be involved
in the progression of many chronic inflammation-related diseases,
including diabetes, multiple fibrosis/sclerosis and also in scar
formation (9–12). Of the members of the TGF-β family,
TGF-β1 and TGF-β2 are believed to play a central role in the
fibrosis and scar formation in tissues (13). During these processes, these were
upregulated, and induced the production of multiple growth factors,
inflammatory cytokines and collagens (14). A previous study on TGF-β1 and
TGF-β2 expression in mice with spinal injury revealed that TGF-β1
was induced at an early stage while TGF-β2 was induced at a later
stage (15). Another study
demonstrated that expression of TGF-β2 but not TGF-β1 was
correlated with the deposition of scar tissue in the spinal lesion
(16). These studies revealed
that TGF-β2 plays a pivotal role in the recovery of patients with
spinal lesions.
Sirtuins (SIRTs) are a highly conserved
NAD+-dependent deacetylase family that plays multiple
roles in metabolism, cell apoptosis, cell fate determination and
lifespan regulation (17). As an
important member of the SIRT family, SIRT6 has been proven to
deacetylate many important proteins involved in physiological and
pathological processes, and plays a role in DNA repair, metabolism
and aging (18). Depletion of
SIRT6 has been shown to result in the suppression of cell
apoptosis, cellular senescence, DNA damage, and telomere
dysfunction in many types of cells (19–21). However, the role and mechanism of
SIRT6 in spinal EF have not been studied in depth.
In the present study, we found that SIRT6 expression
was significantly reduced the lumbar disc of the patients in whom
an epidural scar formed, displaying an opposite expression pattern
to TGF-β2. Overexpression of SIRT6 in primary epidural fibroblasts
suppressed cell proliferation, TGF-β2 and interleukin-1α (IL-1α)
expression, and Col I production. Our results of the bioinformatics
and molecular biological analyses revealed that SIRT6
overexpression suppressed TGF-β2 levels through inducing the
expression of microRNA-21 (miR-21). Finally, a study of pcDNA-SIRT6
vector injection indicated that SIRT6 could suppress EF and
epidural scar formation in vivo.
Patients and methods
Patients and sampling
This study enrolled 96 post-laminectomy patients
with lumbar disc herniation, of which 48 patients developed an
epidural scar and the other 48 patients did not develop an epidural
scar. Their lumbar disc tissues were sampled during the
laminectomy, and the removed tissues were collected, labeled and
saved at −80°C for future use. This study was approved by the
Ethics Committee of Xi'an Red Cross Hospital (Xi'an, China). All
participants provided written informed consent.
Cell culture and transfection
Samples of epidural scar tissues were taken, and
human primary epidural fibroblasts were isolated from these as
described previously (22). The
primary fibroblasts were obtained from the epidural scars using the
enzymatic digestion method in a sterile superclean bench. A total
of 5 mg obtained tissues were cut into small pieces (approximately
1 mm3) and mixed with 3 ml 0.2% collagenase II. The
mixture was incubated at 37°C in a water bath for 50 min. An equal
volume of Dulbecco's modified essential medium (DMEM)/F12 with
fetal bovine serum (FBS; Invitrogen, Grand Island, NY, USA) was
added to terminate the digestion. The mixture was then transferred
to sterile tubes and centrifuged at 500 × g for 7 min. We
resuspended the precipitate with 2 ml medium and repeated the
centrifugation and resuspension twice. The isolated cells were
incubated in DMEM (Life Technologies, Gaithersburg, MD, USA)
containing 10% FBS, 100 µg/ml streptomycin and penicillin.
Fibroblasts from passages 4 to 6 were used in the subsequent
experiments. The cells were incubated in a humidified incubator
with an atmosphere of 95% air and 5% CO2 at 37°C. On
reaching 80% confluence, 1 µg pcDNA-SIRT6 or 1 µg
pcDNA3.1 empty vector (vector), 6 pmol miR-21 mimic or inhibitor
were transfected into the fibroblasts with
Lipofectamine® 3000 (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's instructions.
The medium was changed every 3 days.
The pcDNA-SIRT6 overexpression vector and empty
vector were kindly presented by Professor David Lohnes (Department
of Cellular and Molecular Medicine, University of Ottawa, Ottawa,
ON, Canada). The 2′-OMe modified miR-21 mimic/inhibitor or NC
mimic/inhibitor were synthesized and confirmed to be effective by
Invitrogen Life Technologies.
Cell proliferation and apoptosis
analyses
Cell proliferation was evaluated using an MTT assay
(Sigma, St. Louis, MO, USA). The cells were seeded in 12-well
culture plates at 5×104/well, and then transfected with
the indicated vectors or siRNA. The cells were incubated for 0, 24,
48 and 72 before adding the MTT reagent to each well at a final
concentration of 0.5 mg/ml and incubated at 37°C for 4 h. After
medium removal, 500 µl dimethylsulfoxide was added to each
well. Viable cells were measured at an absorbance of 550 nm
wavelength using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
In the present study, apoptosis was detected using
annexin V/propidium iodide (PI) dual staining, which was followed
by flow cytometric analysis using a flow cytometer [Cytomics™ FC500
(Beckman Coulter Ltd., Brea, CA, USA)].
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies) following the manufacturer's
instructions. The integrity of RNA was checked by electrophoresis
on 1.0% agarose gel with ethidium bromide staining. qPCR reactions
were carried out in a final volume of 25 µl, using SYBR
Premix Ex Taq (Takara Bio, Dalian, China), 0.4 mM of each primer,
and 200 ng of cDNA template. Primers used in the reactions are as
follows: SIRT6 (F: 5′-GTTAGCCATCAAGACGC-3′, R:
5′-TCAGGGATACAGGGATG-3′); TGF-β2 (F: 5′-ACACTCGCTGCGTACTCAG-3′, R:
5′-AGTCTCTCTTGCTGCTGAC-3′); IL-1α (F: 5′-CTAGCTATCAGGAACATTTAT-3′,
R: 5′-TGCTCATGCCTCGTCCT-3′); β-actin (5′-ACGGGACCTAATGAAACTC-3′, R:
5′-CAAGAAGATGCGGCTGT-3′); pri-, pre- and mature miR-21 stem-loop
primer and the quantitative primers and U6 RNA primers were
designed and produced by Guangzhou RiboBio Co., Ltd. (Guangzhou,
China). Each individual sample was run in triplicate wells. The
reactions were initially denatured at 95°C for 3 min followed by 35
cycles of 95°C for 15 sec, 60°C for 1 min. The change of transcript
abundance of all tested genes was calculated using the
2−ΔΔCt method. mRNA was normalized to β-actin; miR-21
transcripts were normalized to U6 RNA.
Western blotting
A total of 50 µg of protein of each sample
were separated by 12% SDS-PAGE and electro-transferred to a PVDF
membrane (Millipore Corp., Billerica, MA, USA) for western
blotting. The following primary antibodies (purchased from Abcam,
Cambridge, UK) were used: anti-Col I (1:300; ab34710), anti-SIRT6
(1:300; ab88494), anti-TGF-β2 (1:500; ab66045) and anti-β-actin
(1:200; ab189073), which was used as the internal reference. After
incubation with the appropriate horseradish peroxidase-conjugated
secondary antibody (goat anti-rabbit IgG, 1:3,000, ab7090; Abcam),
proteins were detected using chemiluminescence reagent (ECL;
Invitrogen Life Technologies) in a ChemiDoc XRS imaging system and
analyzed with Quantity One software (both from Bio-Rad
Laboratories, Inc.).
ChIP analysis
The regulatory association between SIRT6 and miR-21
was calculated using the online platform ChIPBase (http://deepbase.sysu.edu.cn/chipbase/),
which is an integrated resource for decoding transcription factor
binding maps, expression profiles and the transcriptional
regulation of non-coding RNAs and protein-coding genes from
ChIP-seq data. The targeting association between miR-21 and TGF-β2
was predicted with TargetScan (http://www.targetscan.org/), an online resource.
3′-UTR luciferase reporter assay
The 3′-UTR of TGF-β2 mRNA was amplified by PCR,
using primers linked with the XhoI and NotI
restriction sites, respectively. Both the primers were designed and
synthesized by Genscript Co., Ltd., (Nanjing, China). The PCR
products were excised with NotI and XhoI and inserted
into the pGL-2 vector (Promega, Madison, WI, USA) at the 3′-end of
the Renilla gene CDS. Firefly luciferase activity was used
as the internal control. The 3′-UTR dual-luciferase vectors were
transfected or co-transfected with the miR-21 mimic or pcDNA-TGF-β2
expression vector into the primary fibroblasts (100% confluence)
using X-tremeGENE 9 DNA transfection reagent (Roche, Basel,
Switzerland). The medium was changed 6 h later. Cells were
incubated for another 48 h with commercial cell lysis buffer
(Merck, Whitehouse Station, NJ, USA). The luciferase activity was
measured using a luminometer (Promega) according to the
manufacturer's instructions.
Lumbar disc herniation models and
injection
In the present study, a Xenopus tropicalis
model of lumbar disc herniation was established using the method of
caudal vertebral pulposus transplantation, as described previously
(23). The animals (aged
approximately 12 weeks; purchased from the Laboratory Animal
Center, Beijing Institute of Genetics and Development, Chinese
Academy of Sciences, Beijing, China) underwent a laminectomy and
subsequently those which developed epidural fibrotic scars were
selected and used in the following experiment. The Xenopus
tropicalis were sacrificed using a dissecting needle inserted
into the vertebral foramen. For caudal vertebral pulposus
transplantation, the animals were anaesthesized with 40 mg/kg after
adaptive feeding for 1 week. We cut the tail vertebrae at the root
of the tail (about 1 cm from the anus), and the incision was then
sutured. The caudal nucleus pulposus was removed, and we weighed 10
mg of the pulposus with an analytical balance (Pingxuan Scientific
Instrument Co., Ltd., Shanghai, China). We then cut open the back
of the Xenopus tropicalis, removed the spinous processes and
lamina of L4-L6, and gently set the obtained pulposus on the nerve
root. Finally, the incisions were sutured. The miR-21 inhibitor (1
µg/g body weight) or pcDNA-SIRT6 (5 µg/g body weight)
was locally injected into the animals around the surgical site.
Over a 6-week period, the injection was administered once a day.
Subsequently, they were sacrificed, and their epidural scar tissues
were removed carefully, and the mass of total scars and Col I
tissues was examined using an electronic analytical balance
(Pingxuan Scientific Instrument Co., Ltd.), and staining analyses
were then undertaken.
Masson's and immunohistochemical
staining
Masson's trichrome staining was used to analyze the
collagen fibers in the epidural scars in this study. The tissues
were deparaffinized and rehydrated using 100% alcohol, 95% alcohol,
70% alcohol and then washed in distilled water. For formalin-fixed
tissue, these were re-fixed in Bouin's solution for 1 h at 56°C to
improve staining quality although this step is not absolutely
necessary. They were rinsed under running tap water for 5–10 min to
remove the yellow color and then stained using Weigert's iron
hematoxylin working solution for 10 min. The tissues were rinsed in
running warm tap water for 10 min, washed in distilled water and
stained in Biebrich scarlet-acid fuchsin solution for 10–15 min.
They can thus be saved for future use, and our samples were washed
in distilled water. They were differentiated in
phosphomolybdic-phosphotungstic acid solution for 10–15 min or
until the collagen was not red. The sections were transferred
directly (without rinsing) to aniline blue solution and stained for
5–10 min. They were rinsed briefly in distilled water and
differentiated in 1% acetic acid solution for 2–5 min and
subsequently washed in distilled water. These were dehydrated
rapidly using 95% ethyl alcohol, absolute ethyl alcohol (this step
removes Biebrich scarlet-acid fuchsin staining) and cleared in
xylene. They were mounted with resinous mounting medium. Finally,
the stained samples were observed under a microscope (Senben
Electronic Technology Co., Ltd., Shenzhen, China).
Vimentin immunohistochemical staining was used to
evaluate the degree of inflammation in the epidural scars. Vimentin
in the cells was stained using StreptAvidin-Biotin
Complex-myoglobin (SABC-Mb), and the images were captured using a
CQ-L323 phase contrast microscope (Senben Electronic Technology
Co., Ltd.).
Statistical analysis
All data were obtained from ≥3 independent
experiments. Values are expressed as the means ± standard error of
the means. Statistics were calculated using SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA). Multiple comparisons were assessed by one-way
ANOVA followed by Dunnett's tests. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Expression of SIRT6 is reduced in the
lumbar disc tissues of the patients with an epidural scar
As mentioned in Materials and methods, following
laminectomy, the removed lumbar disc tissues were collected,
labeled and saved at −80°C. Two years later, an investigation was
undertaken to confirm whether each patient had an epidural scar and
whether there had been a relapse of lumbar disc herniation. A total
of 48 scar-free patients and 48 patients who developed an epidural
scar were randomly selected. Expression of SIRT6 and TGF-β2 in
their lumbar disc tissues sampled previously was detected using
western blotting, and the results showed that SIRT6 levels in the
patients with epidural scarring were significantly lower than those
of scar-free patients (Fig. 1A).
By contrast, TGF-β2 expression in the patients who suffered an
epidural scar was much higher than that of scar-free patients
(Fig. 1B). These results
suggested that lumbar disc SIRT6 expression is associated with
epidural scar formation and also TGF-β2 expression.
SIRT6 suppresses fibroblast
proliferation, TGF-β2 and IL-1α expression, and Col I
production
In order to explore the role of SIRT6 in EF and scar
formation in vitro, epidural scar samples were taken from
the patients, and primary fibroblasts were isolated using the
collagenase I digestion method. A pcDNA-SIRT6 expression vector was
built and transfected into the primary cells. After incubation for
72 h, the cells were stained with PI/annexin V followed by flow
cytometric analysis. The results showed that the proportion of
viable cells was reduced by pcDNA-SIRT6 transfection but there were
no marked changes in the proportion of early apoptotic cells among
the groups (Fig. 2A). MTT
analysis also demonstrated that pcDNA-SIRT6 transfection reduced
the viability of the cells (Fig.
2B). The levels of TGF-β2 and Col I proteins, as well as IL-1α
mRNA, were determined by western blotting and qPCR, respectively.
The data indicated that SIRT6 overexpression caused a significant
decrease in TGF-β2 and Col I protein levels (Fig. 2C) and the IL-1α mRNA level
(Fig. 2D). These data indicated
that SIRT6 suppressed EF, which manifested as suppression in
fibroblast proliferation, TGF-β2 and IL-1α expression, as well as
Col I production.
TGF-β2 is a target of miR-21, and SIRT6
promotes the expression of miR-21 in primary fibroblasts
Subsequently, the mechanism of SIRT6 suppression of
EF was investigated. Bioinformatics analysis using TargetScan
revealed that there was a complete match between the miR-21 seed
sequence and the 3′UTR of TGF-β2 mRNA, and the match was highly
conserved among species (Fig. 3A and
B). In order to validate the target relationship, the full
length of the 3′UTR of TGF-β2 mRNA was inserted into a pGL3 vector
and a luciferase reporter gene assay was performed. The results
showed that the miR-21 mimic significantly decreased the
fluorescence intensity, while the decrease was rescued by
pcDNA-TGF-β2 transfection (Fig.
3C). In primary fibroblasts, miR-21 mimic transfection caused a
marked reduction in TGF-β2 and Col I protein expression (Fig. 3D). Subsequently, the relationship
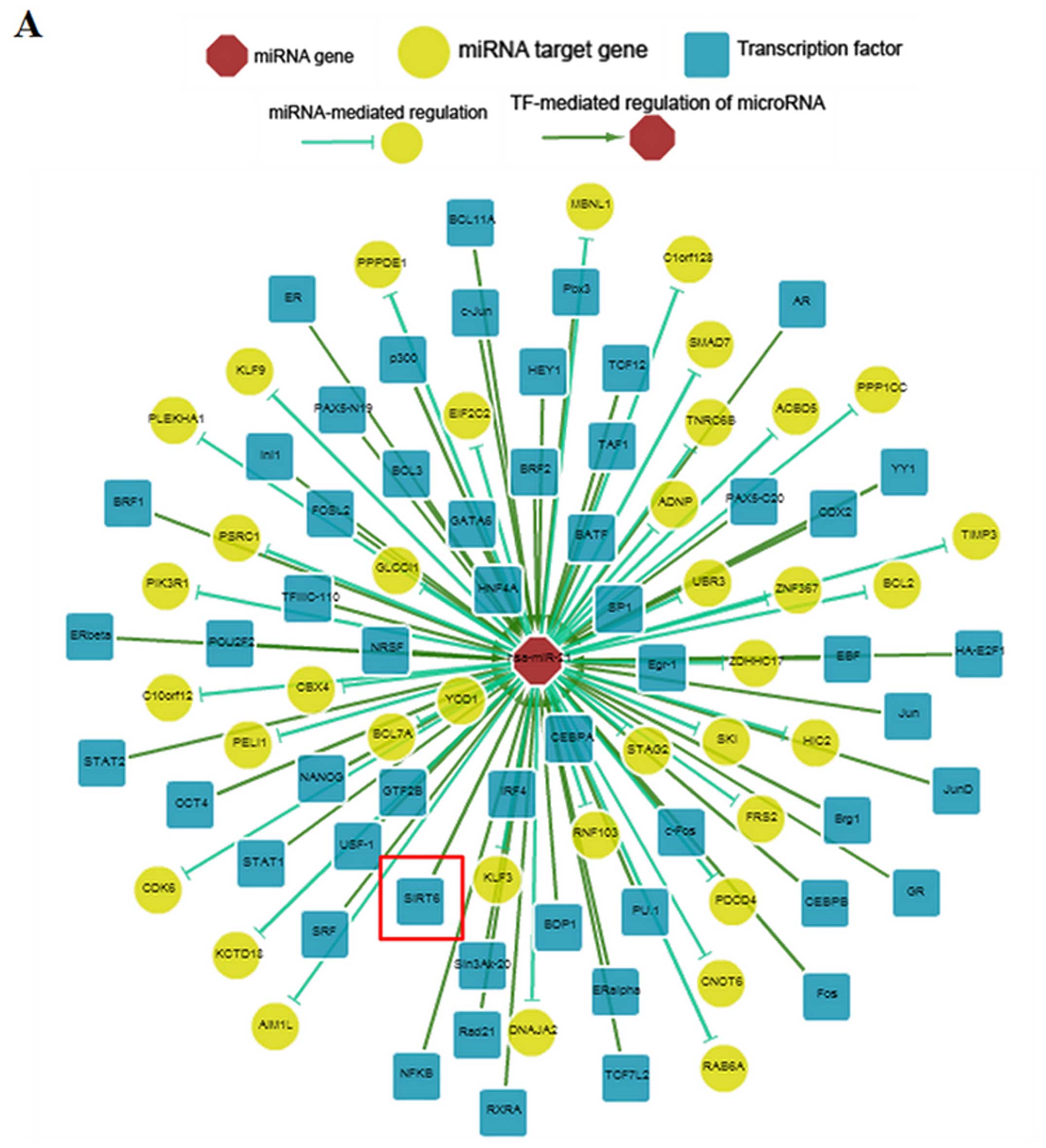
between miR-21 and SIRT6 was predicted using ChIPBase. The results
revealed that SIRT6 was likely to be a transcription factor of
miR-21 (Fig. 4A). In addition,
the overexpression of SIRT6 increased the expression of pri-, pre-
and mature miR-21 transcripts (Fig.
4B). These data demonstrated that SIRT6 negatively regulated
TGF-β2 expression and fibrosis by promoting miR-21 expression.
SIRT6 suppresses EF and scar formation
after laminectomy in the Xenopus tropicalis model of lumbar disc
herniation
The role of SIRT6 in the suppression of EF and scar
formation was investigated in vivo. A Xenopus
tropicalis model of lumbar disc herniation was established with
caudal vertebral pulposus transplantation. The pcDNA-SIRT6 vector
or miR-21 inhibitor were locally injected into the models around
the laminectomy site every day. After 6 weeks, the Xenopus
models were sacrificed and their epidural scar tissues were removed
carefully. The mass of the scars was examined using an electronic
analytical balance. The results showed that pcDNA-SIRT6 injection
significantly reduced the mass of the total scar and also Col I
(Fig. 5A and B). The rate of Col
I/total scar was also reduced by pcDNA-SIRT6 injection (Fig. 5C). By contrast, injection with the
miR-21 inhibitor increased the total mass of the scar, Col I and
the proportion of the Col I/total scar (Fig. 5A–C). Masson′s staining analysis
showed that the fibers in the pcDNA-SIRT6-injected group were more
scattered and slightly stained (Fig.
6A), suggesting a lesser degree of fibrosis. Moreover, the
number of vimentin positive (vimentin+) cells in the
pcDNA-SIRT6 injection group was much smaller than the control
(Fig. 6B). By contrast, injection
of the miR-21 inhibitor exerted the opposite effect to pcDNA-SIRT6
injection (Fig. 6A and B). These
data indicate that SIRT6 had a suppressive effect on EF and scar
formation in vivo.
Discussion
As an important member of the TGF-β family, TGF-β1
has been noted to be the master transcription factor of tissue
fibrosis (24). The role of
TGF-β2 in fibrosis has caught the attention of researchers:
previous studies have indicated that TGF-β2 acted synergistically
with TGF-β1 in the fibrotic pathway in certain types of cells and
even regulated fibrosis-related biological processes, including
spinal fibrosis (16,25,26). In the present study, we found that
TGF-β2 was markedly upregulated in patients who developed epidural
scars, and we noted a novel SIRT6/miR-21 pathway and the inhibition
of EF and scar formation upon SIRT6 vector transfection.
The suppression of cell apoptosis and cellular
senescence means that SIRT6 acts as a critical regulator in growth
inhibition in many types of normal and cancerous cells; it has
previously been noted that SIRT6 plays a protective role in the
progression of many chronic illnesses including obesity,
tumorigenesis, fatty liver, diabetes and cardiac hypertrophy
(27,28). Previous studies have revealed that
SIRT6 also plays a suppressive role in liver and cardiac
inflammation or fibrosis (27,29). However, the effect of SIRT6 on
spinal EF had not received so much attention. In the present study,
we noted a decreased expression of SIRT6 in patients who developed
epidural scars after laminectomy. Our in vitro SIRT6
overexpression study indicated that SIRT6 suppressed epidural
fibrotic scar formation, as was clear from our examination of
fibroblast proliferation, TGF-β2 and IL-1α expression, and Col I
production. To the best of our knowledge, this is the first report
on the role of SIRT6 and its regulation of spinal EF and scar
formation.
The action of SIRT6 in terms of regulating
expression or activity of other genes is largely dependent on its
deacetylase activity. For instance, SIRT6 overexpression has been
shown to induce apoptosis through p53 and p73 activation in many
cancer cells (19). However,
deacetylating proteins at a post-translational level was not the
only functional mechanism of SIRT6. A recent study showed that
SIRT6 promoted FoxO1 nuclear exclusion and had a significant effect
on gluconeogenesis (30). In the
present study, our ChIPBase analysis and detection of miR-21
transcripts after SIRT6 overexpression showed that SIRT6 is likely
a transcription factor for miR-21. We suggest that this is a novel
mechanism for SIRT6 regulation of other genes. Moreover, the
targeting relationship between miR-21 and TGF-β2 is also a novel
finding of this study.
Animal models of lumbar disc herniation are an
important tool for the exploration of treatment methods and
prognosis evaluation. The animal model used in this study was
established using the method of caudal nucleus pulposus
transplantation (31), which is
generally accepted in research on lumbar disc herniation. Although
it would have been optimal to establish the model using animals
which are closer to humans, considering both the animal
experimental cost and the conservation of the TGF-β2 gene, we
finally determined to use Xenopus tropicalis. This is more
convenient for obtaining epidural fibrotic scar tissue.
In conclusion, we examined the role and mechanisms
of SIRT6 in suppressing postoperative epidural scar formation. We
showed that SIRT6 promoted the expression of miR-21 and then
suppressed TGF-β2 expression in a targeted manner.
References
|
1
|
Alkalay RN, Kim DH, Urry DW, Xu J, Parker
TM and Glazer PA: Prevention of postlaminectomy epidural fibrosis
using bioelastic materials. Spine. 28:1659–1665. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Wang H, Liu H, Yin J, Cui L and Chen
Z: The prevention effect of poly (L-glutamic acid)/chitosan on
spinal epidural fibrosis and peridural adhesion in the
post-laminectomy rabbit model. Eur Spine J. 23:2423–2431. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang C, Kong X, Ning G, Liang Z, Qu T,
Chen F, Cao D, Wang T, Sharma HS and Feng S: All-trans retinoic
acid prevents epidural fibrosis through NF-κB signaling pathway in
post-laminectomy rats. Neuropharmacology. 79:275–281. 2014.
View Article : Google Scholar
|
|
4
|
Sharma Manoj, Dahima Rashmi and Gupta Anil
Kumar: A review on role of peridural fibrosis and its diagnosis.
Journal of Biomedical and Pharmaceutical Research. 2:81–87.
2013.
|
|
5
|
Spiegelberg L, Swagemakers SM, Van Ijcken
WF, Oole E, Wolvius EB, Essers J and Braks JA: Gene expression
analysis reveals inhibition of radiation-induced TGFβ-signaling by
hyperbaric oxygen therapy in mouse salivary glands. Mol Med.
20:257–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Y, Yan LQ, Liang Y, Li XL, Cao XJ and
Lu C: Reduction of epidural scar adhesion by topical application of
simvastatin after laminectomy in rats. Eur Rev Med Pharmacol Sci.
19:3–8. 2015.PubMed/NCBI
|
|
7
|
Wang Z, Wang Y, Xie P, Liu W and Zhang S:
Calcium channel blockers in reduction of epidural fibrosis and
dural adhesions in laminectomy rats. Eur J Orthop Surg Traumatol.
24(Suppl 1): S293–S298. 2014. View Article : Google Scholar
|
|
8
|
Chang H, Brown CW and Matzuk MM: Genetic
analysis of the mammalian transforming growth factor-β superfamily.
Endocr Rev. 23:787–823. 2013. View Article : Google Scholar
|
|
9
|
Yan J, Zhang H, Yin Y, Li J, Tang Y,
Purkayastha S, Li L and Cai D: Obesity- and aging-induced excess of
central transforming growth factor-β potentiates diabetic
development via an RNA stress response. Nat Med. 20:1001–1008.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Avouac J, Palumbo K, Tomcik M, Zerr P,
Dees C, Horn A, Maurer B, Akhmetshina A, Beyer C, Sadowski A, et
al: Inhibition of activator protein 1 signaling abrogates
transforming growth factor β-mediated activation of fibroblasts and
prevents experimental fibrosis. Arthritis Rheum. 64:1642–1652.
2012. View Article : Google Scholar
|
|
11
|
Bhattacharyya S, Kelley K, Melichian DS,
Tamaki Z, Fang F, Su Y, Feng G, Pope RM, Budinger GR, Mutlu GM, et
al: Toll-like receptor 4 signaling augments transforming growth
factor-β responses: a novel mechanism for maintaining and
amplifying fibrosis in scleroderma. Am J Pathol. 182:192–205. 2013.
View Article : Google Scholar :
|
|
12
|
Heldin CH and Moustakas A: Role of Smads
in TGFβ signaling. Cell Tissue Res. 347:21–36. 2012. View Article : Google Scholar
|
|
13
|
Huang C and Ogawa R: Fibroproliferative
disorders and their mechanobiology. Connect Tissue Res. 53:187–196.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zgheib C, Xu J and Liechty KW: Targeting
inflammatory cytokines and extracellular matrix composition to
promote wound regeneration. Adv Wound Care (New Rochelle).
3:344–355. 2014. View Article : Google Scholar
|
|
15
|
Joko M, Osuka K, Usuda N, Atsuzawa K,
Aoyama M and Takayasu M: Different modifications of phosphorylated
Smad3C and Smad3L through TGF-β after spinal cord injury in mice.
Neurosci Lett. 549:168–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lagord C, Berry M and Logan A: Expression
of TGFβ2 but not TGFβ1 correlates with the deposition of scar
tissue in the lesioned spinal cord. Mol Cell Neurosci. 20:69–92.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin H: Sirtuins and novel protein post
translational modifications. The FASEB Journal. 29:496.12015.
|
|
18
|
Lombard DB, Schwer B, Alt FW and
Mostoslavsky R: SIRT6 in DNA repair, metabolism and ageing. J
Intern Med. 263:128–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van Meter M, Mao Z, Gorbunova V and
Seluanov A: SIRT6 overexpression induces massive apoptosis in
cancer cells but not in normal cells. Cell Cycle. 10:3153–3158.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwer B, Schumacher B, Lombard DB, Xiao
C, Kurtev MV, Gao J, Schneider JI, Chai H, Bronson RT, Tsai LH, et
al: Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and
causes obesity. Proc Natl Acad Sci USA. 107:21790–21794. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Etchegaray JP, Zhong L and Mostoslavsky R:
The histone deacetylase SIRT6: at the crossroads between
epigenetics, metabolism and disease. Curr Top Med Chem.
13:2991–3000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi K, Wang D, Cao X and Ge Y: Endoplasmic
reticulum stress signaling is involved in mitomycin C (MMC)-induced
apoptosis in human fibroblasts via PERK pathway. PLoS One.
8:e593302013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sekiguchi M, Konno S and Kikuchi S: The
effects of a 5-HT2A receptor antagonist on blood flow in lumbar
disc herniation: application of nucleus pulposus in a canine model.
Eur Spine J. 17:307–313. 2008. View Article : Google Scholar
|
|
24
|
Verrecchia F and Mauviel A: Transforming
growth factor-beta and fibrosis. World J Gastroenterol.
13:3056–3062. 2007.PubMed/NCBI
|
|
25
|
Kamath VV, Krishnamurthy S, Satelur KP and
Rajkumar K: Transforming growth factor-β1 and TGF-β2 act
synergistically in the fibrotic pathway in oral submucous fibrosis:
an immunohistochemical observation. Indian J Med Paediatr Oncol.
36:111–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng Z, Li R, Shi H, Bi W, Hou W and Zhang
X: Combined silencing of TGF-β2 and Snail genes inhibit
epithelial-mesenchymal transition of retinal pigment epithelial
cells under hypoxia. Graefes Arch Clin Exp Ophthalmol. 253:875–884.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao C, Wang R-H, Lahusen TJ, Park O,
Bertola A, Maruyama T, Reynolds D, Chen Q, Xu X, Young HA, et al:
Progression of chronic liver inflammation and fibrosis driven by
activation of c-JUN signaling in Sirt6 mutant mice. J Biol Chem.
287:41903–41913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Swierczynski S, Klieser E, Illig R,
Alinger-Scharinger B, Kiesslich T and Neureiter D: Histone
deacetylation meets miRNA: epigenetics and post-transcriptional
regulation in cancer and chronic diseases. Expert Opin Biol Ther.
15:651–664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pillai VB, Sundaresan NR and Gupta MP:
Regulation of Akt signaling by sirtuins: its implication in cardiac
hypertrophy and aging. Circ Res. 114:368–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang P, Tu B, Wang H, Cao Z, Tang M,
Zhang C, Gu B, Li Z, Wang L, Yang Y, et al: Tumor suppressor p53
cooperates with SIRT6 to regulate gluconeogenesis by promoting
FoxO1 nuclear exclusion. Proc Natl Acad Sci USA. 111:10684–10689.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwashina T, Mochida J, Sakai D, Yamamoto
Y, Miyazaki T, Ando K and Hotta T: Feasibility of using a human
nucleus pulposus cell line as a cell source in cell transplantation
therapy for intervertebral disc degeneration. Spine. 31:1177–1186.
2006. View Article : Google Scholar : PubMed/NCBI
|