Introduction
It is well known that coronary disease is the
leading cause of mortality in the modern world (1). It is characterized by transient or
chronic myocardial ischemia that is caused by reduced coronary
blood flow and tissue hypoxia. Ischemia plays a critical role in
cardiomyocyte necrotic and apoptotic death in the border area close
to a myocardial infarct area and greatly affects left ventricular
remodeling, which causes heart failure and mortality (2). Apoptosis, which is determined by a
balance between pro- and anti-apoptotic factors, is frequently
detected in ischemic heart tissue (3). Attenuating the induction of
apoptosis that is triggered by tissue hypoxia is a major strategy
for treating ischemic heart disease.
One of the several signaling pathways that are
involved in the apoptotic process is glycogen synthase kinase-3β
(GSK-3β), which belongs to a family of conserved serine/threonine
kinases present in eukaryotic cells. Its activity is regulated by
various pathways, including the phosphatidylinositol-3-kinase
(PI3K)/Akt, the extracellular signal-regulated kinase (ERK1/2) and
the Wnt/wingless signaling pathways (4–6).
GSK-3β phosphorylation at Ser9 causes its N-terminal protein tail
to act as a pre-phosphorylated substrate, leading to GSK-3β
inactivation, and thus it differs from other protein kinases
(7). Moreover, the selective
inhibition of GSK-3β has been shown to exert cardioprotective
effects by maintaining mitochondrial function during
ischemia/reperfusion injury (4).
Together with adenomatous polyposis coli (APC) and Axin, GSK-3β
forms a cytoplasmic multiprotein complex that can phosphorylate
β-catenin, an important GSK-3β-regulated protein, leading to
β-catenin degradation and limited β-catenin expression (8).
As with nitric oxide (NO) and carbon monoxide (CO),
hydrosulfide (H2S) is the body's third gaseous signaling
molecule (9–12). There is increasing evidence
indicating that H2S exerts cardioprotective effects
(13,14), such as the relaxation of vascular
smooth muscle, the reduction of blood pressure (15), the inhibition of vascular smooth
muscle cell proliferation, and the regulation of cardiac
contractility (16). Sodium
hydrogen sulfide (NaHS) is frequently used as an H2S
donor (17). In addition, it has
also been proposed that, in a model of myocardial ischemia, the
protective effects of NaHS on myocardial tissues may be associated
with its anti-inflammatory and anti-apoptotic properties (18,19); however, the underlying mechanisms
remain unclear. This study was designed to determine whether NaHS
exerts its cardioprotective effects through the activation of the
GSK-3β/β-catenin signaling pathway. SB216763 was used as a GSK-3β
inhibitor.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (n=96, provided by the
Center of Experimental Animals of Hebei Province, China) with a
body weight of 280–320 g were housed in a temperature-controlled
environment, exposed to a natural photoperiod (light/dark cycle of
12:12 h), and were allowed access to food and water ad
libitum. All rats were housed for 1 week to adapt to their
environment prior to the initiation of the expriments. The study
was approved and carried out in accordance with the guidelines of
Hebei Medical University. The experimental protocols were performed
in adherence with the Institutional Animal Care and Use Committee
of Hebei Medical University.
Chemicals and reagents
NaHS was purchased from Sigma Chemical Co. (St.
Louis, MO, USA). The antibodies against phosphorylated (p-)GSK-3β
(#9323), total (t-)GSK-3β (#12456) and β-catenin (#8480) were
obtained from Cell Signaling Technology (Beverly, MA, USA).
Antibodies against Bax (sc-526), Bcl-2 (sc-492) and β-actin
(sc-7210) were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). SB216763 (purity >98%) was purchased from
Selleck Chemicals (Houston, TX, USA). The TUNEL apoptosis kit was
obtained from Roche Diagnostics (Boehringer Mannheim, Germany). The
lactate dehydrogenase (LDH) assay kit was purchased from Nanjing
Jiancheng Biotechnology (Nanjing, China).
Experimental design and surgical
procedures
A total of 40 rats were randomly assigned to 5
groups as follows: i) the sham-operated group; ii) the group
subjected to ischemia for 2 h (Is2h group); iii) the group
subjected to ischemia for 3 h (Is3h group); iv) the group subjected
to ischemia for 6 h (Is6h group); and v) the group subjected to
ischemia for 9 h (Is9h group). The protein expression levels of
GSK-3β and β-catenin were then determined by western blot analysis
as described below.
The remaining 56 rats were randomly assigned to one
of the following treatment groups (n=8/group) as follows: i) the
sham-operated group; ii) the group subjected to acute myocardial
infarction (AMI; ischemia) and not treated with any agents (AMI
group); iii) the group subjected to AMI and treated with low-dose
NaHS (AMI + NaHS-L group); iv) the group subjected to AMI and
treated with medium-dose NaHS (AMI + NaHS-M group); v) the group
subjected to AMI and treated with high-dose NaHS (AMI + NaHS-H
group); vi) the group subjected to AMI and treated with SB216763
(AMI + SB group); and vii) the group subjected to AMI and treated
with the vehicle (AMI + vehicle group).
The rats were subjected to ligation of the anterior
descending artery as previously described (20,21), resulting in acute myocardial
ischemic injury. In brief, the rats were anesthetized with
ketamine-xylazine (90 and 9 mg/kg, respectively,
intraperitoneally), and intubated with a 14-gauge polyethylene
catheter and ventilated with room air using a small animal
ventilator. The heart was exposed via a left-sided thoracotomy, and
the anterior descending artery was ligated using a 6–0 silk suture
between the pulmonary outflow tract and the left atrium. The
sham-operated rats underwent the same procedure except that the
suture was passed under the coronary artery and then removed. The
rats in the Is2h, Is3h, Is6h and Is9h groups were sacrificed at 2,
3, 6 and 9 h after ischemia, respectively. The rats in the
treatment groups were sacrificed at 6 h after ischemia.
NaHS (0.78, 1.56, 3.12 mg/kg, intraperitoneally) was
respectively administered to the rats in the AMI + low-, medium-,
and high-dose NaHS groups, SB216763 (0.6 mg/kg, dissolved in 1%
dimethylsulfoxide, intravenously) was administered to the rats in
the AMI + SB group, and 1% dimethyl sulfoxide (DMSO; 2 ml/kg,
intravenously) was administered to the rats in the AMI + vehicle
group. The doses of NaHS and SB216763 were established from prior
investigations (22–26).
Determination of cardiac function
The rats were anesthetized, and a polyethylene
Millar catheter was inserted into the right common carotid artery
and then further advanced into the left ventricular chamber, and
the cannula was connected to a pressure transducer. The mean
arterial pressure (MAP), left ventricular developed pressure
(LVDP), the left ventricular end-diastolic pressure (LVEDP) and the
rate of contraction and relaxation (dp/dtmax and
dp/dtmin) were recorded using an 8-channel polygraph
system (Powerlab 8s/30 ADInstruments, Castle Hill, NSW,
Australia).
Measurement of serum LDH levels
Myocardial cellular damage and necrosis were
evaluated by measuring the serum LDH levels. Blood samples were
collected after the measurement of cardiac function. The LDH levels
were measured in a blinded manner using a spectrophotometer
(BioTek, Instruments, Inc., Winooski, VT, USA) in duplicate.
Morphological changes in the
myocardium
The rats were sacrificed by exsanguination (blood
was taken from rats via the right carotid artery). The thoracic
cavity was opened and rat hearts were obtained and washed with cold
physiological saline. The non-infarcted left ventricular tissue was
separated for determination. The non-infarcted left ventricular
tissues were fixed in 4% paraformaldehyde and then dehydrated in
ascending grades of alcohol and embedded in paraffin, and sliced
into 5-µm-thick sections using a microtome (RM2245; Leica
Biosystems, Buffalo Grove, IL, USA). The myocardial sections
(5-µm-thick) were stained with hematoxylin and eosin
(H&E) to analyze the morphological changes in the
myocardium.
Terminal deoxynucleotidyl
transferase-mediated dUTP-biotin nick-end labeling (TUNEL)
assay
The apoptosis of cardiomyocytes was detected by
TUNEL assay. Briefly, the apoptotic cells from the heart tissues
were identified using an in situ cell death detection kit
according to the manufacturer's instructions. Nuclei with brown
staining indicated TUNEL-positive cells. For each group, 20
randomly selected fields (5 hearts/group, 4 fields/heart) were
observed under a microscope. The apoptotic index (AI), namely the
percentage of apoptotic nuclei (TUNEL-positive) vs. the total
number of nuclei was measured.
Western blot analysis of p-GSK-3β, total
GSK-3β, β-catenin, Bax, Bcl-2 and β-actin
The hearts were excised and the left ventricles were
immediately frozen in liquid nitrogen before being stored at −80°C.
Total protein from cardiomyocytes was extracted and the
concentrations were determined by using the BCA protein assay kit
(Boster Biotechnology, Wuhan, China). Proteins were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred onto a polyvinylidene difluoride
(PVDF) membrane, blocked, and probed with primary antibodies
against p-GSK-3β, t-GSK-3β, β-catenin, Bax, Bcl-2 and β-actin. The
membrane was then incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG. Finally, the bound antibody
complexes were detected using the ECL Western blotting detection
kit (Beyotime Institute of Biotechnology, Nanjing, China).
Immunohistochemical staining
Immunohistochemical staining for β-catenin, Bcl-2
and Bax was performed on the myocardial tissue. The non-infarcted
left ventricular tissue was deparaffinized, rehydrated in a graded
series of alcohol solutions, and washed twice with distilled water.
The sections were incubated with endogenous peroxidase blocked in
50 ml of 3% H2O2 at room temperature for 10
min and then washed with phosphate-buffered saline (PBS) (pH 7.4) 3
times for 5 min each. Following incubation in 2% BSA in PBS at room
temperature for 30 min, the sections were washed once with PBS. The
β-catenin, Bcl-2 or Bax antibodies were added, and the sections
were incubated at 4°C overnight. The β-catenin, Bcl-2 and Bax
proteins were assayed with an UltraSensitive SP kit (ZSGB-BIO,
Beijing, China). The sections were counterstained with hematoxylin.
The incubation of the tissue sections with normal rabbit IgG served
as the negative control. The cardiomyocytes were seeded on slides,
and then counterstained with hematoxylin, mounted with DPX
(Solarbio, Beijing, China) and visualized under a microscope
(Olympus BX3-CBH, Olympus, Tokyo, Japan).
Statistical analysis
All data are presented as the means ± standard error
of mean (SEM). Statistical analyses were performed using the SPSS
statistical package (SPSS, version 16.0; SPSS Inc., Chicago, IL,
USA). Differences between the groups of rats were analyzed by
one-way ANOVA. Differences between 2 groups were analyzed using the
Student-Newman-Keuls test. Probability values <0.05 were
considered to indicate statistically significant differences.
Results
Protein expression of GSK-3β and
β-catenin in rats with AMI
As shown by western blot analysis, no significant
difference was observed in the total GSK-3β expression levels
between the rats in the sham-operated group and the rats subjected
to ishemia for various periods of time (Fig. 1A and B). However, the levels of
p-GSK-3β and the p-GSK-3β/t-GSK-3β ratio were significantly
decreased in the rats subjected to ischemia for 2, 3, 6 and 9 h
compared with the rats in the sham-operated group when they were
normalized to the GSK-3β total protein level, as illustrated in
Fig. 1B and C (p<0.01). The
levels of β-catenin, a downstream target of GSK-3β, were also
significantly decreased in the rats subjected to ischemia for 2, 3,
6 and 9 h compared with those of the rats in the sham-operated
group (p<0.05 and p<0.01; Fig.
1D). In addition, H&E staining of the rat hearts from the
Is2h, Is3h, Is6h and Is9h groups showed that cardiomyocyte
mecrosis, edema and inflammatory cell infiltration were observed in
the Is6h and Is9h groups (data not shown). Thus, in the following
experiments, the rats were subjected to ischemia for 3 h and
sacrificed at 6 h after ischemia.
Effect of hydrosulfide on cardiac
function in rats with AMI
The MAP, LVDP and maximal positive and negative rate
of left ventricular pressure (dp/dtmax,
dp/dtmin) were decreased, and LVEDP was increased in the
rats in the AMI group compared with the rats in the sham-operated
group. However, the MAP, LVDP, dp/dtmax and
dp/dtmin were significantly increased, and LVEDP was
significantly decreased in the rats in the AMI + low-, medium- and
high-dose NaHS groups compared with the rats in the AMI group
(p<0.01; Fig. 2). Treatment
with SB216763 had similar effects, increasing MAP, LVDP and
dp/dtmax and dp/dtmin, and decreasing
LVEDP.
 | Figure 2Effect of sodium hydrosulfide on
cardiac function in rats with acute myocardial infarction (AMI).
(A) Mean arterial pressure (MAP), (B) left ventricular developed
pressure (LVDP), (C) left ventricular end diastolic pressure
(LVEDP), and (D) maximal positive and negative rate of left
ventricular pressure (dp/dtmax, dp/dtmin).
Data are expressed as the means ± SEM. (n=8 rats/group).
**p<0.01 vs. sham-operated group;
#p<0.05 and ##p<0.01 vs. AMI group.
Sham, sham-operated group; AMI, rats subjected to AMI (ischemia)
and not treated with any agents; AMI + NaHS-L, rats subjected to
AMI and treated with low-dose (0.78 mg/kg) NaHS; AMI + NaHS-M, rats
subjected to AMI and treated with medium-dose (1.56 mg/kg) NaHS;
AMI + NaHS-H, rats subjected to AMI and treated with high-dose
(03.12 mg/kg) NaHS; AMI + SB, rats subjected to AMI and treated
with the glycogen synthase kinase-3β (GSK-3β) inhibitor, SB216763
(0.6 mg/kg); AMI + vehicle, rats subjected to AMI and treated with
the vehicle (DMSO, 2 ml/kg). |
Effect of hydrosulfide on myocardial
injury (histopathology, necrosis and apoptosis) in rats with
AMI
The disorganization of cell structures, the loss of
cell-cell adherence between cardiomyocytes and abnormally shaped
cell nuclei were observed in the rat hearts from the AMI group
compared with those of the rats in the sham-operated group.
Compared with the hearts of the rats in the AMI group,
cardiomyocyte necrosis, edema and inflammatory cell infiltration
into the myocardium were significantly decreased in the rats in the
AMI + low-, medium-, high-dose NaHS groups (Fig. 3A). Treatment with SB216763 had
similar effects to NaHS, attenuating myocardial injury.
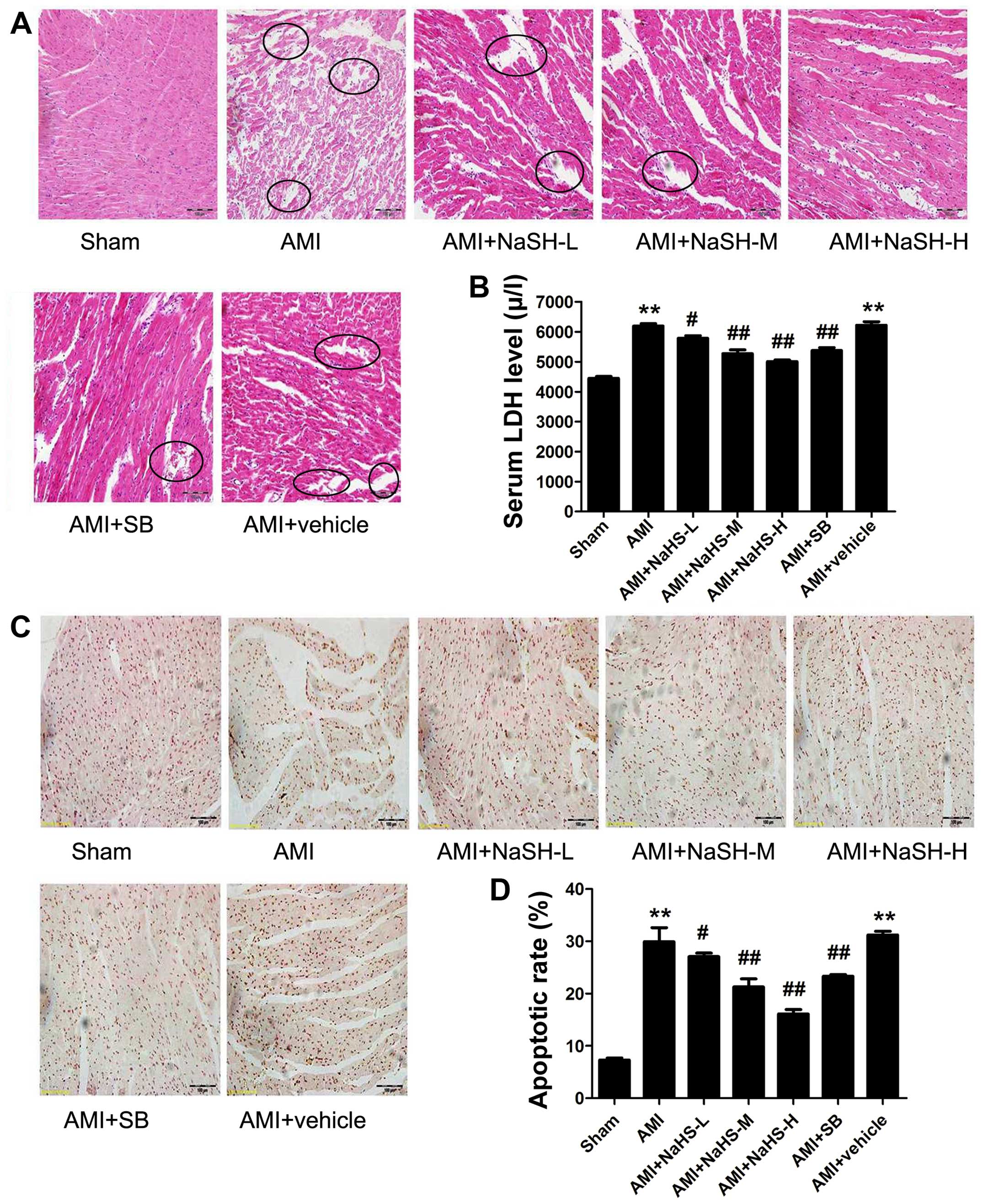 | Figure 3Effect of sodium hydrosulfide on rat
myocardial tissue necrosis and cell apoptosis. (A) Hematoxylin and
eosin (H&E) staining to determine histopathological changes
under a light microscope (×20 magnification; scale bar, 100
µm). Circles indicate the disorganization of cell structures
and inflammatory cell infiltration. (B) Serum lactate dehydrogenase
(LDH) concentration, (C) cardiomyocyte apoptosis evaluated by TUNEL
staining (×20 magnification; scale bar, 100 µm). (D)
Quantification of TUNEL-positive nuclei. Data are expressed as the
means ± SEM. **p<0.01 vs. sham-operated group;
#p<0.05 and ##p<0.01 vs. acute
myocardial infarction (AMI) group. Sham, sham-operated group; AMI,
rats subjected to AMI (ischemia) and not treated with any agents;
AMI + NaHS-L, rats subjected to AMI and treated with low-dose (0.78
mg/kg) NaHS; AMI + NaHS-M, rats subjected to AMI and treated with
medium-dose (1.56 mg/kg) NaHS; AMI + NaHS-H, rats subjected to AMI
and treated with high-dose (03.12 mg/kg) NaHS; AMI + SB, rats
subjected to AMI and treated with the glycogen synthase kinase-3β
(GSK-3β) inhibitor, SB216763 (0.6 mg/kg); AMI + vehicle, rats
subjected to AMI and treated with the vehicle (DMSO, 2 ml/kg). |
The serum LDH level was significantly elevated in
the rats in the AMI group compared with the rats in the
sham-operated group (P<0.01). Compared with the rats in the AMI
group, the serum LDH level was significantly decreased in the rats
in the AMI + low-, medium- and high-dose NaHS groups (P<0.05 and
P<0.01; Fig. 3B). Therefore,
treatment with the H2S donor, NaHS, decreased myocardial
necrosis in vivo post-AMI. Treatment with SB216763 had
similar effects to NaHS, significantly decreasing the serum LDH
level (P<0.01) compared to the rats in the AMI group (Fig. 3B).
The expression of Bcl-2 was significantly increased
and the Bax and Bax/Bcl-2 ratio was significantly decreased in the
rats in the AMI + low-, medium-, high-dose NaHS groups compared
with the rats in the AMI group (P<0.01; Fig. 4A–D). Moreover, the same results
were shown by immunohistochemical analysis for Bax and Bcl-2
(Fig. 4E). Again, similar effects
were observed following treatment with SB216763 (Fig. 4). In addition, as regards
apoptosis, the number of TUNEL-positive cells was significantly
increased in the AMI group compared with that of the sham-operated
group. However, compared with the AMI group, the number of
TUNEL-positive cardiac cells was significantly decreased in the AMI
+ low-, medium- high-dose NaHS groups and in the SB group
(P<0.01; Fig. 3C and D). Taken
together, these data suggest that treatment with the H2S
donor, NaHS, decreases AMI-induced cardiomyocyte apoptosis in
vivo.
 | Figure 4Effect of sodium hydrosulfide on
apoptotic protein in rats with acute myocardial infarctiom (AMI).
(A) Western blot analysis of the indicated proteins. Quantification
of (B) Bax/Bcl-2 ratio, (C) Bax and Bcl-2. (D) Quantification of
Bax and Bcl-2 immunohistochemical analysis, (E) immunohistochemical
analysis of Bax and Bcl-2 (×40 magnification; scale bar, 50
µm). Data are expressed as the means ± SEM.
**p<0.01 vs. sham-operated group;
#p<0.05 and ##p<0.01 vs. AMI group.
Sham, sham-operated group; AMI, rats subjected to AMI (ischemia)
and not treated with any agents; AMI + NaHS-L, rats subjected to
AMI and treated with low-dose (0.78 mg/kg) NaHS; AMI + NaHS-M, rats
subjected to AMI and treated with medium-dose (1.56 mg/kg) NaHS;
AMI + NaHS-H, rats subjected to AMI and treated with high-dose
(03.12 mg/kg) NaHS; AMI + SB, rats subjected to AMI and treated
with the glycogen synthase kinase-3β (GSK-3β) inhibitor, SB216763
(0.6 mg/kg); AMI + vehicle, rats subjected to AMI and treated with
the vehicle (DMSO, 2 ml/kg). |
Effect of hydrosulfide on the
phosphorylation of GSK-3β and the concentration of the downstream
target, β-catenin
The p-GSK-3β/t-GSK-3β ratio and the content of
β-catenin were significantly decreased in the AMI group compared
with the sham-operated group. However, compared with the AMI group,
the p-GSK-3β/t-GSK-3β ratio and the content of β-catenin were
significantly increased in the AMI + low-, medium- and high-dose
NaHS groups (P<0.01; Fig. 5).
It should be noted that treatment with SB216763 had similar effects
to NaHS (Fig. 5).
 | Figure 5Effect of sodium hydrosulfide on
phosphorylation of glycogen synthase kinase-3β (GSK-3β) and
β-catenin expression in rats with acute myocardial infarction
(AMI). (A) Western blot analysis of the indicated proteins.
Quantification of (B) p-GSK-3β and t-GSK-3β, (C) p-GSK-3β/t-GSK-3β
ratio and (D) β-catenin relative to actin. (E) Immunohistochemical
detection of β-catenin (×40 magnification; scale bar, 50
µm). All values are expressed as the means ± SEM.
**p<0.01 vs. sham-operated group;
#p<0.05 and ##p<0.01 vs. AMI group.
Sham, sham-operated group; AMI, rats subjected to AMI (ischemia)
and not treated with any agents; AMI + NaHS-L, rats subjected to
AMI and treated with low-dose (0.78 mg/kg) NaHS; AMI + NaHS-M, rats
subjected to AMI and treated with medium-dose (1.56 mg/kg) NaHS;
AMI + NaHS-H, rats subjected to AMI and treated with high-dose
(03.12 mg/kg) NaHS; AMI + SB, rats subjected to AMI and treated
with the GSK-3β inhibitor, SB216763 (0.6 mg/kg); AMI + vehicle,
rats subjected to AMI and treated with the vehicle (DMSO, 2
ml/kg). |
Role of GSK-3β/β-catenin signaling in
hydrosulfide-induced cardioprotection
To investigate the molecular mechanisms underlying
NaHS-mediated cardioprotection, we measured the levels of p-GSK-3β
and t-GSK-3β, and the p-GSK-3β/t-GSK-3β ratio and the content of
β-catenin by western blot analysis. Compared with the AMI group,
the phosphorylation levels of GSK-3β, the p-GSK-3β/t-GSK-3β ratio,
and the content of β-catenin were significantly increased in the
AMI + SB group (P<0.05 and P<0.01; Fig. 5). In addition, the MAP, LVDP,
dp/dtmax and dp/dtmin were significantly
increased, and the LVEDP, serum LDH level, the Bax/Bcl-2 ratio, and
the number of apoptotic cells were significantly decreased in the
AMI + SB group compared with the AMI group, as shown by our
above-mentioned results (Figs.
2Figure 3–4).
Discussion
In the present study, our data demonstrated that
NaHS attenuated AMI-induced injury in vivo through the
activation of the GSK-3β/β-catenin signaling pathway and exerted
anti-apoptotic effects.
Evidence indicates that H2S plays an
important role in vasodilation in different disease states
(27–30), functions as a neuromodulator
(31), and is also a novel
mediator of lipopolysaccharide-induced inflammation (19,32). Furthermore, it is postulated that
this gaseous signaling molecule acts as a regulator of
physiological cardiac function by exerting antioxidant and
anti-apoptotic effects in cardiovascular and neurological diseases
(33–35). Cardiomyocyte apoptosis is a major
pathogenic mechanism underlying AMI-induced injury; thus,
inhibiting cardiomyocyte apoptosis may effectively minimize cardiac
injury (36). As part of the
intrinsic apoptosis pathway, the Bcl-2 protein family is an
important regulator during cardiomyocyte apoptosis (37). The anti-apoptotic protein, Bcl-2,
and survivin (38) have been
shown to block the release of cytochrome c from the
mitochondria, and to inhibit caspase activity and decrease cell
apoptosis (5,37,39). Therefore, the balance in apoptotic
signaling is influenced by the Bcl-2/Bax ratio. In this study, we
demonstrated that the administration of NaHS and SB216763 (GSK-3β
inhibitor) increased the expression of Bcl-2, decreased the
expression of Bax, and decreased TUNEL-positive staining in rat
heart tissue. The administration of NaHS significantly ameliorated
cardiac function, as reflected by an increase in MAP, LVDP and
dp/dtmax and dp/dtmin, and a decrease in
LVEDP and the serum LDH level. Treatment with NaHS preserved
myocardial structural integrity, thereby effectively restricting
myocardial enzyme leakage.
The serine/threonine survival kinase GSK-3β is a
point of downstream convergence for PI3K/Akt and Wnt signaling. It
is known that ischemic pre- and post-conditioning activate PI3K/Akt
(25,40–42), and promote the phosphorylation of
downstream target molecules, such as GSK-3β, downstream of insulin
receptor substrates (25,43,44). In contrast to other protein
kinases, GSK-3β phosphorylation inhibits GSK-3β activity, thus
inhibiting cardiomyocyte apoptosis, exerting cardioprotective
effects (7,26). Kaga et al (7) reported that the activation of
GSK-3β/β-catenin further increases Bcl-2 and survivin expression,
thereby providing enhanced anti-apoptotic effects in the ischemic
preconditioned myocardium. Therefore, we propose that the
administration of NaHS exerts cardioprotective effects through the
GSK-3β signaling pathway.
In the present study, our results revealed that
AMI-induced injury led to decreased levels of p-GSK-3β and
β-catenin. However, the administration of NaHS increased the
phosphorylation levels of GSK-3β and the expression of β-catenin,
and reduced cardiomyocyte apoptosis by regulating the expression of
the Bcl-2 family proteins. A similar effect was also noted
following the administration of SB216763, a potent inhibitor of the
GSK-3β ATP binding site (45).
In conclusion, our data provide strong supportive
evidence that the phosphorylation of GSK-3β by NaHS and its
inhibitor, SB216763, exert cardioprotective and anti-apoptotic
effects against AMI-induced injury through the activation of the
GSK-3β/β-catenin signaling pathway. However, the specific
subcellular mechanisms responsible for the NaHS-induced
anti-apoptotic effects remain unknown, and this warrants further
investigation.
Acknowledgments
This study was supported by the Basic Research
Project of Hebei Province, China (no. 13967602D) and the Natural
Science Foundation of Hebei Province, China (no. C2009001458).
References
|
1
|
Thom T, Haase N, Rosamond W, Howard VJ,
Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S,
et al: American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics - 2006
update: A report from the American Heart Association Statistics
Committee and Stroke Statistics Subcommittee. Circulation.
113:e85–e151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nabel EG and Braunwald E: A tale of
coronary artery disease and myocardial infarction. N Engl J Med.
366:54–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiong M, Wang ZV, Pedrozo Z, Cao DJ,
Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA and
Lavandero S: Cardiomyocyte death: Mechanisms and translational
implications. Cell Death Dis. 2:e2442011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu
Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, et al:
Glycogen synthase kinase-3beta mediates convergence of protection
signaling to inhibit the mitochondrial permeability transition
pore. J Clin Invest. 113:1535–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nishihara M, Miura T, Miki T, Tanno M,
Yano T, Naitoh K, Ohori K, Hotta H, Terashima Y and Shimamoto K:
Modulation of the mitochondrial permeability transition pore
complex in GSK-3beta-mediated myocardial protection. J Mol Cell
Cardiol. 43:564–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steinbrecher KA, Wilson W III, Cogswell PC
and Baldwin AS: Glycogen synthase kinase 3beta functions to specify
gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol.
25:8444–8455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaga S, Zhan L, Altaf E and Maulik N:
Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and
anti-apoptotic signaling through the induction of VEGF, Bcl-2 and
survivin expression in rat ischemic preconditioned myocardium. J
Mol Cell Cardiol. 40:138–147. 2006. View Article : Google Scholar
|
|
8
|
Liu J, Stevens J, Matsunami N and White
RL: Targeted degradation of beta-catenin by chimeric F-box fusion
proteins. Biochem Biophys Res Commun. 313:1023–1029. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abe K and Kimura H: The possible role of
hydrogen sulfide as an endogenous neuromodulator. J Neurosci.
16:1066–1071. 1996.PubMed/NCBI
|
|
10
|
Wang R: Two's company, three's a crowd:
Can H2S be the third endogenous gaseous transmitter? FASEB J.
16:1792–1798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YH, Yao WZ, Geng B, Ding YL, Lu M,
Zhao MW and Tang CS: Endogenous hydrogen sulfide in patients with
COPD. Chest. 128:3205–3211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Porokhya MV, Abramochkin DV, Abramov AA,
Kuzmin VS and Sukhova GS: Inotropic effects of gaseous transmitters
in isolated rat heart preparation. Bull Exp Biol Med. 153:855–857.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu X, Li T, Bi S, Jin Z, Zhou G, Bai C, Li
L, Cui Q and Liu W: Possible role of hydrogen sulfide on the
preservation of donor rat hearts. Transplant Proc. 39:3024–3029.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lavu M, Bhushan S and Lefer DJ: Hydrogen
sulfide-mediated cardioprotection: Mechanisms and therapeutic
potential. Clin Sci (Lond). 120:219–229. 2011. View Article : Google Scholar
|
|
15
|
Sikora M, Drapala A and Ufnal M: Exogenous
hydrogen sulfide causes different hemodynamic effects in
normotensive and hypertensive rats via neurogenic mechanisms.
Pharmacological Rep. 66:751–758. 2014. View Article : Google Scholar
|
|
16
|
Li L, Liu D, Bu D, Chen S, Wu J, Tang C,
Du J and Jin H: Brg1-dependent epigenetic control of vascular
smooth muscle cell proliferation by hydrogen sulfide. Biochim
Biophys Acta. 1833.1347–1355. 2013.
|
|
17
|
Mostofa MG, Saegusa D, Fujita M and Tran
LS: Hydrogen sulfide regulates salt tolerance in rice by
maintaining Na(+)/K(+) balance, mineral homeostasis and oxidative
metabolism under excessive salt stress. Front Plant Sci.
6(1055)2015. View Article : Google Scholar
|
|
18
|
Sivarajah A, Collino M, Yasin M, Benetti
E, Gallicchio M, Mazzon E, Cuzzocrea S, Fantozzi R and Thiemermann
C: Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide
in a rat model of regional myocardial I/R. Shock. 31:267–274. 2009.
View Article : Google Scholar
|
|
19
|
Rios EC, Szczesny B, Soriano FG, Olah G
and Szabo C: Hydrogen sulfide attenuates cytokine production
through the modulation of chromatin remodeling. Int J Mol Med.
35:1741–1746. 2015.PubMed/NCBI
|
|
20
|
Bhindi R, Witting PK, McMahon AC,
Khachigian LM and Lowe HC: Rat models of myocardial infarction.
Pathogenetic insights and clinical relevance. Thromb Haemost.
96:602–610. 2006.PubMed/NCBI
|
|
21
|
Lee TM, Lin MS and Chang NC: Effect of
ATP-sensitive potassium channel agonists on ventricular remodeling
in healed rat infarcts. J Am Coll Cardiol. 51:1309–1318. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu F, Liu GJ, Liu N, Zhang G, Zhang JX
and Li LF: Effect of hydrogen sulfide on inflammatory cytokines in
acute myocardial ischemia injury in rats. Exp Ther Med.
9:1068–1074. 2015.PubMed/NCBI
|
|
23
|
Johansen D, Ytrehus K and Baxter GF:
Exogenous hydrogen sulfide (H2S) protects against
regional myocardial ischemia-reperfusion injury - Evidence for a
role of K ATP channels. Basic Res Cardiol. 101:53–60. 2006.
View Article : Google Scholar
|
|
24
|
Chen QL, Gu EW, Zhang L, Cao YY, Zhu Y and
Fang WP: Diabetes mellitus abrogates the cardioprotection of
sufentanil against ischaemia/reperfusion injury by altering
glycogen synthase kinase-3β. Acta Anaesthesiol Scand. 57:236–242.
2013. View Article : Google Scholar
|
|
25
|
Gross ER, Hsu AK and Gross GJ: Diabetes
abolishes morphine-induced cardioprotection via multiple pathways
upstream of glycogen synthase kinase-3beta. Diabetes. 56:127–136.
2007. View Article : Google Scholar
|
|
26
|
Obame FN, Plin-Mercier C, Assaly R, Zini
R, Dubois-Randé JL, Berdeaux A and Morin D: Cardioprotective effect
of morphine and a blocker of glycogen synthase kinase 3 beta,
SB216763
[3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione],
via inhibition of the mitochondrial permeability transition pore. J
Pharmacol Exp Ther. 326:252–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao W, Zhang J, Lu Y and Wang R: The
vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP)
channel opener. EMBO J. 20:6008–6016. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Huang H, Liu P, Tang C and Wang
J: Hydrogen sulfide contributes to cardioprotection during
ischemia-reperfusion injury by opening K ATP channels. Can J
Physiol Pharmacol. 85:1248–1253. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen SS, Tang CS, Jin HF and Du JB: Sulfur
dioxide acts as a novel endogenous gaseous signaling molecule in
the cardiovascular system. Chin Med J (Engl). 124:1901–1905.
2011.
|
|
30
|
Yang G, Sun X and Wang R: Hydrogen
sulfide-induced apoptosis of human aorta smooth muscle cells via
the activation of mitogen-activated protein kinases and caspase-3.
FASEB J. 18:1782–1784. 2004.PubMed/NCBI
|
|
31
|
Whiteman M, Cheung NS, Zhu YZ, Chu SH,
Siau JL, Wong BS, Armstrong JS and Moore PK: Hydrogen sulphide: A
novel inhibitor of hypochlorous acid-mediated oxidative damage in
the brain? Biochem Biophys Res Commun. 326:794–798. 2005.
View Article : Google Scholar
|
|
32
|
Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath
RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M and Moore PK:
Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced
inflammation in the mouse. FASEB J. 19:1196–1198. 2005.PubMed/NCBI
|
|
33
|
Tokuda K, Kida K, Marutani E, Crimi E,
Bougaki M, Khatri A, Kimura H and Ichinose F: Inhaled hydrogen
sulfide prevents endotoxin-induced systemic inflammation and
improves survival by altering sulfide metabolism in mice. Antioxid
Redox Signal. 17:11–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang LH, Luo X, He W, Huang XX and Cheng
TT: Effects of exogenous hydrogen sulfide on apoptosis proteins and
oxidative stress in the hippocampus of rats undergoing heroin
withdrawal. Arch Pharm Res. 34:2155–2162. 2011. View Article : Google Scholar
|
|
35
|
Sodha NR1, Clements RT, Feng J, Liu Y,
Bianchi C, Horvath EM, Szabo C and Sellke FW: The effects of
therapeutic sulfide on myocardial apoptosis in response to
ischemia-reperfusion injury. Eur J Cardiothorac Surg. 33:906–913.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tian Y, Zhang W, Xia D, Modi P, Liang D
and Wei M: Postconditioning inhibits myocardial apoptosis during
prolonged reperfusion via a JAK2-STAT3-Bcl-2 pathway. J Biomed Sci.
18(53)2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar D and Jugdutt BI: Apoptosis and
oxidants in the heart. J Lab Clin Med. 142:288–297. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu F, Xing J, Zhang X, Dong S, Zhao Y,
Wang L, Li H, Yang F, Xu C and Zhang W: Exogenous hydrogen sulfide
prevents cardiomyocyte apoptosis from cardiac hypertrophy induced
by isoproterenol. Mol Cell Biochem. 381:41–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cook SA, Sugden PH and Clerk A: Regulation
of bcl-2 family proteins during development and in response to
oxidative stress in cardiac myocytes: Association with changes in
mitochondrial membrane potential. Circ Res. 85:940–949. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bharti S, Golechha M, Kumari S, Siddiqui
KM and Arya DS: Akt/GSK-3β/eNOS phosphorylation arbitrates
safranal-induced myocardial protection against ischemia-reperfusion
injury in rats. Eur J Nutr. 51:719–727. 2012. View Article : Google Scholar
|
|
41
|
Bharti S, Singh R, Chauhan SS, Hussain T,
Al-Attas OS and Arya DS: Phosphorylation of Akt/GSK-3β/eNOS
amplifies 5-HT2B receptor blockade mediated anti-hypertrophic
effect in rats. FEBS Lett. 586:180–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu Y, Zhou J, Xu C, Lin H, Xiao J, Wang Z
and Yang B: JAK/STAT and PI3K/AKT pathways form a mutual
transactivation loop and afford resistance to oxidative
stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem.
21:305–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yadav HN, Singh M and Sharma PL:
Involvement of GSK-3β in attenuation of the cardioprotective effect
of ischemic preconditioning in diabetic rat heart. Mol Cell
Biochem. 343:75–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Desrois M, Sidell RJ, Gauguier D, King LM,
Radda GK and Clarke K: Initial steps of insulin signaling and
glucose transport are defective in the type 2 diabetic rat heart.
Cardiovasc Res. 61:288–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Coghlan MP, Culbert AA, Cross DA, Corcoran
SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee
Cox L, et al: Selective small molecule inhibitors of glycogen
synthase kinase-3 modulate glycogen metabolism and gene
transcription. Chem Biol. 7:793–803. 2000. View Article : Google Scholar : PubMed/NCBI
|



















