Introduction
Chronic myelogenous leukemia (CML) is one of the
most common types of cancer in humans, and multidrug resistance
(MDR) is a major factor that limits the efficacy of
chemotherapeutic agents used to treat CML. MDR is a phenomenon
characterized by the development of resistance in cancer cells to a
variety of unrelated drugs following exposure to a single
chemotherapy drug (1). MDR has
many causes; however, the most widely studied mechanism involves
the increased efflux of cytotoxic drugs, which is mediated by
ATP-binding cassette (ABC) transporters (2). P-glycoprotein (P-gp) and multidrug
resistance protein (MRP)-1 are members of the ABC transporter
family, which are mainly expressed in the plasma membrane and pump
the cytotoxic drugs out of the cells through active transportation.
The increased expression of P-gp and MRP-1 has been demonstrated to
cause reductions in the concentration of chemotherapeutic drugs
inside tumor cells, thus reducing the clinical effectiveness of
chemotherapy drugs (4–10). P-gp, the first member of the
family to be identified, is a 170-kDa energy-dependent drug efflux
pump which mediates resistance to a variety of pharmacologically
unrelated anticancer drugs, such as doxorubicin, epirubicin and
paclitaxel (1–3). MRP-1, a drug-transporting MRP, is a
190-kDa protein and is encoded by the MRP-1 gene, which is located
on chromosome 16p13. MRP-1 overexpression has emerged as an
important contributor to chemoresistance. In tumor cells, MRP-1 was
found to cause resistance not only to doxorubicin, but also to many
other chemotherapeutic drugs, including methotrexate, etoposide and
vincristine (11).
Homeobox (HOX) proteins are homeodomain
transcription factors that are highly conserved in many species,
from Drosophila to humans. The human and murine HOX genes
are found in four groups on four different chromosomes with 9–11
genes in each group (referred to as HOXA-D) (12–15). The HOX genes are important
regulators encoding transcription factors that influence
embryologic development. HOXA10 belongs to the HOX gene family.
HOXA10 is maximally expressed in the primitive hematopoietic cell
compartment and decreases when the cells differentiate. In
addition, it has been demonstrated that the overexpression of
HOXA10 is involved in myelopoiesis and blocks differentiation,
which may ultimately lead to myeloid leukemia (13,16). Previous studies have confirmed
that HOXA10 expression was associated with temozolomide resistance
in glioblastoma (GBM) (17,18). However, the role of HOXA10 in
multidrug-resistant human CML K562/ADM cells was previously
unknown. Thus, in the present study, we explored the effects of
HOXA10 knockdown on multi-drug resistance and the underlying
molecular mechanism.
Materials and methods
Chemicals and reagents
Adriamycin (ADR; Melone Pharm aceutical Co., Ltd.,
Dalian, China) was dissolved at a concentration of 2 g/l with
ddH2O and divided into 25 aliquots. Rabbit polyclonal
antibodies against HOXA10 (cat. no. bs-2502R), P-gp (cat. no.
bs-0563R), MRP-1 (cat. no. bs-0657R) were obtained from
Biosynthesis Biotechnology Co., Ltd. (Beijing, China). Rabbit
polyclonal antibodies against GAPDH were obtained from Hangzhou
Goodhere Biotechnology Co., Ltd. (Hangzhou, China).
Cell lines and cell culture
The human CML cell line, K562, was obtained from the
Key Laboratory of Tumour Molecular Biology of Binzhou Medical
University (Binzhou, China) and its MDR subline, K562/ADM, was
obtained from the Department of Pharmacology at the Institute of
Hematology of the Chinese Academy of Medical Sciences (Tianjin,
China). The cells were cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS) (both from HyClone Laboratories,
Inc., Logan, UT, USA) at 37°C in a humidified atmosphere containing
5% CO2. K562/ADM cells were maintained in the presence
of 4 mg/l ADR. Prior to the experiment, the cells were cultured in
drug-free medium for one week.
Determination of multidrug resistance in
K562/ADM cells
A Cell Counting kit 8 (CCK8; Dojindo Molecular
Technologies, Inc., Shanghai, China) was used to determine the
survival rate of cells incubated with ADR at various concentrations
(0.2–1.6 mg/l for the K562 cells and 16–128 mg/l for the K562/ADM
cells), as previously described (7). After dilution in RPMI-1640 medium
for 24 h, 10 μl CCK8 solution was added to each well and
incubated for 1–4 h. The absorbance was then measured at 570 nm
with a fluorescence spectrofluorometer (F-7000; Hitachi
High-Technologies Corp., Tokyo, Japan). A blank well containing
only medium and ADR was used as a control. The concentration of ADR
causing 50% inhibition of cell growth (IC50) was
calculated (19).
Determination of gene expression by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA), according to the manufacturer's
instructions, followed by the synthesis of first strand cDNA using
2 μg total RNA. The primers (Table I) used in this experiments were
designed using Primer 5 version 5.6.0 software and synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China).
 | Table IPrimers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence | Product length
(bp) |
|---|
| HOXA10 | F:
5′-CTCACGGCAAAGAGTGGTC-3′ | |
| R:
5′-AGTTTCATCCTGCGGTTCTG-3′ | 182 |
| GAPDH | F:
5′-TGACTTCAACAGCGACACCCA-3′ | |
| R:
5′-CACCCTGTTGCTGTAGCCAAA-3′ | 121 |
Reverse transcription was performed with a
PrimeScript™ RT reagent kit with gDNA Eraser (Takara Bio, Inc.,
Otsu, Japan). PCR amplification was performed on an Eppendorf
Mastercycler personal (Eppendorf China Co., Ltd., Shanghai, China)
using Premix Taq™ (Takara Bio, Inc.). The reaction system contained
diethyl pyrocarbonate, forward primer, reverse primer, Premix Taq
and template cDNA. The amplification was as follows: 95°C for 2
min, then 35 cycles of 95°C for 30 sec, 60°C for 30 sec, 72°C for 1
min, followed by a full extension cycle of 72°C for 5 min. The PCR
products were electrophoresed on 1.5% agarose gels (Takara
Biotechnology Co., Ltd., Dalian, China), and stained with ethidium
bromide for 15 min. The images were captured with a Tanon gel
imaging system. The results are expressed for each sample as band
intensity relative to that of GAPDH.
qPCR was performed on an ABI PRISM 7500 real-time
PCR system (Applied Biosystems, Foster City, CA, USA) using a
SYBR-Green reaction kit (Takara Bio, Inc.). The PCR reaction system
consisted of SYBR-Green reagent, forward primer, reverse primer,
template cDNA and nuclease-free distilled water. The PCR conditions
were as follows: 95°C for 30 sec, followed by 45 cycles of 95°C for
5 sec and 60°C for 30 sec. GAPDH was used as an internal control.
qPCR for each gene of each cDNA sample was assayed in triplicate.
The results were calculated using the 2−ΔΔCt method. The
following equations were used: ΔCt = Ct(target gene) - Ct(GAPDH);
ΔΔCt = Ct[short hairpin RNA (shRNA) cells] - Ct(untreated
control).
In vitro transfection with shRNA
The HOXA10-specific shRNA and the control shRNA were
synthesized with recombinant plasmids containing the green
fluorescent protein (GFP) vector, pGPH1, purchased from Shanghai
GenePharma Co., Ltd., (Shanghai, China). The target sequence of
HOXA10 shRNA was as follows: 5′-GCCAAAUUAUCCCACAACA-3′. Prior to
transfection, the cells were cultured in RPMI-1640 medium free of
serum and antibiotics. shRNA transfection (at a final concentration
of 1 μg in all experiments) was performed using
Lipofectamine™ 2000 transfection reagent (Invitrogen) according to
the manufacturer's instructions. Briefly, shRNAs and lipofectamine
(2.5 μl) were diluted in RPMI-1640 separately and incubated
for 5 min at room temperature. The diluted solutions were then
mixed and incubated for 15 min at room temperature. The mixtures
were then added to each well containing cells and medium. In
addition, the cells treated with only Lipofectamine were considered
as the blank control. The cell culture plates were subsequently
incubated for 6 h at 37°C in an incubator. Subsequently, RPMI-1640
medium containing 20% FBS was added and the cells were then
incubated under the abovementioned conditions. Transfection
efficiency was examined under a fluorescence microscope (Olympus
DP71; Olympus, Tokyo, Japan). RT-qPCR and western blot analysis
were performed to determine the inhibitory efficacy. G418 (500
ng/ml; Life Technologies, Carlsbad, CA, USA) was then added to the
medium after 48 h transfection, and the cells were cultured for 1
month to permit selection. The cells successfully transfected with
HOXA10 shRNA and control shRNA were named HOXA10 shRNA and control
shRNA cells.
Assay of the reversal efficacy of HOXA10
knockdown
The K562/ADM cells as well as the cells transfected
with HOXA10 shRNA and the control shRNA were seeded into 96-well
plates in the presence of various concentrations of ADR (0–128
μg/ml) for 24 h at 37°C in 5% CO2, and the
quantity and percentages of viable cells were determined using the
CCK8 assay. Each group consisted of five parallel wells. ADR
IC50 was calculated using the untreated cells as a 100%
viable control. The reversal fold (RF) values, as a measure of the
potency of reversal, were obtained using the following formula: RF
= IC50 of K562/ADM/IC50 of HOXA10 shRNA.
Enhancement of intracellular ADR
accumulation
The intracellular accumulation of ADR was monitored
using a standard procedure. The K562/ADM cells, and cells
transfected with HOXA10 shRNA and control shRNA were incubated for
1 h at 37°C with ADR (3 mg/l). The cells were then harvested by
centrifugation (1,500 rpm for 5 min at 4°C) and washed twice with
ice-cold phosphate-buffered saline (PBS). The cell-associated mean
fluorescence intensity (MFI) of ADR was detected by a flow
cytometer (FACS FC500; Beckman Coulter, Brea, CA, USA) with
excitation/emission wavelengths of 485/585 nm.
Western blot analysis
The cells were harvested, a total of 100 μl
lysis buffer was added and the protein concentration of the lysate
was determined using a bicinchoninic acid protein assay kit
(Beyotime Biotechnology, Shanghai, China). The lysed samples (50
μg) were separated by 6–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Beyotime
Biotechnology) with a constant voltage of 80 V for 0.5 h which was
then switched to 100 V for another 1.5 h. The resolved proteins
were electrophoretically transferred to polyvinylidine difluoride
membranes (EMD Millipore, Bedford, MA, USA) and blocked with 5%
skimmed milk for 2 h. Subsequently, the membranes were incubated
overnight at 4°C with specific antibodies. The primary antibodies
used were rabbit polyclonal antibodies against HOXA10 (1:500), P-gp
(1;500), MRP-1 (1;500) and GAPDH (1;1,000). The following day, the
membranes were incubated in horseradish peroxidase-labeled goat
anti-rabbit immunoglobulin G (1:5,000; cat. no. ZB-5301; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
for 2 h at room temperature. Finally, the images were captured
using a FluorChem FC2 gel imaging system (Alpha Innotech, San
Leandro, CA, USA). The intensity of each band was normalized to
GAPDH for their respective lanes.
Data analysis
Statistical analyses were performed using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). Data are expressed as the
means ± SD. Statistical comparisons were evaluated by one-way
ANOVA. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of multidrug resistance in
K562/ADM cells and K562 cells
Compared with the non-resistant K562 cells, the
K562/ADM cells exhibited significant resistance to ADR. As shown in
Table II, a 31.2747-fold
increase in resistance was observed in the K562/ADM cells in
comparison with that in the non-resistant K562 cells (P<0.05)
(Table II).
 | Table IIDetermination of multidrug resistance
according to the sensitivity of K562/ADM and K562 cells to ADR
(means ± SD of triplicate experiments). |
Table II
Determination of multidrug resistance
according to the sensitivity of K562/ADM and K562 cells to ADR
(means ± SD of triplicate experiments).
| Treatment | K562/ADM
IC50 (μg/ml) | K562 IC50
(μg/ml) | Fold resistance |
|---|
| ADR |
43.6783±0.33096a | 1.3966±0.01526 | 31.2747 |
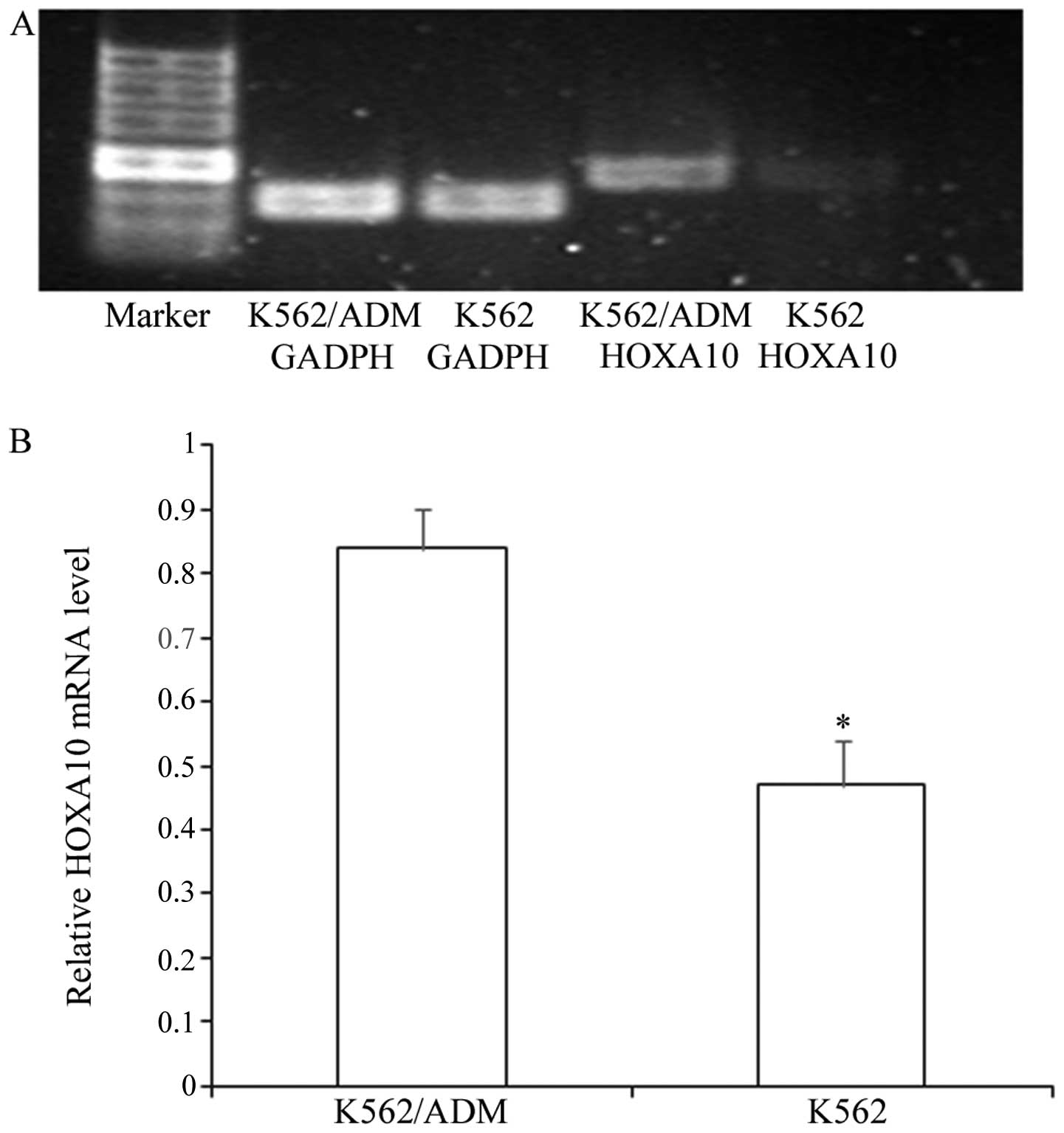
Expression of HOXA10 in K562 cells and
K562/ADM cells
We determined the expression of HOXA10 in the
non-resistant K562 cells and the resistant K562/ADM cells. We
demonstrated that the K562 cells and K562/ADM cells exhibited high
expression levels of HOXA10, while the K562/ADM cells expressed
higher levels of HOXA10 than the K562 cells (Fig. 1) (P<0.05).
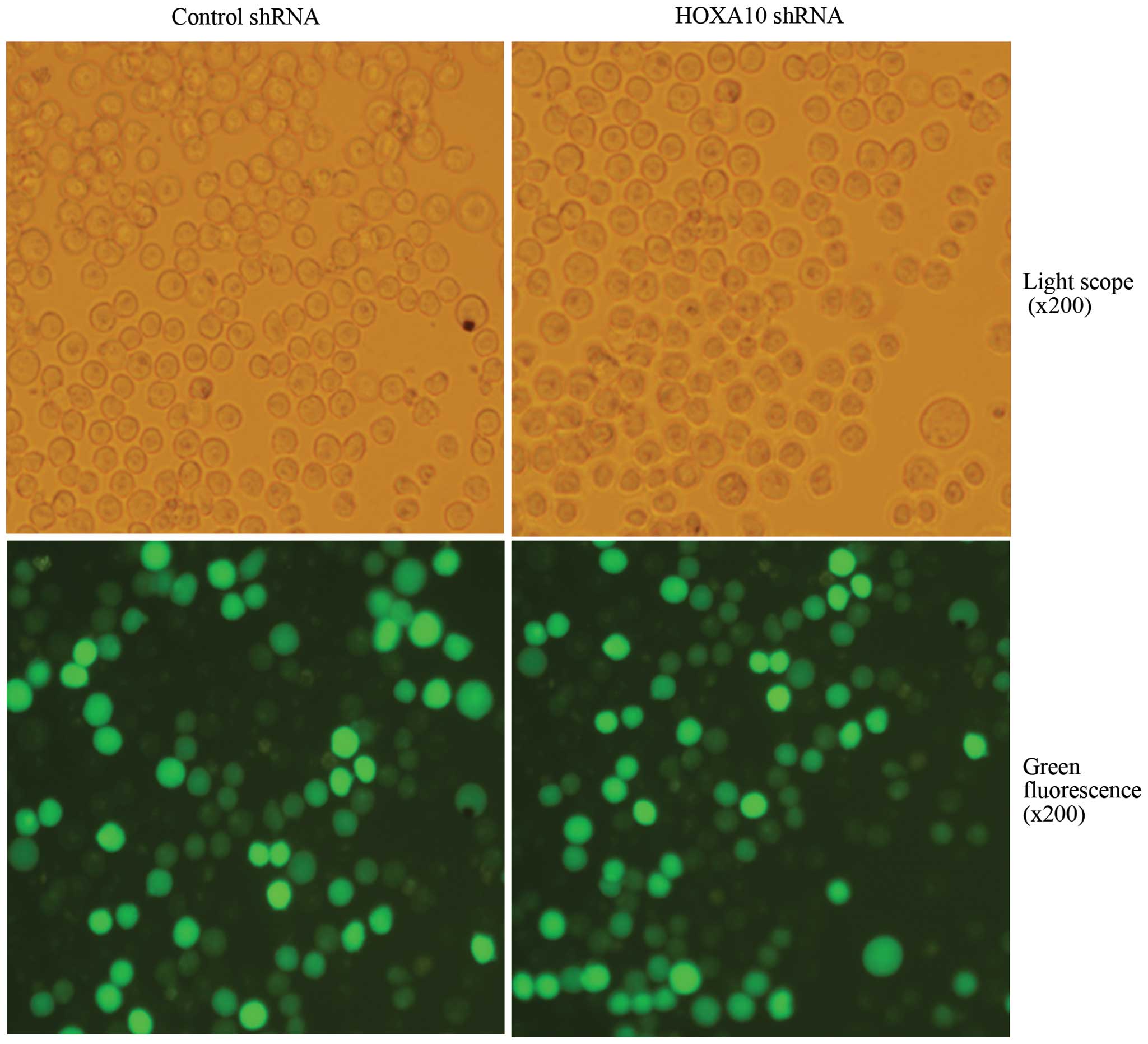
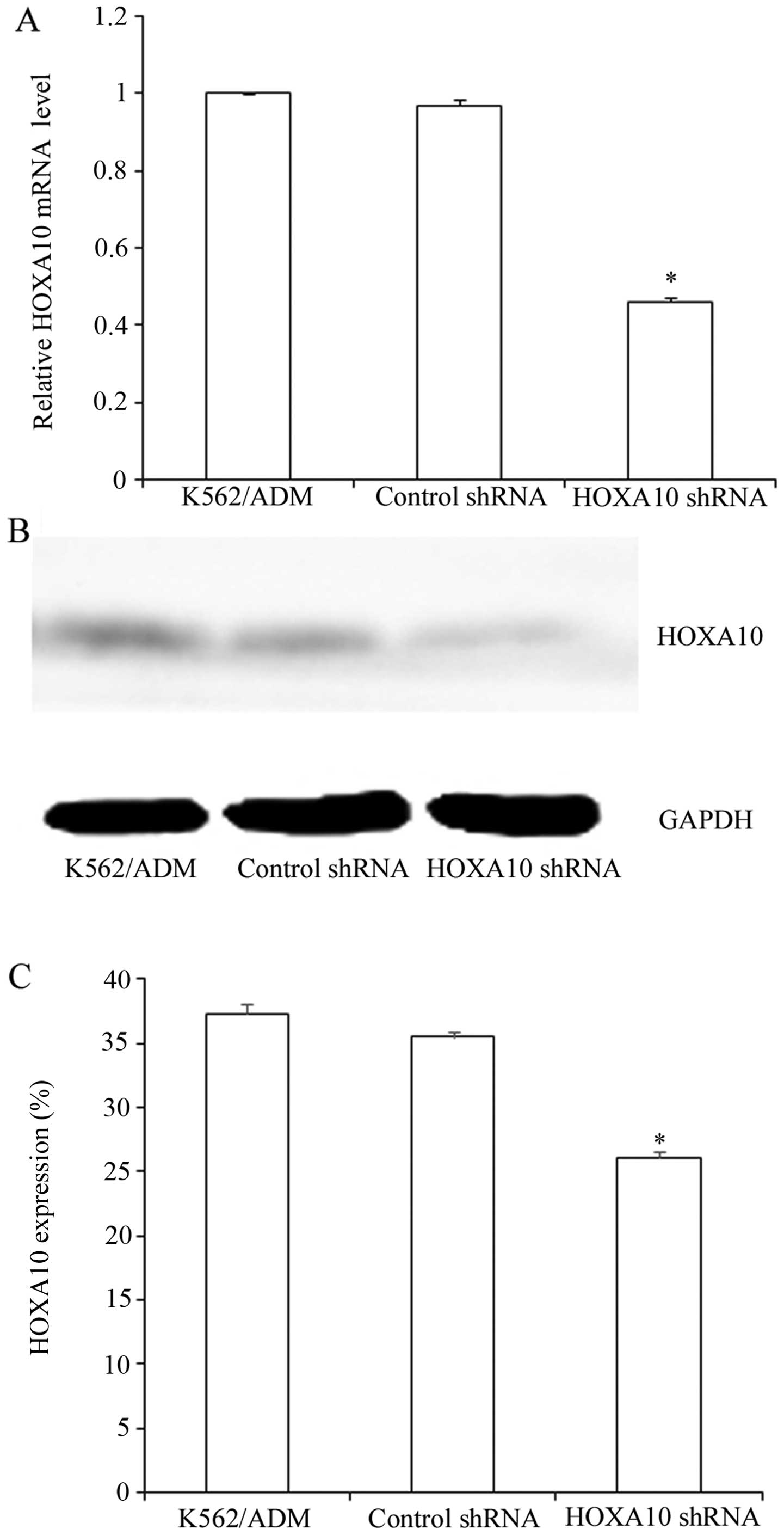
Suppression efficacy of HOXA10 shRNA
After transfection, more than 80% of cells were
GFP-positive, indicating high transfection efficiency (Fig. 2). RT-qPCR analysis revealed that
mRNA levels of HOXA10 in the K562/ADM cells transfected with HOXA10
shRNA decreased by 46.15±1.245%, while the control shRNA had almost
no influence on the HOXA10 mRNA levels in the K562/ADM cells
(P<0.05) (Fig. 3A). Western
blot analysis revealed that transfection with HOXA10 shRNA resulted
in a reduction to 26.1±0.489% compared with that in the parental
K562/ADM cells and the K562/ADM cells transfected with control
shRNA (P<0.05) (Fig. 3B and
C).
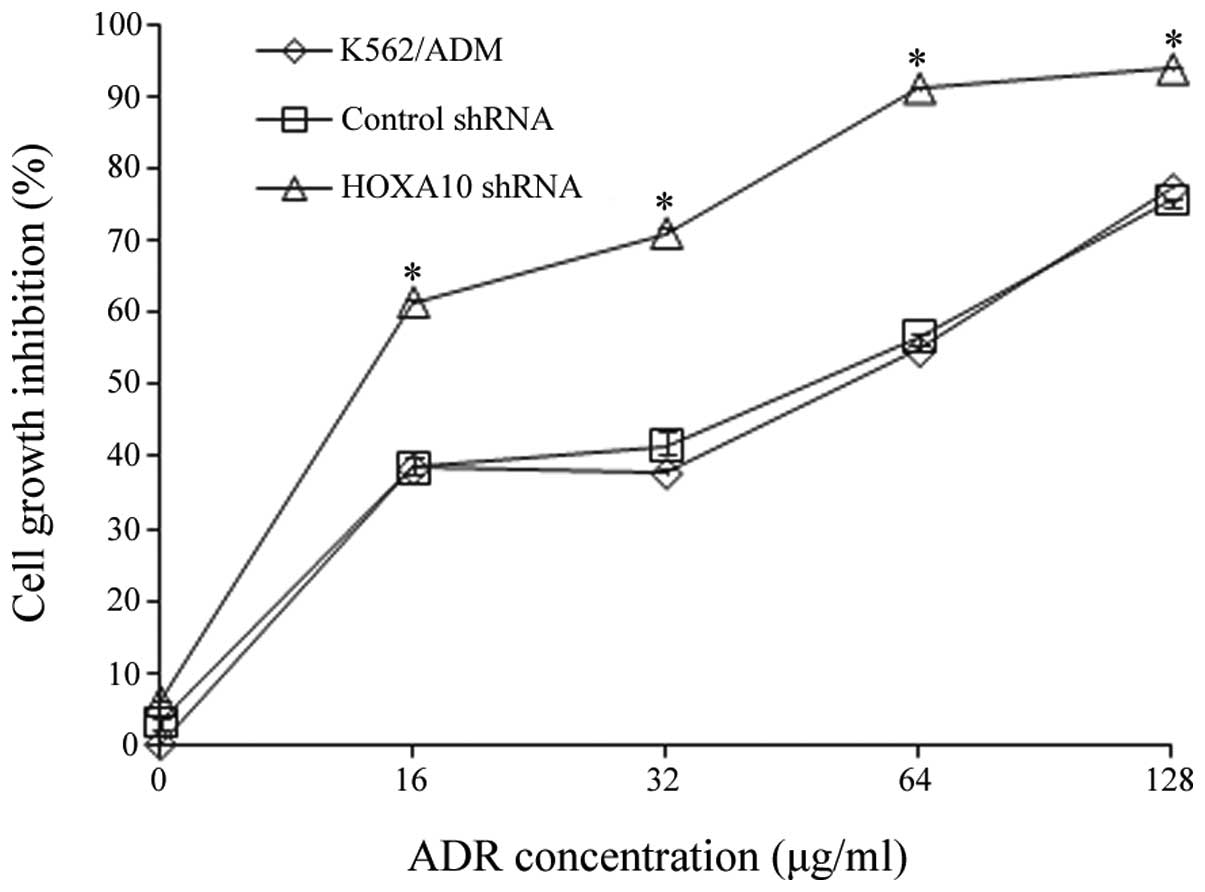
Evaluation of ADR-induced cytotoxicity by
knockdown of HOXA10
To determine whether the downregulation of HOXA10
affected multidrug resistance in vitro, the effect of HOXA10
shRNA on ADR-induced cytotoxicity was assessed by a CCK8 assay. The
results are shown in Table III
and Fig. 4. They indicated that
after 24 h, the K562/ADM cells transfected with HOXA10 shRNA had a
slower rate of cell proliferation compared with the cells
transfected with control shRNA and the parental K562/ADM cells
(P<0.05) (Fig. 4), suggesting
that the transfection of HOXA10 shRNA increased ADR-induced
cytotoxicity in the K562/ADM cells. Knockdown of HOXA10 caused a
2.587-fold reversal in the sensitivity of K562/ADM cells to ADR
according to the results of a CCK8 assay (P<0.05) (Table III).
 | Table IIIEffect of silencing homeobox A10
(HOXA10) on the sensitivity of K562/ADM toward adriamycin (ADR)
determined by CCK8 assay (means ± SD of triplicate
experiments). |
Table III
Effect of silencing homeobox A10
(HOXA10) on the sensitivity of K562/ADM toward adriamycin (ADR)
determined by CCK8 assay (means ± SD of triplicate
experiments).
| Treatment | IC50
(μg/ml) | Reversal fold |
|---|
| ADR + K562/ADM | 44.4435±1.08027 | |
| ADR + control
shRNA |
42.1894±1.03356 | 1.053 |
| ADR + HOXA10
shRNA |
17.1824±0.19211a | 2.587 |
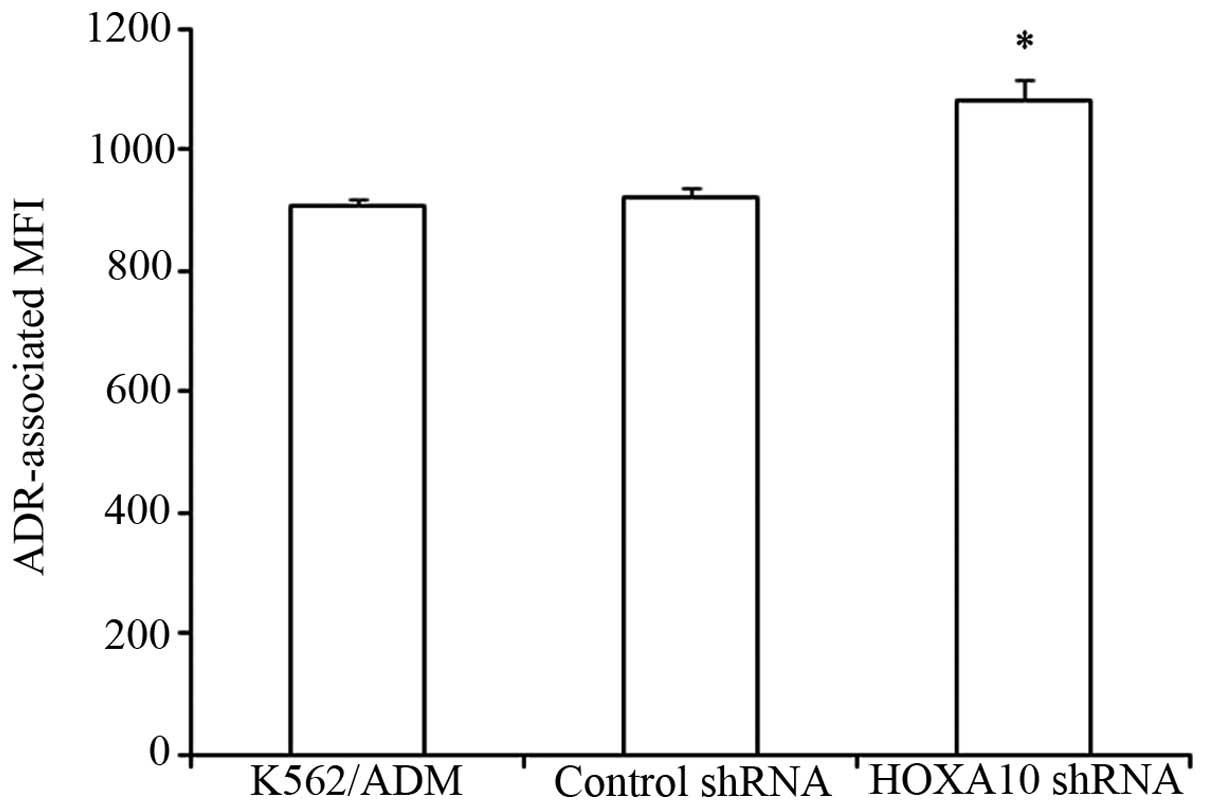
Knockdown of HOXA10 increases
intracellular accumulation of ADR
It was previously noted that the intracellular
accumulation of ADR decreased significantly in K562/ADM cells
compared to the parental K562 cells (7). In the present study, we determined
that knockdown of HOXA10 increased the intracellular accumulation
of ADR in K562/ADM cells in comparison with K562/ADM cells
transfected with control shRNA and parental K562/ADM cells. These
results indicated that knockdown of HOXA10 increased the
sensitivity of the K562/ADM cells to ADR through increasing the
intracellular accumulation of ADR (P<0. 05) (Fig. 5).
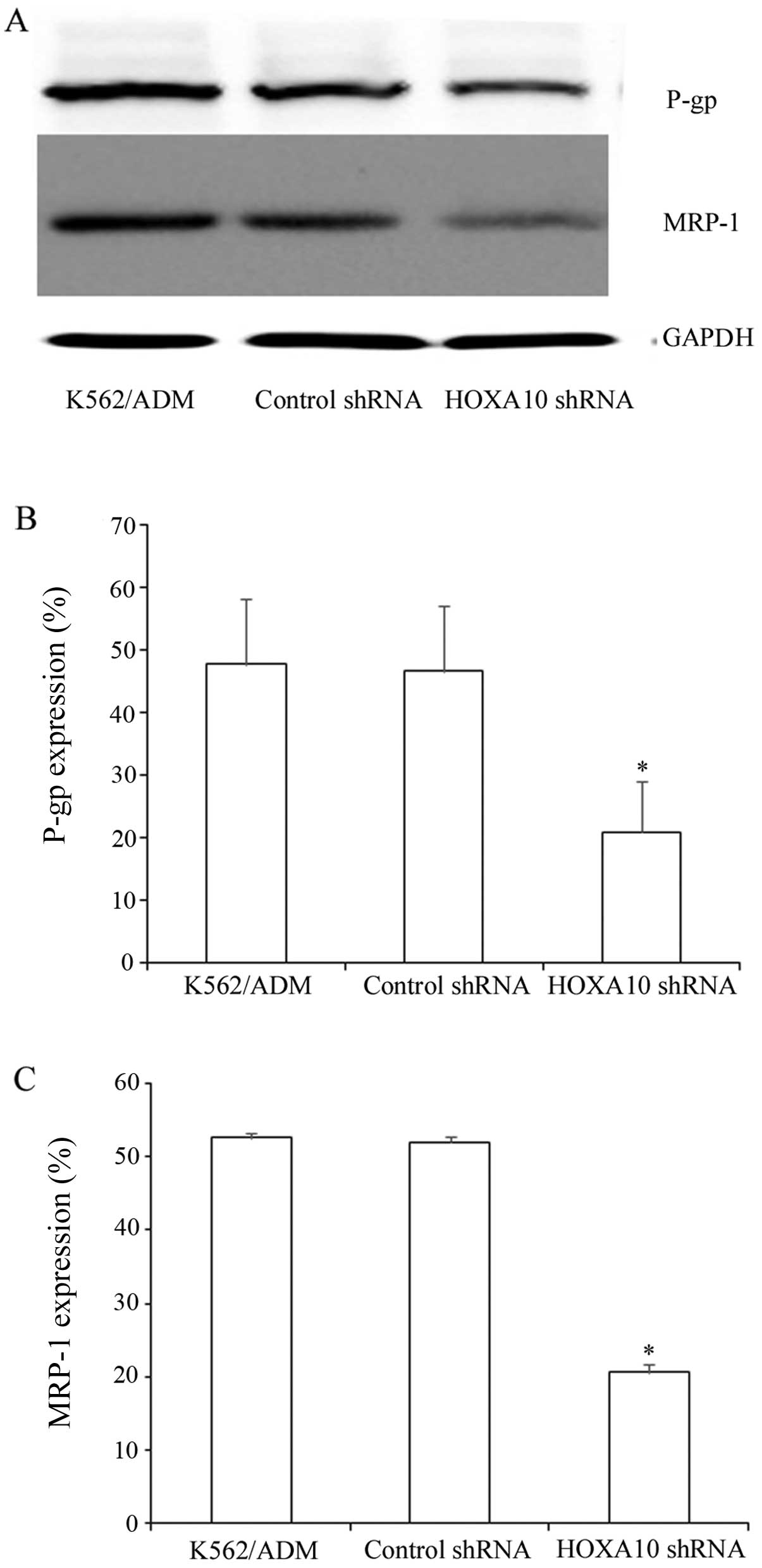
Knockdown of HOXA10 decreases the protein
expression of P-gp and MRP-1 in K562/ADM cells
P-gp and MRP-1 are ABC transporters, which are
overexpressed in many drug-resistant cells (9,11).
In the present study, the K562/ADM cells expressed P-gp and MRP-1
at high levels. Western blot analysis revealed that after knockdown
of HOXA10, P-gp and MRP-1 expression decreased markedly to
20.85±8.258% and 20.55±1.144%, respectively (Fig. 6A–C). These results indicate that
knockdown of HOXA10 decreases the protein expression of P-gp and
MRP-1 by increasing the intracellular accumulation of ADR.
Discussion
We noted that silencing of HOXA10 significantly
increased the cytotoxicity of ADR in K562/ADM cells. The effect of
silencing HOXA10 is associated with the increased intracellular
accumulation of ADR, as well as the inhibition of the expression of
P-gp and MRP-1.
CML is a stem cell disorder characterized by chronic
and blast-crisis phases (20).
Chemotherapy plays a vital role in CML treatment; however, it is
always accompanied by MDR which results in treatment failure. MDR
in tumors is characterized by the ability of the tumor cells to
exhibit simultaneous resistance to a number of structurally and
functionally unrelated chemotherapeutic agents (21). There are multiple mechanisms of
MDR in cancer, including the high expression of members of the ABC
transporter family, and abnormalities in enzymatic systems and
apoptosis. ADR is an effective chemotherapy drug that has been used
extensively in the treatment of CML despite the emergence of MDR,
which considerably limits the therapeutic efficacy of ADR (3). As shown in Table II, the K562/ADM cells
demonstrated significant resistance to ADR-induced cytotoxicity.
Overexpression of P-gp and MRP-1 is one of the best known causes of
MDR. Significant efforts have been made to identify novel
MDR-inhibiting genes, modulating the expression of P-gp and MRP-1.
It has been previously suggested that HOX genes are important
regulators in normal and leukemic stem cells. HOXA10, a member of
the HOX gene family, which has been reported to be an important
regulator in normal and leukemic stem cells, is frequently
over-expressed in myeloid leukemia (22–24). It has been reported that
overexpression of HOXA10 may exert both pro-differentiation and
anti-differentiation effects in a dose-dependent spatiotemporal
manner (25). High levels of
HOXA10 expression were predictive of tumor resistance to treatment.
Studies have demonstrated that HOXA10 is associated with resistance
in GBM (17,18). The inhibition of HOXA10 reinforced
temozolomide sensitivity independent of O6-methylguanine DNA
methyltransferase (MGMT) status in GBM cell lines (17). High expression levels of the
HOXA9/HOXA10 genes were noted in pediatric GBM patient samples as
well as in a TMZ-resistant pediatric GBM cell line (18). Temozolomide resistance in the high
HOXA9/HOXA10-expressing GBM cell line was independent of MGMT
status, and the PI3K pathway was considered to be an upstream
regulator of HOX genes that is targeted to overcome resistance
(18). In the present study, we
determined that the expression of HOXA10 was higher in the K562/ADM
cell line than in the K562 cell line (Fig. 1).
The present study was designed to investigate the
cellular functions of HOXA10 in order to elucidate the mechanism by
which it contributes to reversing MDR of human CML-derived K562/ADM
cell lines. The results revealed that the expression of HOXA10 was
higher in the K562/ADM cells than in the K562 cell lines, which
suggested that HOXA10 was involved in MDR of the K562/ADM cell
lines. This study used shRNA to reduce the expression of HOXA10 in
the human CML K562/ADM cell line. As shown in Fig. 3, the shRNA-containing vector
efficiently suppressed HOXA10 expression at both the mRNA and
protein levels. The effect of HOXA10 knockdown on the cellular
functions of K562/ADM cells was then explored. The results of the
CCK8 assay indicated that ADR-induced cytoxicity in the K562/ADM
cells transfected with HOXA10 shRNA was significantly increased
compared with that in the control cells, which confirmed that
knockdown of HOXA10 was capable of reversing MDR in the K562/ADM
cells (Fig. 4 and Table III). The flow cytometric
analysis indicated that the silencing of HOXA10 increased the
intracellular accumulation of ADR. These results indicated that
knockdown of HOXA10 reversed MDR by increasing the intracellular
accumulation of ADR. To further examine the mechanism by which
knockdown of HOXA10 increased the intracellular accumulation of ADR
within the K562/ADM cells, the expression of P-gp and MRP-1 were
measured. Overexpression of P-gp and MRP-1, members of the ABC
transporter family, lower intracellular drug accumulation and
decrease the cellular toxicity of chemotherapeutic agents, such as
adriamycin, daunorubicin, epirubicin, mitoxantrone, bisantrene,
vincristine, vinblastine, etoposide and paclitaxel (11,25). P-gp is one of the most well-known
MDR-associated proteins, which functions as an ATP-dependent
transmembrane drug transporter that reduces intracellular drug
accumulation by pumping drugs out of cells (3). MRP-1, another energy-dependent drug
pump, plays an important role in MDR by decreasing the
intracellular accumulation of chemotherapeutics agents (5). In the present study, western blot
analysis revealed that transfection with HOXA10 shRNA significantly
decreased the expression of P-gp and MRP-1 compared with the
expression levels in the cells transfected with control shRNA and
the parental K562/ADM cells (Fig.
6). The decreased expression of P-gp and MRP-1 increased the
intracellular accumulation of ADR in the K562/ADM cells. Silencing
of HOXA10 may increase the intracellular accumulation of ADR as a
result of the downregulation of P-gp and MRP-1 proteins. Hence, the
results confirmed that knockdown of HOXA10 reversed MDR through
modulating the expression of P-gp and MRP-1.
In conclusion, HOXA10 was expressed at a high level
in the K562/ADM cells, and knockdown of HOXA10 enhances the
sensitivity of the K562/ADM cells to cytotoxic killing by the
therapeutic drug, ADR, as a result of the increased intracellular
accumulation of ADR. The accumulation of ADR induced by HOXA10
silencing was associated with the downregulation of P-gp and MRP-1
proteins. To the best of our knowledge, we are the first to suggest
that knockdown of HOXA10 is a novel and potent therapeutic target
that may be used for reversing MDR in human CML-derived mylogenous
leukemia K562/ADM cells.
Acknowledgments
This study was supported by the Natural Science
Foundation of Shandong Province (no. ZR2014HL032), the Projects of
Medical and Health Technology Development Program in Shandong
Province (no. 2014WS0183) and the Shandong Science and Technology
Committee (no. 2010GSF10264).
Abbreviations:
|
GFP
|
green fluorescent protein
|
|
PBS
|
phosphate-buffered saline
|
|
SDS-PAGE
|
sodium dodecyl sulphate polyacrylamide
gel electrophoresis
|
|
shRNA
|
short hairpin RNA
|
|
HOXA10 shRNA
|
homeobox A10 short hairpin RNA
|
|
control shRNA
|
control short hairpin RNA
|
References
|
1
|
Li H, Guo K, Wu C, Shu L, Guo S, Hou J,
Zhao N, Wei L, Man X and Zhang L: Controlled and targeted drug
delivery by a UV-responsive liposome for overcoming
chemo-resistance in non-Hodgkin lymphoma. Chem Biol Drug.
86:783–794. 2015. View Article : Google Scholar
|
|
2
|
Nabekura T, Hiroi T, Kawasaki T and Uwai
Y: Effects of natural nuclear factor-kappa B inhibitors on
anticancer drug efflux transporter human P-glycoprotein. Biomed
Pharmacother. 70:140–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ren J, Xu Y, Huang Q, Yang J, Yang M, Hu K
and Wei K: Chabamide induces cell cycle arrest and apoptosis by the
Akt/MAPK pathway and inhibition of P-glycoprotein in K562/ADR
cells. Anticancer Drugs. 26:498–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen X, Zhang HD, Zhao L, Yao YF, Zhao JH
and Tang JH: Ginsenoside Rh2 differentially mediates microRNA
expression to prevent chemoresistance of breast cancer. Asian Pac J
Cancer Prev. 16:1105–1109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao W, Zhu F, Duan Y, Yang Y and Cai H:
HtrA1 resensitizes multidrug-resistant hepatocellular carcinoma
cells by targeting XIAP. Biomed Pharmacother. 70:97–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Yang L, Xia Y, Guo C and Kong L:
Icariin enhances cytotoxicity of doxorubicin in human
multidrug-resistant osteosarcoma cells by inhibition of ABCB1 and
down-regulation of the PI3K/Akt pathway. Biol Pharm Bull.
38:277–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song YN, Guo XL, Zheng BB, Liu XY, Dong X,
Yu LG and Cheng YN: Ligustrazine derivate DLJ14 reduces multidrug
resistance of K562/A02 cells by modulating GSTπ activity. Toxicol
In Vitro. 25:937–943. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma J, Wang T, Guo R, Yang X, Yin J, Yu J,
Xiang Q, Pan X, Tang H and Lei X: Involvement of miR-133a and
miR-326 in ADM resistance of HepG2 through modulating expression of
ABCC1. J Drug Target. 23:519–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang X, Iyer AK, Singh A, Choy E, Hornicek
FJ, Amiji MM and Duan Z: MDR1 siRNA loaded hyaluronic acid-based
CD44 targeted nanoparticle systems circumvent paclitaxel resistance
in ovarian cancer. Sci Rep. 5:85092015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fantappiè O, Sassoli C, Tani A, Nosi D,
Marchetti S, Formigli L and Mazzanti R: Mitochondria of a human
multidrug-resistant hepatocellular carcinoma cell line
constitutively express inducible nitric oxide synthase in the inner
membrane. J Cell Mol Med. 19:1410–1417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu X, Zhang Y, Li W, Miao H, Zhang H, Zhou
Y, Li Z, You Q, Zhao L and Guo Q: Wogonin reverses multi-drug
resistance of human myelogenous leukemia K562/A02 cells via
downregulation of MRP1 expression by inhibiting Nrf2/ARE signaling
pathway. Biochem Pharmacol. 92:220–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bei L, Shah C, Wang H, Huang W, Roy R and
Eklund EA: β-catenin activates the HOXA10 and CDX4 genes in myeloid
progenitor cells. J Biol Chem. 287:39589–39601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Lindsey S, Konieczna I, Bei L,
Horvath E, Huang W, Saberwal G and Eklund EA: Constitutively active
SHP2 cooperates with HoxA10 overexpression to induce acute myeloid
leukemia. J Biol Chem. 284:2549–2567. 2009. View Article : Google Scholar :
|
|
14
|
Vitiello D, Pinard R and Taylor HS: Gene
expression profiling reveals putative HOXA10 downstream targets in
the periimplantation mouse uterus. Reprod Sci. 15:529–535. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bei L, Huang W, Wang H, Shah C, Horvath E
and Eklund E: HoxA10 activates CDX4 transcription and Cdx4
activates HOXA10 transcription in myeloid cells. J Biol Chem.
286:19047–19064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Magnusson M, Brun AC, Miyake N, Larsson J,
Ehinger M, Bjornsson JM, Wutz A, Sigvardsson M and Karlsson S:
HOXA10 is a critical regulator for hematopoietic stem cells and
erythroid/megakaryocyte development. Blood. 109:3687–3696. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JW, Kim JY, Kim JE, Kim SK, Chung HT
and Park CK: HOXA10 is associated with temozolomide resistance
through regulation of the homologous recombinant DNA repair pathway
in glioblastoma cell lines. Genes Cancer. 5:165–174.
2014.PubMed/NCBI
|
|
18
|
Gaspar N, Marshall L, Perryman L, Bax DA,
Little SE, Viana-Pereira M, Sharp SY, Vassal G, Pearson AD, Reis
RM, et al: MGMT-independent temozolomide resistance in pediatric
glioblastoma cells associated with a PI3-kinase-mediated HOX/stem
cell gene signature. Cancer Res. 70:9243–9252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JY, Jia XH, Xing HY, Li YJ, Fan WW,
Li N and Xie SY: Inhibition of Forkhead box protein M1 by
thiostrepton increases chemosensitivity to doxorubicin in T-cell
acute lymphoblastic leukemia. Mol Med Rep. 12:1457–1464.
2015.PubMed/NCBI
|
|
20
|
Sengupta A, Banerjee D, Chandra S, Banerji
SK, Ghosh R, Roy R and Banerjee S: Deregulation and cross talk
among Sonic hedgehog, Wnt, Hox and Notch signaling in chronic
myeloid leukemia progression. Leukemia. 21:949–955. 2007.PubMed/NCBI
|
|
21
|
Abdallah HM, Al-Abd AM, El-Dine RS and
El-Halawany AM: P-glycoprotein inhibitors of natural origin as
potential tumor chemo-sensitizers: a review. J Adv Res. 6:45–62.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sloma I, Imren S, Beer PA, Zhao Y, Lecault
V, Leung D, Raghuram K, Brimacombe C, Lambie K, Piret J, et al: Ex
vivo expansion of normal and chronic myeloid leukemic stem cells
without functional alteration using a NUP98HOXA10homeodomain fusion
gene. Leukemia. 27:159–169. 2013. View Article : Google Scholar :
|
|
23
|
Yao J, Fang LC, Yang ZL, Huang H, Li Y,
Deng J and Zheng J: Mixed lineage leukaemia histone methylases 1
collaborate with ERα to regulate HOXA10 expression in AML. Biosci
Rep. 34:e001562014. View Article : Google Scholar
|
|
24
|
Zhang L, Wan Y, Jiang Y, Ma J, Liu J, Tang
W, Wang X and Cheng W: Upregulation HOXA10 homeobox gene in
endometrial cancer: role in cell cycle regulation. Med Oncol.
31:522014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang XB, Wang SS, Zhang QF, Liu M, Li HL,
Liu Y, Wang JN, Zheng F, Guo LY and Xiang JZ: Inhibition of
tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug
resistant human hepatocellular carcinoma cells. Oncol Rep.
23:211–215. 2010.
|