Introduction
Breast cancer is the most frequently diagnosed type
of cancer and the leading cause of cancer-related mortality in
women worldwide, accounting for 1.7 million cases and 521,900
deaths in 2012 (1). Although
methods for the screening and treatment of breast cancer have
improved over the years (2), the
molecular pathogenesis of breast cancer remains unclear and breast
cancer remains a major public health challenge (3).
MicroRNAs (miRNAs or miRs) are small non-coding RNA
molecules that modulate gene expression by negatively regulating
the stability or translational efficiency of their target mRNAs by
targeting the 3′-untranslated region (3′-UTR) (4). More than 474 miRNA genes have been
identified in the human genome, and over 10% of the protein-coding
mRNAs may be conserved targets of miRNAs (5,6),
which suggests an important role of miRNAs in biological processes
in humans. It has been shown that miRNAs are aberrantly expressed
or mutated in cancer, and the analysis of miRNA profiles can help
to distinguish between normal and cancerous tissue (7). The majority of miRNAs, such as
miR-21 and miR-155, are overexpressed in several human cancers
(8–11), while other miRNAs, such as Let-7
and miR-34, are frequently downregulated in human cancers (12–15); thus, miRNAs can function as either
oncogenes or tumor suppressor genes (16). Various cellular processes,
including proliferation, apoptosis, cell cycle progression,
differentiation and metastasis, have been implicated to be under
the regulation of miRNAs (17–19). All these above-mentioned studies
suggest an important role of miRNAs in the development of cancer. A
complete understanding of miRNAs and cancer may provide new insight
into the molecular mechanisms underlying tumorigenesis.
Breast cancer was one of the first solid tumors in
which miRNA expression was profiled. Iorio et al (20) identified 29 miRNAs whose
expression was significantly dysregulated in breast cancer compared
to normal breast tissues, including miR-10b and miR-125b, which
were downregulated, and miR-145 and miR-21, which were upregulated
in breast cancer. miR-101 was also downregulated in breast cancer
(20), and its established
targets include enhancer of zeste homolog 2 (EZH2), DNA
methyltransferase 3A (DNMT3A), and microphthalmia-associated
transcription factor (MITF) (21–23). However, there are only a few
studies available on the potential function of miR-101 in breast
cancer (20,41).
The eyes absent (Drosophila) (EYA) proteins
are crucial regulators in the development of the eye (24,25). Four homologs of the EYA family
proteins (EYA 1–4) are defined by a conserved 275-amino acid
carboxyl-terminal motif, referred to as the EYA domain (ED). The
overexpression of EYA family proteins has been reported in many
cancer cell lines, including in ovarian and breast cancer cell
lines (26,27), and it has also been reported that
the overexpression of EYA proteins promotes cell proliferation
through the epidermal growth factor receptor
(EGFR)/RAS/mitogen-activated protein kinase (MAPK) and Notch
signaling pathways (28,29). EYA1 is an essential transactivated
gene whose mutation can cause branchio-oto-renal and branchio-oto
syndromes. The overexpression of EYA1 has been observed in cancers
(30,31), and EYA1 can form a transcription
complex with sine oculis homeobox 1 (SIX 1), which regulates
expression of a number of downstream target genes that are
important for cell proliferation, survival and migration (32). Although EYA1 has been shown to
play an important role in cancer development and progression,
whether miRNAs can regulate EYA1 expression has not yet been
reported, at least to the best of our knowledge. Thus, our study
aimed to assess the expression level of miR-101 and to elucidate
the regulatory effects of miR-101 in breast cancer.
Materials and methods
Human tissue specimens
Breast cancer and normal adjacent breast tissues
were obtained from 28 patients with breast cancer who had not
received any pre-operative cancer treatments at the Second
Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China. The
tissue samples obtained from surgery were frozen in liquid nitrogen
immediately. Informed consent was obtained from all patients. This
study was approved by the Ethics Committee of the Second Affiliated
Hospital of Xi'an Jiaotong University.
Cell culture
The breast cancer cell lines, MDA-MB-231, T-47D,
SKBR3, MCF-7, BT474 and HS578T, and the normal breast epithelial
cell line, MCF-10, were all obtained from ATCC (Manassas, VA, USA)
and were cultured in Dulbecco's modified Eagle's medium
(Invitrogen, Carlsbad, CA, USA) containing 1%
penicillin/streptomycin (Sigma, St. Louis, MO, USA) and
supplemented with 10% fetal bovine serum (Invitrogen) in a 5%
CO2 cell culture incubator.
miRNA transfection and small RNA
interference
The SKBR3 cells were seeded in 6-well plates, and
miR-101 mimic (miR-101) or its negative control (NC-miR), and
miR-101 inhibitor (miR-101-in) or its negative control (miR-NC-in)
(Dharmacon, Lafayette, CO, USA), were transfected into the cells
using Lipofectamine 2000 transfection reagent (Invitrogen)
according to the instructions provided by the manufacturer. The
cells were harvested for analysis after being incubated for 48
h.
To knockdown EYA1 expression, siRNA targeting EYA1
(EYA1-siRNA) and its negative control (EYA1-NC) (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) were transfected into the SKBR3
cells. Following culture for 48 h, the cells were harvested and the
expression level of miR-101 and EYA1 was measured as described
below.
Cell proliferation assay
Cell proliferation was determined using CCK-8
reagent (Beyotime, Nantong, China) according to the manufacturer's
instructions. The cells were plated into a 96-well plate at
5×104 cells/well. After 1, 2, 3, 4 and 5 days, the cells
were treated with CCK-8 reagent and incubated for 2 h. The
absorbance was measured using a Bio-Rad microplate reader (Bio-Rad,
Hercules, CA, USA) at a wavelength of 450 nm.
Apoptosis assay
Apoptotic cells were detected using the Annexin
V-FITC/PI apoptosis detection kit (Abcam, Cambridge, UK). In brief,
1×105 cells were harvested and incubated with Annexin
V-FITC for 5 min in the dark, and then stained with PI for a
further 5 min. The apoptotic cells were analyzed using a
FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA,
USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
To determine the abundance of miR-101, total miRNAs
were extracted from the SKBR3 cells using the miRcute miRNA
isolation kit (Tiangen, Beijing, China), and cDNA was synthesized
using the miRcute miRNA First-Strand cDNA synthesis kit (Tiangen).
qPCR was performed using the miScript SYBR Green PCR kit (Qiagen,
Valencia, CA, USA) according to the manufacturers instructions. U6
was used as a quantitative and qualitative control to normalize
miRNA expression. The RT-PCR primers for miR-101 were forward,
5′-TGGGCTACAGTACT GTGATA-3′ and reverse, 5′-TGCGTGTCGTGGAGTC-3′ as
previously described (36).
To determine the mRNA expression level of EYA1,
total RNA was extracted from the SKBR3 cells using TRIzol reagent
(Invitrogen), and cDNA was synthesized using MMLV reverse
transcriptase (Clontech, Palo Alto, CA, USA). qPCR was performed
using TaqMan PCR Master Mix and an ICycler system (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). β-actin was
used as a negative control. The RT-PCR primers for EYA1 [as
previously described (33)] were
forward, 5′-TAACGGACAGGACCTAAGCA-3′ and reverse,
5′-TTTCTCATCCAgTCCACACC-3′.
Target prediction
To determine whether EYA1 is a direct tareget of
miR-101, we searched for the potential targets of miR-101 using the
prediction programs, microRNA.org
(http://www.microrna.org/microrna/home.do), TargetScan
(http://www.targetscan.org/vert_60/)
and PicTar (http://www.pictar.org/cgi-bin/PicTar_vertebrate.cgi).
Dual-luciferase reporter assay
The SKBR3 cells co-transfected with miR-101 mimic
and luciferase reporter constructs containing a wild-type or mutant
EYA1 3′-UTR (Promega, Madison, WI, USA) were seeded into a 24-well
plate. Forty-eight hours later, the cells were harvested and the
luciferase activity was analyzed using the dual-luciferase reporter
assay kit (Promega). All transfection experiments were reproduced
at least 3 times.
Western blot analysis
The SKBR3 cells were lysed with RIPA buffer
(Beyotime, Bejing, China) to extract total protein, and the protein
concentration was quantified using the Bradford assay (Beyotime). A
total of 20 µg protein was separated by electrophoresis on a
12% SDS-PAGE gel. Proteins were transferred onto nitrocellulose
membranes (Bio-Rad), and then incubated with 2% non-fat milk to
block non-specific binding for 1 h at 37°C. The membranes were then
incubated with the following primary antibodies: rabbit anti-EYA1
(ab85009), rabbit anti-jagged1 (ab7771), rabbit anti-Hey1 (ab22614)
and rabbit anti-Hes1 (ab71559) polyclonal antibodies (Abcam)
overnight at 4°C. The membranes were washed with TAS-Tween (TBST) 3
times, and then incubated with secondary antibody horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (ab6762; Abcam)
for 4 h at 37°C. Proteins were detected using an enhanced
chemiluminescence detection system (Amersham, Piscataway, NJ, USA)
after washing with TBST 3 times.
Statistical analysis
Data are presented as the means ± standard deviation
(SD) and the statistically significant differences between
different groups were analyzed using SPSS 18.0 software (SPSS nc.,
Chicago, IL, USA) and the Student's t-test. A value of p<0.05
was considered to indicate a statistically significant
difference.
Results
miR-101 is downregulated in breast cancer
tissues and cell lines
The results of RT-qPCR revealed that miR-101 was
significantly downregulated in the breast cancer tissues compared
with the normal adjacent breast tissues, and miR-101 could not be
detected in 6 of the 28 breast cancer tissues (Fig. 1A, p<0.05). The expression of
miR-101 was also examined in 6 breast cancer cell lines, and the
results revealed that miR-101 was markedly downregulated in all 6
breast cancer cell lines compared with the normal breast epithelial
cell line, MCF-10 (Fig. 1B,
p<0.05).
miR-101 regulates cell proliferation and
apoptosis
The downregulation or even the loss of miR-101 in
breast cancer suggested that it may play a role in the development
and progression of breast cancer. To determine the role of miR-101
in breast cancer, we transfected miR-101, miR-101-in or their
negative control into the SKBR3 cells, and examined the effects of
miR-101 on breast cancer cell proliferation by CCK-8 assay. The
results revealed that transfection with miR-101 significantly
upregulated miR-101 expression, whereas transfection with
miR-101-in significantly downregulated miR-101 expression (Fig. 2A, p<0.05). The overexpression
of miR-101 significantly decreased the proliferation of the SKBR3
cells, and the decreased expression of miR-101 promoted the
proliferation of the SKBR3 cells in a time-dependent manner
(Fig. 2B, p<0.05). To
determine whether miR-101 also regulates the apoptosis of breast
cancer cells, we examined the apoptosis of the SKBR3 cells
transfected with miR-101 or miR-101-in using an Annexin V-FITC
assay. A marked increase in apoptosis was induced by transfection
with miR-101 mimic in the SKBR3 cells (Fig. 2C, p<0.05).
miR-101 directly targets EYA1 in breast
cancer cells
To elucidate the potential molecular mechanisms
responsible for the inhibitory effects of miR-101 on the
proliferation of breast cancer cells and its apoptosis promoting
effects, we searched for the potential targets of miR-101 using the
prediction programs microRNA.org,
TargetScan and PicTar. Two conserved pairing target regions
(position 198–204 and 1899–1906) between the EYA1 3′-UTR and
miR-101 were found, and according to the context++ score, position
1899–1906 was selected for further analysis (Fig. 3A), suggesting that EYA1 may be a
potential target of miR-101. To determine whether miR-101 directly
targets the 3′-UTR region of EYA1, a dual-luciferase reporter assay
was performed. Transfection with miR-101 significantly reduced the
luciferase reporter activity of the wild-type EYA1 3′-UTR compared
with the luciferase reporter activity of the mutant 3′-UTR.
Conversely, miR-101 caused no significant changes in the expression
of the transcript containing the mutant 3′-UTR of EYA1. The
luciferase activity of the reporter plasmid was also not affected
by transfection with miR-101 inhibitor (Fig. 3B).
As miR-101 suppressed the luciferase transcript
containing the wild-type EYA1 3′-UTR, we speculated that miR-101
may regulate the expression of EYA1 in breast cancer. To verify
this, we examined the potential regulatory effect of miR-101 on the
expression of EYA1. miR-101 or miR-101-in was transfected into the
SKBR3 cells. Western blot analysis revealed a significantly
decreased protein level of EYA1 in the SKBR3 cells transfected with
miR-101 compared with the controls (Fig. 3C, p<0.05).
EYA1 is overexpressed in breast cancer
tissues and cell lines
As EYA1 was found to be a direct target of miR-101
and miR-101 expression was found to be downregulated in breast
cancer, we examined the expression level of EYA1 in the breast
cancer tissues and cell lines. The expression of EYA1 was
significantly higher in the breast cancer tissues than the adjacent
normal breast tissues (Fig. 4A,
p<0.05). RT-qPCR analysis of the breast cancer cell lines also
revealed that EYA1 expression in the breast cancer cell lines,
apart from the T-47D cell line, was significantly upregulated
(Fig. 4B, p<0.05).
EYA1 is involved in the regulation of
cell proliferation and apoptosis by miR-101
EYA1 is a crucial regulator of cell growth in
cancers (31). Our study explored
the role of EYA1 in the regulatory effects of miR-101 in breast
cancer. The expression of EYA1 was silenced using EYA1-siRNA
(p<0.05, Fig. 5A). CCK-8 assay
was then performed to examine the proliferation of the SKBR3 cells
transfected with miR-101, miR-101-in, or EYA1-siRNA, or
co-transfected with EYA1-siRNA and miR-101 inhibitor. The inhibitor
of miR-101 (miR-101-in) promoted cell proliferation. When the cells
were transfected with miR-101 or EYA1-siRNA, cell proliferation
decreased markedly (p<0.05, Fig.
5B). Transfection with EYA1-siRNA also significantly inhibited
the increased proliferation and decreased the apoptosis that was
induced by the miR-101-inhibitor. The Annexin V-FITC assay
indicated that miR-101 promoted apoptosis and that miR-101-in
inhibited apoptosis, whereas EYA1-siRNA and co-transfection with
EYA1-siRNA and miR-101-in promoted apoptosis (p<0.05, Fig. 5C).
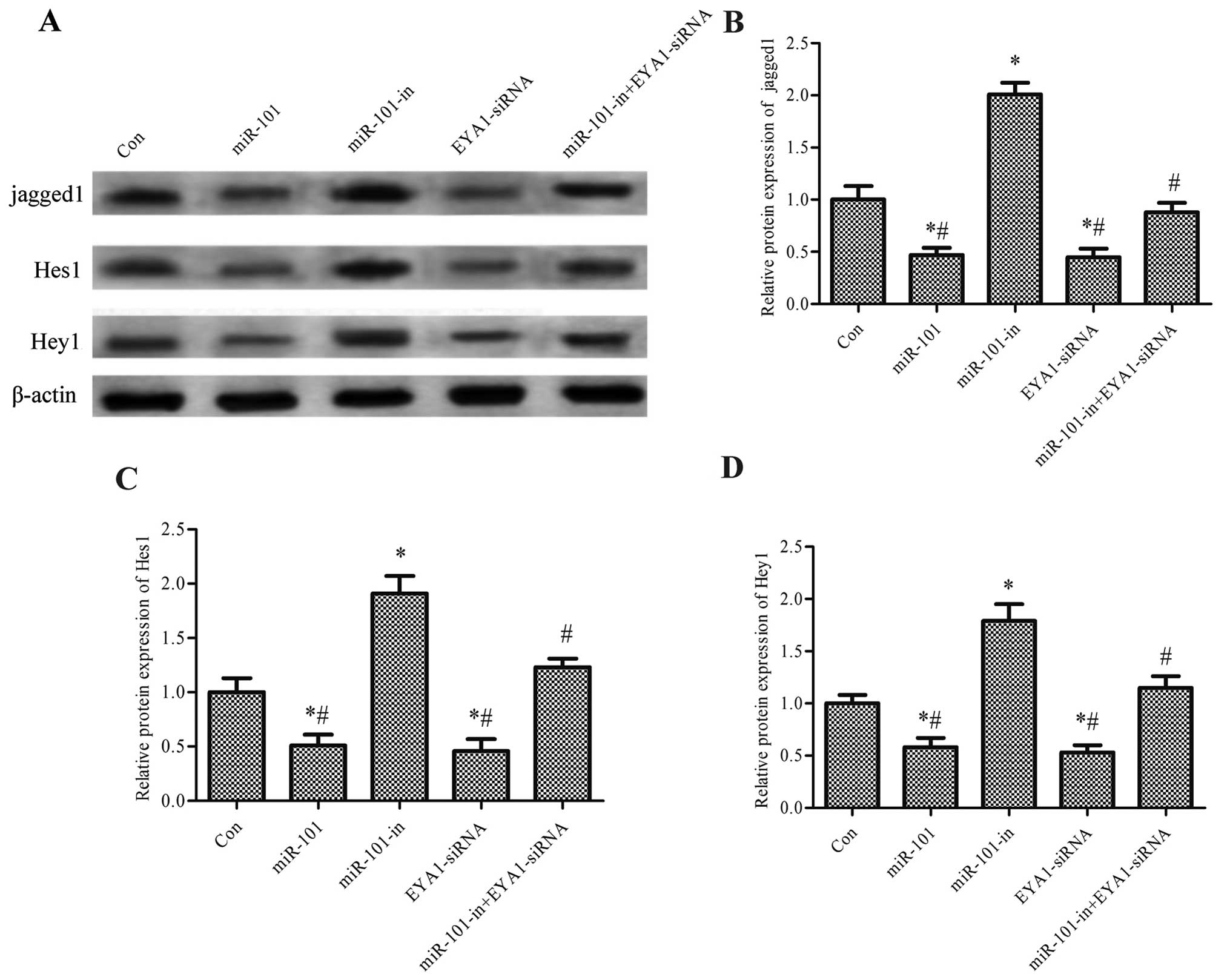
miR-101 regulates the Notch signaling
pathway through EYA1
To determine the signaling pathway involved in the
regulatory effects of miR-101 in breast cancer, we analyzed the
Notch signaling pathway. The expression level of jagged1, one of
the important ligands of Notch, and Hes1 and Hey1, the downstream
genes of the Notch pathway, were analyzed in SKBR3 cells
transfected with miR-101, miR-101-in or EYA1-siRNA. The protein
expression levels of jagged1, Hes1 and Hey1 were significantly
decreased following transfection with miR-101 and EYA1-siRNA, but
were increased following transfection with miR-10-in (p<0.05).
Furthermore, the elevated protein expression levels of jagged1,
Hes1 and Hey1 induced by miR-101-in in the SKBR3 cells were
significantly decreased by transfection with EYA1-siRNA (p<0.05,
Fig. 6).
Discussion
The dysregulation of miRNAs has frequently been
reported in several types of cancer (34). Increasing evidence has
demonstrated a cancerocidal or carcinogenic role of miRNAs in
mediating the process of tumorigenesis and the behavior of cancer
cells (35). In the present
study, we confirmed that miR-101 expression was downregulated or
even lost in human cancer tissues and cell lines. We also found
that miR-101 inhibited cell growth and promoted apoptosis in
vitro. We further demonstrated that EYA1 is a functional target
of miR-101, and that miR-101 regulates cell behavior by targeting
EYA1 through the Notch signaling pathway.
miRNA-101 has been shown to be downregulated in
gastric cancer, glioblastoma, prostate cancer, colon cancer and
hepatocellular carcinoma, and is involved in cell migration,
invasion, proliferation and apoptosis (36–40). The decreased expression of miR-101
has also been detected in human breast cancer tissues (20). In this study, we detected the
expression of miR-101 in human breast cancer tissues and cell
lines, and found that miR-101 was downregulated in both tissues and
cell lines compared with adjacent normal tissues and cells,
respectively. Indeed, the expression of miR-101 was not detectable
in 6 of the 28 breast cancer tissues, which is in accordance with
the study by Varambally et al (41), who found that 6 of 29 breast
cancer tissues were negative for miR-101. Thus, we speculated that
miR-101 may function as a tumor suppressor gene in breast cancer.
To verify this, we examined cell proliferation and apoptosis
following the up- or downregulation of miR-101 by transfecting the
cells with miR-101 or miR-101-in, respectively. The overexpression
of miR-101 inhibited cell proliferation and promoted apoptosis,
whereas the downregulation of miR-101 exerted an opposite effect,
indicating that miR-101 may serve as a novel therapeutic marker in
breast cancer patients.
Cancer is a complex disease that is strongly
dependent on the regulation of gene expression. mRNAs exert their
regulatory effects by base-pairing with their target mRNAs, and a
single miRNA can regulate multiple downstream target mRNAs.
Although proto-oncogene MYCN, histone methyltransferase EZH2 and
DNM3A have been reported to be targets of miR-101 in cancers
(22,41,42), the regulatory effects of miR-101
in cancer are not yet fully understood. Thus, in the present study,
we searched for the target gene of miR-101 on microRNA.org, TargetScan and PicTar. Two conserved
pairing target regions between the EYA1 3′-UTR and miR-101 were
found. Position 1899–1906 was selected for further analysis through
luciferase reporter assays. The results confirmed that EYA1 is a
direct target of miR-101, and furthermore, miR-101 downregulated
the protein expression of EYA1. EYA1 is an important factor of the
retinal determination gene network involved in organismal
development (43). The
dysregulation of EYA1 has been reported in many types of cancer in
recent years (33,44,45); however, the role of EYA1 in tumor
growth may vary in different types of cancer. The decreased
expression of EYA1 in gastric tumor samples has been described, and
EYA1 has been shown to negatively correlate with tumor size,
lymphatic invasion and distant metastasis (33).
Wu et al (31) investigated the expression of EYA1
and showed that it was upregulated both in breast cancer tissues
and cell lines, and that EYA1 contributed to cancer cell growth by
promoting cell proliferation and DNA synthesis, and also reduced
apoptosis. Our study demonstrated that the expression level of EYA1
was significantly upregulated in breast cancer tissues and cells,
and that the knockdown of EYA1 inhibited the effects of miR-101 on
cell proliferation and apoptosis. Taken together, our study
indicates that the regulatory effects of miR-101 on the growth and
development of breast cancer may be partly mediated by targeting
EYA1.
Notch is one of the fundamental signaling pathways
that regulates embryonic cell fate decisions, differentiation,
proliferation and patterning (46,47). The dysregulation of Notch
signaling was discovered in various types of cancer and has been
shown to correlate with cell cycle progression, proliferation and
apoptosis (48,49). The aberrant activation of the
Notch pathway leads to adenocarcinoma of the mammary gland
(50). Stylianou et al
(51) demonstrated that activated
Notch signaling protects breast cancer cells from apoptosis. Our
study revealed that Notch was the downstream signaling pathway of
EYA1, which was under the regulatory effect of miR-101.
In conclusion, our results demonstrate that miR-101
is downregulated both in breast cancer tissues and cell lines, and
that it directly downregulates the expression of EYA1 at the
post-translational level. The Notch signaling pathway is also
proposed to play an important role in the regulatory effects of
miR-101. Considering these results, miR-101 may be used as a
potential therapeutic marker in breast cancer.
Abbreviations:
|
miRNA or miR
|
microRNA
|
|
3′-UTR
|
3′-untranslated region
|
|
EYA1
|
eyes absent homolog 1
(Drosophila)
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rhodes DJ, O'Connor MK, Phillips SW, Smith
RL and Collins DA: Molecular breast imaging: a new technique using
technetium Tc 99m scintimammography to detect small tumors of the
breast. Mayo Clin Proc. 80:24–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gompel A: Breast cancer incidence rates in
US women are no longer declining. Climacteric. 14:690–691.
2011.
|
|
4
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer. 96(Suppl
96): R40–R44. 2007.PubMed/NCBI
|
|
5
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: miRBase: microRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34:D140–D144. 2006.
View Article : Google Scholar :
|
|
6
|
Griffiths-Jones S: The microRNA Registry.
Nucleic Acids Res. 32:D109–D111. 2004. View Article : Google Scholar :
|
|
7
|
Blenkiron C, Goldstein LD, Thorne NP,
Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE,
Green AR, Ellis IO, et al: MicroRNA expression profiling of human
breast cancer identifies new markers of tumor subtype. Genome Biol.
8:R2142007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frezzetti D, De Menna M, Zoppoli P, Guerra
C, Ferraro A, Bello AM, De Luca P, Calabrese C, Fusco A, Ceccarelli
M, et al: Upregulation of miR-21 by Ras in vivo and its role in
tumor growth. Oncogene. 30:275–286. 2011. View Article : Google Scholar
|
|
9
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang M, Shen H, Qiu C, Ni Y, Wang L, Dong
W, Liao Y and Du J: High expression of miR-21 and miR-155 predicts
recurrence and unfavourable survival in non-small cell lung cancer.
Eur J Cancer. 49:604–615. 2013. View Article : Google Scholar
|
|
11
|
Shibuya H, Iinuma H, Shimada R, Horiuchi A
and Watanabe T: Clinicopathological and prognostic value of
microRNA-21 and microRNA-155 in colorectal cancer. Oncology.
79:313–320. 2010. View Article : Google Scholar
|
|
12
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J and Song E: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corney DC, Hwang CI, Matoso A, Vogt M,
Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH,
Hermeking H and Nikitin AY: Frequent downregulation of miR-34
family in human ovarian cancers. Clin Cancer Res. 16:1119–1128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang R, Ma J, Wu Q, Xia J, Miele L, Sarkar
FH and Wang Z: Functional role of miR-34 family in human cancer.
Curr Drug Targets. 14:1185–1191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akao Y, Nakagawa Y and Naoe T: let-7
microRNA functions as a potential growth suppressor in human colon
cancer cells. Biol Pharm Bull. 29:903–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iorio MV, Ferracin M, Liu C-G, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lei Q, Shen F, Wu J, Zhang W, Wang J and
Zhang L: MiR-101, downregulated in retinoblastoma, functions as a
tumor suppressor in human retinoblastoma cells by targeting EZH2.
Oncol Rep. 32:261–269. 2014.PubMed/NCBI
|
|
22
|
Wei X, Xiang T, Ren G, Tan C, Liu R, Xu X
and Wu Z: miR-101 is down-regulated by the hepatitis B virus x
protein and induces aberrant DNA methylation by targeting DNA
methyltransferase 3A. Cell Signal. 25:439–446. 2013. View Article : Google Scholar
|
|
23
|
Luo C, Merz PR, Chen Y, Dickes E, Pscherer
A, Schadendorf D and Eichmüller SB: MiR-101 inhibits melanoma cell
invasion and proliferation by targeting MITF and EZH2. Cancer Lett.
341:240–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Popov VM, Wu K, Zhou J, Powell MJ, Mardon
G, Wang C and Pestell RG: The Dachshund gene in development and
hormone-responsive tumorigenesis. Trends Endocrinol Metab.
21:41–49. 2010. View Article : Google Scholar :
|
|
25
|
Voas MG and Rebay I: Signal integration
during development: Insights from the Drosophila eye. Dev Dyn.
229:162–175. 2004. View Article : Google Scholar
|
|
26
|
Zhang L, Yang N, Huang J, Buckanovich RJ,
Liang S, Barchetti A, Vezzani C, O'Brien-Jenkins A, Wang J, Ward
MR, et al: Transcriptional coactivator Drosophila eyes absent
homologue 2 is up-regulated in epithelial ovarian cancer and
promotes tumor growth. Cancer Res. 65:925–932. 2005.PubMed/NCBI
|
|
27
|
Li Z, Hu J, Sun Y, Li S, Pestell RG and Wu
K: EYA promotes proliferation through up-regulation of cyclin D1.
Cancer Res. 71(Suppl 8): abs. 2942 Proceedings: AACR 102nd Annual
Meeting 2011, April 2–6 2011; http://cancerres.aacrjournals.org/content/71/8_Supplement/2942.short.
|
|
28
|
Pignoni F, Hu B, Zavitz KH, Xiao J,
Garrity PA and Zipursky SL: The eye-specification proteins So and
Eya form a complex and regulate multiple steps in Drosophila eye
development. Cell. 91:881–891. 1997. View Article : Google Scholar
|
|
29
|
Kumar JP and Moses K: EGF receptor and
Notch signaling act upstream of Eyeless/Pax6 to control eye
specification. Cell. 104:687–697. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li C-M, Guo M, Borczuk A, Powell CA, Wei
M, Thaker HM, Friedman R, Klein U and Tycko B: Gene expression in
Wilms' tumor mimics the earliest committed stage in the metanephric
mesenchymal-epithelial transition. Am J Pathol. 160:2181–2190.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu K, Li Z, Cai S, Tian L, Chen K, Wang J,
Hu J, Sun Y, Li X, Ertel A and Pestell RG: EYA1 phosphatase
function is essential to drive breast cancer cell proliferation
through cyclin D1. Cancer Res. 73:4488–4499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Y, Kaneko S and Li X and Li X: The
PI3K/Akt signal hyperactivates Eya1 via the SUMOylation pathway.
Oncogene. 34:2527–2537. 2015. View Article : Google Scholar
|
|
33
|
Nikpour P, Emadi-Baygi M, Emadi-Andani E
and Rahmati S: EYA1 expression in gastric carcinoma and its
association with clinicopathological characteristics: A pilot
study. Med Oncol. 31:9552014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sassen S, Miska EA and Caldas C: MicroRNA:
implications for cancer. Virchows Arch. 452:1–10. 2008. View Article : Google Scholar
|
|
36
|
Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT,
Xia YJ, Ye ZY and Tao HQ: MicroRNA-101 is down-regulated in gastric
cancer and involved in cell migration and invasion. Eur J Cancer.
46:2295–2303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ambs S, Prueitt RL, Yi M, Hudson RS, Howe
TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, et al:
Genomic profiling of microRNA and messenger RNA reveals deregulated
microRNA expression in prostate cancer. Cancer Res. 68:6162–6170.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Strillacci A, Valerii MC, Sansone P,
Caggiano C, Sgromo A, Vittori L, Fiorentino M, Poggioli G, Rizzello
F, Campieri M and Spisni E: Loss of miR-101 expression promotes
Wnt/β-catenin signalling pathway activation and malignancy in colon
cancer cells. J Pathol. 229:379–389. 2013. View Article : Google Scholar
|
|
39
|
Smits M, Nilsson J, Mir SE, van der Stoop
PM, Hulleman E, Niers JM, de Witt Hamer PC, Marquez VE, Cloos J,
Krichevsky AM, et al: miR-101 is down-regulated in glioblastoma
resulting in EZH2-induced proliferation, migration, and
angiogenesis. Oncotarget. 1:710–720. 2010. View Article : Google Scholar
|
|
40
|
Xu L, Beckebaum S, Iacob S, Wu G, Kaiser
GM, Radtke A, Liu C, Kabar I, Schmidt HH, Zhang X, et al:
MicroRNA-101 inhibits human hepatocellular carcinoma progression
through EZH2 downregulation and increased cytostatic drug
sensitivity. J Hepatol. 60:590–598. 2014. View Article : Google Scholar
|
|
41
|
Varambally S, Cao Q, Mani RS, Shankar S,
Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, et al:
Genomic loss of microRNA-101 leads to overexpression of histone
methyltransferase EZH2 in cancer. Science. 322:1695–1699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Buechner J, Tømte E, Haug BH, Henriksen
JR, Løkke C, Flægstad T and Einvik C: Tumour-suppressor microRNAs
let-7 and miR-101 target the proto-oncogene MYCN and inhibit cell
proliferation in MYCN-amplified neuroblastoma. Br J Cancer.
105:296–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Graziussi DF, Suga H, Schmid V and Gehring
WJ: The 'eyes absent' (eya) gene in the eye-bearing hydrozoan
jellyfish Cladonema radiatum: conservation of the retinal
determination network. J Exp Zoolog B Mol Dev Evol. 318:257–267.
2012. View Article : Google Scholar
|
|
44
|
Li X, Wang S and Schor N: Methylase meets
phosphatase: roles of PRMT and EYA1 in neuroblastoma. Cancer Res.
74(Suppl 19): abs. 464. 2014.
|
|
45
|
Wang J, Cai S, Chen K, Li S, Pestell R and
Wu K: Regulation of AR transcriptional activity and prostate cancer
cellular proliferation by DACH1/Eya1/Six1 pathway. Cancer Res.
73(Suppl 8): abs. 1319. 2013.
|
|
46
|
Bolós V, Grego-Bessa J and de la Pompa JL:
Notch signaling in development and cancer. Endocr Rev. 28:339–363.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Allenspach EJ, Maillard I, Aster JC and
Pear WS: Notch signaling in cancer. Cancer Biol Ther. 1:466–476.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Miele L, Golde T and Osborne B: Notch
signaling in cancer. Curr Mol Med. 6:905–918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Z, Li Y, Banerjee S, Kong D, Ahmad A,
Nogueira V, Hay N and Sarkar FH: Down-regulation of Notch-1 and
Jagged-1 inhibits prostate cancer cell growth, migration and
invasion, and induces apoptosis via inactivation of Akt, mTOR, and
NF-kappaB signaling pathways. J Cell Biochem. 109:726–736.
2010.PubMed/NCBI
|
|
50
|
Kiaris H, Politi K, Grimm LM, Szabolcs M,
Fisher P, Efstratiadis A and Artavanis-Tsakonas S: Modulation of
notch signaling elicits signature tumors and inhibits hras1-induced
oncogenesis in the mouse mammary epithelium. Am J Pathol.
165:695–705. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stylianou S, Clarke RB and Brennan K:
Aberrant activation of notch signaling in human breast cancer.
Cancer Res. 66:1517–1525. 2006. View Article : Google Scholar : PubMed/NCBI
|