Introduction
Inflammatory bowel disease (IBD) is a chronic and
recurrent disorder with unknown etiology (1,2).
IBD occurs mostly in young individuals, interfering with their
education, working abilities and social life (3). IBD comprises ulcerative colitis (UC)
and Crohn's disease (CD), which have different clinical
manifestations, courses and prognoses (1,4,5).
Their clinical courses can vary from frequent relapses or chronic
active disease to years of virtually complete remission (3). IBD affects 1.4 million individuals
in North America and 2.2 millions in Europe, and the reported
incidence has a range of 3–20/100,000 individuals per year
(6–8). Although the incidence of IBD is low
in Asia compared with North America and Europe (2,9,10),
recent studies have indicated that the incidence of IBD in Asia is
increasing (2,10).
There is as yet no ideal treatment for IBD, and the
treatment options available today comprise 5-aminosalicylates,
corticosteroids, immunosuppressants such as thiopurine analogs,
methotrexate and biological agents such as antibodies against tumor
necrosis factor α (TNFα) (1,4,5,11–13). While 5-aminosalicylates and
corticosteroids are beneficial for many patients with IBD, they are
not effective in the long term for most patients (1,4,13),
and the short- and long-term side-effects of immunosuppressive
drugs limit their use (1,4,12).
Furthermore, although anti-TNFα therapy can be effective in IBD,
only approximately 65% of patients respond to this treatment
(3,11,12,14–20).
There are two novel potential therapeutic candidates
for the treatment of IBD:
3-[(dodecylthiocarbonyl)-methyl]-glutarimide (DTCM-G) and
dehydroxymethylepoxyquinomicin (DHMEQ). DTCM-G is a synthetic
derivative of 9-methylstreptimidone isolated from
Streptomyces spp. that has been shown to possess potent
anti-inflammatory effects and found to inhibit the
lipopolysaccharide-induced activation of macrophages, possibly via
the suppression of activator protein-1 (AP-1) (21,22). DHMEQ is a newly designed
low-molecular-weight nuclear factor-κB (NF-κB) inhibitor that has
also demonstrated potent anti-inflammatory activity in many animal
models (23,24).
Animal models of IBD do not reproduce exactly the
conditions in human IBD, but they are valuable for testing the
efficacy of anti-inflammatory agents (25). Dextran sulfate sodium
(DSS)-induced colitis has been considered to closely mimic the
clinical and morphological features of human UC (25). The aim of this study was to
elucidate the anti-inflammatory effects of the two novel
anti-inflammatory substances, DTCM-G and DHMEQ, on DSS-induced
colitis in rats.
Materials and methods
Rats
Male Wistar rats (Hannover GALAS; Taconic Farms,
Lille Skensved, Denmark) with a mean body weight of 279.2 g (range,
228–382 g) were housed in Macrolon III cages with water and food
available ad libitum. The standard diet provided to the rats
(B&K Universal, Nittedal, Norway) consisted of cereal products
(88.5%), soy protein (6%), animal protein (2.5%), soy oil (0.5%),
and vitamins, minerals and amino-acid supplements (2.5%). The
animals were maintained under a controlled environment at 21±1°C, a
relative humidity of 55±5% and under a 12/12 h light/dark
cycle.
The study was carried out in accordance with the
Directive for the Protection of Vertebrate Animals used for
Experimental and Other Scientific Purposes of the European Union
(86/609/EEC), in compliance with the Declaration of Helsinki. The
local ethics committee for experimental animals approved the study
protocols.
Study design
Thirty animals were allowed to acclimatize in the
animal house under the aforementioned conditions for 7 days prior
to the commencement of the experiments. Colitis was induced in
these rats by the administration of DSS for 7 days (as described
below). The animals were then randomized into 3 groups with 10
animals in each group according to the planned treatments, which
were administered intraperitoneally (i.p.), twice daily for 5 days
in all groups, as follows: i) the control group received 0.5 ml of
0.5% carboxymethyl cellulose (CMC; vehicle), ii) the DTCM-G group
received 20 mg/kg body weight DTCM-G in 0.5% CMC, and iii) the
DHMEQ group received 15 mg/kg DHMEQ in 0.5% CMC. The methods used
to synthesize DTCM-G and DHMEQ are described elsewhere (21,26). At the end of the 5-day treatment
period, the animals were sacrificed by CO2 inhalation,
and a postmortem laparotomy was carried out in which the abdomen
and colon were examined. Tissue samples were taken from the lower
part of the colon for further, histological examination (Fig. 1).
Induction of colitis by DSS
Colitis was induced by the administration of DSS as
previously described (27,28).
Briefly, the normal drinking water was replaced with distilled
water containing 5% DSS (mol. wt. 40 kD; TdB Consultancy, Uppsala,
Sweden) for 7 days. The DSS solution was prepared daily, and the
amount consumed by the rats was measured. The animals were
monitored twice daily and were weighed on a daily basis. Animals
with any signs of pain were injected subcutaneously with 1 ml of
Temgesic solution (containing 0.3 g/ml Temgesic; Merck
Pharmaceutical, Darmstadt, Germany).
The disease activity index (DAI)
The DAI was used to measure the severity of the
induced colitis. To this end, the animals were weighed, and the
fecal consistency and presence and degree of occult or gross rectal
bleeding were recorded daily. The DAI was determined (as described
in detail elsewhere) (29,30)
by rating the percentage body weight loss (0, no body weight loss;
1, 1–5%; 2, 6–10%; 3, 11–15%; 4, 16%), fecal consistency (0,
normal; 2, loose; 4, diarrhea) and the degree of rectal bleeding
(0, normal; 2, occult bleeding; and 4, gross bleeding). The DAI was
estimated as the sum of all of these scores divided by 3.
Histopathology and
immunohistochemistry
The tissue samples taken from the colon during
postmortem laparotomy were fixed overnight in 4% buffered
paraformaldehyde, embedded in paraffin, and then cut into
5-µm-thick sections. The sections were deparaffinized and then
stained with hematoxylin and eosin (H&E) or else immunostained
using the ultraView Universal DAB Detection kit (v1.02.0018) and
the BenchMark Ultra IHC/ISH staining module (both from Venata
Medical Systems, Basel, Switzerland). For immunostaining, the
sections were incubated with one of the primary antibodies for 32
min at 37°C. The primary antibodies used were monoclonal mouse
antihuman CD45 (code no. M0701), monoclonal mouse anti-human CD47
(code no. I5647), monoclonal mouse antihuman CD68 (code no. M0814)
and monoclonal mouse antihuman mast cell tryptase (code no. M7052)
(all from Dako, Glostrup, Denmark). CD45 is considered as a common
leukocyte antigen and is expressed exclusively on cells of the
hematopoietic system and their progenitors. CD57 is expressed by
subsets of natural killer cells and CD8+ lymphocytes,
and by a small proportion of CD4+/CD45R0+ T
lymphocytes. CD68 labels human monocytes, macrophages and myeloid
cells. Human mast cell tryptases comprise a family of trypsin-like
neutral serine proteases that are expressed predominantly in mast
cells.
Histological grading of colitis
The histological grading of DSS-induced colitis was
performed using the H&E-stained sections by the same
investigator (M.E.-S.) in a blinded manner as described previously
(31). The following parameters
were examined and graded: degree of inflammation (0, none; 1,
slight; 2, moderate; 3, severe), extent of inflammation (0, none;
1, mucosa; 2, mucosa and submucosa; 3, transmural), regeneration
(4, no tissue repair; 3, surface epithelium not intact; 2,
regeneration with crypt depletion; 1, almost complete regeneration;
0, complete regeneration or normal tissue), crypt damage (0, none;
1, basal one-third damaged; 2, basal two-thirds damaged; 3, only
surface epithelium intact; 4, entire crypt and epithelium lost) and
the percentage involvement (1, 1–25%; 2, 26–50%; 3, 1–75%; 4,
76–100%).
Quantification of immune cells
The immune cells were quantified by counting
immunopositive cells in 10 randomly selected microscopic fields for
each immunostained immune cell type (i.e., leukocytes, lymphocytes,
macrophages/monocytes and mast cells). Measurements were performed
on a computer linked to a microscope (BX 43) that was equipped with
a digital camera (DP 26) (both from Olympus, Tokyo, Japan), and
using Olympus cellSens imaging software (version 1.7). The number
of immune cells in the submucosa of each field was counted manually
by pointing and clicking the computer mouse. A ×40 objective was
used, for which each frame (field) on the monitor represented a
tissue area of 0.035 mm2. The data are presented as
density measurements (i.e., the number of immune cells per field).
Immunostained sections were coded and mixed, and measurements were
made by the same investigator (M.E.-S.), who was blinded to the
identity of the sections (i.e., the treatment group from which they
were taken).
Statistical analysis
Differences between the control, DTCM-G and DHMEQ
groups were tested using the Kruskal-Wallis non-parametric test,
with Dunn's test as a post-test. The data are presented as the mean
± SEM values, and the threshold for statistical significance was
set at P<0.05.
Results
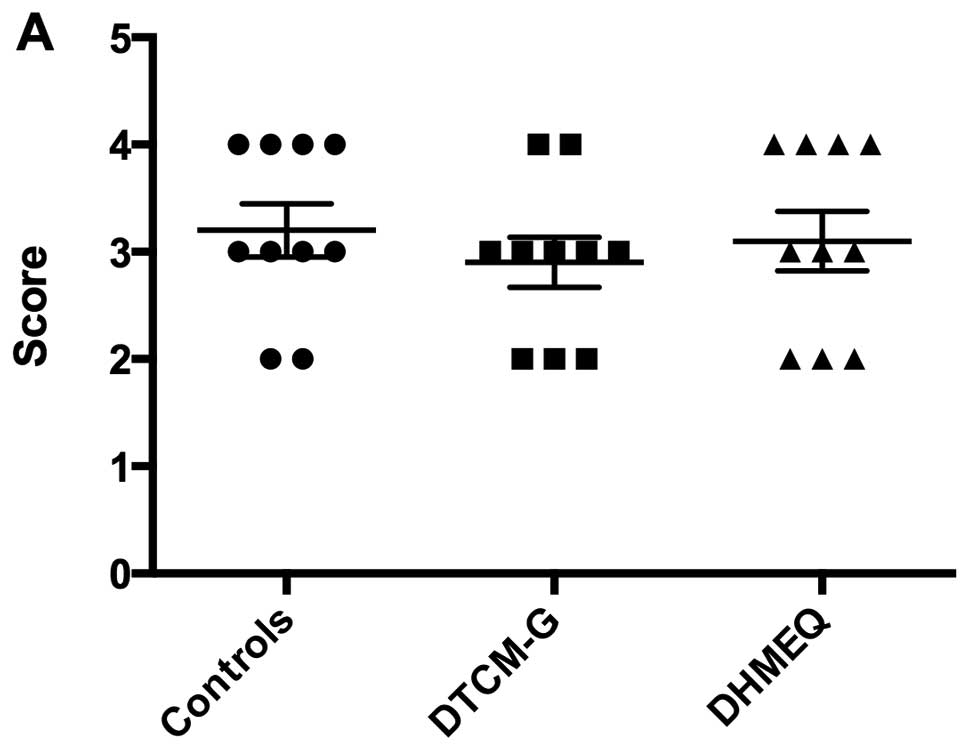
DAI
The DAI values before treatment (i.e., at baseline)
were 3.2±0.2, 2.9±0.2 and 3.1±0.3 in the control, DTCM-G and DHMEQ
groups, respectively; the baseline DAI did not differ significantly
between the 3 groups (P=0.7; Fig.
2). After the 5 days of treatment, the DAI values were
significantly lower in the DTCM-G (0.5±0.2) and DHMEQ (0.6±0.2)
groups than in the control group (3.4±0.2; P<0.0001 for
both).
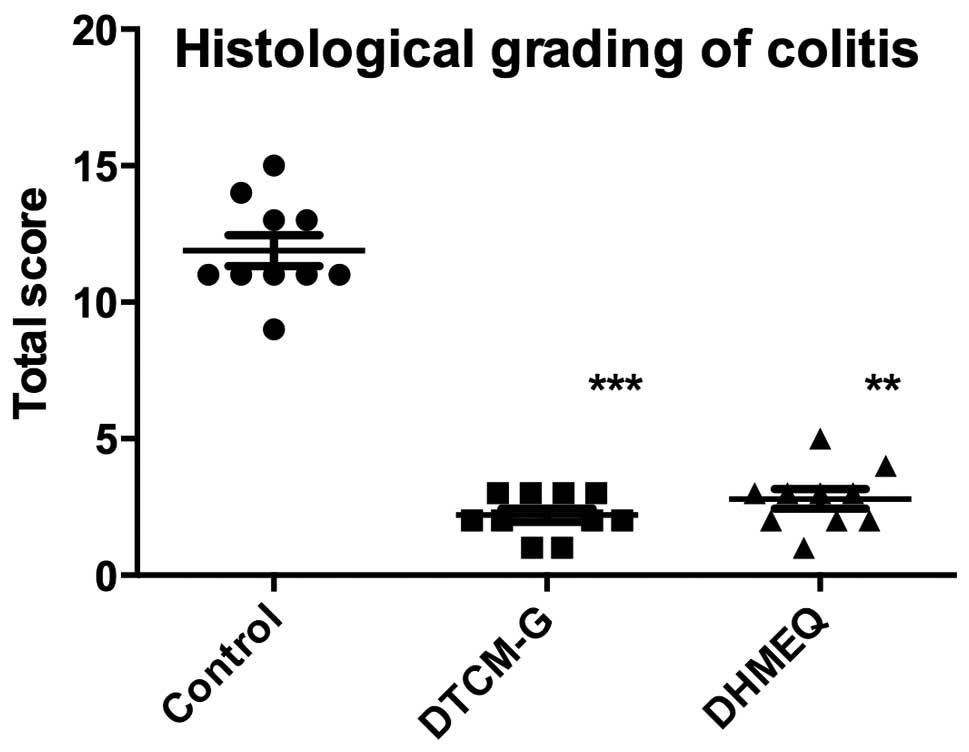
Histological grading of colitis
At the endpoint of the experiment, histopathological
examination of the colonic tissues revealed that the untreated
control group had severe-to-moderate inflammation and disturbed
mucosal architecture, crypt abscesses, edema, bleeding and
infiltration of immune cells into the mucosa and submucosa. The
only sign of inflammation observed in the animals treated with
either DTCM-G or DHMEQ was a slight infiltration of immune cells
into the submucosa (Fig. 3). The
total scores for the histological grading of colitis were
significantly lower in the DTCM-G (2.2±0.2) and DHMEQ (2.8±0.4)
groups than in the control group (11.9±0.6; P<0.0001 and
P<0.001, respectively) (Fig.
4).
Quantification of immune cells
The densities of submucosal leukocytes were
23.2±2.4, 7.1±0.9 and 5.9±0.8 cells/field in the control, DTCM-G
and DHMEQ groups, respectively (Kruskal-Wallis test was significant
at P<0.0001) (Figs. 5 and
6). The densities of submucosal
leukocytes were significantly lower in the DTCM-G and DHMEQ groups
than in the control group (P<0.001 and <0.0001,
respectively).
The densities of submucosal lymphocytes were
26.2±3.1, 8.1±1.2 and 7.8±0.8 cells/field in the control, DTCM-G
and DHMEQ groups, respectively (Kruskal-Wallis multiple comparison
test was significant at P<0.0001) (Figs. 5 and 7). The density of lymphocytes was
significantly lower in the submucosa of the DTCM-G- and
DHMEQ-treated animals than in their vehicle-treated, control
counterparts (P<0.0001 for both).
The submucosal densities of macrophages/monocytes in
the control, DTCM-G and DHMEQ groups were 21.8±2.2, 6.4±0.9 and
6.9±0.4 cells/field, respectively (Figs. 5 and 8) (Kruskal-Wallis test was significant
at P<0.0001). The densities of macrophages/monocytes in the
submucosa were significantly lower in the DTCM-G- and DHMEQ-treated
animals than in the control animals (P<0.0001 for both).
The densities of mast cells in the submucosa of the
control, DTCM-G and DHMEQ groups were 26.2±5.1, 7.4±1.0 and 6.6±0.9
cells/field (Figs. 5 and 9). There were significant differences
between the 3 groups, as revealed by the Kruskal-Wallis test. The
densities of mast cells in the submucosa were significantly lower
in the DTCM-G- and DHMEQ-treated animals than in the control
animals (P<0.001 and P<0.0001, respectively).
Discussion
DSS-induced colitis closely mimics human UC and is a
useful model for studying the inflammatory/recovery processes and
for testing potential therapies (31). Similar to human UC, the animals
suffer from diarrhea and rectal bleeding. The colitis induced by
DSS is caused by a chemical injury to the intestinal epithelium,
which results in the exposure of the lamina propria and the
submucosa to luminal antigens and enteric bacteria, triggering
inflammation (32). However, one
limitation of this animal model is that it lacks the chronic
changes seen in human UC (32).
DTCM-G and DHMEQ are novel anti-inflammatory agents
with different modes of action: DTCM-G is an AP-1 inhibitor that
inhibits the activation of macrophages and pro-inflammatory
cytokines (22,33), while DHMEQ inhibits the nuclear
translocation of NF-κB by binding to the Rel-family components and
inhibiting their DNA-binding activity (34-36). The migration of immune cells to
the site of inflammation, and their subsequent activation are
regulated by different cytokines and chemokines, which in turn are
regulated by the transcription factors, AP-1 and NF-κB (37–39). DTCM-G and DHMEQ have been found to
have a high potency for suppressing inflammation in animal models
of various inflammatory diseases including IBD (23,24).
In the present study, 5 days of treatment with
either DTCM-G or DHMEQ reduced the inflammation observed in the
rats with DSS-induced colitis, as indicated by the reduction in the
DAI values, the histological grading score for colitis, and the
infiltration of immune cells in the animals treated with these 2
agents compared to their vehicle-treated, control counterparts.
These observations are in line with the previously reported effects
of DHMEQ in a murine model of (DSS-induced) colitis, whereby
pro-inflammatory cytokines such as interleukin (IL)-1β, TNFα, IL-6,
IL-12p40, IL-17 and monocyte chemotactic protein-1 were suppressed
following the administration of DHMEQ (23).
In addition to its anti-inflammatory effects, the
administration of DHMEQ either i.p. or intravenously does not
result in a detectable concentration in the blood; although there
is a high concentration in the peritoneal cavity within 5 min
following the i.p. administration of DHMEQ, and a rapid decrease 30
min thereafter, the drug cannot be detected in the bloodstream
(34). Umezawa has proposed that
DHMEQ exerts its effects locally via DHMEQ uptake by immune cells
in the peritoneal cavity prior to their migration to sites of
inflammation (34). This may
explain the low toxicity of this agent observed in experimental
animals.
There is considerable concern regarding the use of
azathioprine and anti-TNFα antibodies, which are used in the
clinical setting for the treatment of IBD, due to the possible
increased risk of developing cancer when they are used on a
long-term basis (3,4,11,12). By contrast, both DTCM-G and DHMEQ
exhibit anticancer activities against various types of cancers
(40–49). The demonstrated anti-inflammatory
and anticancer effects of DTCM-G and DHMEQ, and the absence of any
apparent associated toxicity render them excellent therapeutic
candidates for clinical use in the treatment of IBD.
Acknowledgments
The study was supported by grants from Helse-Vest
(grant no. 911978) and Helse-Fonna (grant no. 40415).
References
|
1
|
Prantera C and Marconi S:
Glucocorticosteroids in the treatment of inflammatory bowel disease
and approaches to minimizing systemic activity. Therap Adv
Gastroenterol. 6:137–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cosnes J, Gower-Rousseau C, Seksik P and
Cortot A: Epidemiology and natural history of inflammatory bowel
diseases. Gastroenterology. 140:1785–1794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carter MJ, Lobo AJ and Travis SP: IBD
Section, British Society of Gastroenterology: Guidelines for the
management of inflammatory bowel disease in adults. Gut. 53(Suppl
5): V1–V16. 2004. View Article : Google Scholar
|
|
4
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Podolsky DK: The current future
understanding of inflammatory bowel disease. Best Pract Res Clin
Gastroenterol. 16:933–943. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loftus EV Jr: Clinical epidemiology of
inflammatory bowel disease: Incidence, prevalence, and
environmental influences. Gastroenterology. 126:1504–1517. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loftus EV Jr and Sandborn WJ: Epidemiology
of inflammatory bowel disease. Gastroenterol Clin North Am.
31:1–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Betteridge JD, Armbruster SP, Maydonovitch
C and Veerappan GR: Inflammatory bowel disease prevalence by age,
gender, race, and geographic location in the U.S. military health
care population. Inflamm Bowel Dis. 19:1421–1427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang SK, Loftus EV Jr and Sandborn WJ:
Epidemiology of inflammatory bowel disease in Asia. Inflamm Bowel
Dis. 7:260–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goh K and Xiao SD: Inflammatory bowel
disease: A survey of the epidemiology in Asia. J Dig Dis. 10:1–6.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sands BE: New therapies for the treatment
of inflammatory bowel disease. Surg Clin North Am. 86:1045–1064.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sands BE: The risks and benefits of early
immunosuppression and biological therapy. Dig Dis. 30(Suppl 3):
100–106. 2012. View Article : Google Scholar
|
|
13
|
Prantera C, Pallone F, Brunetti G, Cottone
M and Miglioli M; The Italian IBD Study Group: Oral
5-aminosalicylic acid (Asacol) in the maintenance treatment of
Crohn's disease. Gastroenterology. 103:363–368. 1992.PubMed/NCBI
|
|
14
|
Lopez A, Billioud V, Peyrin-Biroulet C and
Peyrin-Biroulet L: Adherence to anti-TNF therapy in inflammatory
bowel diseases: A systematic review. Inflamm Bowel Dis.
19:1528–1533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danese S: Anti TNF-alpha treatment for
Crohn' disease: 'ménage a trois'. Curr Drug Targets. 11:136–137.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Danese S and Angelucci E: New and emerging
biologics in the treatment of inflammatory bowel disease: Quo
vadis? Gastroenterol Clin Biol. 33(Suppl 3): S217–S227. 2009.
View Article : Google Scholar
|
|
17
|
Danese S, Angelucci E, Malesci A and
Caprilli R: Biological agents for ulcerative colitis: Hypes and
hopes. Med Res Rev. 28:201–218. 2008. View Article : Google Scholar
|
|
18
|
Danese S, Colombel JF, Peyrin-Biroulet L,
Rutgeerts P and Reinisch W: Review article: The role of anti-TNF in
the management of ulcerative colitis - past, present and future.
Aliment Pharmacol Ther. 37:855–866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Danese S, Colombel JF, Reinisch W and
Rutgeerts PJ: Review article: Infliximab for Crohn's disease
treatment - shifting therapeutic strategies after 10 years of
clinical experience. Aliment Pharmacol Ther. 33:857–869. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Danese S, Semeraro S, Armuzzi A, Papa A
and Gasbarrini A: Biological therapies for inflammatory bowel
disease: Research drives clinics. Mini Rev Med Chem. 6:771–784.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ota E, Takeiri M, Tachibana M, Ishikawa Y,
Umezawa K and Nishiyama S: Synthesis and biological evaluation of
molecular probes based on the 9-methylstreptimidone derivative
DTCM-glutarimide. Bioorg Med Chem Lett. 22:164–167. 2012.
View Article : Google Scholar
|
|
22
|
Shibasaki S, Yamashita K, Goto R, Wakayama
K, Tsunetoshi Y, Zaitsu M, Igarashi R, Haga S, Ozaki M, Umezawa K
and Todo S: Immunosuppressive effects of DTCM-G, a novel inhibitor
of the mTOR downstream signaling pathway. Transplantation.
95:542–550. 2013. View Article : Google Scholar
|
|
23
|
Funakoshi T, Yamashita K, Ichikawa N,
Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T,
Ichihara S, et al: A novel NF-κB inhibitor,
dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic
injury in mice. J Crohn's Colitis. 6:215–225. 2012. View Article : Google Scholar
|
|
24
|
El-Salhy M, Umezawa K, Gilja OH, Hatlebakk
JG, Gundersen D and Hausken T: Amelioration of severe TNBS induced
colitis by novel AP-1 and NF-κB inhibitors in rats.
ScientificWorldJournal. 2014:8138042014. View Article : Google Scholar
|
|
25
|
Elson CO, Sartor RB, Tennyson GS and
Riddell RH: Experimental models of inflammatory bowel disease.
Gastroenterology. 109:1344–1367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsumoto N, Ariga A, To-e S, Nakamura H,
Agata N, Hirano S, Inoue J and Umezawa K: Synthesis of NF-kappaB
activation inhibitors derived from epoxyquinomicin C. Bioorg Med
Chem Lett. 10:865–869. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grimstad T, Bjørndal B, Cacabelos D,
Aasprong OG, Omdal R, Svardal A, Bohov P, Pamplona R, Portero-Otin
M, Berge RK and Hausken T: A salmon peptide diet alleviates
experimental colitis as compared with fish oil. J Nutr Sci.
2:e22013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stucchi AF, Shofer S, Leeman S, Materne O,
Beer E, McClung J, Shebani K, Moore F, O'Brien M and Becker JM:
NK-1 antagonist reduces colonic inflammation and oxidative stress
in dextran sulfate-induced colitis in rats. Am J Physiol
Gastrointest Liver Physiol. 279:G1298–G1306. 2000.PubMed/NCBI
|
|
29
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
30
|
Mao JW, Huang YS, Tang HY, Bi J, Liu YF
and Wang YD: Flt3/Flt3L participates in the process of regulating
dendritic cells and regulatory T cells in DSS-induced colitis.
Gastroenterol Res Pract. 2014:4835782014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dieleman LA, Palmen MJ, Akol H, Bloemena
E, Peña AS, Meuwissen SG and Van Rees EP: Chronic experimental
colitis induced by dextran sulphate sodium (DSS) is characterized
by Th1 and Th2 cytokines. Clin Exp Immunol. 114:385–391. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Low D, Nguyen DD and Mizoguchi E: Animal
models of ulcerative colitis and their application in drug
research. Drug Des Devel Ther. 7:1341–1357. 2013.PubMed/NCBI
|
|
33
|
Takeiri M, Tachibana M, Kaneda A, Ito A,
Ishikawa Y, Nishiyama S, Goto R, Yamashita K, Shibasaki S, Hirokata
G, et al: Inhibition of macrophage activation and suppression of
graft rejection by DTCM-glutarimide, a novel piperidine derived
from the antibiotic 9-methylstreptimidone. Inflamm Res. 60:879–888.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Umezawa K: Possible role of peritoneal
NF-κB in peripheral inflammation and cancer: Lessons from the
inhibitor DHMEQ. Biomed Pharmacother. 65:252–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamamoto M, Horie R, Takeiri M, Kozawa I
and Umezawa K: Inactivation of NF-kappaB components by covalent
binding of (−)-dehydroxymethylepoxyquinomicin to specific cysteine
residues. J Med Chem. 51:5780–5788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ariga A, Namekawa J, Matsumoto N, Inoue J
and Umezawa K: Inhibition of tumor necrosis factor-alpha-induced
nuclear translocation and activation of NF-kappa B by
dehydroxymethy-lepoxyquinomicin. J Biol Chem. 277:24625–24630.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schonthaler HB, Guinea-Viniegra J and
Wagner EF: Targeting inflammation by modulating the Jun/AP-1
pathway. Ann Rheum Dis. 70(Suppl 1): i109–i112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsushima A, Kaisho T, Rennert PD, Nakano
H, Kurosawa K, Uchida D, Takeda K, Akira S and Matsumoto M:
Essential role of nuclear factor (NF)-kappaB-inducing kinase and
inhibitor of kappaB (IkappaB) kinase alpha in NF-kappaB activation
through lymphotoxin beta receptor, but not through tumor necrosis
factor receptor I. J Exp Med. 193:631–636. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Umezawa K, Ariga A and Matsumoto N:
Naturally occurring and synthetic inhibitors of NF-kappaB
functions. Anticancer Drug Des. 15:239–244. 2000.
|
|
40
|
Brassesco MS, Roberto GM, Morales AG,
Oliveira JC, Delsin LE, Pezuk JA, Valera ET, Carlotti CG Jr, Rego
EM, de Oliveira HF, et al: Inhibition of NF-κB by
dehydroxymethylepoxyquinomicin suppresses invasion and
synergistically potentiates temozolomide and γ-radiation
cytotoxicity in glioblastoma cells. Chemother Res Pract.
2013:5930202013.
|
|
41
|
Celegato M, Borghese C, Umezawa K,
Casagrande N, Colombatti A, Carbone A and Aldinucci D: The NF-κB
inhibitor DHMEQ decreases survival factors, overcomes the
protective activity of microenvironment and synergizes with
chemotherapy agents in classical Hodgkin lymphoma. Cancer Lett.
349:26–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fukushima T, Kawaguchi M, Yorita K, Tanaka
H, Takeshima H, Umezawa K and Kataoka H: Antitumor effect of
dehydroxymethylepoxyquinomicin, a small molecule inhibitor of
nuclear factor-κB, on glioblastoma. Neurooncol. 14:19–28. 2012.
|
|
43
|
Kozakai N, Kikuchi E, Hasegawa M, Suzuki
E, Ide H, Miyajima A, Horiguchi Y, Nakashima J, Umezawa K,
Shigematsu N and Oya M: Enhancement of radiosensitivity by a unique
novel NF-κB inhibitor, DHMEQ, in prostate cancer. Br J Cancer.
107:652–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lampiasi N, Azzolina A, Umezawa K,
Montalto G, McCubrey JA and Cervello M: The novel NF-κB inhibitor
DHMEQ synergizes with celecoxib to exert antitumor effects on human
liver cancer cells by a ROS-dependent mechanism. Cancer Lett.
322:35–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lampiasi N, Umezawa K, Montalto G and
Cervello M: Poly (ADP-ribose) polymerase inhibition synergizes with
the NF-κB inhibitor DHMEQ to kill hepatocellular carcinoma cells.
Biochim Biophys Acta. 1843:2662–2673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miyanishi N, Suzuki Y, Simizu S, Kuwabara
Y, Banno K and Umezawa K: Involvement of autocrine CXCL12/CXCR4
system in the regulation of ovarian carcinoma cell invasion.
Biochem Biophys Res Commun. 403:154–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mino K, Ozaki M, Nakanishi K, Haga S, Sato
M, Kina M, Takahashi M, Takahashi N, Kataoka A, Yanagihara K, et
al: Inhibition of nuclear factor-kappaB suppresses peritoneal
dissemination of gastric cancer by blocking cancer cell adhesion.
Cancer Sci. 102:1052–1058. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Suzuki K, Aiura K, Matsuda S, Itano O,
Takeuchi O, Umezawa K and Kitagawa Y: Combined effect of
dehydroxymethylepoxyquinomicin and gemcitabine in a mouse model of
liver metastasis of pancreatic cancer. Clin Exp Metastasis.
30:381–392. 2013. View Article : Google Scholar
|
|
49
|
Yasuda A, Kondo S, Nagumo T, Tsukamoto H,
Mukudai Y, Umezawa K and Shintani S: Anti-tumor activity of
dehydroxymethylepoxyquinomicin against human oral squamous cell
carcinoma cell lines in vitro and in vivo. Oral Oncol. 47:334–339.
2011. View Article : Google Scholar : PubMed/NCBI
|