Introduction
Population aging is a major problem in China and it
is also a global issue. Degenerative spinal disorders are caused by
pathological changes resulting from lumbar disc degeneration, such
as lumbar spondylolisthesis, lumbar spinal stenosis and lumbar disc
disease, and have gained increasing attention from researchers
(1). Lumbar disc degeneration in
the early stages, does not cause pain in the waist or leg; it is
only when lumbar disc degeneration has progressed to a particular
point that spine-related diseases develop, and pressure on the
spinal cord or nerve root results in symptoms including paraplegia,
bladder dysfunction, lumbar scoliosis, back pain, sciatica, loss of
bilateral or unilateral lower limb muscle strength and paresthesia
(2). Approximately 90% of the
worldwide population has suffered from pains in the waist or leg,
and some individuals may experience permanent incapacitation from
the pain (3). Epidemiological
data has also shown that the incidence of lumbar disc degeneration
is rising, and it is developing in younger patients (4). A high morbidity as well as a trend
towards lumbar disc degeneration in younger patients has gained the
attention of experts, and research into the etiology, pathogenesis
and treatment of disc degeneration is ongoing (5).
A number of degenerative spinal diseases and
secondary lesions caused by lumbar disc degeneration are commonly
seen in clinical practice, and the cause and the exact mechanisms
responsible for these conditions remains unclear. The excessive
apoptosis of disc cells directly leads to a reduction in the number
of disc cells, resulting in lumbar disc degeneration (6). Nucleus pulposus cells (NPCs) play an
important role in maintaining and repairing the environment within
the normal intervertebral disc (7). Thus, the excessive apoptosis of NPCs
is a direct cause of lumbar disc degeneration (8). Apoptosis may be triggered by the
mitochondrial-dependent and the non-mitochondrial-dependent
apoptotic signaling pathways (9).
The mitochondrial-dependent pathway may be activated by hydrogen
peroxide (H2O2)-mediated oxidative stress,
leading to apoptosis (10).
Plumbago zeylanica has been used in
traditional Chinese medicine, as it possesses various
pharmacological activities including anti-inflammatory,
antibacterial and antitumor effects, as well as the ability to
inhibit glycolysis and to stimulate the central nervous system
(3,11). Plumbagin
(5-hydroxy-2-methyl-1,4-naphthoquinone) is one of the active
constituents responsible for the various biological activities of
P. zeylanica (chemical structure shown in Fig. 1). Plumbagin belongs to the class
of compounds known as napthoquinones, which are phenolic compounds,
and a phenolic hydroxyl group with a quinone ring structure is the
principal active antioxidant group (12). As the phenolic hydroxyl group is
an active hydrogen donor, it provides a hydrogen atom to the
peroxide free radical of unsaturated fatty acids, and thereby
prevents the formation of new radicals, and interrupts the process
of fat oxidation (13). The
present study was designed to determine whether plumbagin exerts
protective effects against oxidative stress in
H2O2-exposed NPCs as well as to elucidate the
underlying mechansim responsible for these effects.
Materials and methods
Animals and cell culture
Male Sprague-Dawley (SD) rats (8 weeks of age)
weighing 200±30 g were housed in animal quarters at a temperature
of 23±1°C and 60–70% relative humidity on a 12 h light/dark cycle
(8:00; 20:00). The experimental study was conducted in accordance
with the Guide for the Care and Use of Laboratory Animals prepared
by Soochow University (Suzhou, China) and received ethics approval
from the Ethics Committee of The 100th Hospital of Chinese People's
Liberation Army (Suzhou, China). The SD rats were injected with an
overdose of chloral hydrate, and then the L1–L6 lumbar
intervertebral discs were collected from the spinal column. The
gel-like nucleus pulposus (NP) tissues were harvested from the
discs and immediately placed into 25 cm2 plastic bottles
containing Dulbecco's modified Eagle's medium (DMEM)/F-12, and 10%
fetal bovine serum (FBS). The NP tissue samples were cultured for
1–2 h and then digested with 0.01% trypsin (Beyotime Institute of
Biotechnology, Haimen, China) for 30 min at room temperature.
Following the removal of trypsin, the NP tissue samples were washed
twice with phosphate-buffered saline (PBS) and digested with 0.5%
collagenase II (Beyotime Institute of Biotechnology) for 4 h at
room temperature. The NPCs were then filtered through a
200-µm mesh strainer and incubated with DMEM/F-12, 10% FBS,
and antibiotics (1% penicillin/streptomycin) at 37°C in a
humidified 5% CO2 atmosphere. After 2 days of
incubation, the NPCs were washed with PBS and incubated with fresh
medium containing H2O2 (200 µM) at
37°C in a humidified 5% CO2 atmosphere for 6 h.
Cell viability
The NPCs (5,000 cells in 100 µl of medium)
were seeded in 96-well plates and cultured with plumbagin
(Sigma-Aldrich China, Inc., Shanghai, China) at various
concentrations (0, 0.5, 1, 2, 5, 10 and 20 µM) at 37°C in a
humidified 5% CO2 atmosphere for 24 h. Subsequently, 10
µl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was added into each well and the cells were incubated
at 37°C in a humidified 5% CO2 atmosphere for 4 h. The
culture medium was then removed, and 150–200 µl dimethyl
sulfoxide (DMSO) was added into each well and shaken for 20 min.
The absorbance was read at 540 nm using a Labsystems Multiskan MS
Plate Reader (Synergy2; BioTek Instruments, Winooski, VT, USA).
Detection of reactive oxygen species
(ROS)
The NPCs (5,000 cells in 100 µl of medium)
were seeded in 96-well plates and cultured with plumbagin (0, 2, 5
and 10 µM) at 37°C in a humidified 5% CO2
atmosphere for 24 h. ROS activity was examined as previously
described by Pi et al (14) using 2′,7′-dichlorofluorescein
diacetate (DCF-DA; Beyotime Institute of Biotechnology) and
measured using a Labsystems Multiskan MS Plate Reader (Synergy2;
BioTek Instruments) at an excitation wavelength of 485 nm and an
emission wavelength of 528 nm.
Detection of lipid peroxidation
The NPCs (5,000 cells in 100 µl of medium)
were seeded in 96-well plates and cultured with plumbagin (0, 2, 5
and 10 µM) at 37°C in a humidified 5% CO2
atmosphere for 24 h. Lipid peroxidation was examined as previously
described by Pi et al (14) using a lipid peroxidation kit
(Beyotime Institute of Biotechnology) and measured using Labsystems
Multiskan MS Plate Reader (Synergy2; BioTek Instruments) at 532 nm
and the results are expressed as nM thiobarbituric acid reactive
substances (TBARS)/mg of protein.
Detection of oxidative stress
The NPCs (5,000 cells in 100 µl of medium)
were seeded in 96-well plates and cultured with plumbagin (0, 2, 5
and 10 µM) at 37°C in a humidified 5% CO2
atmosphere for 24 h. The glutathione (GSH) content, as well as the
activity of catalase (CAT), superoxide dismutase (SOD) and
glutathione peroxdiase (GSH-Px) were detected using commercial kits
(Beyotime Institute of Biotechnology) and absornace was measured
using a Labsystems Multiskan MS Plate Reader (Synergy2; BioTek
Instruments).
Detection of inflammatory cytokines
The NPCs (5,000 cells in 100 µl of medium)
were seeded in 96-well plates and cultured with plumbagin (0, 2, 5
and 10 µM) at 37°C in a humidified 5% CO2
atmosphere for 24 h. The levels of tumor necrosis factor-α (TNF-α),
interleukin (IL)-1β and IL-6 were detected using commercial kits
(Beyotime Institute of Biotechnology) and absorbance was measured
using a Labsystems Multiskan MS Plate Reader (Synergy2; BioTek
Instruments).
Detection of apoptosis
The NPCs (1×105 cells in 100 µl of
medium) were seeded in 6-well plates and cultured with plumbagin
(0, 2, 5 and 10 µM) at 37°C in a humidified 5%
CO2 atmosphere for 24 h. The NPCs were washed twice with
cold PBS, and and resuspended in 500 µl of binding buffer
(BestBio, Inc., Shanghai, China). Thereafter, 5 µl of
Annexin V-FITC was added and incubated for 30 min at 4°C in the
dark. Then, 5 µl of PI was added in the dark and immediately
analyzed on a FACScan flow cytometer (BD Biosciences, San Jose, CA,
USA).
Detection of caspase-9 and -3
activity
The NPCs (1×105 cells in 100 µl of
medium) were seeded in 6-well plates and cultured with plumbagin
(0, 2, 5 and 10 µM) at 37°C in a humidified 5%
CO2 atmosphere for 24 h. The NPCs were harvested and
resuspended using lysis buffer (Beyotime Institute of
Biotechnology) for 30–60 min on ice. The NPCs were harvested by
centrifugation (10,000 x g for 10 min at 4°C), and the protein
concentration was determined using a bicinchoninic acid protein
assay (BCA; KeyGen, Nanjing, China). The activity of caspase-9 and
-3 was detected at a wavelength of 405 nm using caspase-3 and -9
colorimetric assay kits (Beyotime Institute of Biotechnology) on a
FACScan flow cytometer (BD Biosciences).
Western blot analysis of nuclear
factor-κB (NF-κB) and nuclear factor erythroid 2-related factor 2
(Nrf-2)
The NPCs (1×105 cells in 100 µl of
medium) were seeded in 6-well plates and cultured with plumbagin
(0, 2, 5 and 10 µM) at 37°C in a humidified 5%
CO2 atmosphere for 24 h. The NPCs were harvested and
resuspended using lysis buffer (Beyotime Institute of
Biotechnology) for 30–60 min on ice. The NPCs were harvested by
centrifugation (10,000 × g for 10 min at 4°C), and the protein
concentration was determined using a BCA assay(KeyGEN). Sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was
performed with approximately 50 µg of sample proteins which
were loaded onto 12% SDS-polyacrylamide gel and then transferred to
a nitrocellulose membrane (Bio-Rad Laboratories GmbH, Munich,
Germany). The nitrocellulose membrane was blocked with
Tris-buffered saline (pH 7.4) containing 0.1% Tween-20 (TBST) and
5% non-fat milk for 2 h. The membrane was incubated with anti-NF-κB
(3035) and anti-Nrf-2 (12721) (1:1,000; American Diagnostica Inc.,
Stamford, CT, USA) and anti-β-actin (A01011, 1:5,000; BestBio,
Inc.) overnight at 4°C. The membrane was washed twice with PBS and
incubated with a secondary antibody, horseradish peroxidase-linked
anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for
2 h. The membrane was then incubated with chemiluminescence reagent
(ECL Plus Western Blotting Detection system; GE Healthcare,
Waukesha, WI, USA). The relative quantity of protein was measured
using AlphaEase FC (FluorChem FC2) software (Cell Biosciences Inc.,
Santa Clara, CA, USA).
Statistical analysis
The results are expressed as the means ± SEM and
were analyzed using the Student's t-test or analysis of variance
(ANOVA) as appropriate. A p-value <0.05 was considered to
indicate a statistically significant difference.
Results
Protective effect of plumbagin increases
the viability of NPCs
We demonstrated that the viability of NPCs was
increased by treatment with plumbagin in a dose-dependent manner.
Particularly, cell viability was significantly increased following
treatment with plumbagin at concentrations of 2 to 20 µM,
compared with that in the 0 µM plumbagin-treated group
(Fig. 2).
Protective effect of plumbagin decreases
the generation of ROS in NPCs
We next examined whether plumbagin exerted a
protective effect against the H2O2-induced
generation of ROS in the NPCs. The generation of ROS was highest in
the 0 µM plumbagin-treated group and there was a significant
reduction in ROS levels in the plumbagin-treated groups (2–10
µM) (Fig. 3).
Protective effect of plumbagin decreases
lipid peroxidation in NPCs
We examined whether plumbagin exerted a protective
effect against lipid peroxidation in the
H2O2-exposed NPCs. The results showed that
treatment with plumbagin (2–10 µM) significantly inhibited
H2O2-induced lipid peroxidation in the NPCs
compared with that in the 0 µM plumbagin-treated group
(Fig. 4).
Protective effect of plumbagin decreases
oxidative stress in NPCs
We examined whether plumbagin exerted a protective
effect against the H2O2-induced oxidative
stress in the NPCs. As compared with the 0 µM
plumbagin-treated groups, GSH content as well as the activity of
CAT, SOD and GSH-Px in the plumbagin-treated groups (2–10
µM) was significantly increased in the
H2O2-exposed NPCs (Fig. 5).
Protective effect of plumbagin decreases
the levels of inflammatory cytokines in NPCs
In order to further explore the protective effects
of plumbagin, we examined whether plumbagin decreased the levels of
inflammatory cytokines in the H2O2-exposed
NPCs. The administration of plumbagin (2–10 µM)
significantly decreased the levels of TNF-α, IL-1β and IL-6 in the
H2O2-exposed NPCs compared with those in the
0 µM plumbagin-treated groups (Fig. 6).
Protective effect of plumbagin decreases
the apoptosis of NPCs
In the next experiment, we examined the
anti-apoptotic effect of plumbagin in the
H2O2-exposed NPCs. As shown in Fig. 7, plumbagin treatment (2–10
µM) significantly decreased the cell apoptosis rate in the
H2O2-exposed NPCs, compared with that in the
0 µM plumbagin-treated group (Fig. 7).
Protective effect of plumbagin decreases
the activity of caspase-9 and -3 in NPCs
In order to further examine the anti-apoptotic
effect of plumbagin in the H2O2-exposed NPCs,
the activity of caspase-9 and -3 was also measured. Caspase-9 and
-3 activity was significantly inhibited in the plumbagin-treated
groups (2–10 µM), compared with those in the 0 µM
plumbagin-treated groups (Fig.
8).
Protective effect of plumbagin decreases
NF-κB protein expression in NPCs
To explore the anti-inflammatory effect of plumbagin
in the H2O2-exposed NPCs, NF-κB protein
expression was evaluated using western blot analysis. NF-κB protein
expression was significantly suppressed by the administration of
plumbagin (2–10 µM), compared with that in the 0 µM
plumbagin-treated group (Fig.
9).
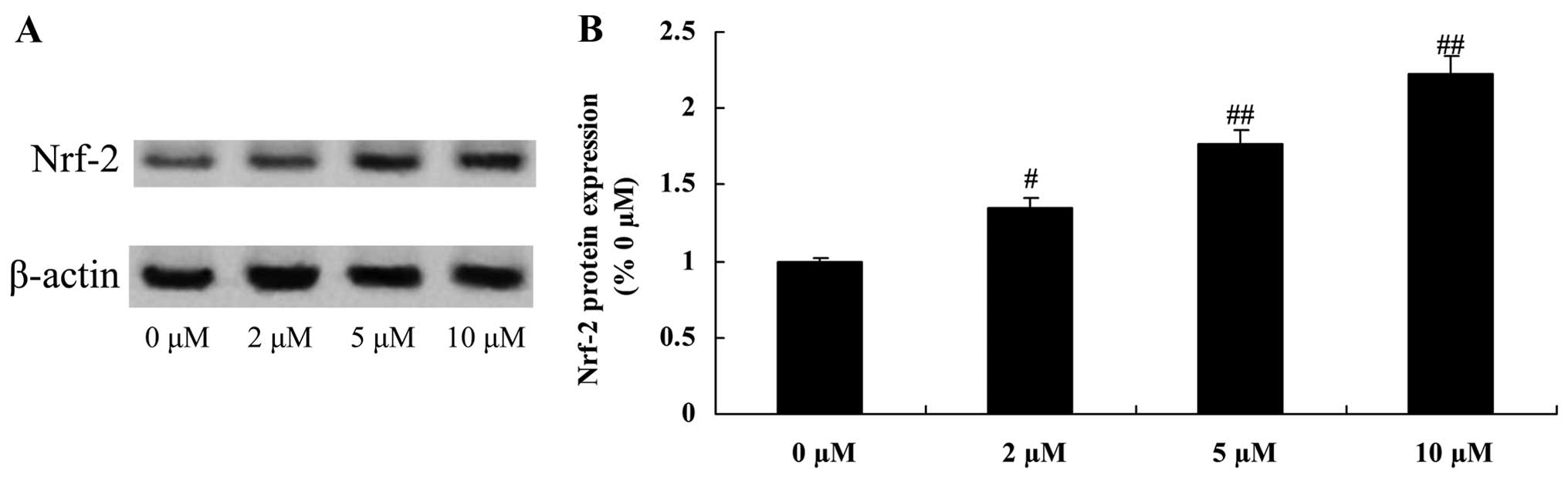
Protective effect of plumbagin increases
Nrf-2 protein expression in NPCs
To examine the anti-inflammatory effect of plumbagin
in the H2O2-exposed NPCs, Nrf-2 protein
expression was evaluated using western blot analysis. When compared
with the 0 µM plumbagin-treated group, Nrf-2 protein
expression was significantly increased by treatment with plumbagin
at concentrations of 2–10 µM (Fig. 10).
Discussion
Approximately 90% of the worldwide population has
suffered from pains in the waist or leg to varying extents, and
some individuals may experience permanent incapacitation from the
pain (15). Epidemiological data
also shows that the incidence of lumbar disc degeneration is rising
and it is developing in younger patients (16). With the changes in human longevity
and lifestyle, degenerative spinal disorders caused by lumbar disc
degeneration, such as lumbar spondylolisthesis, lumbar spinal
stenosis and lumbar disc disease have gained increasing attention
(15,17). However, it is proving difficult to
treat interveterbral disc degeneration in clinical practice
(18). In the present study, we
found that treatment with plumbagin significantly increased the
viability of H2O2-exposed NPCs in a
dose-dependent manner. Zhang et al suggested that plumbagin
protects against spinal cord injury through the suppression of
oxidative stress and inflammation through Nrf-2 upregulation in
Wistar rats (19). Wang et
al reported that plumbagin inhibits lipopolysaccharide-induced
inflammation in RAW 264.7 cells (20).
Oxidative stress refers to the process of tissue
damage caused by ROS accumulation in the body or cells as well as
the cell toxicity that results from a serious imbalance between
free radicals production and the antioxidant defenses when the
cells are subjected to harmful stimuli (21). ROS are a class of free radicals
which cause oxidative stress, (including O2−,
−OH, H2O2 and NO, etc.,) and may
be produced by mitochondria in human cells, and also induced by
environmental stresses (22).
Oxidative stress damage in the body is revealed by lipid
peroxidation, intracellular protein and enzyme denaturation as well
as DNA damage, resulting in abnormal cell function, which
ultimately leads to cell death or apoptosis (23). ROS may cause degradation of the
extracellular matrix through the inhibition of proteoglycan
synthesis and the destruction of extracellular matrix protein
structure and protease activity. With increasing age, oxidative
stress levels in the body gradually increase as ROS accumulate,
causing the destruction of the extracellular matrix in disc tissues
and the death or apoptosis of NPCs, thus causing disc degeneration
(24). In the present study,
treatment with plumbagin significantly reduced the
H2O2-induced generation of ROS as well as
lipid peroxidation. GSH content as well as the activity of CAT, SOD
and GSH-Px were increased in the NPCs. Zhang et al suggested
that plumbagin protects against spinal cord injury through the
suppression of oxidative stress,as well as the inhibition of ROS
and lipid peroxidation in Wistar rats (19). Checker et al demonstrated
that plumbagin inhibits lipopolysaccharide-induced oxidative stress
and inflammation (25).
Evidence indicates that the essential pathological
changes responsible for lumbar disc degeneration include not only
morphological and histological changes, but also a series of
changes in biochemical properties (26). Trauma may cause early disc
degeneration, which in turn induces the expression of cytokines;
the secondary inflammation induces further damage, and moreover
increases the severity of lumbar disc degeneration (27). IL-1β, IL-6 and TNF-α are important
cytokines, which may be involved in the pathogenesis of lumbar disc
degeneration (26). The results
of this study showed that the administration of plumbagin
significantly decreased the levels of TNF-α, IL-1β and IL-6 in the
H2O2-exposed NPCs. A study by Sandur et
al indicated that plumbagin abrogated the expression of
NF-κB-regulated gene products which led to the potentiation of
apoptosis induced by cytokine and chemotherapeutic agents (28). Luo et al demonstrated that
plumbagin inhibits NF-κB activation and therefore has the potential
to be developed as a novel anti-inflammatory agent (29).
Apoptosis may occur under physiological and
pathological conditions and circumstances, subject to a variety of
factors including caspases. Caspases belong to a family of cysteine
hydrolases, which play a central role in apoptosis. Caspase-3 is an
effector of apoptosis (30). NPCs
exposed to H2O2 were used in the present
study, and researchers have found that oxidative stress leads to
the activation of apoptotic signaling pathways, which include not
only the intrinsic apoptotic pathway (such as caspase-9), but also
includes the extrinisc apoptotic pathway (caspase-8) as well as the
common pathway of both channels (such as caspase-3) (31). This evidence further proves that
oxidative stress accelerates the apoptosis of NP cells and causes
disc degeneration (32). We found
that plumbagin significantly inhibited the activity of caspase-9
and -3 in the H2O2-exposed NPCs. Checker
et al demonstrated that plumbagin inhibits
lipopolysaccharide-induced oxidative stress, inflammation and
caspase-3 (25).
The Nrf-2-antioxidant response element (ARE)
signaling pathway regulates the encoding of antioxidant proteins
through various interactions, and it is the central regulator of
cellular antioxidant responses (33). The ARE, which is a cisacting
enhancer sequence of many antioxidant enzyme/protein gene upstream,
is one of the defense mechanisms which enables the body to deal
with oxidative stress (34).
Previous findings have shown that Nrf-2 adjusts the encoding of
antioxidant genes by interacting with ARE. Under normal conditions,
Nrf-2 is localized in the cytoplasm, combined with the cytoplasmic
protein Keap1; when subjected to attack from ROS, Nrf-2 dissociates
from Keap1, translocates into the nucleus, binds to Maf protein in
order to form a heterodimer, which binds to ARE in the gene to
activate targeted gene expression as well as to regulate
antioxidant enzyme/protein transcription (34,35). Thus, Nrf-2 is a receptor for
oxidative stress, which plays an important role in the protection
of cells against oxidative stress and it is one of the principal
defense mechanisms induced by exposure to exogenous toxic
substances (19). The findings of
the present study revealed that plumbagin significantly
downregulated NF-κB expression and upregulated Nrf-2 expression in
the H2O2-exposed NPCs. Zhang et al
suggested that plumbagin protects against spinal cord injury
through the suppression of oxidative stress and inflammation
through Nrf-2 upregulation in Wistar rats (19).
In conclusion, our findings have demonstrated that
plumbagin exerts neuroprotective effects in NPCs by attenuating
H2O2-induced oxidative stress, inflammation
and apoptosis. Moreover, we have provided evidence that NF-κB/Nrf-2
are potential targets for plumbagin in intervertebral discs. These
findings suggest that plumbagin may be of potential therapeutic
value in the treatment of various neurological diseases.
References
|
1
|
Merlini L and Donati U: Auto-immunity and
intervertebral disc disease. Ital J Orthop Traumatol. 6:427–432.
1980.PubMed/NCBI
|
|
2
|
van den Eerenbeemt KD, Ostelo RW, van
Royen BJ, Peul WC and van Tulder MW: Total disc replacement surgery
for symptomatic degenerative lumbar disc disease: a systematic
review of the literature. Eur Spine J. 19:1262–1280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saritha K, Rajesh A, Manjulatha K, Setty
OH and Yenugu S: Mechanism of antibacterial action of the alcoholic
extracts of Hemidesmus indicus (L.) R. Br. ex Schult, Leucas aspera
(Wild.), Plumbago zeylanica L., and Tridax procumbens (L.) R. Br.
ex Schult. Front Microbiol. 6:5772015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castaño-Betancourt MC, Oei L, Rivadeneira
F, de Schepper EI, Hofman A, Bierma-Zeinstra S, Pols HA,
Uitterlinden AG and Van Meurs JB: Association of lumbar disc
degeneration with osteoporotic fractures; the Rotterdam study and
meta-analysis from systematic review. Bone. 57:284–289. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen S, Liao M, Li J, Peng H and Xiong M:
The correlation between microvessel pathological changes of the
endplate and degeneration of the intervertebral disc in diabetic
rats. Exp Ther Med. 5:711–717. 2013.PubMed/NCBI
|
|
6
|
Liu ZH, Huo JL, Wu ZG, Sun Z, Bai F,
Samartzis D, Gantenbein B, Fan SD and Wang HQ: RASSF7 expression
and its regulatory roles on apoptosis in human intervertebral disc
degeneration. Int J Clin Exp Pathol. 8:16097–16103. 2015.
|
|
7
|
Sun W, Zhang K, Zhao CQ, Ding W, Yuan JJ,
Sun Q, Sun XJ, Xie YZ, Li H and Zhao J: Quantitative T2 mapping to
characterize the process of intervertebral disc degeneration in a
rabbit model. BMC Musculoskelet Disord. 14:3572013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Li JM and Hu YG: Transplantation of
gene-modified nucleus pulposus cells reverses rabbit intervertebral
disc degeneration. Chin Med J (Engl). 124:2431–2437. 2011.
|
|
9
|
Wang J, Tang T, Yang H, Yao X, Chen L, Liu
W and Li T: The expression of Fas ligand on normal and stabbed-disc
cells in a rabbit model of intervertebral disc degeneration: a
possible pathogenesis. J Neurosurg Spine. 6:425–430. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu ZH, Sun Z, Wang HQ, Ge J, Jiang TS,
Chen YF, Ma Y, Wang C, Hu S, Samartzis D and Luo ZJ: FasL
expression on human nucleus pulposus cells contributes to the
immune privilege of intervertebral disc by interacting with
immunocytes. Int J Med Sci. 10:1053–1060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai Y, Hou LF, Chan YP, Cheng L and But
PP: Inhibition of immediate allergic reactions by ethanol extract
from Plumbago zeylanica stems. Biol Pharm Bull. 27:429–432. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar S, Gautam S and Sharma A:
Antimutagenic and antioxidant properties of plumbagin and other
naphthoquinones. Mutat Res. 755:30–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen JW, Ni BB, Zheng XF, Li B, Jiang SD
and Jiang LS: Hypoxia facilitates the survival of nucleus pulposus
cells in serum deprivation by down-regulating excessive autophagy
through restricting ROS generation. Int J Biochem Cell Biol.
59:1–10. 2015. View Article : Google Scholar
|
|
14
|
Pi H, Xu S, Reiter RJ, Guo P, Zhang L, Li
Y, Li M, Cao Z, Tian L, Xie J, et al: SIRT3-SOD2-mROS-dependent
autophagy in cadmium-induced hepatotoxicity and salvage by
melatonin. Autophagy. 11:1037–1051. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Jiang X and Wang X: Spinal
pleomorphic xanthoastrocytoma companied with periventricular tumor.
Int J Clin Exp Pathol. 8:1036–1040. 2015.PubMed/NCBI
|
|
16
|
Black RC, Gardner VO, Armstrong GW, O'Neil
J and George MS: A contoured anterior spinal fixation plate. Clin
Orthop Relat Res. 227:135–142. 1988.PubMed/NCBI
|
|
17
|
Liang QQ, Ding DF, Xi ZJ, Chen Y, Li CG,
Liu SF, Lu S, Zhao YJ, Shi Q and Wang YJ: Protective effect of
ligustrazine on lumbar intervertebral disc degeneration of rats
induced by prolonged upright posture. Evid Based Complement
Alternat Med. 2014:5084612014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teichtahl AJ, Urquhart DM, Wang Y, Wluka
AE, Heritier S and Cicuttini FM: A Dose-response relationship
between severity of disc degeneration and intervertebral disc
height in the lumbosacral spine. Arthritis Res Ther. 17:2972015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Cheng L, Hou Y, Si M, Zhao YP and
Nie L: Plumbagin protects against spinal cord injury-induced
oxidative stress and inflammation in Wistar rats through Nrf-2
upregulation. Drug Res (Stuttg). 65:495–499. 2015.
|
|
20
|
Wang T, Wu F, Jin Z, Zhai Z, Wang Y, Tu B,
Yan W and Tang T: Plumbagin inhibits LPS-induced inflammation
through the inactivation of the nuclear factor-kappa B and mitogen
activated protein kinase signaling pathways in RAW 264.7 cells.
Food Chem Toxicol. 64:177–183. 2014. View Article : Google Scholar
|
|
21
|
Yu JH and Kim H: Oxidative stress and
inflammatory signaling in cerulein pancreatitis. World J
Gastroenterol. 20:17324–17329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Ge Y, Li W and Hu Y: Diversities
of interaction of murine macrophages with three strains of Candida
albicans represented by MyD88, CARD9 gene expressions and ROS,
IL-10 and TNF-α secretion. Int J Clin Exp Med. 7:5235–5243.
2014.
|
|
23
|
Mohammad NS, Yedluri R, Addepalli P,
Gottumukkala SR, Digumarti RR and Kutala VK: Aberrations in
one-carbon metabolism induce oxidative DNA damage in sporadic
breast cancer. Mol Cell Biochem. 349:159–167. 2011. View Article : Google Scholar
|
|
24
|
Cheng YH, Yang SH and Lin FH:
Thermosensitive chitosan-gelatin-glycerol phosphate hydrogel as a
controlled release system of ferulic acid for nucleus pulposus
regeneration. Biomaterials. 32:6953–6961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Checker R, Patwardhan RS, Sharma D, Menon
J, Thoh M, Sandur SK, Sainis KB and Poduval TB: Plumbagin, a
vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative
stress, inflammation and endotoxic shock via NF-κB suppression.
Inflammation. 37:542–554. 2014. View Article : Google Scholar
|
|
26
|
Valdes AM, Hassett G, Hart DJ and Spector
TD: Radiographic progression of lumbar spine disc degeneration is
influenced by variation at inflammatory genes: a candidate SNP
association study in the Chingford cohort. Spine. 30:2445–2451.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Omair A, Holden M, Lie BA, Reikeras O and
Brox JI: Treatment outcome of chronic low back pain and
radiographic lumbar disc degeneration are associated with
inflammatory and matrix degrading gene variants: a prospective
genetic association study. BMC Musculoskelet Disord. 14:1052013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sandur SK, Ichikawa H, Sethi G, Ahn KS and
Aggarwal BB: Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone)
suppresses NF-kappaB activation and NF-kappaB-regulated gene
products through modulation of p65 and IkappaBalpha kinase
activation, leading to potentiation of apoptosis induced by
cytokine and chemotherapeutic agents. J Biol Chem. 281:17023–17033.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo P, Wong YF, Ge L, Zhang ZF, Liu Y, Liu
L and Zhou H: Anti-inflammatory and analgesic effect of plumbagin
through inhibition of nuclear factor-κB activation. J Pharmacol Exp
Ther. 335:735–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SE, Shin WT, Park C, Hong SH, Kim GY,
Kim SO, Ryu CH, Hong SH and Choi YH: Induction of apoptosis in
MDA-MB-231 human breast carcinoma cells with an ethanol extract of
Cyperus rotundus L. by activating caspases. Oncol Rep.
32:2461–2470. 2014.PubMed/NCBI
|
|
31
|
Piao CS, Loane DJ, Stoica BA, Li S,
Hanscom M, Cabatbat R, Blomgren K and Faden AI: Combined inhibition
of cell death induced by apoptosis inducing factor and caspases
provides additive neuroprotection in experimental traumatic brain
injury. Neurobiol Dis. 46:745–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen JW, Ni BB, Li B, Yang YH, Jiang SD
and Jiang LS: The responses of autophagy and apoptosis to oxidative
stress in nucleus pulposus cells: implications for disc
degeneration. Cell Physiol Biochem. 34:1175–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li
W, Zhou ML and Wang XL: Astaxanthin activates nuclear factor
erythroid-related factor 2 and the antioxidant responsive element
(Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in
rats and attenuates early brain injury. Mar Drugs. 12:6125–6141.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaplan O, Meriç M, Acar Z, Kale A,
Demircan S, Yılmaz O, Demircan G and Yılmaz Miroğlu Y: The effect
of exercise and antioxidant enzyme levels in syndrome X and
coronary slow flow phenomenon: an observational study. Anadolu
Kardiyol Derg. 13:641–646. 2013.PubMed/NCBI
|
|
35
|
Delgado-Buenrostro NL, Medina-Reyes EI,
Lastres-Becker I, Freyre-Fonseca V, Ji Z, Hernández-Pando R,
Marquina B, Pedraza-Chaverri J, Espada S, Cuadrado A and Chirino
YI: Nrf2 protects the lung against inflammation induced by titanium
dioxide nanoparticles: a positive regulator role of Nrf2 on
cytokine release. Environ Toxicol. 30:782–792. 2015. View Article : Google Scholar
|