Introduction
Valeriana is the main genus in the family
Valerianaceae, and valerian root extracts have been used as a
traditional herbal medicine for centuries (1). The genus Valeriana contains
>250 species and many subspecies (2). Valeriana fauriei Briq. (VF)
has been used to treat humans for hundreds of years (3). In certain countries, it is primarily
sold as a sleeping aid, and in Europe it is used to treat
restlessness, tremors and anxiety (4–8).
Valeriana officinalis has been used as a sedative and to
treat anxiety and sleep disorders (9,10).
Various effects of V. officinalis have been reported; it has
been suggested that Valeriana exerts its effects through
gamma-aminobutyric acid (GABA) ergic mechanisms (11). In a previous study, V.
officinalis exerted antioxidant effects and decreased lipid
peroxidation induced by quinolinic acid (12). In addition, V. officinalis
has been reported to exert neuroprotective effects in several
neurodegenerative diseases, such as Parkinson's and Alzheimer's
disease (13–15).
Prenatal stress (PNS) during the critical period of
fetal brain development is an important environmental risk factor
for the development of human psychiatric disorders, such as
schizophrenia, in the adult offspring, and the second trimester of
pregnancy in humans seems to be the most vulnerable period for
insult (16–21).
Additionally, previous studies have demonstrated
that PNS elevates glucocorticoids during gestation and is
associated with biochemical, physiological and behavioral changes
in the offspring, including reduced birth weight, cardiovascular
and neuroendocrinological abnormalities, attention dysfunction,
enhanced anxiety-related behaviors and cognitive deficits (22–31). Thus, the pregnant rats in the
present study were exposed to stressful manipulations during the
third week of pregnancy, which is similar to the second trimester
of human gestation (28–30). Previous studies have shown that
PNS decreases dendritic length, spine density, the number of
neurons, and diminishes the number of hippocampal synapses as
compared with non-stressed (NS) controls (32,33). PNS also causes various changes in
gene expression, including the expression of genes associated with
neural development, cell differentiation, and neurotransmitter
function in the brains of rats (27,34,35).
To the best of our knowledge, no previous studies
have examined the effects of VF on PNS or neurodegeneration. In the
present study, behavioral patterns and changes in protein levels
were examined in the prefrontal cortex of the offspring of rats
exposed to PNS, and we subsequently determined whether the changes
caused by PNS were affected by treatment with VF.
Materials and methods
Preparation of VF extracts and
administration to rats
VF root extract was purchased from Yunpung
(Chungbuk, Korea), and the specimens were identified taxonomically
by an Oriental medicine physician at the National Institute of
Horticultural and Herbal Science [Rural Development Administration
(RDA); Wanju, Jeonbuk, Korea]. The voucher specimen (HPR-207) was
deposited in the herbarium of the Herbal Crop Research Institute
(Eumseong, Korea).
Drugs and animals
VF was dissolved in water, and the drug was
administered on postnatal day 35 for 3 weeks until postnatal day
56, as previously described (31). It was provided to the rats in
regular drinking bottles (100 mg/kg/day) to avoid preadolescent
stress exposure resulting from the administration of repeated
injections.
Prenatal stress procedures
Prenatal stress procedures. Pregnant Sprague-Dawley
rats (n=6 in each group) were purchased from Central Lab Animal,
Inc. (Seoul,Korea) and arrived at the animal facility on day 7 of
gestation. The rats were housed under standard conditions with a
12/12-h light/dark cycle (lights on at 06:30) with free access to
food and water. All animal procedures were performed in accordance
with the Guidelines for the Care and Use of Laboratory Animals of
the US National Institutes of Health. All experimental procedures
were reviewed and approved by the institutional Review Board for
Animal Welfare at Soonchunhyang University. The rat model of PNS
was established as described in our previous studies (36,37). Briefly, PNS exposure was initiated
on day 14 of gestation and continued until day 21, and consisted
of: i) 1 h restraint in a well-ventilated, cylindrical Plexiglas
restraint device (Braintree Scientific, Inc. Braintree, MA, USA),
ii) 6 h of exposure to a cold environment (4°C), iii) overnight
fasting, iv) 15 min of swimming stress in room-temperature water,
v) reversal of the light-dark cycle, and/or vi) social stress
induced by overcrowded housing conditions during the dark phase
(28,29). Pregnant dams used as controls
remained in the animal housing room during gestational days 14–21
and were exposed to only normal animal husbandry procedures.
All groups contained litters of between 8 and 15
pups with similar numbers of males and females, extremely large or
small litters being eliminated. The offspring were weaned 21 days
after birth and group-housed. The male offspring were selected and
used for further experiments. Thus, 3 experimental groups were
tested as adults: the 'control' (n=16 male offspring) group were
offspring of unstressed mothers; the prenatal stress group 'PNS'
(n=16 male offspring) were offspring of mothers subjected to stress
before parturition; and the VF group, VF administration in a group
subjected to prenatal stress (n=16 male offspring).
Behavioral tests
Modified behavioral tests, including the forced swim
test (FST), the open field test (OFT), a social interaction test
(SIT), and the prepulse inhibition (PPI) test were performed as
previously described (28,37–40).
PPI test
Briefly, an automated startle reflex system (SR-Lab,
San Diego Instruments, San Diego, CA, USA) was used to measure PPI.
The system consisted of a startle chamber housed in a
sound-attenuated isolation cabinet equipped with an internal fan
and light. A cylindrical, transparent, acrylic holding apparatus
resting on a four-pegged platform within the isolation chamber was
used to hold each subject throughout the testing session (subject
age, 56 days). Background noise and acoustic stimuli were
controlled via the SR Lab microcomputer and interface assembly, and
were delivered through a speaker mounted above the cylindrical
holding apparatus. All test chambers were located in a
sound-attenuated experimental room to minimize external noise, as
previously described (41).
Background noise of 70 dB was present throughout the test session.
After a 5-min acclimation period to the background noise, each
subject was presented with a series of 60 acoustic stimuli trials.
The trials were presented in pseudorandom order, namely the
individual startle trials (single acoustic stimulus delivered at
120 dB for 40 msec), the prepulse stimulus trials (a single
prepulse stimulus presented at 15 dB above background for 20 msec),
the non-stimulus trials (not following any noise), and the prepulse
stimulus trials with acoustic stimuli (a single prepulse stimulus
presented at 15 dB above background, followed 20 msec later by a
startle stimulus presented at 120 dB for 40 msec). The inter-trial
interval was 15 msec, and each session lasted 22 min. The holding
chambers were cleaned with 75% ethanol between each test session.
Prepulse inhibition was presented as the percentage decrease in
startle amplitude as a function of the magnitude of the prepulse
stimulus using the following formula: percentage decrease = 100×
[(acoustic stimuli trial) − (the prepulse stimulus trials +
acoustic stimuli)] (42).
FST
The FST was performed (subject age, 57 days) as
previously described (37–39).
The rats were lowered individually into a cylinder filled with
fresh warm tap water (25±2°C). The rat was removed after 15 min and
wiped with a clean towel to remove excess water before being
returned to its home cage. Each rat was placed in the cylinder
again for 5 min the following day, and swimming, climbing and
immobility behaviors were recorded with a video camera (Samsung
HMX-T10) and by an observer with a stopwatch.
OFT
The OFT was performed in order to assess exploratory
activity and reactivity to a novel environment. The subjects were
removed from their home cage on the day of the test (subject age,
59 days) and placed individually in an open-field start box for 5
min. The apparatus was constructed from black polygal panels (Dowin
Polychem Co., Ltd., Seoul, Korea), and no background noise was
provided. The experimenter exited the room, and the behavior of the
subject was recorded, as previously described (37–39).
SIT
The SIT was adapted from previous studies (28,39,40) (subject age, 58 days). The social
interaction partners were same-sex siblings that resided in the
same cage after weaning and were of approximately equal body
weight. Each session lasted 20 min and was scored in terms of total
duration of social play and the number and type of
interactions.
Measurement of corticosterone
Following the behavior tests, the adult (59 days of
age) male offspring from each group were moved to the laboratory.
At approximately 16:00 on the last behavior test day, the
experimental subjects were placed in cylindrical plastic restraint
tubes for 60 min (n=5–6 animals/group). The restraint stressed male
offspring (n=5–6 animals/group) were sacrificed by decapitation.
Some of the rats were anesthetized with ethyl ether and perfused
with 4% paraformaldehyde. (n=5–6 animals/group). Trunk blood was
collected immediately in plastic tubes. The blood was centrifuged
at 13,000 rpm, and the serum was placed in a fresh tube. The brains
were removed rapidly from the skull and the prefrontal cortex and
hippocampus were separated, placed in a fresh tube, and frozen in
liquid nitrogen. The brain tissue and serum were stored at −80°C
until use. Serum corticosterone levels were determined by
immunoassay using the Rat Cortisol ELISA kit purchased from
MyBioSource (cat no. MBS023335; San Diego, CA, USA). Assays were
conducted according to the manufacturer's instructions, as
previously described (43).
Western blot analysis
Prefrontal cortical tissues were lysed in RIPA
buffer containing protease inhibitors and centrifuged at 14,000 rpm
for 10 min at 4°C. To detect dihydropyrimidi-nase-like 2 (Dpysl2)
and the neurofilament protein, 80 µg lysed protein was
subjected to 10 and 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to a polyvinylidene difluoride
membrane (Millipore, Milford, MA, USA). After blocking with 5% skim
milk, the membranes were probed with anti-Dpysl2 (1:1,000; #9393,
Cell Signaling Technology, Danvers, MA, USA), anti-LIM and SH3
protein 1 (Lasp1; 1:2,000; MAB8991, Millipore), anti-neurofilament
M (Nefm; 1:1,000; #2838, Cell Signaling Technology), anti-PSD95
[discs, large homolog 4 (Dlg4); 1:1,000; #3450, Cell Signaling
Technology] or anti-β-actin (Actb; 1:1,000; sc-81178, Santa Cruz
Biotechnology, Inc., CA, USA) antibodies overnight at 4°C and then
with peroxidase-conjugated secondary antibody (1:10,000; N4142,
Sigma, St. Louis, MO, USA) for 1 h at room temperature.
Immunoreactive bands were detected using an enhanced
chemiluminescence kit (Elpis-Biotech, Inc., Daejeon, Korea), and
quantitative measurements of Dpysl2, Lasp1, Nefm, Dlg4 and Actb
proteins were calculated using ImageJ software.
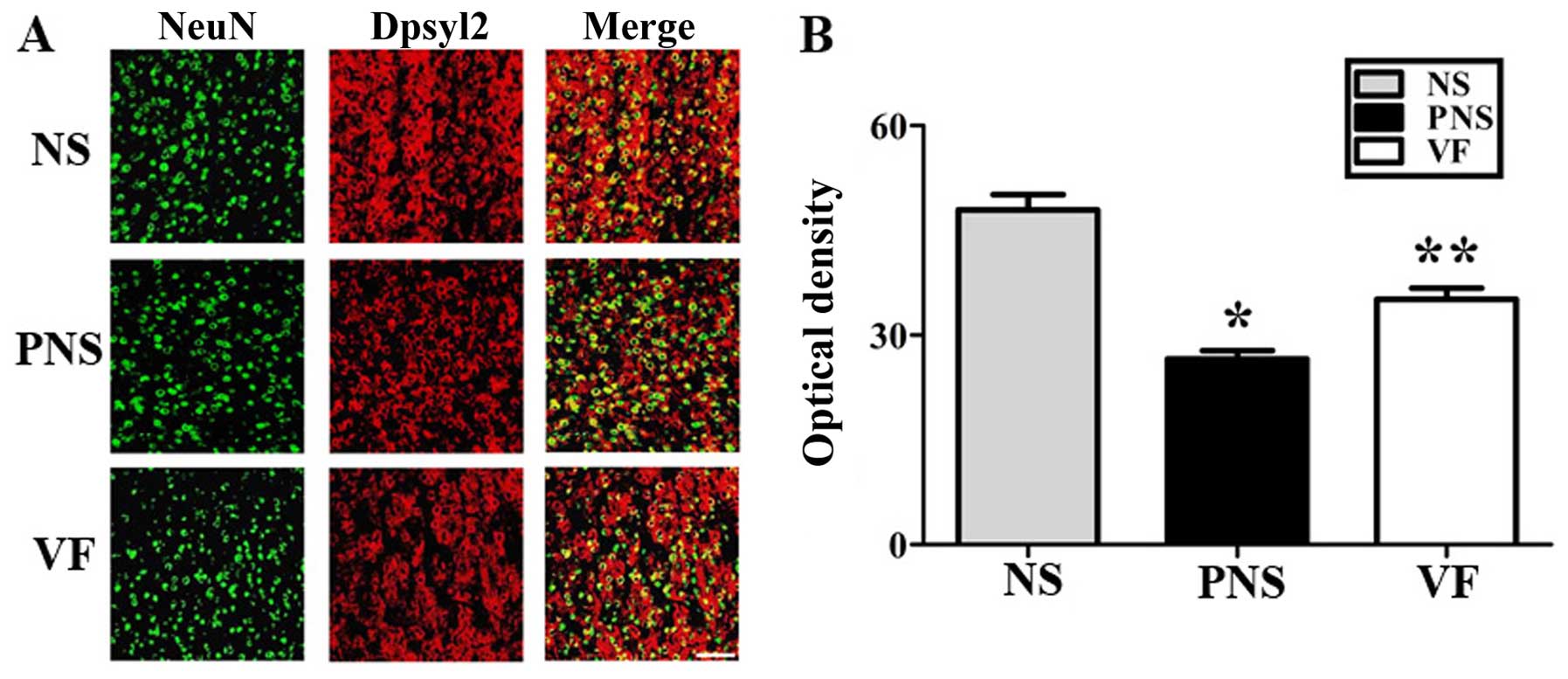
Immunohistochemistry
The rats were anesthetized with ethyl ether and
perfused with 4% paraformaldehyde. The fixed brains were removed,
frozen and cut into 30-µm sections. Frozen sections from the
prefrontal cortex were blocked with normal horse serum, incubated
with anti-Dpysl2 (1:1,000; HPA002381, Atlas Antibodies AB,
Stockholm, Sweden), Nefm (1:100; #2838, Cell Signaling Technology)
and anti-NeuN (1:100; MAB377, Millipore) and then incubated with
Cy3-conjugated anti-rabbit and mouse secondary antibodies (1:500
and 1:800; 715-545-151, 111-165-003, Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA). Nuclei staining was
carried out using 4′,6-diamidino-2-phenylindole (DAPI) staining
(Sigma). Fluorescent images were subsequently captured using a
confocal laser scanning microscope (FV10-ASW; Olympus, Tokyo,
Japan), and the images were quantified with Image J software
according to a protocol described previously with minor
modifications (44).
Statistical analysis
All data are expressed as the means ± standard
deviation and/or standard error of the means and compared using the
Student's t-test. All statistical analyses were performed using IBM
SPSS Statistics 19 software (SPSS Inc., Chicago, IL, USA). A
p-value <0.05 was considered to indicate a statistically
significant difference.
Results
We established a rat model of variable and
unpredictable PNS in order to evaluate the extent to which VF
treatment altered behavioral patterns and protein expression in the
prefrontal cortex. Thus, we aimed to examine the effects of VF on
the pathophysiology of stress-induced psychiatric disorders caused
by maternal stress during E14 to E21 of pregnancy.
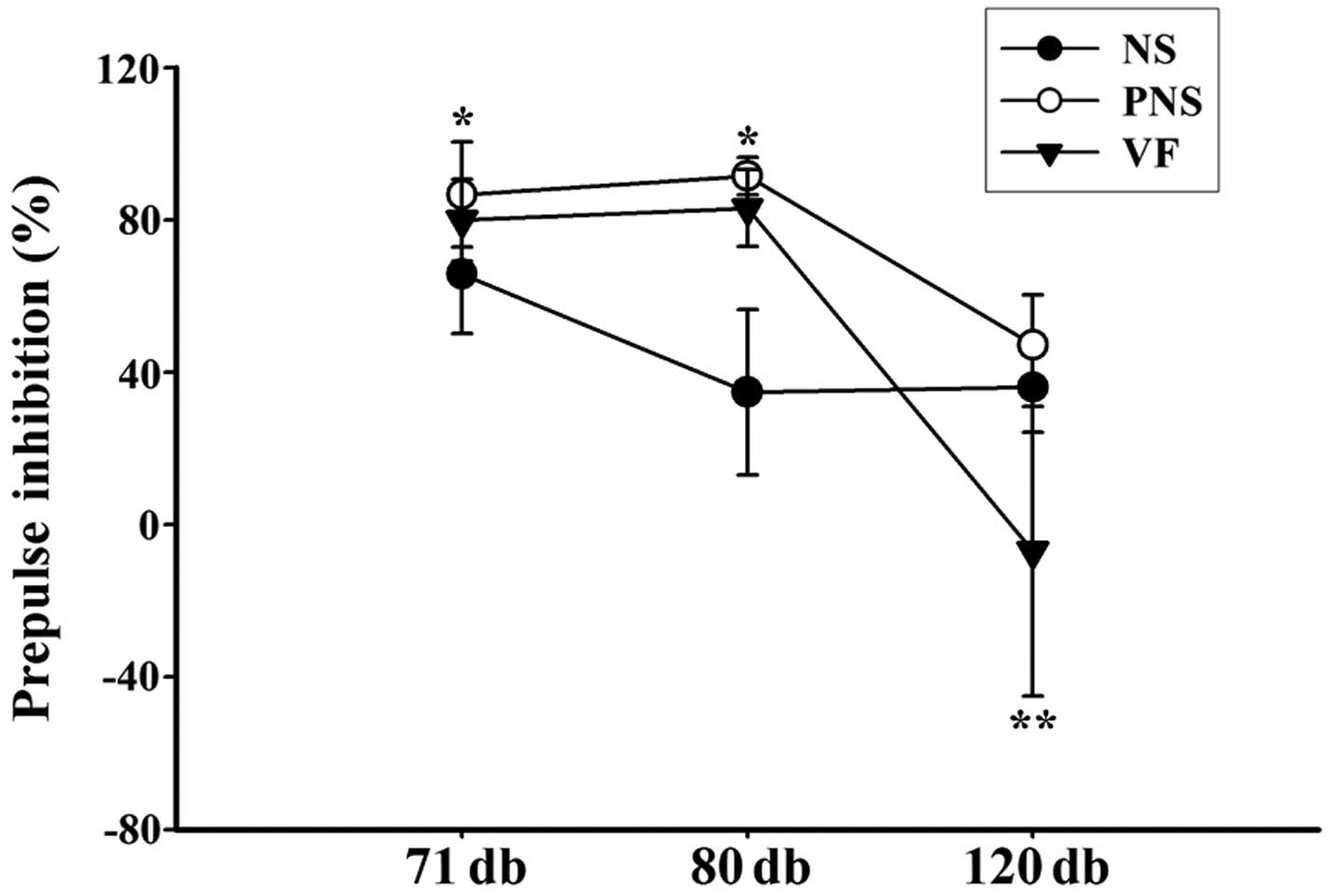
PPI test
Prenatal stress exposure during the third week of
gestation significantly affected sensorimotor gating. Significant
prepulse facilitation was detected in offspring of
prenatally-stressed rats (PNS group) at lower prepulse stimulus
intensities, whereas no change in the startle response was detected
in the offspring of NS rats (NS group) (Fig. 1). In order to examine the effect
of VF on sensorimotor gating function, we measured the PPI level
using the acoustic startle response test in the NS, PNS and VF
groups. The results revealed a significantly different effect on
PPI between the NS and PNS groups. The results indicate that PNS
significantly altered the PPI level at prepulse stimulus levels of
1 and 10 dB above background between the PNS and NS offspring
(p<0.05) but not at 50 dB above background (p>0.05). VF
treatment affected the PPI level at 71 and 80 dB pulse levels
(p>0.05); however, VF treatment decreased the PPI level at 120
dB pulse level (p<0.001).
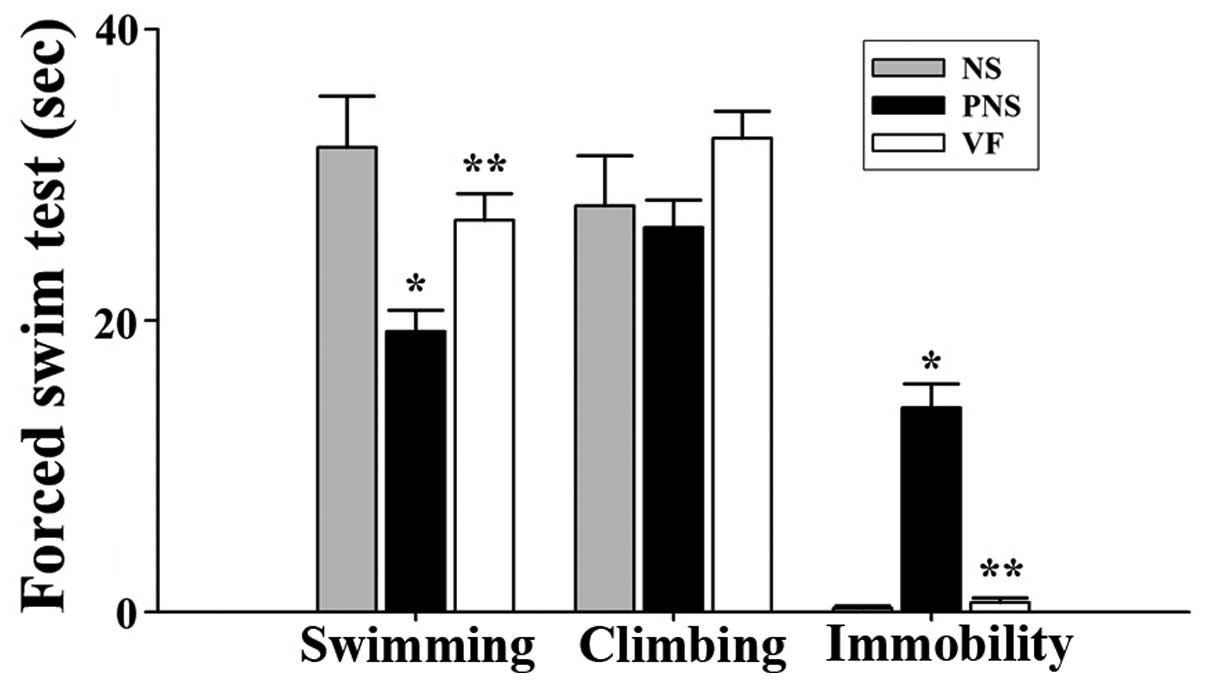
FST
We noted significant differences among the NS, PNS,
and VF-treated groups in the FST (Table I). The offspring of rats exposed
to PNS exhibited decreased swimming ability and increased
immobility compared with the offspring of NS rats (p<0.05,
Fig. 2). The changed behavior
recovered after VF treatment (p<0.05, Fig. 2).
 | Table IBehavior of the offspring of
prenatally-stressed and non-stressed rats as well as of the
offspring of prenatally-stressed rats treated with Valeriana
fauriei extract in a forced swim test. |
Table I
Behavior of the offspring of
prenatally-stressed and non-stressed rats as well as of the
offspring of prenatally-stressed rats treated with Valeriana
fauriei extract in a forced swim test.
| Behavior | NS (sec) | PNS (sec) | VF (sec) |
|---|
| Swimminga,b | 31.88±3.53 | 19.25±1.44 | 26.88±1.83 |
| Climbingb | 27.88±3.44 | 26.38±1.89 | 32.50±1.87 |
| Immobilitya,b | 0.25±0.16 | 14.00±1.65 | 0.63±0.32 |
OFT
The offspring from the NS and PNS groups were tested
with the OFT for 20 min. The PNS group had a significantly
decreased number of central entries and line crossings as well as a
decreased number and duration of rearing behaviors; these scores
recovered to normal levels following VF treatment (p<0.05,
Table II). In the PNS group, we
noted a significant increase in the number and duration of grooming
episodes and immobility behaviors; these scores also recovered to
the normal level following VF treatment (p<0.001, Table II).
 | Table IIBehavior of the offspring of
prenatally-stressed and non-stressed rats as well as of the
offspring of prenatally-stressed rats treated with Valeriana
fauriei extract in an open field test. |
Table II
Behavior of the offspring of
prenatally-stressed and non-stressed rats as well as of the
offspring of prenatally-stressed rats treated with Valeriana
fauriei extract in an open field test.
| Behavior | NS | PNS | VF |
|---|
| Centrala,b | 6.14±0.94 | 1.14±0.46 | 9.43±2.29 |
| Line
crossinga,b | 3.43±0.53 | 0.00±0.00 | 3.42±0.75 |
| Run(n) | 0.14±0.14 | 0.00±0.00 | 0.00±0.00 |
| Run(s) | 0.14±0.14 | 0.00±0.00 | 0.00±0.00 |
| Rear(n)a,b | 72.71±5.83 | 18.43±3.08 | 68.29±5.27 |
| Rear(s)a,b | 296.14±29.18 | 78.85±16.88 | 338.43±32.49 |
| Grooming(n)a | 9.57±1.57 | 21.00±3.29 | 14.71±1.15 |
| Grooming(s)a | 121.57±18.19 | 262.86±39.75 | 189.86±33.59 |
| Cage(n) | 107.43±6.40 | 78.00±33.01 | 78.57±5.43 |
| Cage(s)a | 313.29±17.42 | 206.86±15.09 | 241.29±21.98 |
| Immobile(n)a,b | 0.00±0.00 | 45.86±6.04 | 0.00±0.00 |
| Immobile(s)a,b | 0.00±0.00 | 248.00±53.46 | 0.00±0.00 |
SIT
Certain behavior scores measured during the SIT
decreased significantly in the PNS group, namely, sniffing,
following and grooming the partner. These decreased scores
increased towards normal levels following VF treatment (p<0.05,
Table III). We also noted that
certain scores increased in the PNS group: fighting, aggressive
grooming (the partner) and biting. These increased scores also
returned to normal levels following VF treatment.
 | Table IIIBehavior of the offspring of
prenatally-stressed and non-stressed rats as well as of the
offspring of prenatally-stressed rats treated with Valeriana
fauriei extract in a social interaction test. |
Table III
Behavior of the offspring of
prenatally-stressed and non-stressed rats as well as of the
offspring of prenatally-stressed rats treated with Valeriana
fauriei extract in a social interaction test.
| Behavior | NS | PNS | VF |
|---|
| Sniffing(n)a,b | 84.57±8.20 | 42.29±4.03 | 76.57±5.22 |
| Sniffing(s)a,b | 176.71±6.28 | 57.71±7.92 | 145.57±11.40 |
|
Following(n)a,b | 22.00±5.42 | 7.57±1.21 | 17.71±3.29 |
|
Following(s)a,b | 82.14±32.44 | 11.00±2.57 | 38.00±10.13 |
| Grooming(n)a,b | 7.00±1.69 | 0.00±0.00 | 5.00±1.09 |
| Grooming(s)a,b | 37.57±13.07 | 0.00±0.00 | 19.57±6.59 |
| Fight(n)a,b | 1.57±0.57 | 10.14±1.79 | 1.71±0.64 |
| Fight(s)a,b | 1.57±0.57 | 12.43±2.66 | 2.00±0.90 |
|
Aggressive(n)a,b | 1.43±0.48 | 3.29±0.57 | 0.43±0.43 |
|
Aggressive(s)b | 3.71±1.39 | 7.71±1.35 | 0.71±0.71 |
| Biting(n)a,b | 0.00±0.00 | 7.57±1.48 | 0.14±0.14 |
| Biting(s)a,b | 0.00±0.00 | 9.86±2.04 | 0.14±0.14 |
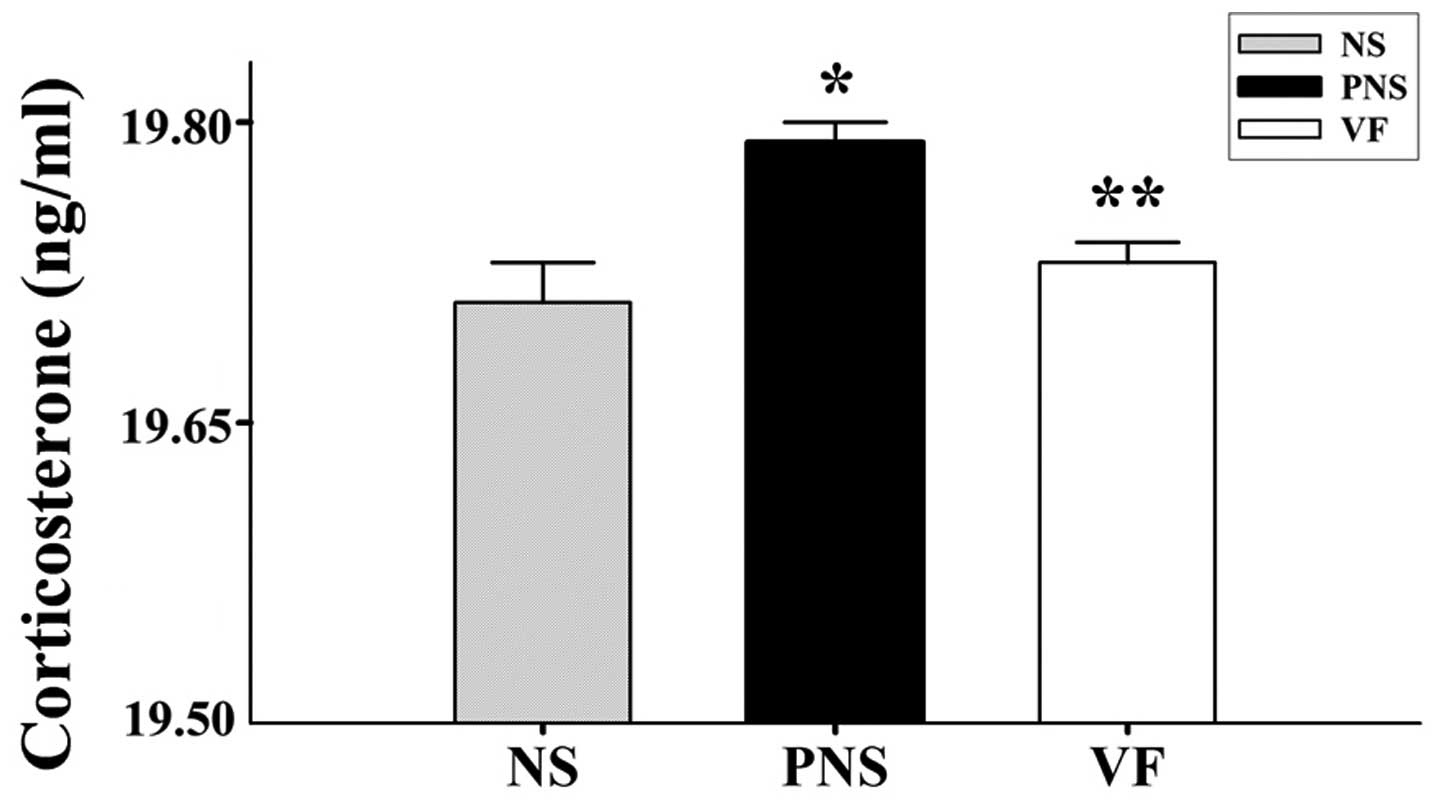
Corticosterone
In the PNS group, we noted increased stress-induced
corticosterone levels (p<0.05, Fig. 3) compared with the NS group. The
corticosterone level in the VF treatment group decreased and was
close to the control level (p<0.05, Fig. 3).
Western blot analysis and
immunohistochemistry
To examine the PNS-induced downregulation of several
neurodevelopmental proteins, namely Lasp1, Dpysl2, Dlg4 and the
Nefm proteins, we performed western blot analysis (Fig. 4) and immunohistochemical analyses
(Figs. 5Figure 6–7) of the prefrontal cortex areas of the
brains of rats in the NS, PNS and VF-treated groups. Western blot
analysis revealed that the quantities of these four proteins in the
prefrontal cortex were significantly lower in the PNS group than in
the NS group (p<0.05; Fig. 4).
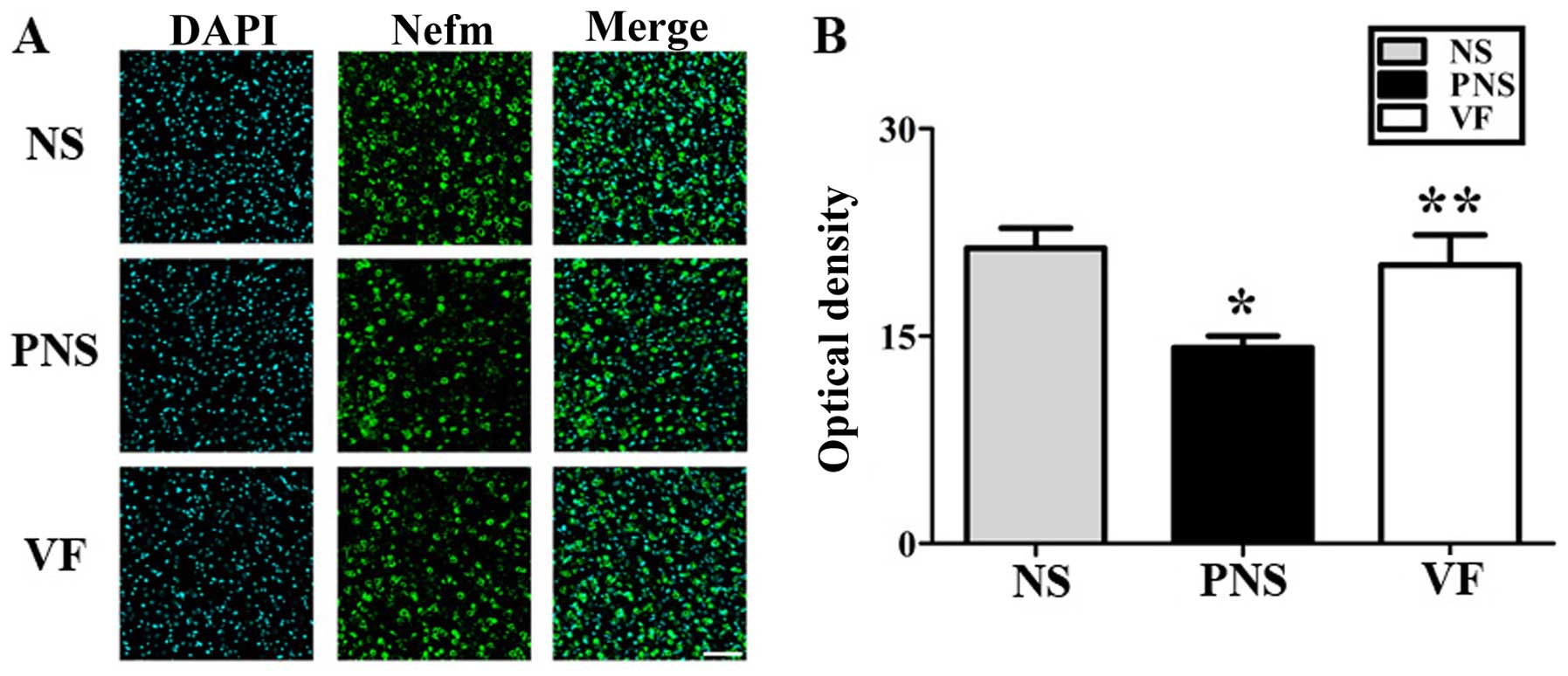
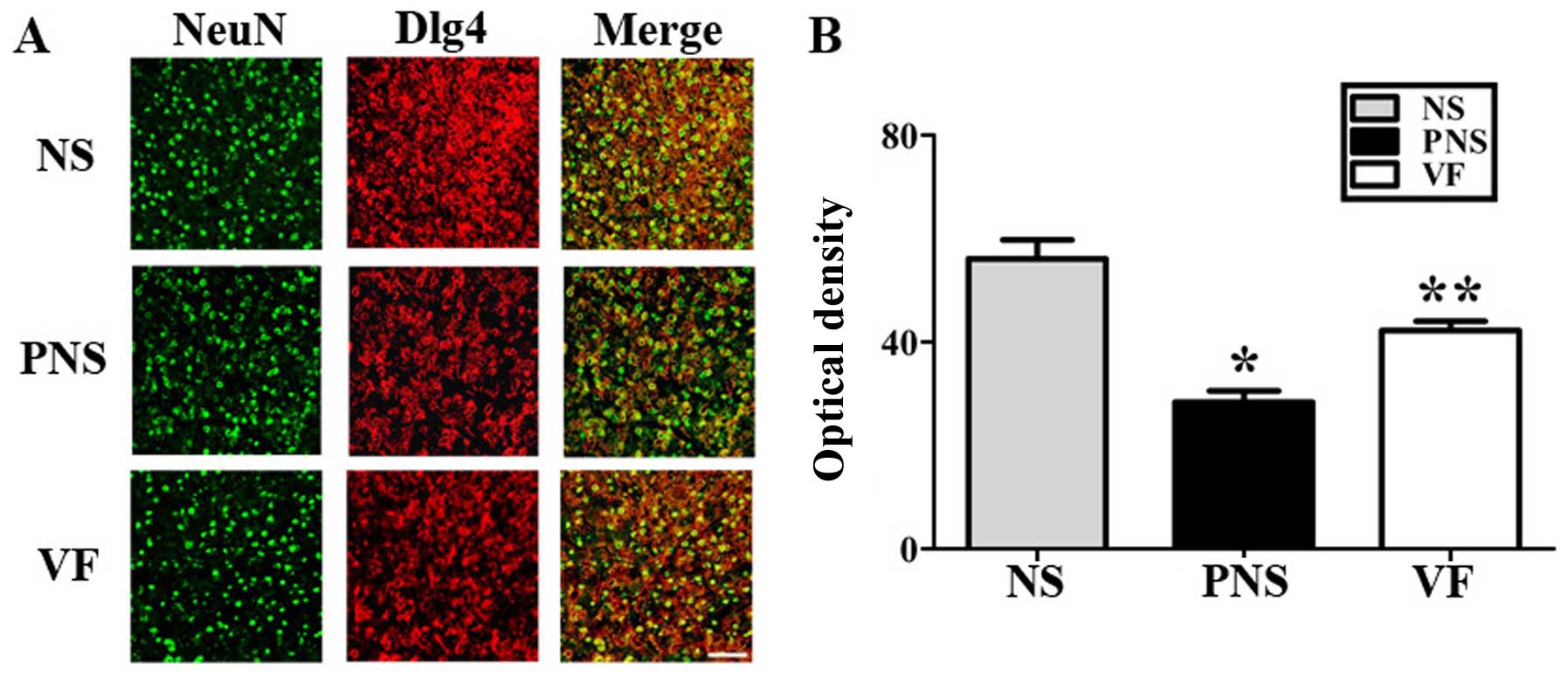
These changes were reversed by VF treatment (p<0.05; Fig. 4). Dpysl2, Dlg4 and Nefm were
differentially expressed, as shown in the immunofluorescent-stained
brain images of the NS, PNS and VF-treated groups as well as in the
immunohistochemical staining intensity values (p<0.05, Figs. 5Figure 6–7).
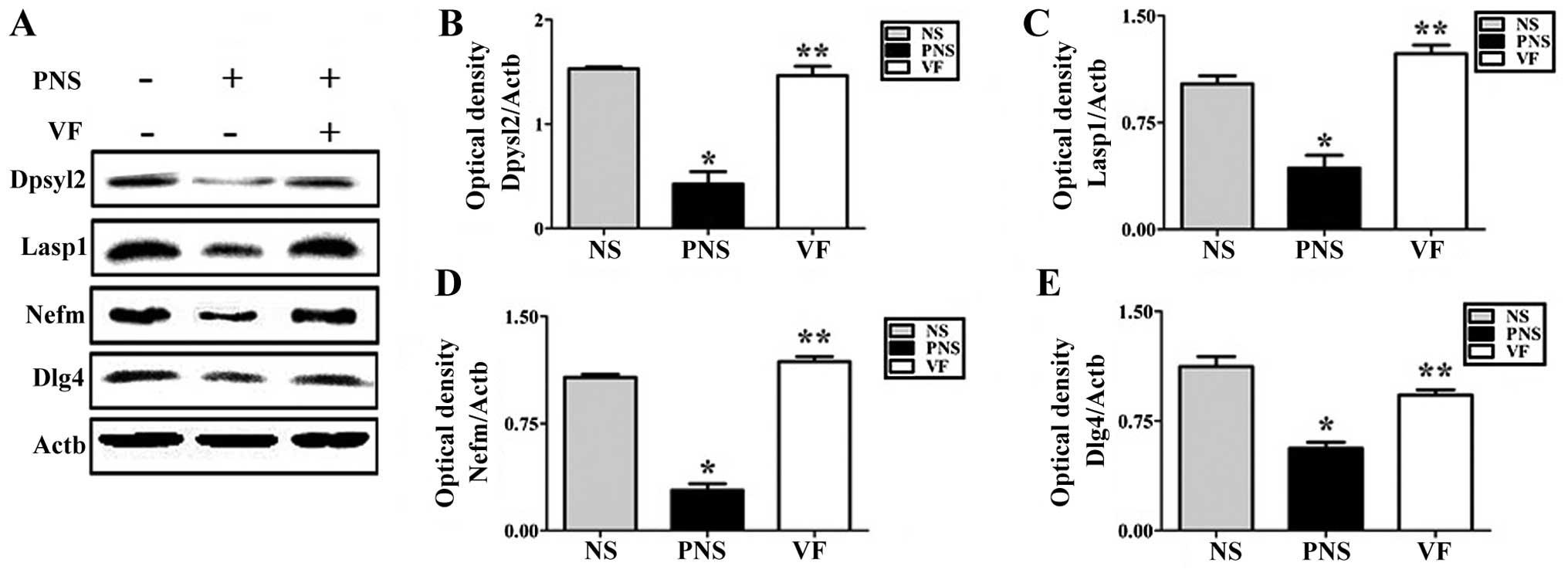 | Figure 4Western blot analysis of
dihydropyrimidinase-like 2 (Dpysl2), LIM and SH3 protein 1 (Lasp1),
neurofilament M (Nefm) and discs, large homolog 4 (Dlg4) expression
in the brains of the offspring of rats exposed to prenatal stress
(PNS). (A) Dpysl2, Lasp1, Nefm and Dlg4 expression was detected by
western blot analysis; Actb was used as the internal control. Rats
in the PNS group exhibited decreased Dpysl2, Lasp1, Nefm and Dlg4
expression in the prefrontal cortex. Decreased Dpysl2, Lasp1, Nefm
and Dlg4 expression recovered after Valeriana fauriei
treatment. Quantitative analysis of (B) Dpysl2 expression, (C)
Lasp1 expression, (D) Nefm expression and (E) Dlg4 expression. The
data in the graphs represent the means ± SEM.
*P<0.05, comparison between NS and PNS groups;
**P<0.05, comparison between PNS and VF groups. NS,
offspring of non-stressed rats; PNS, offspring of
prenatally-stressed rats; VF, Valeriana fauriei-treated
offspring. |
Discussion
In this study, we performed behavioral tests as well
as protein expression analyses in a rat model of PNS in order to
examine the potential preventive effects of VF on the
pathophysiology of stress-related psychiatric disorders, such as
depression and schizophrenia, according to neurodevelopmental
theory (50).
Certain preclinical studies have focused on the
antidepressant-like effect of the Valeriana species
(45), and the alterations in
cerebral Na+/K+-ATPase activity caused by
Valerian species in other psychiatric disorders (46,47). Additionally, valerian root extract
has been proven to exert neuroprotective effects in several
neurodegenerative diseases, such as Parkinson's and Alzheimer's
disease (13–15). It has also been reported that the
effects of valerian and its active component valerenic acid are
closely related to the GABA system (11,48). GABA-induced depolarization
activates cAMP response element-binding signaling, which promotes
neuronal survival by activating downstream survival genes (49).
Experimental manipulation of the PNS model
interrupts early brain and central nervous system development, thus
inducing significant neurodevelopmental changes, increasing
maternal stress hormones, and altering responses to prenatal
stressors (23,50). Impaired social behavior and
interaction was observed in prepubertal rats at 56 days of age as
well as in young adult rats and all these rats were the offspring
of PNS-exposed rats (56). One of
the first clinical signs associated with human schizophrenia is
social withdrawal during adolescence (51–53). The emergence of social withdrawal
in adolescent rats in the PNS group appears to be consistent with
the literature on clinical schizophrenia and further supports the
theory that this model is relevant to studies of the schizophrenia
phenotype. This diminution of social behavior and interaction may
reflect the increased anxiety experienced by the offspring of the
prenatally-stressed rats (24).
In the present study, PNS-induced decreases in non-aggressive
behavior and increases in aggressive behavior returned towards
normal levels following VF treatment. Additionally, certain
behavioral patterns noted in the FST and OFT, which assess
depressive behavior, were recovered following VF treatment.
We investigated sensorimotor gating, as reflected by
PPI (54,55). This gating has been reported to be
disrupted in patients with schizophrenia (56), but de Bruin et al (57) demonstrated that these forms of
gating are independent central nervous system (CNS) phenomena.
Previous studies have noted PPI deficits in a neonatal rat model of
ventral hippocampal lesions (58), heterozygous reeler mice (59), rats reared in isolation (60), and rats which received a prenatal
immune challenge (61), and a
mouse model of maternal influenza (62). However, Lehmann et al
failed to generate PPI deficits by repeatedly stressing Wistar
female rats during the final week of gestation (63). We suggest that several factors
including the rat strain (64,65) and the homotypic stress paradigm
used by Lehmann et al (63) contributed to the different
outcomes. We report in the present study that repeated exposure to
various stressors disrupted the gating, as reflected by deficits in
PPI, and this was affected by VF treatment.
The decrease in the expression of neurofilament and
Dpysl2 proteins in the offspring of rats with PNS was affected by
VF treatment. Our previous studies have shown that Dpysl2 and
neurofilament protein levels decreased in the offspring of rats
exposed to PNS (36,37). Furthermore, according to our
unpublished data, Lasp1 protein was also downregulated in the PNS
group. To confirm these results and to verify the effect of VF on
the expression of the proteins, in the present study we examined
protein expression in the brains of the offspring of the VF-treated
group. We detected the decreased expression of a postsynaptic
protein associated with the development of the dendritic spine
called Dlg4 (also known as PSD95) for the first time, to the best
of our knowledge, in the rat model of PNS. Thus, we conclude that
PNS induced decreases in the expression of several
neurodevelopmental proteins and the expression levels were
increased following VF treatment. These changes in protein
expression may affect brain development and may have influenced the
behavioral changes in the offpsring of the prenatally-stressed
rats.
Our results illustrate the beneficial effect which
VF exerts, and we suggest that it would be useful in the treatment
of psychiatric disorders such as schizophrenia. However, further
research using cellular and animal model systems with a single
extract component is necessary in order to characterize the
potential pharmacological functions of VF in models of
schizophrenia and depression-like behavior.
Acknowledgments
The present study was performed with the support of
the 'Cooperative Research Program for Agriculture Science and
Technology Development (project no. PJ0115822016)' of the Rural
Development Administration, Korea.
References
|
1
|
Stevinson C and Ernst E: Valerian for
insomnia: a systematic review of randomized clinical trials. Sleep
Med. 1:91–99. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Circosta C, De Pasquale R, Samperi S, Pino
A and Occhiuto F: Biological and analytical characterization of two
extracts from Valeriana officinalis. J Ethnopharmacol. 112:361–367.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JS, Ahn JD and Cho SI: Effects of
Valerianae Radix et Rhizoma extract on psychological stress in
mice. Pharmacogn Mag. 11:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barton DL, Atherton PJ, Bauer BA, Moore DF
Jr, Mattar BI, Lavasseur BI, Rowland KM Jr, Zon RT, Lelindqwister
NA, Nagargoje GG, et al: The use of Valeriana officinalis
(Valerian) in improving sleep in patients who are undergoing
treatment for cancer: a phase III randomized, placebo-controlled,
double-blind study (NCCTG Trial, N01C5). J Support Oncol. 9:24–31.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rezvani ME, Roohbakhsh A, Allahtavakoli M
and Shamsizadeh A: Anticonvulsant effect of aqueous extract of
Valeriana officinalis in amygdala-kindled rats: possible
involvement of adenosine. J Ethnopharmacol. 127:313–318. 2010.
View Article : Google Scholar
|
|
6
|
Murphy K, Kubin ZJ, Shepherd JN and
Ettinger RH: Valeriana officinalis root extracts have potent
anxiolytic effects in laboratory rats. Phytomedicine. 17:674–678.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang PC, Ran XH, Chen R, Luo HR, Liu YQ,
Zhou J and Zhao YX: Germacrane-type sesquiterpenoids from the roots
of Valeriana officinalis var. latifolia. J Nat Prod. 73:1563–1567.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Letchamo W, Ward W, Heard B and Heard D:
Essential oil of Valeriana officinalis L. cultivars and their
antimicrobial activity as influenced by harvesting time under
commercial organic cultivation. J Agric Food Chem. 52:3915–3919.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leuschner J, Müller J and Rudmann M:
Characterisation of the central nervous depressant activity of a
commercially available valerian root extract.
Arzneimittelforschung. 43:638–641. 1993.PubMed/NCBI
|
|
10
|
Hadley S and Petry JJ: Valerian. Am Fam
Physician. 67:1755–1758. 2003.PubMed/NCBI
|
|
11
|
Yuan CS, Mehendale S, Xiao Y, Aung HH, Xie
JT and Ang-Lee MK: The gamma-aminobutyric acidergic effects of
valerian and valerenic acid on rat brainstem neuronal activity.
Anesth Analg. 98:353–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sudati JH, Fachinetto R, Pereira RP,
Boligon AA, Athayde ML, Soares FA, de Vargas Barbosa NB and Rocha
JB: In vitro antioxidant activity of Valeriana officinalis against
different neurotoxic agents. Neurochem Res. 34:1372–1379. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Oliveria DM, Barreto G, De Andrade DVG,
Saraceno E, Aon-Bertolino L, Capani F, Dos Santos El, Bachá R and
Giraldez LD: Cytoprotective effect of Valeriana officinalis extract
on an in vitro experimental model of Parkinson disease. Neurochem
Res. 34:215–220. 2009. View Article : Google Scholar
|
|
14
|
Malva JO, Santos S and Macedo T:
Neuroprotective properties of Valeriana officinalis extracts.
Neurotox Res. 6:131–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pereira RP, Fachinetto R, de Souza Prestes
A, Wagner C, Sudati JH, Boligon AA, Athayde ML, Morsch VM and Rocha
JBT: Valeriana officinalis ameliorates vacuous chewing movements
induced by reserpine in rats. J Neural Transm Vienna.
118:1547–1557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown AS, van Os J, Driessens C, Hoek HW
and Susser ES: Further evidence of relation between prenatal famine
and major affective disorder. Am J Psychiatry. 157:190–195. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sullivan PF: The genetics of
schizophrenia. PLoS Med. 2:e2122005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huttunen MO and Niskanen P: Prenatal loss
of father and psychiatric disorders. Arch Gen Psychiatry.
35:429–431. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
King M, Nazroo J, Weich S, McKenzie K,
Bhui K, Karlsen S, Stansfeld S, Tyrer P, Blanchard M, Lloyd K, et
al: Psychotic symptoms in the general population of England - a
comparison of ethnic groups (The EMPIRIC study). Soc Psychiatry
Psychiatr Epidemiol. 40:375–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
King S, Laplante D and Joober R:
Understanding putative risk factors for schizophrenia:
retrospective and prospective studies. J Psychiatry Neurosci.
30:342–348. 2005.PubMed/NCBI
|
|
21
|
Lim C, Chong SA and Keefe R: Psychosocial
factors in the neurobiology of schizophrenia: a selective review.
Ann Acad Med Singapore. 38:402–406. 2009.PubMed/NCBI
|
|
22
|
Imamura Y, Nakane Y, Ohta Y and Kondo H:
Lifetime prevalence of schizophrenia among individuals prenatally
exposed to atomic bomb radiation in Nagasaki City. Acta Psychiatr
Scand. 100:344–349. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meyer U and Feldon J: Epidemiology-driven
neurodevelopmental animal models of schizophrenia. Prog Neurobiol.
90:285–326. 2010. View Article : Google Scholar
|
|
24
|
Weinstock M: The long-term behavioural
consequences of prenatal stress. Neurosci Biobehav Rev.
32:1073–1086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seckl JR: Prenatal glucocorticoids and
long-term programming. Eur J Endocrinol. 151(Suppl 3): U49–U62.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Kloet ER, Sibug RM, Helmerhorst FM and
Schmidt MV: Stress, genes and the mechanism of programming the
brain for later life. Neurosci Biobehav Rev. 29:271–281. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beydoun H and Saftlas AF: Physical and
mental health outcomes of prenatal maternal stress in human and
animal studies: a review of recent evidence. Paediatr Perinat
Epidemiol. 22:438–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee PR, Brady DL, Shapiro RA, Dorsa DM and
Koenig JI: Prenatal stress generates deficits in rat social
behavior: reversal by oxytocin. Brain Res. 1156:152–167. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kinnunen AK, Koenig JI and Bilbe G:
Repeated variable prenatal stress alters pre- and postsynaptic gene
expression in the rat frontal pole. J Neurochem. 86:736–748. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koenig JI, Elmer GI, Shepard PD, Lee PR,
Mayo C, Joy B, Hercher E and Brady DL: Stress during gestation
produces alterations in adult rat behavior: relevance to
schizophrenia. Soc Neurosci abs. 495:pp. 6http://www.sfn.org/Annual-Meeting/Past-and-Future-Annual-Meetings/Abstract-Archive/Abstract-Archive-Detail?AbsYear=2002&AbsID=7512.
|
|
31
|
Koenig JI, Elmer GI, Shepard PD, Lee PR,
Mayo C, Joy B, Hercher E and Brady DL: Prenatal exposure to a
repeated variable stress paradigm elicits behavioral and
neuroendocrinological changes in the adult offspring: potential
relevance to schizophrenia. Behav Brain Res. 156:251–261. 2005.
View Article : Google Scholar
|
|
32
|
Lemaire V, Koehl M, Le Moal M and Abrous
DN: Prenatal stress produces learning deficits associated with an
inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci
USA. 97:11032–11037. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martínez-Téllez RI, Hernández-Torres E,
Gamboa C and Flores G: Prenatal stress alters spine density and
dendritic length of nucleus accumbens and hippocampus neurons in
rat offspring. Synapse. 63:794–804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Van den Hove DL, Kenis G, Brass A,
Opstelten R, Rutten BP, Bruschettini M, Blanco CE, Lesch KP,
Steinbusch HW and Prickaerts J: Vulnerability versus resilience to
prenatal stress in male and female rats; implications from gene
expression profiles in the hippocampus and frontal cortex. Eur
Neuropsychopharmacol. 23:1226–1246. 2013. View Article : Google Scholar
|
|
35
|
Mairesse J, Vercoutter-Edouart AS,
Marrocco J, Zuena AR, Giovine A, Nicoletti F, Michalski JC, Maccari
S and Morley-Fletcher S: Proteomic characterization in the
hippocampus of prenatally stressed rats. J Proteomics.
75:1764–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee H, Joo J, Nah SS, Kim JW, Kim HK, Kwon
JT, Lee HY, Kim YO and Kim HJ: Changes in Dpysl2 expression are
associated with prenatally stressed rat offspring and
susceptibility to schizophrenia in humans. Int J Mol Med.
35:1574–1586. 2015.PubMed/NCBI
|
|
37
|
Kim YO, Lee HY, Won H, Nah SS, Lee HY, Kim
HK, Kwon JT and Kim HJ: Influence of Panax ginseng on the offspring
of adult rats exposed to prenatal stress. Int J Mol Med.
35:103–109. 2015.
|
|
38
|
Dulawa SC, Holick KA, Gundersen B and Hen
R: Effects of chronic fluoxetine in animal models of anxiety and
depression. Neuropsychopharmacology. 29:1321–1330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schroeder M, Sultany T and Weller A:
Prenatal stress effects on emotion regulation differ by genotype
and sex in prepubertal rats. Dev Psychobiol. 55:176–192. 2013.
View Article : Google Scholar
|
|
40
|
Becker A, Peters B, Schroeder H, Mann T,
Huether G and Grecksch G: Ketamine-induced changes in rat
behaviour: a possible animal model of schizophrenia. Prog
Neuropsychopharmacol Biol Psychiatry. 27:687–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koenig JI, Elmer GI, Shepard PD, Lee PR,
Mayo C, Joy B, Hercher E and Brady DL: Prenatal exposure to a
repeated variable stress paradigm elicits behavioral and
neuroendocrinological changes in the adult offspring: potential
relevance to schizophrenia. Behav Brain Res. 156:251–261. 2005.
View Article : Google Scholar
|
|
42
|
Chang CH, Hsiao YH, Chen YW, Yu YJ and
Gean PW: Social isolation-induced increase in NMDA receptors in the
hippocampus exacerbates emotional dysregulation in mice.
Hippocampus. 25:474–485. 2015. View Article : Google Scholar
|
|
43
|
Morley-Fletcher S, Darnaudery M, Koehl M,
Casolini P, Van Reeth O and Maccari S: Prenatal stress in rats
predicts immobility behavior in the forced swim test. Effects of a
chronic treatment with tianeptine. Brain Res. 989:246–251. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Joo J, Lee S, Nah SS, Kim YO, Kim DS, Shim
SH, Hwangbo Y, Kim HK, Kwon JT, Kim JW, et al: Lasp1 is
down-regulated in NMDA receptor antagonist-treated mice and
implicated in human schizophrenia susceptibility. J Psychiatr Res.
47:105–112. 2013. View Article : Google Scholar
|
|
45
|
Liu XG, Gao PY, Wang GS, Song SJ, Li LZ,
Li X, Yao XS and Zhang ZX: In vivo antidepressant activity of
sesquiterpenes from the roots of Valeriana fauriei Briq.
Fitoterapia. 83:599–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ortiz JG, Nieves-Natal J and Chavez P:
Effects of Valeriana officinalis extracts on [3H]flunitrazepam
binding, synaptosomal [3H] GABA uptake, and hippocampal [3H]GABA
release. Neurochem Res. 24:1373–1378. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Patočka and Jakl J: Biomedically relevant
chemical constituents of Valeriana officinalis. J Appl Biomed.
8:11–18. 2010. View Article : Google Scholar
|
|
48
|
Santos MS, Ferreira F, Cunha AP, Carvalho
AP and Macedo T: An aqueous extract of valerian influences the
transport of GABA in synaptosomes. Planta Med. 60:278–279. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Merz K, Herold S and Lie DC: CREB in adult
neurogenesis - master and partner in the development of adult-born
neurons? Eur J Neurosci. 33:1078–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Charil A, Laplante DP, Vaillancourt C and
King S: Prenatal stress and brain development. Brain Res Brain Res
Rev. 65:56–79. 2010. View Article : Google Scholar
|
|
51
|
Kelley ME, Gilbertson M, Mouton A and van
Kammen DP: Deterioration in premorbid functioning in schizophrenia:
a developmental model of negative symptoms in drug-free patients.
Am J Psychiatry. 149:1543–1548. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Møller P and Husby R: The initial prodrome
in schizophrenia: searching for naturalistic core dimensions of
experience and behavior. Schizophr Bull. 26:217–232. 2000.
View Article : Google Scholar
|
|
53
|
Cornblatt BA: The New York high risk
project to the Hillside recognition and prevention (RAP) program.
Am J Med Genet. 114:956–966. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Geyer MA, Krebs-Thomson K, Braff DL and
Swerdlow NR: Pharmacological studies of prepulse inhibition models
of sensorimotor gating deficits in schizophrenia: a decade in
review. Psychopharmacology (Berl). 156:117–154. 2001. View Article : Google Scholar
|
|
55
|
Swerdlow NR and Geyer MA: Using an animal
model of deficient sensorimotor gating to study the pathophysiology
and new treatments of schizophrenia. Schizophr Bull. 24:285–301.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Braff DL: Information processing and
attention dysfunctions in schizophrenia. Schizophr Bull.
19:233–259. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
de Bruin NM, van Luijtelaar EL, Cools AR
and Ellenbroek BA: Auditory information processing in rat genotypes
with different dopaminergic properties. Psychopharmacology (Berl).
156:352–359. 2001. View Article : Google Scholar
|
|
58
|
Lipska BK and Weinberger DR: To model a
psychiatric disorder in animals: schizophrenia as a reality test.
Neuropsychopharmacology. 23:223–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tueting P, Costa E, Dwivedi Y, Guidotti A,
Impagnatiello F, Manev R and Pesold C: The phenotypic
characteristics of heterozygous reeler mouse. Neuroreport.
10:1329–1334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Day-Wilson KM, Jones DN, Southam E, Cilia
J and Totterdell S: Medial prefrontal cortex volume loss in rats
with isolation rearing-induced deficits in prepulse inhibition of
acoustic startle. Neuroscience. 141:1113–1121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Borrell J, Vela JM, Arévalo-Martin A,
Molina-Holgado E and Guaza C: Prenatal immune challenge disrupts
sensorimotor gating in adult rats. Implications for the
etiopathogenesis of schizophrenia. Neuropsychopharmacology.
26:204–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shi L, Fatemi SH, Sidwell RW and Patterson
PH: Maternal influenza infection causes marked behavioral and
pharmacological changes in the offspring. J Neurosci. 23:297–302.
2003.PubMed/NCBI
|
|
63
|
Lehmann J, Stöhr T and Feldon J: Long-term
effects of prenatal stress experiences and postnatal maternal
separation on emotionality and attentional processes. Behav Brain
Res. 107:133–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Faraday MM, O'Donoghue VA and Grunberg NE:
Effects of nicotine and stress on startle amplitude and sensory
gating depend on rat strain and sex. Pharmacol Biochem Behav.
62:273–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Stöhr T, Schulte Wermeling D, Szuran T,
Pliska V, Domeney A, Welzl H, Weiner I and Feldon J: Differential
effects of prenatal stress in two inbred strains of rats. Pharmacol
Biochem Behav. 59:799–805. 1998. View Article : Google Scholar : PubMed/NCBI
|