Introduction
Bone tissue is subjected to constant remodelling
processes mediated by bone-forming osteoblasts and bone-resorbing
osteoclasts. According to Wolff's law, these processes are not
random, but are a consequence of bone tissue responding to
mechanical stress (1). Fukada and
Yasuda demonstrated the piezoelectric properties of bone and showed
that mechanical stress induces the formation of endogenous electric
fields within the tissue (2).
Several studies have been conducted to investigate whether bone
healing can be influenced by electrical stimulation. Although the
underlying mechanisms of electrically induced osteogenesis are not
yet completely understood, previous findings have demonstrated the
benefit of applying electromagnetic fields on bone regeneration. An
in vitro study by Icaro Cornaglia et al using the
human osteosarcoma cell line, SAOS-2, showed significantly
increased matrix calcification following stimulation with
electromagnetic fields (3). In
other studies, an association between the use of low-frequency
electromagnetic fields and enhanced collagen synthesis in mouse
osteoblasts was observed (4).
Electric (5,6) and electromagnetic (7–9)
fields even support the differentiation of human mesenchymal stem
cells into the osteoblastic phenotype. These effects are mediated
through direct effects on intracellular and transmembrane channels
(10), as well as through
indirect effects through the inverse piezoelectric effect (11,12).
It has also been shown in vitro and in
vivo, that biophysical stimulation via the application of
electric currents enhances bone healing and restores structural
strength (13–15). Based on these findings, electrical
bone growth stimulators have been developed for clinical
application (16,17). These systems provide external
stimulation that imitates endogenous electric fields in order to
activate bone regeneration. Common bone stimulator techniques
represent a promising therapeutic approach for diseases, such as
osteoarthritis and osteoporosis, as well as for complicated
fractures, including delayed unions, non-unions and stress
fractures (18). A particular
technique for the application of electromagnetic fields with an
additional alternating electric field (EMF + EF) is the ASNIS-III s
screw system (Stryker GmbH, Duisburg, Germany). This system is
based on the bipolar induction screw system (BISS) (19) that is derived from the stimulation
method developed by Mittelmeier et al. This semi-invasive
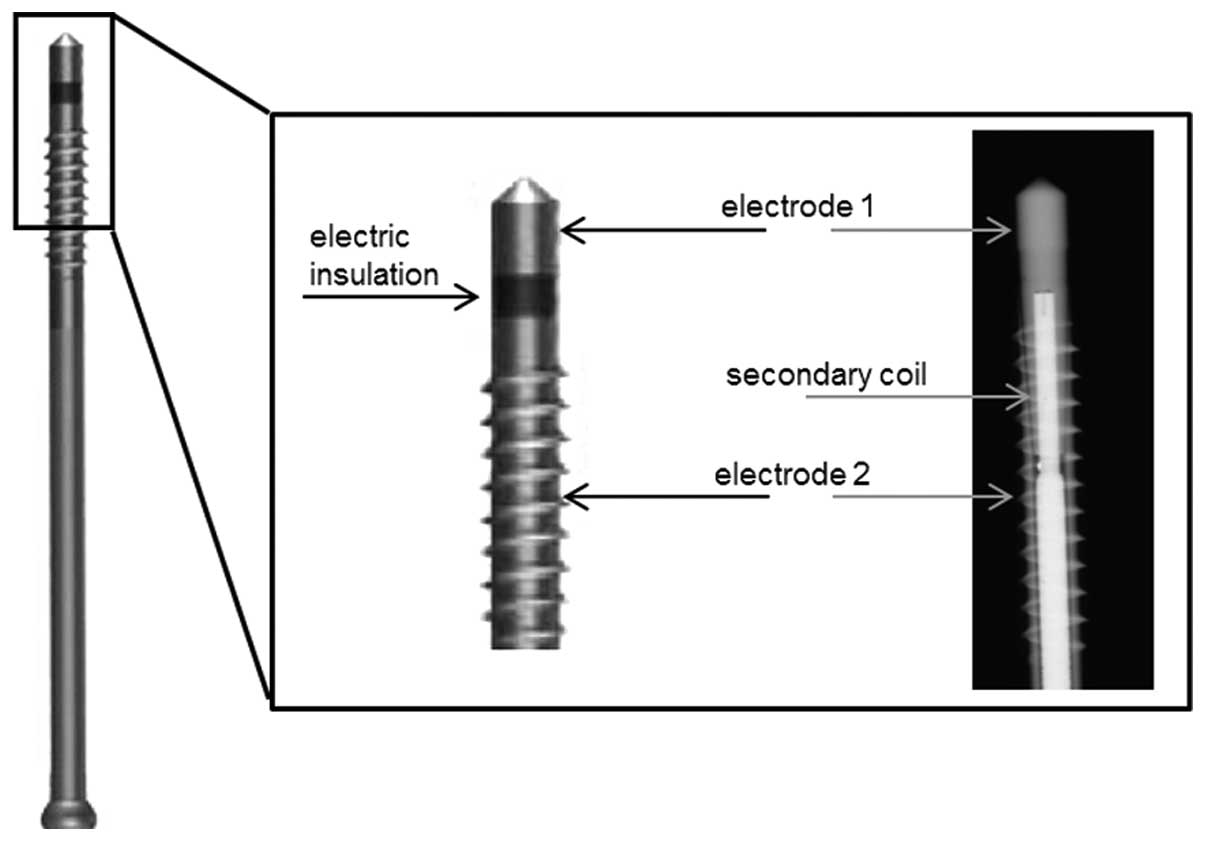
method has been described in detail previously (12,19). The ASNIS screw is implanted at the
site of the bone defect. A magnetic field of 3–5 mT oscillating at
a 20 Hz sine wave, generated by an external primary coil induces
voltage within the secondary coil inside the ASNIS screw. This
secondary coil is connected to two electrodes in the screw tip and
shaft, which are separated by electrical insulation. As a result,
an electric field is created between the two electrodes. The
maximum root mean square (RMS) electric potential on the surface of
the electrodes is 700 mV (Fig.
1).
The ASNIS-III s screw system is a bone-stimulating
implant that is already being applied in clinical practice for the
treatment of avascular necrosis of the femoral head, fracture of
the femur neck and subtalar arthrodeses. The ASNIS-III s screw
system applies electromagnetic fields and an additional electric
field by a single screw and directly stimulates the adjacent bone
tissue which is supposed to accelerate bone regeneration. Although
the ASNIS-III s system is already being used clinically, optimal
parameters of stimulation (electric field strength, frequency and
stimulation periods) to further enhance the effects of
electromagnetic stimulation are still unknown. Therefore, we
developped a three-dimensional (3D) in vitro test set-up
using the technical equipment of the ASNIS-III s screw system
(12). In this set-up, the
influence of EMF, as well as EMF + EF on bone cells on different
biomaterials was investigated. This in vitro study showed an
early shift of the osteoblasts towards differentiation after a
stimulation period of 3 days when seeded on collagen scaffolds,
indicating the influence of piezoelectric materials on the
stimulatory effects. However, several studies stimulating cells on
non-piezoelectric materials have also demonstrated the effects of
electromagnetic stimulation (20–22). Therefore, in the present study, we
focused on the electromagnetic stimulation of human osteoblasts
(hOBs) in the absence of a matrix displaying piezoelectric
properties to reveal the direct effect of electromagnetic
stimulation on cells. Hence, in the present study, hOBs were
integrated in agarose gels enabling the stimulation of osteoblasts
in a 3D matrix and facilitating RNA isolation for subsequent gene
expression analysis. In such a set-up, the influence of EMF and EMF
+ EF on the viability and differentiation capacity of primary hOBs
was analysed.
Materials and methods
Isolation of hOBs and embedding in
agarose scaffold
Primary hOBs were isolated under sterile conditions
from the femoral heads of patients undergoing a primary total hip
replacement as previously described (23). The samples were collected with the
consent of patients and after approval by the local ethics
committee (registration number: A 2010-10).
Isolated cells were cultured in 25 cm2
flasks with 8 ml of Dulbecco's modified Eagle's medium (Biochrom,
Berlin, Germany) containing 10% fetal calf serum (FCS), 1%
amphotericin B, 1% penicillin-streptomycin and 1% HEPES buffer
under standard cell culture conditions (5% CO2 and
37°C). Osteogenic differentiation was induced by ascorbic acid (50
µg/ml), β-glycerophosphate (10 mM) and dexamethasone (100
nM), and verified by the immunhistochemical detection of the enzyme
alkaline phosphatase (ALP) using the Fuchsin+ Substrate Chromogen
System (Dako, Hamburg, Germany) according to the manufacturer's
instructions.
At passage 3 the hOBs were embedded in an agarose
scaffold using low gelling agarose (Sigma-Aldrich, Seelze,
Germany). The solid agarose was dissolved with distilled water and
sterilised at 135°C for 1 h. After cooling down for 24 h at 37°C,
the agarose solution was diluted with cell culture medium,
resulting in a 1% agarose solution.
The cultured cells were detached with trypsin/EDTA
solution and centrifuged to a pellet at 118 × g. Subsequently,
8.25×105 cells were resuspended in 3 ml liquid 1%
agarose solution and transferred into inserts for 6-well cell
culture plates (ThinCert™; Greiner Bio-One, Frickenhausen,
Germany). Cell-agarose solution was gelled at 4°C for 3 min.
Afterwards, an agarose scaffold measuring 30 mm in diameter and 5
mm in height was prepared. The centre of the cell-agarose scaffold
was cut out in order to position the ASNIS screw in the middle of
the scaffold. The stimulation of 7.5×105 hOBs required
the use of 10% cell excess to compensate the loss of scaffold
during the preparation of the experimental set-up.
Experimental set-up for magnetic and
electromagnetic stimulation
EMF and EMF + EF were applied in vitro using
the ASNIS-III s-series screw system (Stryker GmbH and Co. KG,
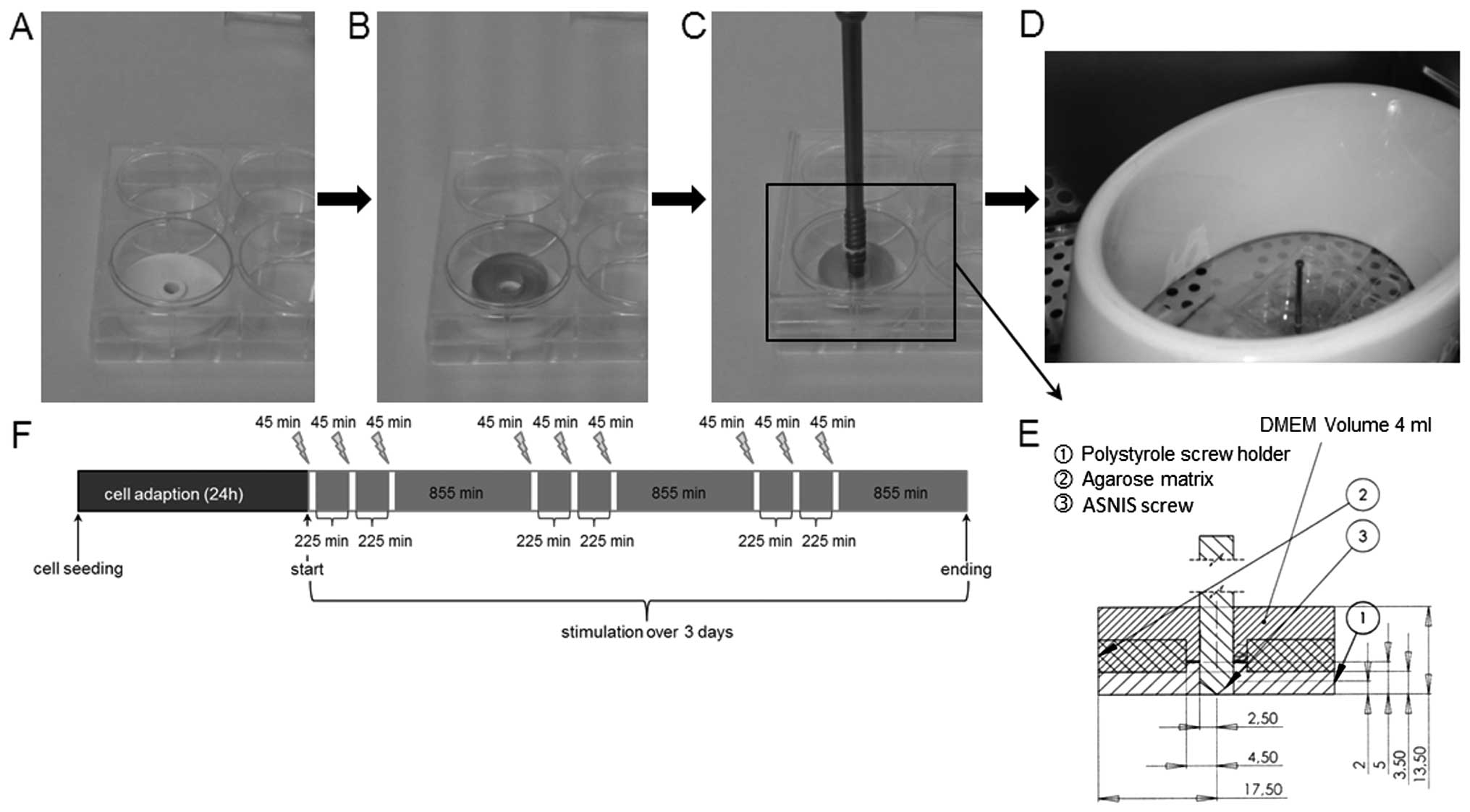
Duisburg, Germany) as previously described (12). Custom-made polystyrole screw
holders with a diameter of 3.5 cm were placed in 6-well cell
culture plates to enable the stable alignment of the ASNIS screw.
The screw holder was covered by the agarose scaffold and the
construct was overlaid with 6 ml cell culture medium containing
supplements for osteogenic differentiation as mentioned above. The
ASNIS screw was adjusted within the centre of the agarose scaffold
and screw holder by screwing them through a pre-drilled hole in the
lid of the 6-well plate (Fig.
2A–E). The cells were incubated for 24 h to ensure cell
adaption within the scaffold.
EMF stimulation was performed by positioning the
cell culture plate with agarose-cell scaffolds and custom screw
holders (Fig. 2B) within the
primary magnetic coil. The coil generated a sinusoidal oscillating
magnetic field with a frequency of 20 Hz and a magnetic flux
density of 3 mT. The addition of the ASNIS screw (Fig. 2C) enabled the application of EMF +
EF on the hOBs. The maximum induced voltage between the electrodes
of the ASNIS screw was 700 mV, as previously described (12). The cells were stimulated (EMF and
EMF + EF) 3 times per day for 45 min over a period of 3 days
(Fig. 2F). The cell culture
conditions were 37°C and 5% CO2 in a humidified
atmosphere. Unstimulated cells embedded in an agarose scaffold with
(EMF + EF control) and without (EMF control) the ASNIS screw served
as the controls and were located in a separate incubator to avoid
influences from the magnetic field generated by the external
magnetic coil.
Determination of cell viability
The metabolic activity of the cells was examined
using the water-soluble tetrazolium salt (WST)-1 assay (Roche,
Berlin, Germany). This colorimetric assay is based on the reduction
of water soluble tetrazolium salt into formazan salt catalyzed by
mitochondrial dehydrogenases in intact cells. The amount of
formazan correlates with the enzymatic activity and reflects the
metabolic activity. Following incubation with a mix of the WST
assay reagent and cell culture medium at a ratio of 1:10 for 120
min at 37°C, the absorbance was measured at 450 nm (reference, 630
nm) using an Opsys MR microplate reader (Dynex Technologies,
Denkendorf, Germany).
The viability of the osteoblasts was assessed using
a LIVE/DEAD© assay kit (Life Technologies, Carlsbad, CA,
USA). The two-color assay discriminates live from dead cells by
simultaneously staining with green fluorescent (494–517 nm)
calcein-acetoxymethyl (calcein-AM), indicating intracellular
esterase activity and red-fluorescent (528–617 nm) ethidium
homodimer-1, indicating the loss of plasma membrane integrity. The
assay was performed as recommended by the manufacturer. Images of
the cells were acquired using a fluorescence microscope (Nikon Type
120) and evaluated with NIS-Elements software (version D 3.2) (both
from Nikon Instruments, Tokyo, Japan). Separate images of live and
dead cells were taken in the same position. Afterwards, the images
were overlaid using image editor software (GIMP2.8.14, The GIMP
Team).
Gene expression analysis
After a stimulation period of 3 days, all samples
were frozen at −70°C for subsequent gene expression analysis. The
samples were thus quickly covered with liquid nitrogen and
homogenised using a pre-cooled pestle and mortar. The fine powder
was transferred into a centrifuge tube; subsequently, TRI
Reagent® (Zymo Research, Freiburg, Germany) and Buffer
QG (Qiagen, Leipzig, Germany) were added. After mixing and
incubating at room temperature for 5 min, the samples were
centrifuged for 2 min at 12 000 × g. The supernatants were used to
perform RNA isolation by column purification using the Direct-zol™
RNA MiniPrep kit (Zymo Research) according to the manufacturer's
instructions.
Single-stranded cDNA was synthesised from total RNA
with a T-Personal Thermocycler (Biometra, Göttingen, Germany) using
the High Capacity cDNA reverse transcription kit following the
manufacturer's instructions (Applied Biosystems, Forster City, CA,
USA). The synthesised cDNA was used as a template for
semi-quantitative real-time polymerase chain reaction (RT-qPCR)
using the innuMIX qPCR MasterMix SyGreen and qTower 2.0 (Analytik
Jena AG, Jena, Germany). The cycling conditions used for
amplification were 95°C for 2 min, 40 cycles of 95°C for 5 sec and
65°C for 25 sec. The sequences of the forward and reverse primers
are shown in Table I. The
expression of all genes was normalised to the expression of the
corresponding housekeeping gene, hypoxanthine
phosphoribosyltransferase 1 (HPRT). The relative amount of
target mRNA in thye unstimulated cells and treated cells was
analysed using the ΔΔCq method, where ΔΔCq =
ΔCqstimulaion−ΔCqcontrol, as previously
described (24).
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | Direction | Primer nucleotide
sequence |
|---|
| Alkaline
phosphatase (ALP) | Forward |
5′-CATTGTGACCACCACGAGAG-3′ |
| Reverse |
5′-CCATGATCACGTCAATGTCC-3′ |
| Bone sialoprotein
(BSP) | Forward |
5′-ATTTTGGGAATGGCCTGTGC-3′ |
| Reverse |
5′-GTCACTACTGCCCTGAACTGG-3′ |
| Collagen type 1
(Col1A1) | Forward |
5′-ACGAAGACATCCCACCAATC-3′ |
| Reverse |
5′-AGATCACGTCATCGCACAAC-3′ |
|
Hypoxanthine-guanine
phosphoribosyltransferase (HPRT) | Forward |
5′-CCCTGGCGTCGTGATTAGTG-3′ |
| Reverse |
5′-TCGAGCAAGACGTTCAGTCC-3′ |
| Osteocalcin
(OC) | Forward |
5′-GGTGCAGCCTTTGTGTCC-3′ |
| Reverse |
5′-TCAGCCAACTCGTCACAGTC-3′ |
| Runt-related
transcription factor 2 (RUNX-2) | Forward |
5′-CGCCTCACAAACAACCACAG-3′ |
| Reverse |
5′-ACTGCTTGCAGCCTTAAATGAC-3′ |
Determination of pro-collagen type I
protein content
The rate of synthesis of the pro-collagen type I
rate was measured using an enzyme-linked immunosorbent assay
(ELISA) (MicroVue™ CICP EIA; Quidel Corporation, San Diego, USA).
The C-terminal pro-peptide of pro-collagen is considered to
correlate with collagen expression. For the analysis, supernatants
of each stimulation experiment were collected and stored at −20°C.
The assay was performed according to the manufacturer's
specifications. The absorbance was measured at a wavelength of 405
nm using an Opsys MR microplate reader (Dynex Technologies). With
the help of a standard curve, the protein content was determined
and set in relation to the respective controls.
Data illustration and statistical
analysis
Data are represented in a box plot. Each box shows
the median, as well as the 25th and 75th percentile. Dots point out
mean values. The whiskers indicate the minimum and maximum data
values. A minimum of 4 independent experiments were performed for
statistical analysis. The Kolmogorov-Smirnov-test was conducted to
assess the distribution of data. As the data sets were found to be
distributed normally, the statistical significance of differences
between groups was calculated by one-way ANOVA (with the Bonferroni
post hoc test) using SPSS Statistics 2.0 software (IBM, Ehningen,
Germany). The level of significance was set to p<0.05.
Results
Viability of hOBs following stimulation
with EMF and EMF + EF
In this study, we examined the influence of EMF and
EMF + EF on the survival and differentiation of hOBs using the
ASNIS-III s screw system. Using the WST assay, the metabolic
activity of the hOBs was detected and represented in relation to
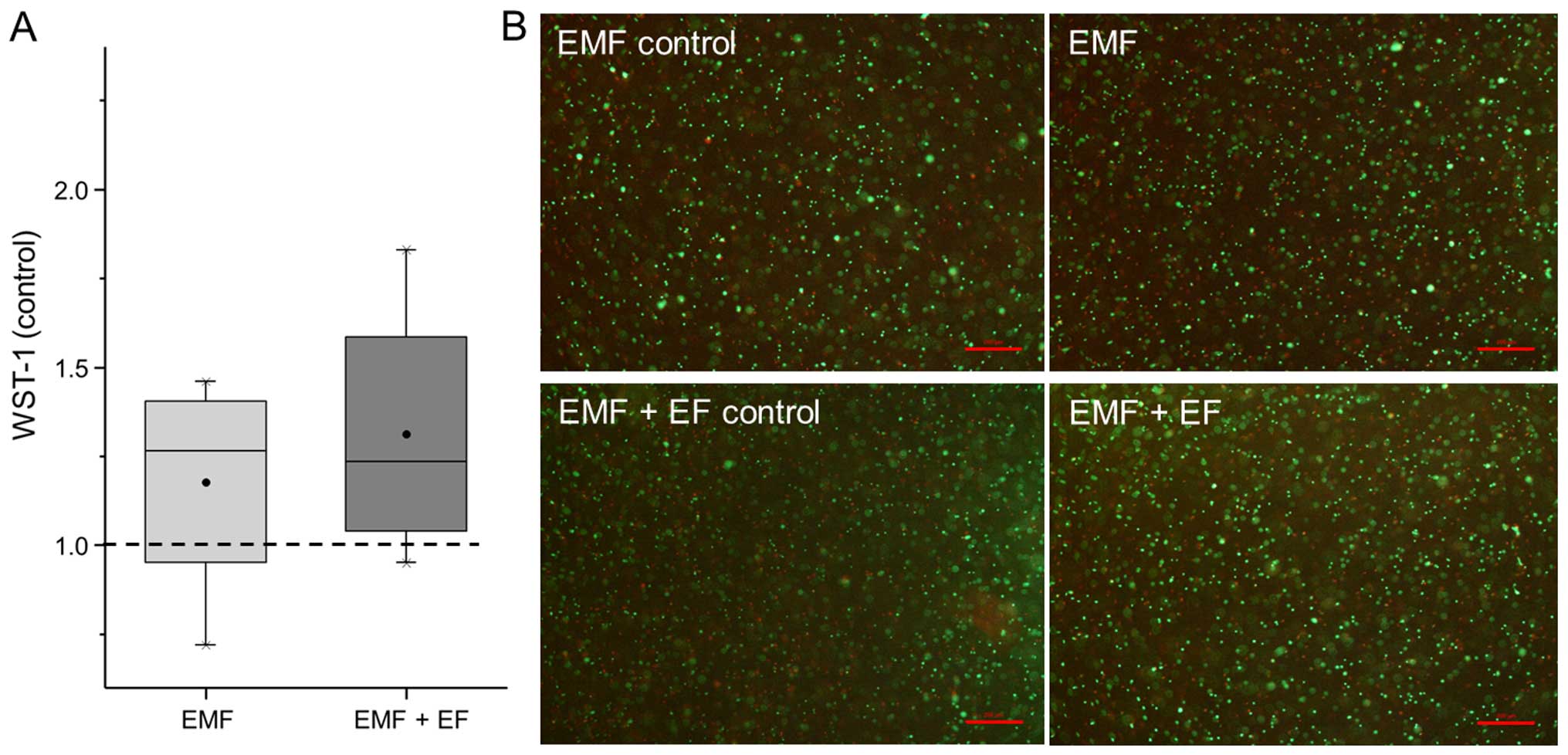
the respective unstimulated controls (Fig. 3A). Accordingly, EMF + EF
(1.24-fold) and EMF (1.27-fold) increased the metabolic activity of
the hOBs compared to the unstimulated cells. Furthermore, the
viability of the hOBs was detected by live/dead staining.
Osteoblasts stimulated with EMF and EMF + EF as well as the
respective unstimulated controls displayed a large number of green
fluorescent, viable cells. Additionally, dead cells (red
fluorescence) were observed in each stimulation group (Fig. 3B). No differences in the
proportion of living and dead cells were detected between EMF, EMF
+ EF and the controls.
Alteration of osteogenic differentiation
in hOBs induced by EMF and EMF + EF
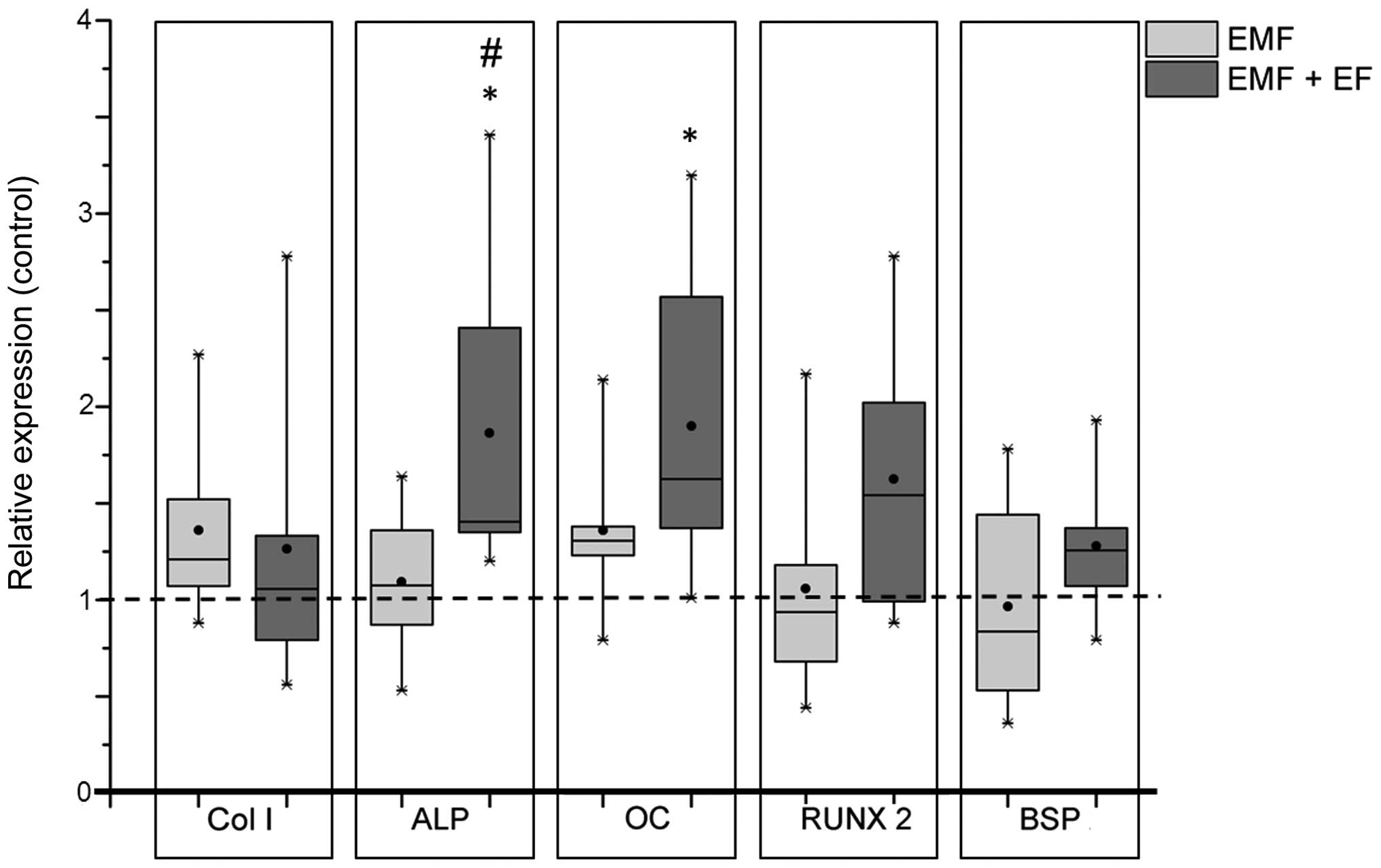
Gene expression analyses of commonly used osteogenic
markers were performed to investigate the differentiation of hOBs
following stimulation with EMF and EMF + EF. During exposure to
EMF, the expression of collagen type 1 (Col1A1; 1.21-fold)
and osteocalcin (OC; 1.31-fold) slightly increased compared
to the corresponding unstimulated controls, whereas the expression
of runt-related transcription factor 2 (RUNX-2; 0.93-fold)
and bone sialoprotein (BSP; 0.84-fold) was slightly
downregulated. However, stimulation with EMF + EF resulted in a
significant increase in the expression of ALP compared to
both EMF (p=0.048) and the unstimulated control (1.4-fold,
p=0.036). Moreover, OC expression was significantly enhanced
following stimulation with EMF + EF (1.6-fold, p=0.017) compared to
the unstimulated cells. The transcription factor, RUNX-2,
exhibited an increased expression level (1.5-fold), while
Col1A1 was only marginally influenced (Fig. 4).
Effect of EMF and EMF + EF on the
synthesis of C1CP
Regarding the synthesis capacity of specific
extracellular matrix components, we determined the content of
collagen type 1 using the pro-collagen type 1 (C1CP) ELISA. The
C1CP concentrations of EMF, EMF + EF and the respective controls
were measured after 3 days in the supernatant of the cells. The
C1CP content of each stimulation group was set in relation to the
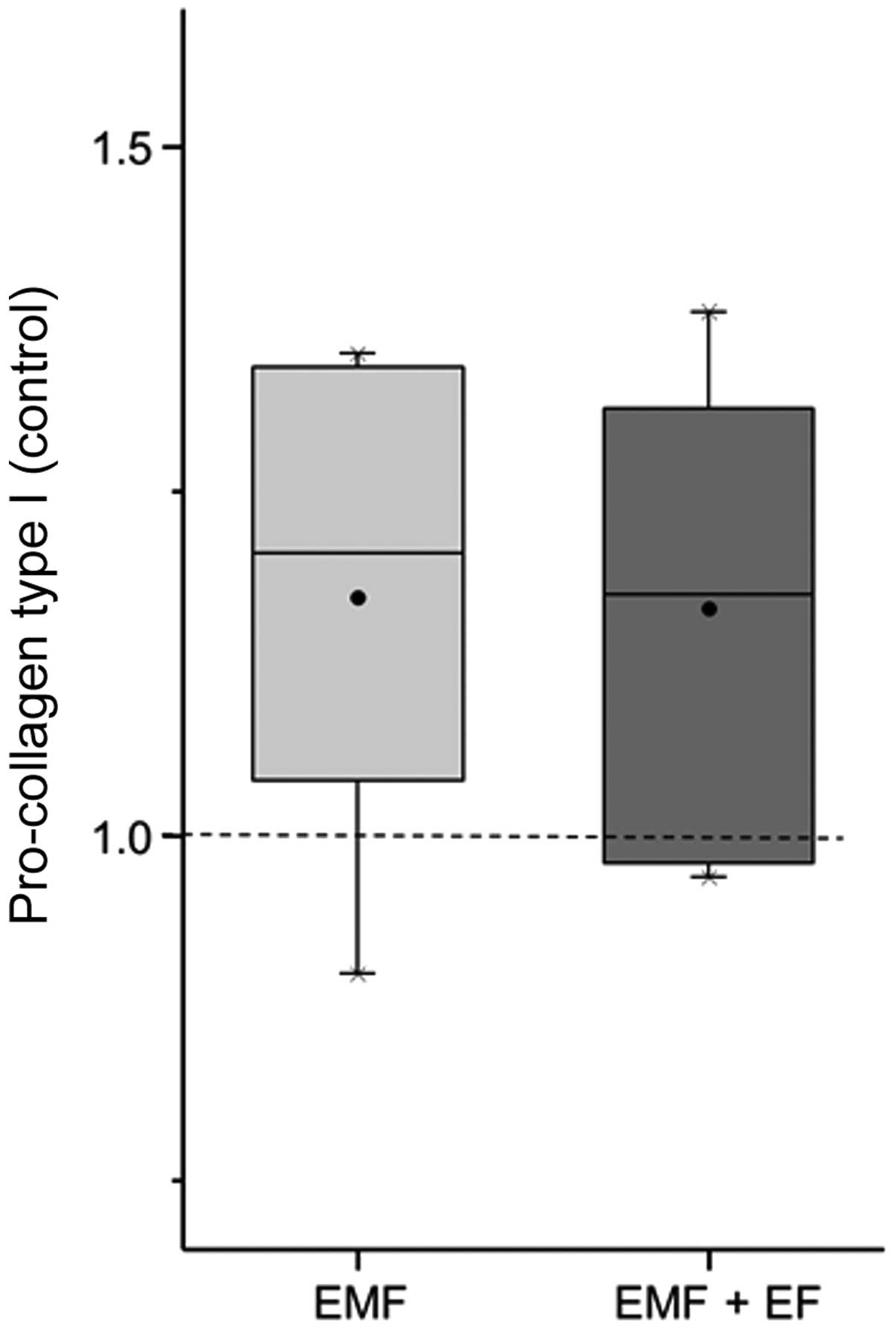
corresponding control. C1CP expression was enhanced to a slightly
greater extent by EMF (1.21-fold) than EMF + EF (1.18-fold)
(Fig. 5).
Discussion
Bone tissue has the ability to regenerate after
lesions. This regeneration process is complex and bone regeneration
of large defects after trauma, tumour development and avascular
necrosis is one of the key challenges in reconstructive bone
surgery (25). Current clinical
approaches for the enhancement of bone regeneration are diverse;
however, the use of bone grafting methods is considered the 'gold
standard'. As these common treatments do not always achieve the
success desired, the application of biophysical stimulation in
combination with bone grafting is a promising therapeutic approach
(9). Hence, the ASNIS-III
s-series screw system, an electro-inductive bone stimulating
system, was introduced to enhance bone regeneration in the case of
avascular necrosis of the femoral head (19).
In a previous study, we established an in
vitro test set-up for the stimulation of hOBs growing on 3D
scaffolds made of collagen and calcium phosphate using the
ASNIS-III s-series screw system (12). In the present study, we used this
test set-up to investigate the influence of EMF and EMF + EF on the
osteogenic differentiation of primary hOBs in the absence of
extracellular matrix molecules. Therefore, we embedded hOBs in
agarose gels to investigate the direct effects of electromagnetic
stimulation. Agarose is highly biocompatible and its fibrous
structure provides high porosity and similarity to physiological
extracellular matrix (26). The
suitability of agarose gels for the culture and differentiation of
osteoblasts has been previously demonstrated for the mouse
osteoblast cell line, MC3T3-E1 (27). Other groups have successfully
implanted hydroxyapatite/agarose hydrogels as bone graft material,
proving osteoconductive properties of composites (26,28). Furthermore, the osteogenic
differentiation of dental stromal cells under mechanical
stimulation in agarose gels has been recently described (29). The ASNIS-III s screw was set in
the centre of the 3D osteoblast-agarose scaffold to expose the bone
cells to the EMF + EF. This set-up has the benefit of mimicking the
situation in vivo, where the ASNIS-III s screw is implanted
in the bone tissue to stimulate bone regeneration.
The ASNIS-III s treatment protocol for patients with
avascular necrosis of the femoral head involves bone stimulation
for 45 min 3 times per day for several months. For our in
vitro experiments, similar stimulation intervals over a
stimulation period of 3 days were used to analyse the initial
effects of electromagnetic stimulation, as done in a previous study
by Grunert et al (12). In
this study, no cytotoxic effects of the stimulation method or the
screw material were determined. We approved the absence of the
negative influence of the implant system, as there was no increase
in cell death (assessed by live/dead staining) using the ASNIS
screw compared to set-ups without the ASNIS screw. This confirms
the biocompatibility of the Ti6Al4V alloy used for the electrodes
(30–32) and rules out any negative effect of
both EMF and EMF + EF. The exclusion of cytotoxic effects is a
notable aspect, given the clinical application.
Our investigations indicate that hOBs cultured in
agarose are sensitive to both EMF and EMF + EF, as for both
conditions, metabolic activity was similarly enhanced compared to
the unstimulated controls. In this study, cell survival and
proliferation, assessed by live/dead staining, were comparable in
all samples. However, other research groups have shown that EMF
positively affects the proliferation of primary osteoblasts and
osteoblast-like cell lines in non-piezoelectric scaffolds (20,33).
Therapeutic success depends, not only on
proliferation and cell viability, but also on the differentiation
of osteoblasts with enhanced synthesis of extracellular matrix
components required for regeneration processes. The osseous
extracellular matrix is mainly composed of collagen type 1. The
analysis of the synthesised collagen type 1 levels showed a
moderate increase following exposure to EMF and EMF + EF.
Furthermore, gene expression analyses of important osteogenic
differentiation markers were performed. Boxplots depicting gene
expression demonstrate the variability of osteoblast donor
susceptibility to electromagnetic stimulation which has to be taken
into account for the evaluation of stimulatory effects and the
potential outcome of EMF therapy. The stimulation of hOBs with EMF
resulted in a slight increase in Col1A1 expression. This is
consistent with the data of previous studies showing that
electromagnetic stimulation alters the expression of Col1A1
and thus affects the osteoblastic proliferation phase (20,34). The influence on differentiation
was also displayed by a slight increase in OC mRNA levels
following stimulation with EMF. However, these findings were trends
and did not reach a level of significance, whereas EMF + EF using
the ASNIS-III s screw system resulted, not only in a significant
enhancement of OC and ALP mRNA expression, but also
in the increased expression of RUNX-2. These findings
support those of previous studies showing that electrical
stimulation promotes extracellular matrix maturation and
mineralization (35,36). Applied electric fields enhanced
the production of important extracellular matrix proteins and
further altered the gene regulation in terms of RUNX-2
expression, a key transcription factor associated with osteoblast
differentiation (37). In
conclusion, gene expression analyses showed a more pronounced
effect of EMF + EF on differentiation-associated markers compared
to EMF alone.
The results of the present study emphasise the
capability of EMF + EF to enhance osteoblastic differentiation, and
thus are consistent with those of our previous study that showed
changes in collagen type 1 synthesis (12). However, the exposure of hOBs to
electromagnetic fields with an additional electric field resulted
in a significant and more pronounced elevation of synthesised
collagen type 1 in a respective study, indicating the influence of
scaffold composition on the response of cells to biophysical
stimuli, as we and other study groups have proposed previously
(12,38). The agarose-gel-scaffold used in
the present study did not contain collagen fibres as opposed to the
one used in our previous study. Collagen supports osteogenic
differentiation by promoting extracellular matrix protein
expression (39,40). Additionally, the deformation of
collagen fibres as a response to electromechanical coupling
(inverse piezoelectric effect) may result in mechanically
stimulated cell differentiation, thereby influencing bone
remodelling (12,22,41). The existence of the inverse
piezoelectric effect in collagen fibres and its resulting deforming
magnitude being sufficient to prompt mechanically induced
stimulation was recently proven by our group through scanning X-ray
diffraction experiments (11).
Thus, the scaffold material may account for variances between our
present and previous studies, indicating the beneficial but not the
crucial effects of piezoelectric matrix components for the
differentiation of hOBs following exposure to electromagnetic
fields as previously demonstrated (21,34,42). The distribution of the electric
field generated by the ASNIS-III s screw derived from the numerical
simulation study showed a considerable gradient within the
scaffolds (12), limiting the
intensity of EMF + EF in the periphery. Cells seeded in agarose are
distributed equally throughout the gel-scaffold; therefore, more
cells were subjected to a lower electric field in the outer edges,
as opposed to cells seeded in the point-wise method used in our
previous study which could also account for the differences between
both studies. The positive stimulatory effects in the absence of
extracellular matrix components prove the direct effect of electric
fields on osteoblasts. The potential mechanisms involved include
the release of calcium from intracellular stores and the activation
of cytoskeletal calmodulin without the involvement of the inositol
phosphate pathway or voltage gated calcium channels and
phospholipase A2 activation as previously proposed
(10).
In conclusion, in this study, we demonstrate the
positive influence of EMF and EMF + EF on bone cell viability and
differentiation even following short-term stimulation.
Differentiation markers were significantly enhanced by stimulation
with EMF + EF. These results emphasise the effectiveness of the
clinically used implant system for promoting bone regeneration.
Future studies regarding EMF + EF on hOBs with extended stimulation
periods for the analysis of long-term effects and variation of
parameters, including frequency, stimulation intervals and maximum
electric field strength, may further optimise existing stimulation
systems.
Acknowledgments
The authors would like to thank the German Research
Foundation (DFG) for the financial support of this study via the
grant BA 3347/2-2 and the GRK 1501 welisa.
References
|
1
|
Wolf JH: Julis Wolff and his 'law of bone
remodeling'. Orthopade. 24:378–386. 1995.In German. PubMed/NCBI
|
|
2
|
Fukada E and Yasuda I: On the
Piezoelectric Effect of Bone. J Phys Soc Jpn. 12:1158–1162. 1957.
View Article : Google Scholar
|
|
3
|
Icaro Cornaglia A, Casasco M, Riva F,
Farina A, Fassina L, Visai L and Casasco A: Stimulation of
osteoblast growth by an electromagnetic field in a model of
bone-like construct. Eur J Histochem. 50:199–204. 2006.PubMed/NCBI
|
|
4
|
Soda A, Ikehara T, Kinouchi Y and
Yoshizaki K: Effect of exposure to an extremely low
frequency-electromagnetic field on the cellular collagen with
respect to signaling pathways in osteoblast-like cells. J Med
Invest. 55:267–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hess R, Neubert H, Seifert A, Bierbaum S,
Hart DA and Scharnweber D: A novel approach for in vitro studies
applying electrical fields to cell cultures by transformer-like
coupling. Cell Biochem Biophys. 64:223–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hess R, Jaeschke A, Neubert H, Hintze V,
Moeller S, Schnabelrauch M, Wiesmann HP, Hart DA and Scharnweber D:
Synergistic effect of defined artificial extracellular matrices and
pulsed electric fields on osteogenic differentiation of human MSCs.
Biomaterials. 33:8975–8985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hronik-Tupaj M, Rice WL, Cronin-Golomb M,
Kaplan DL and Georgakoudi I: Osteoblastic differentiation and
stress response of human mesenchymal stem cells exposed to
alternating current electric fields. Biomed Eng Online. 10:92011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jansen JH, van der Jagt OP, Punt BJ,
Verhaar JA, van Leeuwen JP, Weinans H and Jahr H: Stimulation of
osteogenic differentiation in human osteoprogenitor cells by pulsed
electromagnetic fields: An in vitro study. BMC Musculoskelet
Disord. 11:1882010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saino E, Fassina L, Van Vlierberghe S,
Avanzini MA, Dubruel P, Magenes G, Visai L and Benazzo F: Effects
of electromagnetic stimulation on osteogenic differentiation of
human mesenchymal stromal cells seeded onto gelatin cryogel. Int J
Immunopathol Pharmacol. 24(Suppl 2): 1–6. 2011.PubMed/NCBI
|
|
10
|
Brighton CT, Wang W, Seldes R, Zhang G and
Pollack SR: Signal transduction in electrically stimulated bone
cells. J Bone Joint Surg Am. 83-A:1514–1523. 2001.PubMed/NCBI
|
|
11
|
Wieland DCF, Krywka C, Mick E,
Willumeit-Römer R, Bader R and Kluess D: Investigation of the
inverse piezoelectric effect of trabecular bone on a micrometer
length scale using synchrotron radiation. Acta Biomater.
25:339–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grunert PC, Jonitz-Heincke A, Su Y,
Souffrant R, Hansmann D, Ewald H, Krüger A, Mittelmeier W and Bader
R: Establishment of a novel in vitro test setup for electric and
magnetic stimulation of human osteoblasts. Cell Biochem Biophys.
70:805–817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balint R, Cassidy NJ and Cartmell SH:
Electrical stimulation: A novel tool for tissue engineering. Tissue
Eng Part B Rev. 19:48–57. 2013. View Article : Google Scholar
|
|
14
|
Kang KS, Hong JM, Jeong YH, Seol YJ, Yong
WJ, Rhie JW and Cho DW: Combined effect of three types of
biophysical stimuli for bone regeneration. Tissue Eng Part A.
20:1767–1777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puricelli E, Dutra NB and Ponzoni D:
Histological evaluation of the influence of magnetic field
application in autogenous bone grafts in rats. Head Face Med.
5:12009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldstein C, Sprague S and Petrisor BA:
Electrical stimulation for fracture healing: Current evidence. J
Orthop Trauma. 24(Suppl 1): S62–S65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuzyk PR and Schemitsch EH: The science of
electrical stimulation therapy for fracture healing. Indian J
Orthop. 43:127–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niethard FU and Pfeil J: Duale Reihe
Orthopädie. 5 Aufl. Thieme; Stuttgart: 2005
|
|
19
|
Mittelmeier W, Lehner S, Kraus W, Matter
HP, Gerdesmeyer L and Steinhauser E: BISS: Concept and
biomechanical investigations of a new screw system for
electromagnetically induced internal osteostimulation. Arch Orthop
Trauma Surg. 124:86–91. 2004. View Article : Google Scholar
|
|
20
|
Fassina L, Visai L, Benazzo F, Benedetti
L, Calligaro A, De Angelis MG, Farina A, Maliardi V and Magenes G:
Effects of electromagnetic stimulation on calcified matrix
production by SAOS-2 cells over a polyurethane porous scaffold.
Tissue Eng. 12:1985–1999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Griffin M, Sebastian A, Colthurst J and
Bayat A: Enhancement of differentiation and mineralisation of
osteoblast-like cells by degenerate electrical waveform in an in
vitro electrical stimulation model compared to capacitive coupling.
PLoS One. 8:e729782013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gillespie PG and Walker RG: Molecular
basis of mechanosensory transduction. Nature. 413:194–202. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lochner K, Fritsche A, Jonitz A, Hansmann
D, Mueller P, Mueller-Hilke B and Bader R: The potential role of
human osteoblasts for periprosthetic osteolysis following exposure
to wear particles. Int J Mol Med. 28:1055–1063. 2011.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Dimitriou R, Jones E, McGonagle D and
Giannoudis PV: Bone regeneration: Current concepts and future
directions. BMC Med. 9:662011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Watanabe J, Kashii M, Hirao M, Oka K,
Sugamoto K, Yoshikawa H and Akashi M: Quick-forming
hydroxyapatite/agarose gel composites induce bone regeneration. J
Biomed Mater Res A. 83:845–852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanazaki Y, Ito D, Furusawa K, Fukui A and
Sasaki N: Change in the viscoelastic properties of agarose gel by
HAp precipitation by osteoblasts cultured in an agarose gel matrix.
J Biorheol. 1–2:21–28. 2013. View Article : Google Scholar
|
|
28
|
Tabata M, Shimoda T, Sugihara K, Ogomi D,
Ohgushi H and Akashi M: Apatite formed on/in agarose gel as a
bone-grafting material in the treatment of periodontal infrabony
defect. J Biomed Mater Res B Appl Biomater. 75:378–386. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji J, Sun W, Wang W, Munyombwe T and Yang
XB: The effect of mechanical loading on osteogenesis of human
dental pulp stromal cells in a novel in vitro model. Cell Tissue
Res. 358:123–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bordji K, Jouzeau JY, Mainard D, Payan E,
Netter P, Rie KT, Stucky T and Hage-Ali M: Cytocompatibility of
Ti-6Al-4V and Ti-5Al-2.5Fe alloys according to three surface
treatments, using human fibroblasts and osteoblasts. Biomaterials.
17:929–940. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenbarth E, Velten D, Müller M, Thull R
and Breme J: Biocompatibility of β-stabilizing elements of titanium
alloys. Biomaterials. 25:5705–5713. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Long M and Rack HJ: Titanium alloys in
total joint replacement - a materials science perspective.
Biomaterials. 19:1621–1639. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fassina L, Visai L, De Angelis MG, Benazzo
F and Magenes G: Surface modification of a porous polyurethane
through a culture of human osteoblasts and an electromagnetic
bioreactor. Technol Health Care. 15:33–45. 2007.PubMed/NCBI
|
|
34
|
Lohmann CH, Schwartz Z, Liu Y, Guerkov H,
Dean DD, Simon B and Boyan BD: Pulsed electromagnetic field
stimulation of MG63 osteoblast-like cells affects differentiation
and local factor production. J Orthop Res. 18:637–646. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meng S, Rouabhia M and Zhang Z: Electrical
stimulation modulates osteoblast proliferation and bone protein
production through heparin-bioactivated conductive scaffolds.
Bioelectromagnetics. 34:189–199. 2013. View Article : Google Scholar
|
|
36
|
Meng S, Zhang Z and Rouabhia M:
Accelerated osteoblast mineralization on a conductive substrate by
multiple electrical stimulation. J Bone Miner Metab. 29:535–544.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vimalraj S, Arumugam B, Miranda PJ and
Selvamurugan N: Runx2: Structure, function, and phosphorylation in
osteoblast differentiation. Int J Biol Macromol. 78:202–208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dubey AK, Gupta SD and Basu B:
Optimization of electrical stimulation parameters for enhanced cell
proliferation on biomaterial surfaces. J Biomed Mater Res B Appl
Biomater. 98:18–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Salasznyk RM, Klees RF, Hughlock MK and
Plopper GE: ERK signaling pathways regulate the osteogenic
differentiation of human mesenchymal stem cells on collagen I and
vitronectin. Cell Commun Adhes. 11:137–153. 2004. View Article : Google Scholar
|
|
40
|
Salasznyk RM, Williams WA, Boskey A,
Batorsky A and Plopper GE: Adhesion to vitronectin and collagen I
promotes osteogenic differentiation of human mesenchymal stem
cells. J Biomed Biotechnol. 2004:24–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bistolfi F: Evidence of interlinks between
bioelectromagnetics and biomechanics: From biophysics to medical
physics. Phys Med. 22:71–95. 2006. View Article : Google Scholar
|
|
42
|
Kim IS, Song JK, Zhang YL, Lee TH, Cho TH,
Song YM, Kim K, Kim SJ and Hwang SJ: Biphasic electric current
stimulates proliferation and induces VEGF production in
osteoblasts. Biochim Biophys Acta. 1763:907–916. 2006. View Article : Google Scholar : PubMed/NCBI
|