Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is the
major system involved in the stress response, and the dysregulation
of the HPA axis is associated with depression (1). The activation of the HPA axis may
lead to the release of glucocorticoid (GC) from the adrenal glands
(2,3). GCs regulate stress responses,
including the successful adaptation to stress through the negative
feedback regulation of the HPA axis by binding to the
glucocorticoid receptor (GR) (4).
Under pathological conditions, the impairment of the GR-mediated
negative feedback system of the HPA axis results in constant HPA
axis hyperactivity and chronically high GC levels, leading to the
development of depressive disorders (5,6).
FK506 binding protein 5 (FKBP5), which promotes GR stability and
reduces GR sensitivity to GC, is a negative modulator of GR
activity that may inhibit the negative feedback loop of the HPA
axis (7,8). Serum- and glucocorticoid-inducible
kinase 1 (SGK1) has also been implicated in the cellular stress
response, as well as in neuronal function; it is a modulator of GC
effects on neurogenesis and GR function, particularly in depression
(9–11). The hippocampus and the prefrontal
cortex are involved in the negative feedback regulation of the HPA
axis and in the pathogenesis of depression (1,4,12).
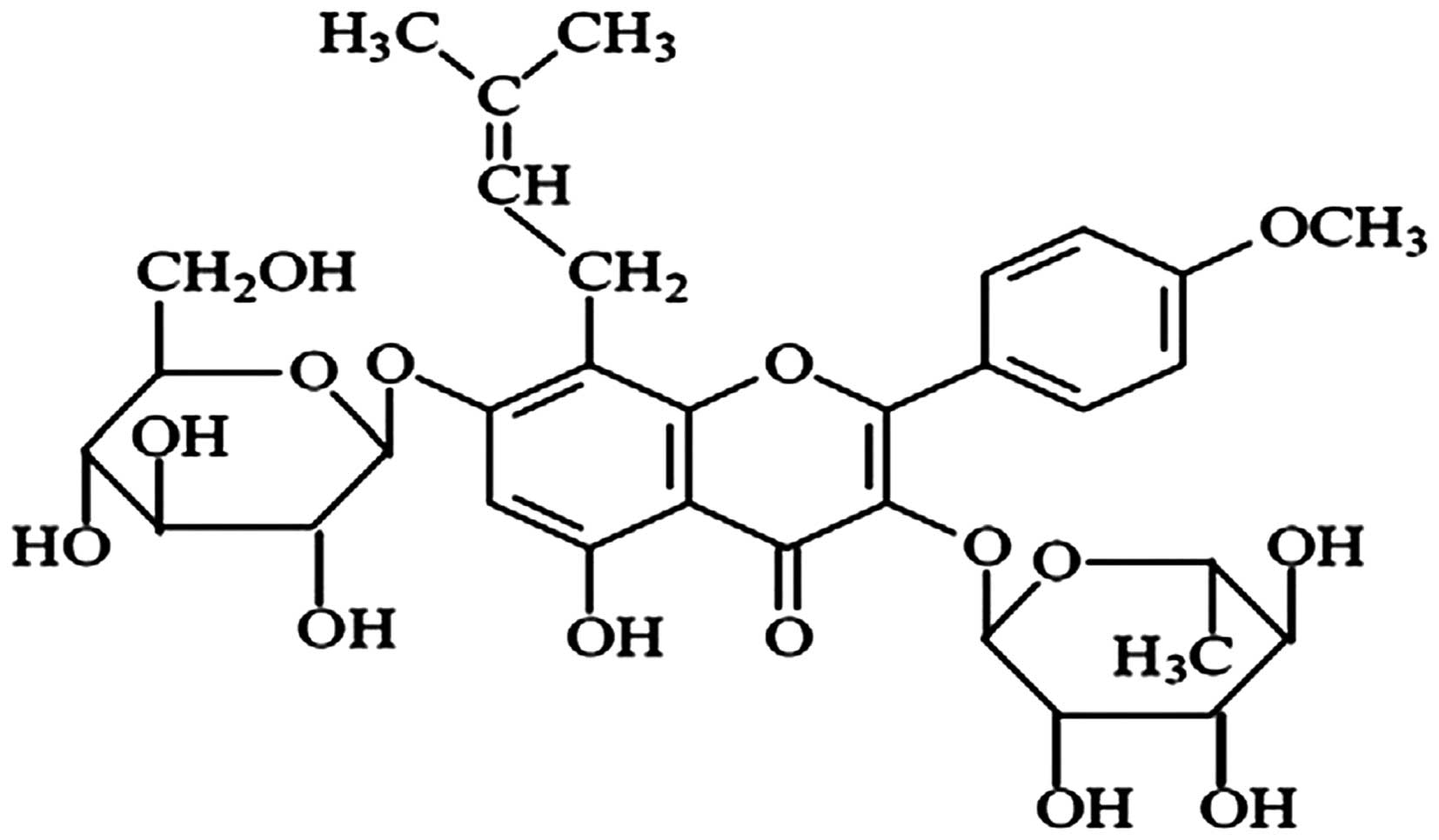
Icariin
[2-(4′-methoxylphenyl)-3-rhamnosido-5-hydroxyl-7-glucosido-8-(3′-methyl-2-butylenyl)-4-chromanone]
(Fig. 1), a flavonoid, is a major
constituent of Herba Epimedii, and exerts a wide range of
pharmacological activities. Our previous studies demonstrated that
icariin may potentially function as a novel antidepressant
(13–15), which exerts an antidepressant
effect by inhibiting neuroinflammation (15), protecting against
corticosterone-induced apoptosis in rat hippocampal neurons
(16), as well as by regulating
the function of the HPA axis (14,17–19). In the present study, a chronic
mild stress (CMS) protocol was used in order to establish a rat
model of depression, which mimics many of the symptoms of
depression in humans (20). We
examined the therapeutic effects of icariin administration on
depression-like behaviors, the mRNA and protein expression of GR,
FKBP5 and SGK1, as well as the distribution of GR in the cytoplasm
and the nucleus in both the hippocampus and the prefrontal cortex
induced by exposure to CMS.
Materials and methods
Reagents
Icariin, [98.93% purity, as verified by
high-performance liquid chromatography (HPLC)] was purchased from
the Shanghai Ronghe Medical Science Co., Ltd. (Shanghai, China).
Fluoxetine was obtained from Eli Lilly Co. (Suzhou, China) and
diluted in saline to a final concentration of 10 mg/ml. A nuclear
and cytoplasmic protein extraction kit, BCA kit and loading buffer
were purchased from the Beyotime Institute of Biotechnology
(Haimen, China). The rat corticosterone ELISA kit was obtained from
eBioscience (San Diego, CA, USA). The primers for the nuclear
receptor subfamily 3, group C, member 1 (glucocorticoid receptor)
(Nr3c1), FKBP5, SGK1 and β-actin genes encoding rat GR, FKBP5, SGK1
and β-actin, respectively, were supplied by Sangon Technologies
(Shanghai, China).
Animals and ethics approval
Fifty male Sprague-Dawley (SD) rats (5 weeks old,
weighing 120–140 g) were purchased from the Shanghai Laboratory
Animal Co. (SLAC; Shanghai, China). All the rats were individually
housed in a laminar-flow room under specific pathogen-free (SPF)
conditions and reared under the following conditions: an average
room temperature of 22±1°C, a relative humidity of 50–60%, a 12 h
light/dark cycle (lights on from 6:00 a.m. to 6:00 p.m.) and access
to food and water ad libitum. The rats were allowed to
acclimatize for at least 7 days prior to the commencement of the
experiments. All experiments were conducted in accordance with the
ethical standards of the Animal Care and Use Committee at Huashan
Hospital of Fudan University (Shanghai, China). All efforts were
made to minimize the number of animals used and their
suffering.
Chronic stress paradigm and drug
treatment
The CMS-exposed and the control groups were housed
in separate rooms under similar standard conditions. The procedure
for the induction of CMS consisted of once or twice daily exposure
to various unpredictable mild stressors in a random order for 5
weeks according to our standard protocol (15), which may lead to a chronic
depression-like state that develops gradually over time (21). The stressors included: food
deprivation (24 h), overnight water deprivation (18 h) followed by
1 h of empty water bottle replacement, cage tilt (45°) for 18 h,
overnight illumination (60 W lamp) for 13 h, soiled cage with 200
ml water in 100 g sawdust bedding for 21 h, forced swimming at 12°C
for 5 min, physical restraint in a clear plastic tube with air
vents at the nasal end for 2 h and pair housing for 24 h. The rats
in the different groups received either the vehicle (saline 1
ml/100 g for both the control and CMS groups), icariin (20 and 40
mg/kg) or fluoxetine (10 mg/kg) by oral administration once daily
for 35 days. The body weight of all the rats was weighed and
recorded on the 1st and 35th days of the experiment using a
platform scale. The behavioral tests were performed at least 16–18
h after the final treatment in order to avoid the acute effects of
drug treatments.
Sucrose preference test (SPT)
All the rats were initially trained to consume
palatable sucrose solution (1%, w/v) by providing it as the only
drinking fluid for 48 h in order to avoid neophobia. On the 36th
day, all the rats were deprived of water for 23 h prior to the
test. Sucrose preference was then determined by giving the rats a
free choice for 1 h between 2 identical bottles placed in the cage
and filled with either 1% sucrose solution or tap water. The SPT is
used to determine deppressive states, as the animals are observed
to examine their interest in seeking out a sweet rewarding drink as
opposed to plain drinking water. The selection of the sweetened
drink is typical of normal behaviour, and the non-selection of the
sweetened drink is indicative of depression. Sucrose preference was
defined as (ml sucrose/total ml consumed) ×100%.
Collection of brain tissue
In order to avoid fluctuations in hormone levels,
all the rats were decapitated between 11:00 a.m. and 1:00 p.m. on
the 37th day. The entire hippocampus and prefrontal cortex of each
rat brain were rapidly dissected on ice, frozen in liquid nitrogen
and stored at −80°C for further analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the rat hippocampus and
prefrontal cortex using TRIzol reagent followed by treatment with
RNase-free DNase I (both from Invitrogen Life Technologies,
Carlsbad, CA, USA). Reverse transcription was performed using the
One-Step RNA-PCR kit (Takara, Dalian, China), according to the
manufacturer's instructions. Quantitative (real-time) PCR was
performed on a 7300 Real-Time PCR system using SYBR-Green PCR
Master Mix (Sangon Technologies). Following the addition of the
primers (Table I) and template
DNA in duplicate, PCR amplification was performed as follows: 95°C
for 10 min, 40 cycles of 60°C for 60 sec and 95°C for 15 sec, and a
melting curve from 60 to 95°C to ensure the amplification of a
single product. The cycle passing threshold (Ct) was recorded for
each mRNA, and β-actin was used as the endogenous control for data
normalization. The relative expression levels were calculated using
the 2−ΔΔCt method.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | GenBank accession
no. | Sequence
(5′→3′) |
|---|
| Nr3c1 | NM_012576.2 | F:
GGGACACGAATGAGGATTG |
| R:
CACACTGCTGGGACTTGAT |
| FKBP5 | NM_001012174.1 | F:
GTTCAGCTGTGCAATCCAGA |
| R:
AGGGTGTTCTGTGCTCTTCAA |
| SGK1 | NM_001193568.1 | F:
GCCTGCCTCCGTTCTACA |
| R:
GCCTTGCTGAGTTGGTGAT |
| β-actin | NM_031144.2 | F:
CCTCTATGCCAACACAGT |
| R:
AGCCACCAATCCACACAG |
Western blot analysis
The proteins were extracted from the rat hippocampus
and prefrontal cortex using a nuclear and cytoplasmic protein
extraction kit (Beyotime Institute of Biotechnology). The protein
concentrations were quantified using the BCA method. The protein
samples (40–80 µg) were dissolved with an equal volume of
loading buffer, separated on 10% SDS-PAGE and then
electrotransferred at 90V to PVDF membranes. The membranes were
blocked with TBST containing 5% non-fat milk for 1 h at room
temperature followed by incubation with primary antibodies in the
refrigerator overnight at 4°C. The following primary antibodies
were used: rabbit anti-GR (1:3,000, ab109022), rabbit anti-FKBP5
(1:3,000, ab2901), rabbit anti-SGK1 (1:1,000, ab59337) (all from
Abcam, Cambridge, MA, USA) and rabbit anti-β-actin, rabbit
anti-lamin B (1:1,000; Bioworld Technology Co., Ltd., Nanjing,
China). The blots were washed extensively with TBST and incubated
with secondary antibodies in TBST/5% non-fat milk for 1 h at room
temperature. Subsequently, the signal was detected using an
enhanced chemiluminescence method (ECL kit; Millipore, Billerica,
MA, USA). Finally, the membranes were imaged and analyzed using
Quantity One Image Analysis Software (Syngene, Cambridge, UK).
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software. All data in the figures are presented as the means ± SD.
One-way analysis of variance (ANOVA) was performed if the data
followed a normal distribution and the variances were homogeneous;
the least significant difference (LSD) test was used for further
pairwise comparison. The non-parametric test was adopted if the
data did not follow a normal distribution or the variances were not
homogeneous; the Games-Howell test was used for further pairwise
comparison. A value of p<0.05 was considered to indicate a
statistically significant difference.
Results
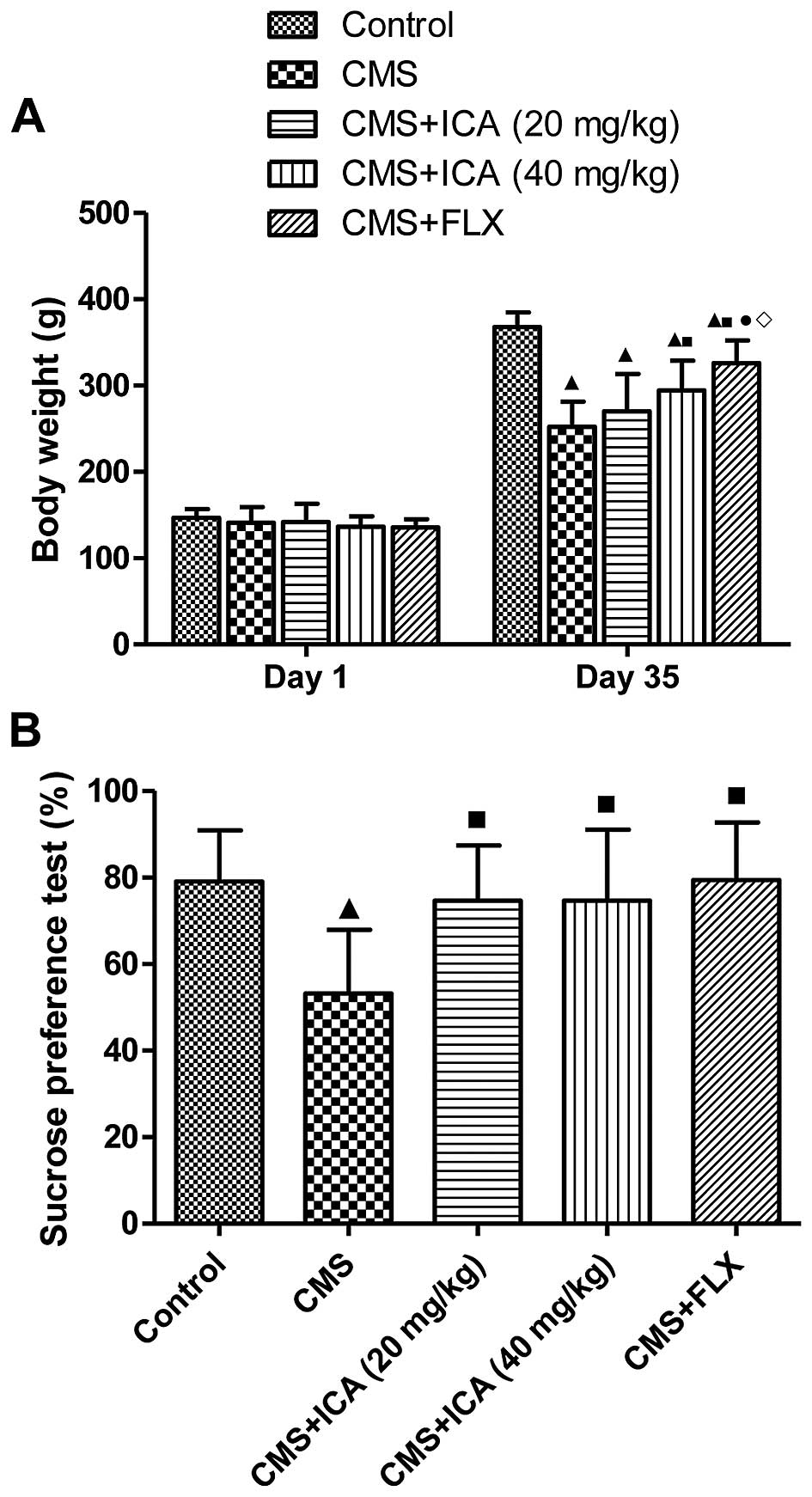
Effects of icariin on body weight and
sucrose preference rate in rats exposed to CMS
Fig. 2A shows that
the body weight of the rats exposed to CMS for 5 weeks was
significantly lower than that of the control animals (p<0.01),
and it was markedly increased by treatment with icariin (40 mg/kg)
and fluoxetine (10 mg/kg) compared with the CMS group (p<0.01).
The body weight of the rats in the fluoxetine group was higher than
that in the icariin (40 mg/kg) group (p<0.05) on day 35.
Moreover, as shown in Fig. 2B,
the rats exposed to CMS for 5 weeks displayed a significantly
reduced preference for sucrose solution (p<0.01) compared with
the control rats. Treatment with icariin (20 and 40 mg/kg) and
fluoxetine (10 mg/kg) reversed the CMS-induced reduction in sucrose
preference (p<0.01). Thus, icariin may be useful in reducing
depression-like behaviours.
Effects of icariin on mRNA Nr3c1
expression and on cytosolic and nuclear GR levels in the
hippocampus and prefrontal cortex of rats exposed to CMS
As shown in Fig. 3A
and B, exposure to CMS slightly decreased the mRNA expression
of Nr3c1 in the hippocampus (p>0.05); however, the mRNA
expression of Nr3c1 was significantly increased in the prefrontal
cortex (p<0.01) of rats exposed to CMS, compared with the rats
in the control group. Following treatment with icariin (20 and 40
mg/kg) and fluoxetine (10 mg/kg), the mRNA expression of Nr3c1 in
the prefrontal cortex was markedly decreased (p<0.01; levels
were similar to those of control group). Compared with the control
group, the expression of cytosolic GR was significantly upregulated
by exposure to CMS in both the hippocampus and the prefrontal
cortex (p<0.05 and p<0.01, respectively; Fig. 3C and D), whereas treatment with
icariin (20 and 40 mg/kg) and fluoxetine (10 mg/kg) significantly
decreased the expression of cytosolic GR in the hippocampus
(p<0.01, p<0.01 and p<0.05, respectively), compared with
the CMS group. The upregulated cytosolic GR expression in the
prefrontal cortex was only downregulated by icariin (20 mg/kg)
(p<0.01), whereas the expression level was further increased in
the icariin (40 mg/kg) group (p<0.01). As demonstrated in
Fig. 3E and F, nuclear GR
expression in the hippocampus was not affected by exposure to CMS
(p>0.05); however, it was upregulated in the prefrontal cortex
by exposure to CMS (p<0.05), compared with the control group.
Treatment with icariin (20 mg/kg) significantly reduced the
distribution of nuclear GR in the prefrontal cortex (p<0.01),
compared with the CMS group, whereas icariin (40 mg/kg) and
fluoxetine (10 mg/kg) had no effect on nuclear GR expression in the
prefrontal cortex.
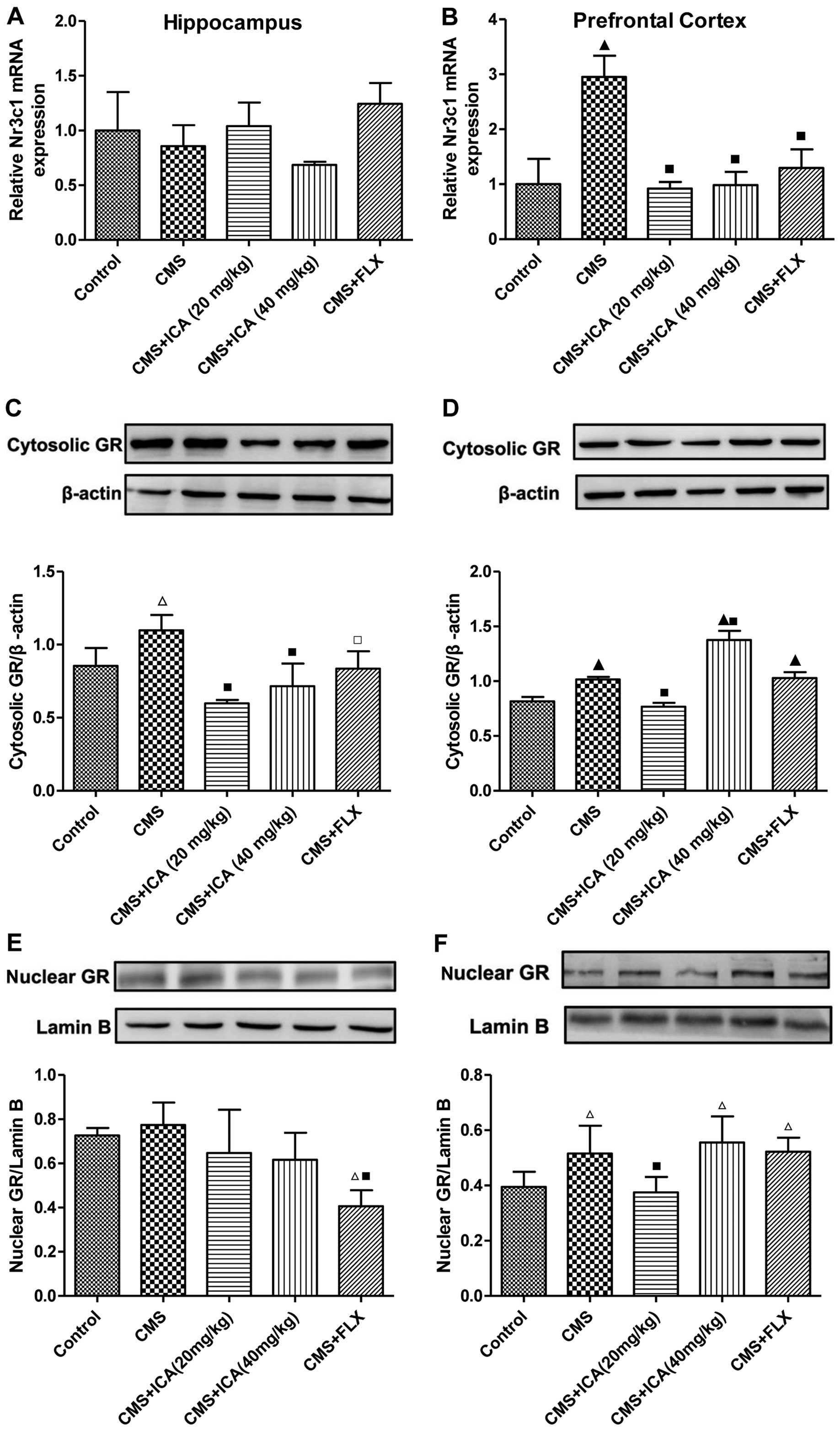 | Figure 3Icariin treatment normalizes the mRNA
expression of Nr3c1 and GR distribution in the hippocampus and the
prefrontal cortex of CMS-exposed rats. (A) CMS induced a slight
decrease in the mRNA expression of Nr3c1 in the hippocampus and (B)
a significant increase in the mRNA expression of Nr3c1 in the
prefrontal cortex, which was significantly decreased by icariin. (C
and D) CMS induced an increase in cytosolic GR expression in the
hippocampus and the prefrontal cortex, and icariin reduced the
accumulation of cytosolic GR in the hippocampus and in the
prefrontal cortex at a dose of 20mg/kg. (E and F) Nuclear GR
expression was only upregulated by exposure to CMS in the
prefrontal cortex, and icariin (20mg/kg) reversed this effect. Data
represent the means ± SD, n=3 rats/group. ▲p<0.01 and
△p<0.05 vs. control; ■p<0.01 and
□p<0.05 vs. CMS. Nuclear receptor subfamily 3, group
C, member 1 (glucocorticoid receptor), Nr3c1; CMS, chronic mild
stress; ICA, icariin; FLX, fluoxetine; GR, glucocortcoid
receptor. |
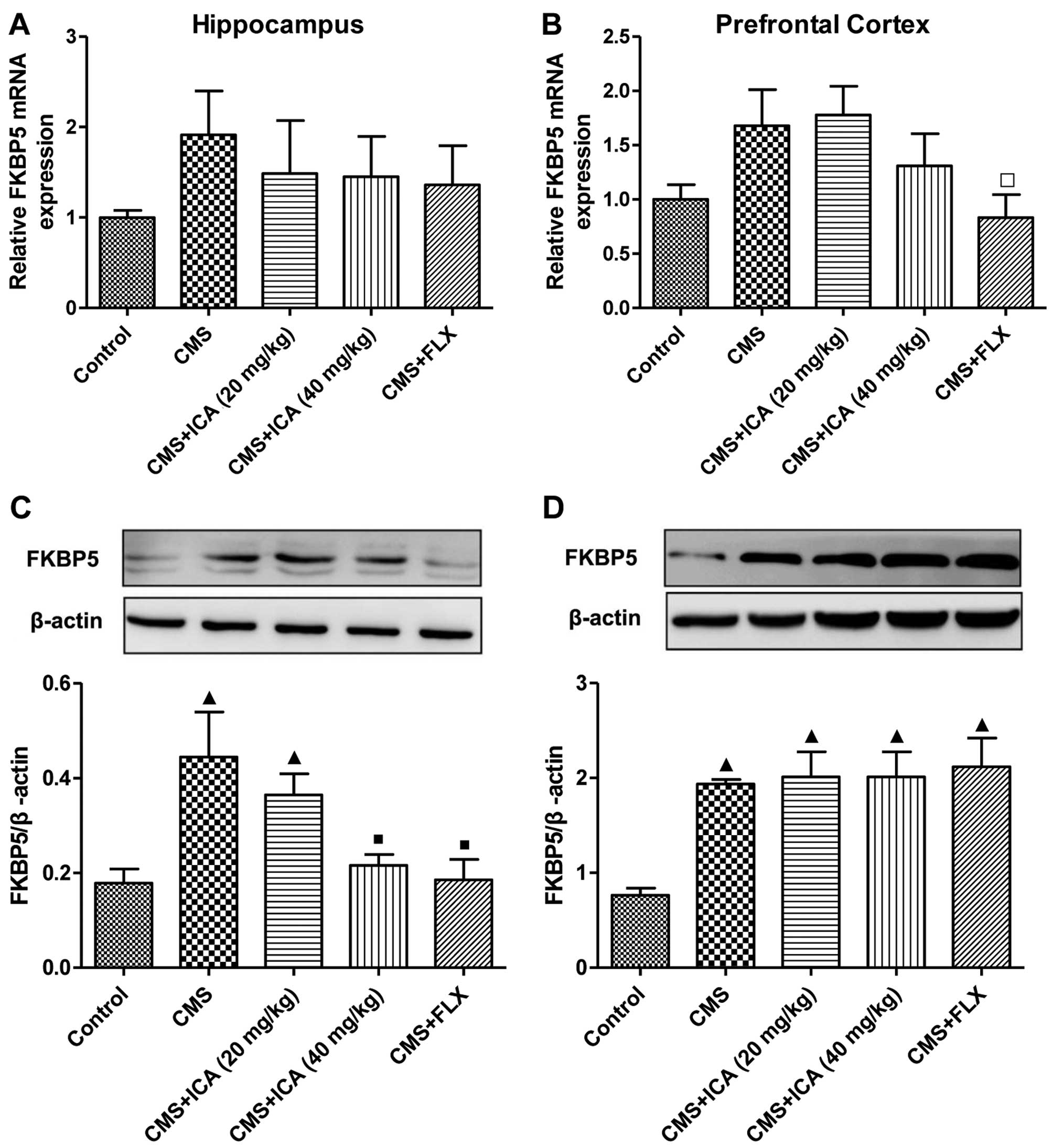
Effects of icariin on the expression of
FKBP5 in the hippocampus and prefrontal cortex of rats exposed to
CMS
As shown in Fig.
4, the mRNA expression of FKBP5 was increased (although not
signifiantly) in both regions of the brain following exposure to
CMS (p>0.05), whereas the protein expression of FKBP5 was
significantly upregulated (p<0.01) in both the hippocampus and
the prefrontal cortex of the rats in the CMS group, compared with
those in the control group. Treatment with icariin (40 mg/kg) and
fluoxetine (10 mg/kg) markedly abrogated the upregulation in the
expression of FKBP5 in the hippocampus (p<0.01; the levels were
close to those of the control group), whereas they had no effect on
FKBP5 protein expression in the prefrontal cortex. Moreover,
fluoxetine (10 mg/kg) also significantly downregulated the mRNA
expression of FKBP5 in the prefrontal cortex (p<0.05), compared
with the CMS group.
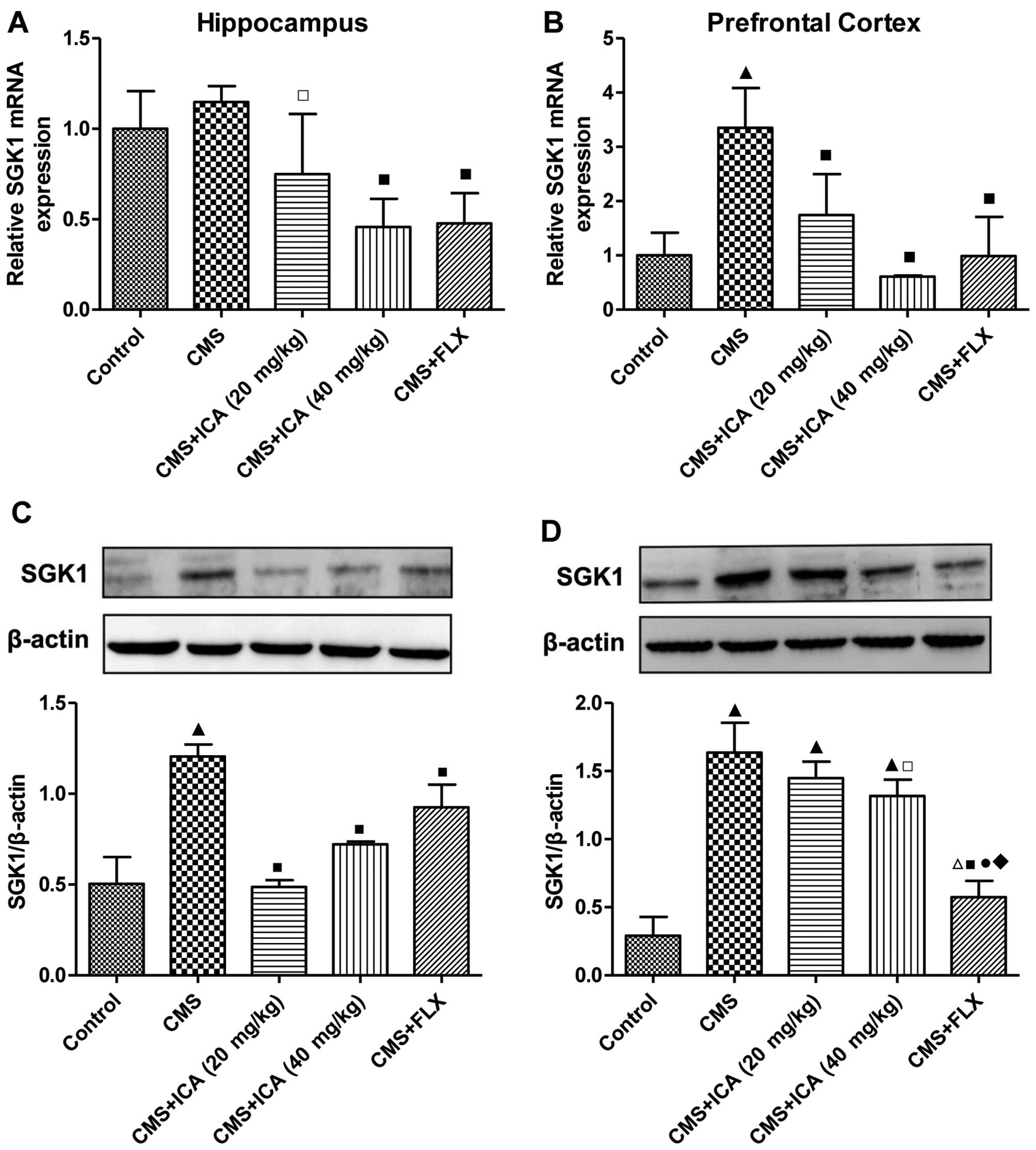
Effects of icariin on the expression of
SGK1 in the hippocampus and prefrontal cortex of rats exposed to
CMS
As shown in Fig. 5A
and B, the mRNA expression of SGK1 was significantly increased
(p<0.01) in the prefrontal cortex of the CMS-exposed rats,
whereas there was no significant difference in the hippocampus,
compared with the control group rats. Following treatment with
icariin (20 and 40 mg/kg) and fluoxetine (10 mg/kg), the mRNA
expression of SGK1 was markedly decreased in the prefrontal cortex
(p<0.01; levels were close to those of the control group), and
was also decreased in the hippocampus (p<0.05, p<0.01 and
p<0.01, respectively), compared with the CMS group. As shown in
Fig. 5C and D, the protein
expression of SGK1 was upregulated by exposure to CMS in both the
hippocampus and prefrontal cortex (p<0.01), as compared with the
control group. Treatment with icariin (20 and 40 mg/kg) and
fluoxetine (10 mg/kg) significantly decreased the expression of
SGK1 in the hippocampus (p<0.01), and in the prefrontal cortex
following treatment with the higher dose of icariin and fluoxetine
(p<0.05 for 40 mg/kg icariin, p<0.01 for 10 mg/kg
fluoxetine), compared with the CMS group. Furthermore, fluoxetine
(10 mg/kg) decreased SGK1 expression in the prefrontal cortex to a
lower level than in the icariin treatment groups (p<0.01).
Discussion
Upon binding to GC, the GR undergoes a
conformational change, which allows it to translocate to the
nucleus (7). In the nucleus, the
GR binds to glucocorticoid response elements (GRE) on the target
DNA as a positive or negative transcription factor in order to
regulate the transcription of GR-responsive genes (12,22,23). The decreased mRNA expression of GR
has been found in the frontal cortex of post-mortem tissue from
patients with schizophrenia and mood disorders (24), and in the hippocampus in rodent
models of depression induced by social defeat (13) or CMS (25,26), as well as in the hippocampus and
the prefrontal cortex of mice receiving chronic dexamethasone
treatment (27). Moreover, it has
been demonstrated that the cytosolic GR levels in the ventral
hippocampus and the prefrontal cortex are significantly increased
following exposure to CMS, and they may be completely normalized
(mainly in the prefrontal cortex) by the administration of
duloxetine (1). Previous research
has also demonstrated that the novel antidepressant icaritin
exerted therapeutic effects by increasing the downregulated mRNA
expression of GR in the hippocampus of socially-defeated mice
(13); emodin opposed
depression-like behaviors in CMS-exposed mice by upregulating the
mRNA expression of hippocampal GR (26). In the present study, the GR mRNA
expression in the hippocampus was slightly decreased by exposure to
CMS; however, this effect was not significant, which is a similar
trend to that observed in previous research (25,26). Notably, in contrast to the
findings of Skupio et al (27), in this study, the GR mRNA
expression was markedly upregulated by exposure to CMS in the
prefrontal cortex, which was decreased by icariin and fluoxetine
treatment. Cytosolic GR expression in both the hippocampus and the
prefrontal cortex was increased by exposure to CMS, as has been
demonstrated by previous research (1), which indicated decreases in GC
sensitivity of the GR in these brain regions. Icariin and
fluoxetine normalized the increased expression of cytosolic GR in
hippocampus, and only icariin (20 mg/kg) downregulated the
expression of cytosolic GR in the prefrontal cortex, which was
unexpectedly upregulated by icariin (40 mg/kg). The expression of
nuclear GR in the hippocampus exhibited no obvious change following
exposure to CMS, although fluoxetine decreased its expression. In
the prefrontal cortex, exposure to CMS upregulated the expression
of nuclear GR, which was only decreased by icariin at 20 mg/kg, but
not icariin at 40 mg/kg or fluoxetine. Previous research has
confirmed that GR mitochondrial translocation also exists in the
hippocampus and cortex of rats (28), and chronic high-dose
corticosterone treatment may decrease the GR levels in the
mitochondria in both primary cortical neurons and rodent prefrontal
cortex (29). As serum
corticosterone levels were upregulated in CMS-exposed rats in this
study (data not shown), decreased mitochondrial GR in the
hippocampus may also exist, which may be one reason for the slight
decrease in the GR mRNA and the increased level of cytosolic GR in
the hippocampus of the CMS-exposed rats. Following exposure to CMS
in the present study, the observed increase in GR mRNA expression
in the prefrontal cortex was contrary to previous findings
(27). Moreover, the increased
levels of cytosolic and nuclear GR in the prefrontal cortex were
not in line with the increase in GR mRNA (2–3-fold increase).
Further studies are warranted in order to explore the reason for
these unexpected results. In the prefrontal cortex, the opposing
effects of icariin (20 mg/kg) and icariin (40 mg/kg) on the level
of cytosolic GR, as well as the unaltered level of nuclear GR in
the icariin (40 mg/kg) group may indicate that the
antidepressant-like effects exerted by icariin are not strictly
dose-dependent.
As a negative modulator of GR activity, the mRNA and
protein expression of FKBP5 may be induced by GR activation
(7). When FKBP5 is bound to GRs,
the GR has a lower binding affinity for GC and is retained in the
cytoplasm. FKBP5 also enhances the stability of the GR, potentially
protecting it from proteolysis, and reduces GR sensitivity to GCs
(7). It has been found that CMS
or chronic dexamethasone treatment may increase the mRNA and/or
protein expression of FKBP5 in the brain of rodents, particularly
in the hippocampus and the prefrontal cortex (1,27,30,31). The upregulation of the mRNA and
protein expression of FKBP5 in the frontal cortex has also been
associated with the depression status (3). Antidepressants may significantly
reverse increases in the mRNA and protein expression of FKBP5 in
the hippocampus and/or prefrontal cortex of CMS-exposed rats
(1,31). In line with previous research,
this study found that exposure to CMS had a tendency to increase
the mRNA expression of FKBP5, but markedly upregulated FKBP5
protein expression in the hippocampus and the prefrontal cortex.
Icariin (40 mg/kg) and fluoxetine reversed the CMS-induced increase
in FKBP5 protein expression in the hippocampus, whereas the protein
expression of FKBP5 in the prefrontal cortex was not affected by
either icariin or fluoxetine, which provides evidence for the
specific targets of icariin in the treatment of depression.
SGK1, another GR target gene, which may be induced
at both the mRNA and protein levels by GC, may act as a modulator
of GC for neurogenesis and GR function. Therefore, it is involved
in the pathogenesis of depression and may serve as a target for
antidepressants (9–11,32). It has been demonstrated that SGK1
is involved in the GC-induced reduction of the proliferation and
differentiation of human hippocampal progenitor cells, by acting
both downstream of GR activation (through SGK1-dependent inhibition
of the Hedgehog pathway) and upstream of GR activation (through
SGK1-dependent GR phosphorylation and nuclear translocation)
(11). Hedgehog signaling
promotes neuronal differentiation (33), and therefore the SGK1-dependent
inhibition of this pathway results in decreased neurogenesis. The
mRNA expression of SGK1 has been found to be significantly
increased (positive correlation with mRNA expression of FKBP5) in
the peripheral blood of drug-free depressed patients, as well as in
the hippocampus of rats subjected to CMS (11). In addition, GC treatment clearly
increased the expression of SGK1 in human neural stem cells, as did
chronic restraint in the hippocampus and the prefrontal cortex in
rodent models of depression (27,34). Consistently, in the present study,
exposure to CMS significantly increased the mRNA expression of SGK1
in the prefrontal cortex, and the protein expression of SGK1 in
both the hippocampus and the prefrontal cortex. In the hippocampus,
there was a slight increase in the mRNA expression of SGK1
following exposure to CMS; however, this effect was not
significant. The administration of icariin and fluoxetine markedly
suppressed the increase in the mRNA expression of SGK1 in the
prefrontal cortex and the protein expression of SGK1 in both the
hippocampus and the prefrontal cortex, among which fluoxetine
decreased SGK1 to a lower level than icariin did in the prefrontal
cortex. As regards the mRNA and protein expression of SGK1 in the
prefrontal cortex, dose-dependent effects were observed between the
icariin treatment groups. In addition, the increased mRNA and
protein expression of SGK1 in the hippocampus and/or prefrontal
cortex following exposure to CMS revealed that SGK1 may mainly
participate in the downstream activation of GR in this study, which
suggests that the inhibition of the Hedgehog pathway may also play
a role in this model of experimental depression induced by CMS.
Further studies are warranted in order to confirm this
hypothesis.
To the best of our knowledge, this is the first
study to demonstrate that icariin treatment may reverse
depression-like behaviors in rodent models by downregulating the
expression of FKBP5 and SGK1 in the hippocampus and/or the
prefrontal cortex, and by normalizing the GR distribution between
the cytoplasm and the nucleus, which are similar to the effects of
fluoxetine to a certain degree. These data extend the mechanisms of
icariin in treating depression, and provide molecular evidence that
icariin may serve as a potentially effective antidepressant with
specific targets in different regions of the brain, which may aid
in the development of novel pharmacotherapeutic approaches that
selectively target these important molecules. However, further
experimental research with more samples, as well as large-scale
randomized controlled clinical trials are necessary in the future
in order to elucidate the molecular mechanisms underlying the
pathogenesis of depression, and to examine in detail the mechanisms
responsible for the antidepressant-like effects of icariin from
experimental and clinical perspectives.
In conclusion, the present study demonstrated that
the antidepressant-like effects of icariin are at least partially
attributed to the normalization of GR distribution between the
cytoplasm and the nucleus, as well as to decreases in the
expression of FKBP5 and SGK1 in the hippocampus and/or prefrontal
cortex, which may restore the normal negative feedback regulation
of the HPA axis and normal neurogenesis in related brain
regions.
Acknowledgments
The present study was funded by grants from the
National Natural Science Foundation of China (nos. 81102562 and
81173390), the National Basic Science Program of China (no.
2009CB523000), the Chinese Ministry of Education Fund for Doctor
Discipline Scientific Research (no. 20110071120072), and the
Development Project of Shanghai Peak Disciplines-Integrative
Medicine.
References
|
1
|
Guidotti G, Calabrese F, Anacker C,
Racagni G, Pariante CM and Riva MA: Glucocorticoid receptor and
FKBP5 expression is altered following exposure to chronic stress:
modulation by antidepressant treatment. Neuropsychopharmacology.
38:616–627. 2013. View Article : Google Scholar :
|
|
2
|
McEwen BS, Gould EA and Sakai RR: The
vulnerability of the hippocampus to protective and destructive
effects of glucocorticoids in relation to stress. Br J Psychiatry
Suppl. 15:18–23. 1992.PubMed/NCBI
|
|
3
|
Tatro ET, Everall IP, Kaul M and Achim CL:
Modulation of glucocorticoid receptor nuclear translocation in
neurons by immunophilins FKBP51 and FKBP52: implications for major
depressive disorder. Brain Res. 1286:1–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adzic M, Djordjevic J, Djordjevic A,
Niciforovic A, Demonacos C, Radojcic M and Krstic-Demonacos M:
Acute or chronic stress induce cell compartment-specific
phosphorylation of glucocor-ticoid receptor and alter its
transcriptional activity in Wistar rat brain. J Endocrinol.
202:87–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sapolsky RM, Romero LM and Munck AU: How
do glucocorticoids influence stress responses? Integrating
permissive, suppressive, stimulatory, and preparative actions.
Endocr Rev. 21:55–89. 2000.PubMed/NCBI
|
|
6
|
Pariante CM: Risk factors for development
of depression and psychosis. Glucocorticoid receptors and pituitary
implications for treatment with antidepressant and glucocorticoids.
Ann NY Acad Sci. 1179:144–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Binder EB: The role of FKBP5, a
co-chaperone of the glucocorticoid receptor in the pathogenesis and
therapy of affective and anxiety disorders.
Psychoneuroendocrinology. 34(Suppl 1): S186–S195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wochnik GM, Rüegg J, Abel GA, Schmidt U,
Holsboer F and Rein T: FK506-binding proteins 51 and 52
differentially regulate dynein interaction and nuclear
translocation of the glucocorticoid receptor in mammalian cells. J
Biol Chem. 280:4609–4616. 2005. View Article : Google Scholar
|
|
9
|
Miyata S, Koyama Y, Takemoto K, Yoshikawa
K, Ishikawa T, Taniguchi M, Inoue K, Aoki M, Hori O, Katayama T and
Tohyama M: Plasma corticosterone activates SGK1 and induces
morphological changes in oligodendrocytes in corpus callosum. PLoS
One. 6:e198592011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lang F, Strutz-Seebohm N, Seebohm G and
Lang UE: Significance of SGK1 in the regulation of neuronal
function. J Physiol. 588:3349–3354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anacker C, Cattaneo A, Musaelyan K,
Zunszain PA, Horowitz M, Molteni R, Luoni A, Calabrese F, Tansey K,
Gennarelli M, et al: Role for the kinase SGK1 in stress,
depression, and glucocorticoid effects on hippocampal neurogenesis.
Proc Natl Acad Sci USA. 110:8708–8713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Djordjevic A, Adzic M, Djordjevic J and
Radojcic MB: Stress type dependence of expression and
cytoplasmic-nuclear partitioning of glucocorticoid receptor, hsp90
and hsp70 in Wistar rat brain. Neuropsychobiology. 59:213–221.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu X, Wu J, Xia S, Li B and Dong J:
Icaritin opposes the development of social aversion after defeat
stress via increases of GR mRNA and BDNF mRNA in mice. Behav Brain
Res. 256:602–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li B, Duan X, Xu C, Wu J, Liu B, Du Y, Luo
Q, Jin H, Gong W and Dong J: Icariin attenuates glucocorticoid
insensitivity mediated by repeated psychosocial stress on an
ovalbumin-induced murine model of asthma. Int Immunopharmacol.
19:381–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu B, Xu C, Wu X, Liu F, Du Y, Sun J, Tao
J and Dong J: Icariin exerts an antidepressant effect in an
unpredictable chronic mild stress model of depression in rats and
is associated with the regulation of hippocampal neuroinflammation.
Neuroscience. 294:193–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu B, Zhang H, Xu C, Yang G, Tao J, Huang
J, Wu J, Duan X, Cao Y and Dong J: Neuroprotective effects of
icariin on corticosterone-induced apoptosis in primary cultured rat
hippocampal neurons. Brain Res. 1375:59–67. 2011. View Article : Google Scholar
|
|
17
|
Pan Y, Wang FM, Qiang LQ, Zhang DM and
Kong LD: Icariin attenuates chronic mild stress-induced
dysregulation of the LHPA stress circuit in rats.
Psychoneuroendocrinology. 35:272–283. 2010. View Article : Google Scholar
|
|
18
|
Pan Y, Zhang WY, Xia X and Kong LD:
Effects of icariin on hypothalamic-pituitary-adrenal axis action
and cytokine levels in stressed Sprague-Dawley rats. Biol Pharm
Bull. 29:2399–2403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan Y, Kong LD, Li YC, Xia X, Kung HF and
Jiang FX: Icariin from Epimedium brevicornum attenuates chronic
mild stress-induced behavioral and neuroendocrinological
alterations in male Wistar rats. Pharmacol Biochem Behav.
87:130–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hill MN, Hellemans KG, Verma P, Gorzalka
BB and Weinberg J: Neurobiology of chronic mild stress: parallels
to major depression. Neurosci Biobehav Rev. 36:2085–2117. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calabrese F, Guidotti G, Molteni R,
Racagni G, Mancini M and Riva MA: Stress-induced changes of
hippocampal NMDA receptors: modulation by duloxetine treatment.
PLoS One. 7:e379162012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maccari S, Mormède P, Piazza PV, Simon H,
Angelucci L and Le Moal M: Hippocampal type I and type II
corticosteroid receptors are modulated by central noradrenergic
systems. Psychoneuroendocrinology. 17:103–112. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pavlides C, Watanabe Y, Magariños AM and
McEwen BS: Opposing roles of type I and type II adrenal steroid
receptors in hippocampal long-term potentiation. Neuroscience.
68:387–394. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Webster MJ, Knable MB, O'Grady J, Orthmann
J and Weickert CS: Regional specificity of brain glucocorticoid
receptor mRNA alterations in subjects with schizophrenia and mood
disorders. Mol Psychiatry. 7:985–994. 9242002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng H, Liu Y, Li W, Yang B, Chen D, Wang
X, Jiang Z, Wang H, Wang Z, Cornelisson G and Halberg F: Beneficial
effects of exercise and its molecular mechanisms on depression in
rats. Behav Brain Res. 168:47–55. 2006. View Article : Google Scholar
|
|
26
|
Li M, Fu Q, Li Y, Li S, Xue J and Ma S:
Emodin opposes chronic unpredictable mild stress induced
depressive-like behavior in mice by upregulating the levels of
hippocampal glucocorticoid receptor and brain-derived neurotrophic
factor. Fitoterapia. 98:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skupio U, Tertil M, Sikora M, Golda S,
Wawrzczak-Bargiela A and Przewlocki R: Behavioral and molecular
alterations in mice resulting from chronic treatment with
dexamethasone: relevance to depression. Neuroscience. 286:141–150.
2015. View Article : Google Scholar
|
|
28
|
Moutsatsou P, Psarra AM, Tsiapara A,
Paraskevakou H, Davaris P and Sekeris CE: Localization of the
glucocorticoid receptor in rat brain mitochondria. Arch Biochem
Biophys. 386:69–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du J, Wang Y, Hunter R, Wei Y, Blumenthal
R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, et al:
Dynamic regulation of mitochondrial function by glucocorticoids.
Proc Natl Acad Sci USA. 106:3543–3548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing Y, He J, Hou J, Lin F, Tian J and
Kurihara H: Gender differences in CMS and the effects of
antidepressant venlafaxine in rats. Neurochem Int. 63:570–575.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xing Y, Hou J, Meng Q, Yang M, Kurihara H
and Tian J: Novel antidepressant candidate RO-05 modulated
glucocorticoid receptors activation and FKBP5 expression in chronic
mild stress model in rats. Neuroscience. 290:255–265. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Webster MK, Goya L, Ge Y, Maiyar AC and
Firestone GL: Characterization of sgk, a novel member of the
serine/threonine protein kinase gene family which is
transcriptionally induced by glucocorticoids and serum. Mol Cell
Biol. 13:2031–2040. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai C, Thorne J and Grabel L: Hedgehog
serves as a mitogen and survival factor during embryonic stem cell
neurogenesis. Stem Cells. 26:1097–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng Z, Liu L, Zhang C, Zheng T, Wang J,
Lin M, Zhao Y, Wang X, Levine AJ and Hu W: Chronic restraint stress
attenuates p53 function and promotes tumorigenesis. Proc Natl Acad
Sci USA. 109:7013–7018. 2012. View Article : Google Scholar : PubMed/NCBI
|