Introduction
Diabetes mellitus (DM) is characterized by
hyperglycemia caused by a lack of or resistance to insulin, and it
affects approximately 422 million individuals worldwide (1). In addition, it is well known that DM
is an important metabolic disease which causes a variety of
complications, including certain types of cancer and hepatic
glucose production (2–4). Although the precise mechanisms
involved remain poorly understood, oxidative stress is believed to
be one of the major factors contributing to DM. Several previous
studies have demonstrated that excessive oxidative stress is
generated in response to pancreatic β-cell death in animal models
of streptozotocin (STZ)-induced diabetes (5–7).
Antioxidant 1 (ATOX1) is abundantly expressed in
various areas of the brain, including the cerebral cortex and
hippocampus, where it plays important roles in copper homeostasis
and acts as a copper chaperone. Additionally, ATOX1 is known to
function as an antioxidant which protects against oxidative stress
and as a copper-dependent transcription regulator, which also
involves the regulation of cell proliferation, the cell cycle and
superoxide dismutase 3, extracellular (SOD3) (8–10).
Copper is a co-factor required in various biological processes;
however, excess copper causes toxicity in cells (11–13). Previous studies have demonstrated
that ATOX1 plays an important role in cell survival in various
diseases, including cancer, hypertension, cardiovascular disease
and neuronal diseases, suggesting that ATOX1 is a potential
therapeutic target molecule for the use in the management of
patients suffering from disorders of copper metabolism and
oxidative stress-induced diseases (14–19).
Despite ATOX1 having the potential to be used as a
therapeutic target molecule in the fight against a variety of
diseases, the applications of ATOX1 are limited by an inability to
deliver it into cells or tissues. Protein transduction domains
(PTDs) or cell-penetrating peptides (CPPs) have helped to overcome
this limitation by allowing target molecules to be transduced into
cells and tissue. Tat peptide is comprised of 9 amino acids
(RKKRRQRRR), and it delivers proteins into cells and tissues such
as brain cells (20–22). Several studies, including those
undertaken by our group, have demonstrated that various Tat-fused
proteins transduce into cells and protect against cell death in
various diseases (23–29).
In the present study, we examined the protective
effects of Tat-ATOX1 protein in STZ-exposed RINm5F cells and in a
mouse model of STZ-induced diabetes. We found that Tat-ATOX1
protein transduced into the RINm5F cells and pancreatic tissues.
Transduced Tat-ATOX1 protein markedly protected against STZ-induced
cell death in vitro and in vivo. Therefore, we
suggest that Tat-ATOX1 protein has potential applications as a
therapeutic agent for oxidative stress-related diseases including
DM.
Materials and methods
Materials
Tat peptide was chemically synthesized by Peptron
(Daejeon, Korea). The rat pancreatic insulinoma cell line RINm5F
was purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). STZ was purchased from Sigma-Aldrich (St.
Louis, MO, USA). An Ni2+-nitrilotriacetic acid Sepharose
superflow was purchased from Qiagen (Valencia, CA, USA). RPMI-1640
medium and antibiotics were obtained from Invitrogen (Carlsbad, CA,
USA). Fetal bovine serum (FBS) was purchased from Lonza (Basel,
Switzerland). Primary antibodies [histidine (sc-804), insulin
(sc-8033), Akt (9273), p-Akt (4058), JNK (9258), p-JNK (9251), p38
(9212), p-p38 (4631), beta-actin (4967) and HRP-conjugated
antibodies (7074)] were obtained from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA) and Cell Signaling Technology (Beverly,
MA, USA). Human ATOX1 cDNA was isolated using the PCR technique.
All other chemicals and reagents were of the highest quality grade
available.
Purification of Tat-ATOX1 protein
The Tat expression vector was prepared in our
laboratory as described previously (24). Briefly, the cDNA sequence for
human ATOX1 was PCR amplified using the sense and antisense
primers: ATOX1 sense, 5′-CTCGAGATGCCGAAGCACG-3′ and antisense,
5′-GGATCCCTACTCAAGGCCAAGG-3′. The PCR products were then sub-cloned
into a TA vector (Promega, Madison, WI, USA) and ligated into the
Tat expression vector to generate the Tat-ATOX1 expression vector.
We generated ATOX1 protein without the Tat peptide as a control.
The recombinant Tat-ATOX1 plasmid was transformed into
Escherichia coli BL21 (DE3; Novagen, Madison, WI, USA) and
cultured in 0.5 mM isopropyl-β-D-thiogalactoside (IPTG; Duchefa,
Haarlem, The Netherlands) at 18°C overnight. The harvested cells
were purified using an Ni2+-nitrilotriacetic acid
Sepharose affinity column and PD-10 column chromatography
(Amersham, Piscataway, NY, USA) (29,30). The concentration of purified
protein was measured using the Bradford assay (Bio-Rad
Laboratories, Hercules, CA, USA), as previously described (31).
Cell culture and transduction of
Tat-ATOX1 protein
The RINm5F cells were cultured in RPMI-1640 medium
containing 2 mM glutamine, 10% FBS and antibiotics (100
µg/ml streptomycin, 100 U/ml penicillin) at 37°C in a
humidified incubator containing 5% CO2 and 95% air.
To examine the transduction ability of Tat-ATOX1 and
control ATOX1 protein, the cells were treated with various doses of
Tat-ATOX1 protein (0.5–3 µM) for 30 min and over various
periods of time (5–30 min) with 3 µM of Tat-ATOX1 protein.
Following treatment with Tat-ATOX1 protein, the cells were washed
three times with phosphate-buffered saline (PBS) and trypsin-EDTA.
The cells were then harvested and western blot analysis was
performed.
Western blot analysis
Equal amounts of proteins were resolved by 15%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE). Following electrophoresis, the proteins were
transferred from the gel to a nitrocellulose membrane. The membrane
was blocked with TBS-T buffer (25 mM Tris-HCl, 140 mM NaCl, 0.1%
Tween-20, pH 7.5) containing 5% non-fat dry milk or bovine serum
albumin (BSA). The membrane was incubated with primary and
HRP-conjugated antibodies (26,27,32). The bands were identified using
chemiluminescent reagents according to the manufacturer's
instructions (Amersham, Franklin Lakes, NJ, USA).
Confocal fluorescence microscopy
Confocal fluorescence microscopy was performed, as
described previously (27,33).
Briefly, the RINm5F cells were grown on coverslips treated with
Tat-ATOX1 protein (3 µM) for 30 min, and the cells were
washed with PBS twice and fixed with 4% paraformaldehyde for 3 min.
The cells were then treated with the primary antibody
(anti-histidine; 1:2,000) and Alexa Fluor 488-conjugated secondary
antibody (1:15,000; Invitrogen). After the nuclei were stained with
DAPI (1 µg/ml; Roche Applied Science, Mannheim, Germany) for
3 min, the cells were observed under a confocal fluorescence
microscope (FV-300; Olympus, Tokyo, Japan).
Cell viability assay
The RINm5F cells were plated at a confluence of 80%
on a 96-well plate and treated with various amounts of Tat-ATOX1
proteins (1–3 µM) for 30 min. The cells were incubated with
3 mM STZ for 12 h. Subsequently, cell viability was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Duchefa, Haarlem, The Netherlands). The absorbance was
measured at 570 nm using an ELISA microplate reader (Labsystems
Multiskan MCC/340; Labsystem Oy, Helsinki, Finland), and cell
viability is presented as a percentage of the control cells, as
previously described (30,34).
Measurement of DNA damage
8-Hydroxy-2′-deoxyguanosine (8-OHdG; Santa Cruz
Biotechnology, Inc.) and terminal deoxynucleotidyl transferase
(TdT)-mediated biotinylated dUTP nick end labeling (TUNEL; Roche
Applied Science) staining was performed as described previously
(27). The RINm5F cells were
pre-treated with Tat-ATOX1 protein (3 µM) for 30 min, and
then the cells were incubated with 3 mM STZ for 24 h. Images were
analyzed using a fluorescence microscope (Nikon Eclipse 80i; Nikon,
Tokyo, Japan). The fluorescence intensity was measured using a
Fluoroskan ELISA plate reader (Labsystems Oy).
Animals and experimental protocol
Male 5-week-old ICR mice were housed at 23°C and 60%
humidity under a fixed 12-h light/dark cycle with free access to
food and water. All experimental procedures involving the animals
and their care conformed to the Guide for the Care and Use of
Laboratory Animals of the National Veterinary Research and
Quarantine Service of Korea and were approved by the Hallym Medical
Center Institutional Animal Care and Use Committee.
The ICR mice were divided into the following six
groups (n=10/group): i) untreated normal control group, ii)
STZ-injected group, iii) STZ + Tat-ATOX1 protein (2 mg/kg) injected
group, iv) STZ + ATOX1 protein (2 mg/kg) injected group, v) STZ +
Tat peptide (2 mg/kg) injected group, and vi) STZ + insulin (0.02
IU; Sigma-Aldrich) injected group. To induce diabetes, the mice
were injected intraperitoneally with STZ (Sigma-Aldrich) dissolved
in 50 mM sodium citrate buffer solution (pH 4.5; Sigma-Aldrich) at
a dose of 70 mg/kg body weight. The untreated normal control mice
were injected with the same volume of sodium citrate buffer. The
mice received 6 injections of Tat-ATOX1 protein, control ATOX1
protein, Tat peptide, and insulin at weekly intervals. The mice
were sacrificed by cervical dislocation 8 weeks after inducing
diabetes with STZ. Mice were perfused transcardially with
phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in
0.1 M phosphate buffer (PB, pH 7.4) under urethane anesthesia (1.5
g/kg, i.p.). The pancreatic tissues were removed for histological
examinations. In order to analyze the pancreatic β-cells in the
present study, tissue sections were incubated with either an
anti-mouse insulin IgG (dilution 1:300; InnoGenex, San Ramon, CA,
USA) or anti-His (dilution 1:200). The pancreatic tissue sections
were stained with a peroxidas e/3,3′-diaminobenzidine (DAB) system
kit (Dako EnVision kit; Dako, Glostrup, Denmark) or hematoxylin and
eosin (H&E; Sigma-Aldrich), as previously described (6).
Biochemical analysis
Changes in body weight and blood glucose levels were
monitored weekly over the 8-week study period. Blood glucose levels
were analyzed using an Accu-Chek glucose strip machine (Roche
Applied Science) (35). To
examine changes to blood glycated hemoglobin A1c (HbA1c) levels,
blood samples were collected from the tail vein after 8 weeks.
HbA1c levels were then measured using a glycohemoglobin analyzer
(HLCr-723GHb; Tosoh Corp., Kyoto, Japan).
Statistical analysis
All experiments were performed three times. Data
were analyzed using analysis of variance (ANOVA) and the Student's
t-test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Construction and purification of
Tat-ATOX1 protein
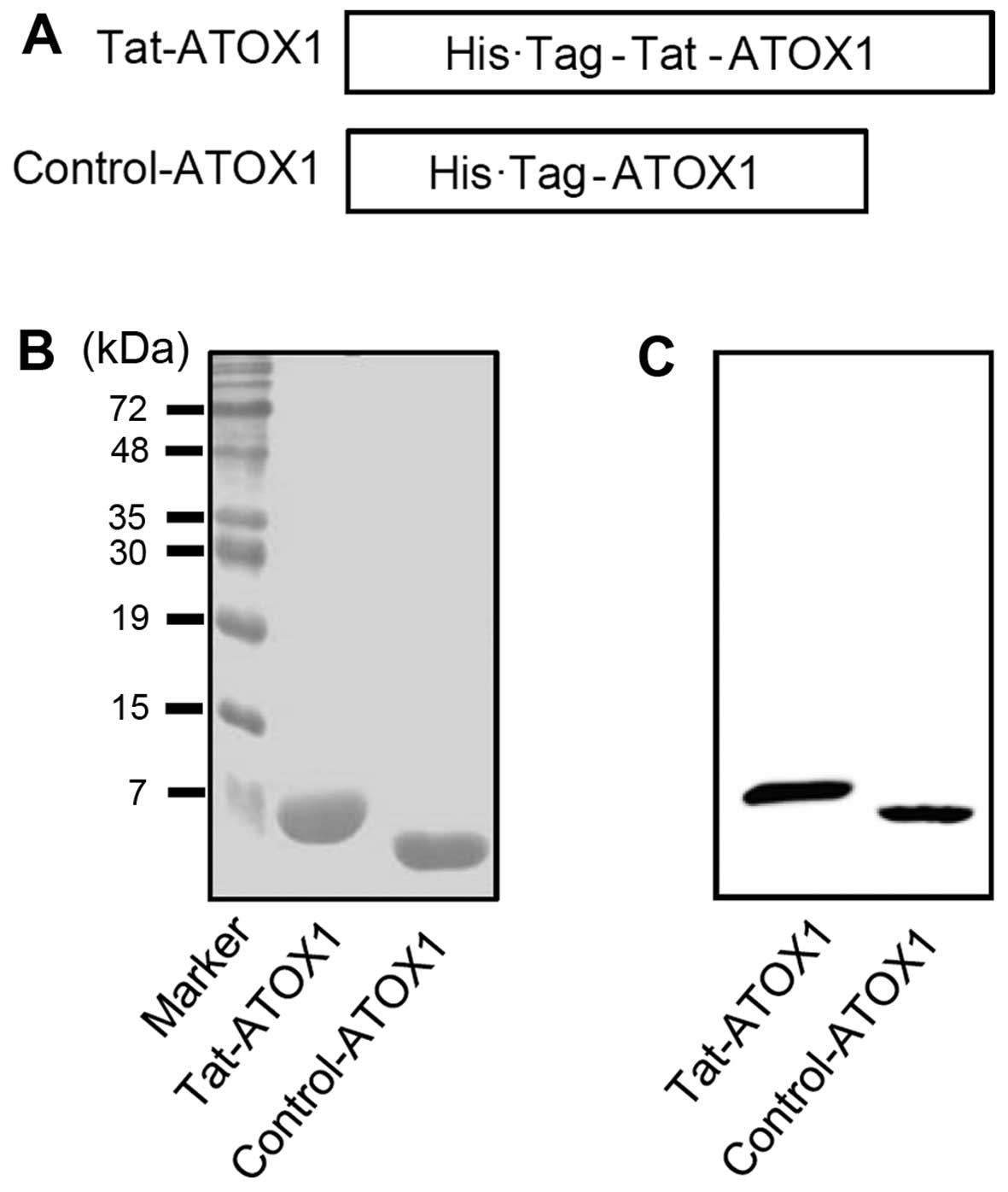
The human ATOX1 gene was inserted into a pET-15b
plasmid containing a Tat peptide PTD in order to generate a
recombinant Tat-ATOX1 protein expression plasmid. The resulting
recombinant Tat-ATOX1 plasmid was confirmed by automated sequencing
analysis. The Tat-ATOX1 protein expression plasmid consisted of
human ATOX1 cDNA, a Tat peptide, and a His tag with 6-histidine
residues (Fig. 1A). The control
ATOX1 protein expression plasmid containing no Tat peptide was also
generated.
Following the culture of the Tat-ATOX1 and control
protein in 0.5 mM IPTG overnight at 18°C, the proteins were
purified using an Ni2+-nitrilotriacetic acid Sepharose
affinity column and PD-10 column chromatography. The purified
proteins were confirmed by SDS-PAGE (Fig. 1B). We also identified the proteins
by performing western blot analysis with an anti-histidine antibody
(Fig. 1C). These results indicate
that we successfully expressed and purified Tat-ATOX1 and control
ATOX1 proteins.
Transduction of Tat-ATOX1 protein into
RINm5F cells
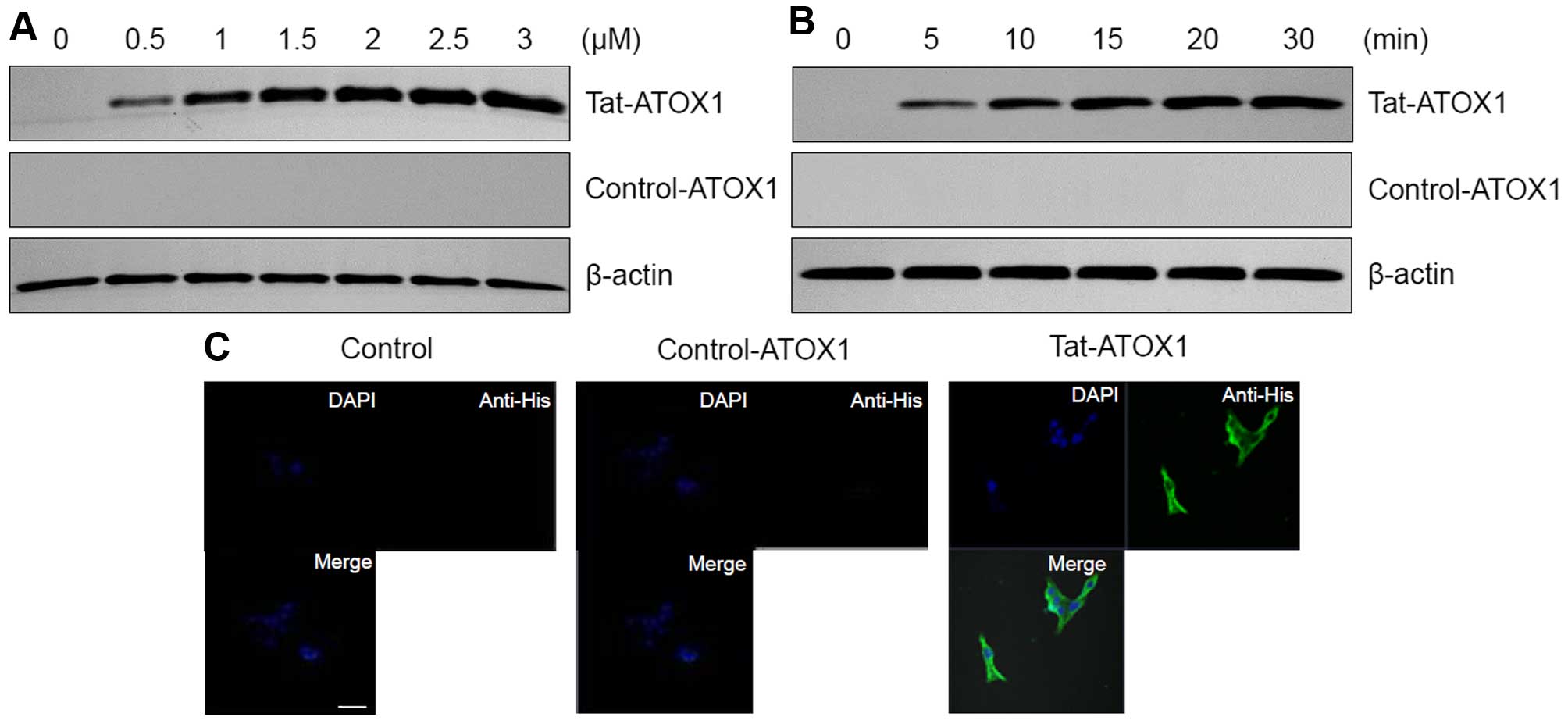
The transduction ability of Tat-ATOX1 protein into
the RINm5F cells was determined by western blot analysis and
confocal microscopy. The RINm5F cells were treated with different
concentrations of Tat-ATOX1 protein (0.5–3 µM) for 30 min
and over different periods of time (5–30 min) with 3 µM
Tat-ATOX1 protein. The transduction efficiency of Tat-ATOX1 protein
was assessed by western blot analysis. As shown in Fig. 2A and B, we found that Tat-ATOX1
protein transduced into the RINm5F cells in a concentration- and
time-dependent manner, whereas control ATOX1 protein did not
transduce into the cells.
We further confirmed Tat-ATOX1 protein transduction
by direct DAPI- and Alexa Fluor 488-staining using fluorescence
confocal microscopy (Fig. 2C). In
the cells treated with Tat-ATOX1 protein, green fluorescence was
clearly visible in the nucleus and cytoplasm. By contrast, the
cells treated with control ATOX1 protein did not exhibit green
fluorescence. These results further confirm that Tat-ATOX1 protein
transduced into the RINm5F cells.
Effect of transduced Tat-ATOX1 protein on
STZ-induced cell damage
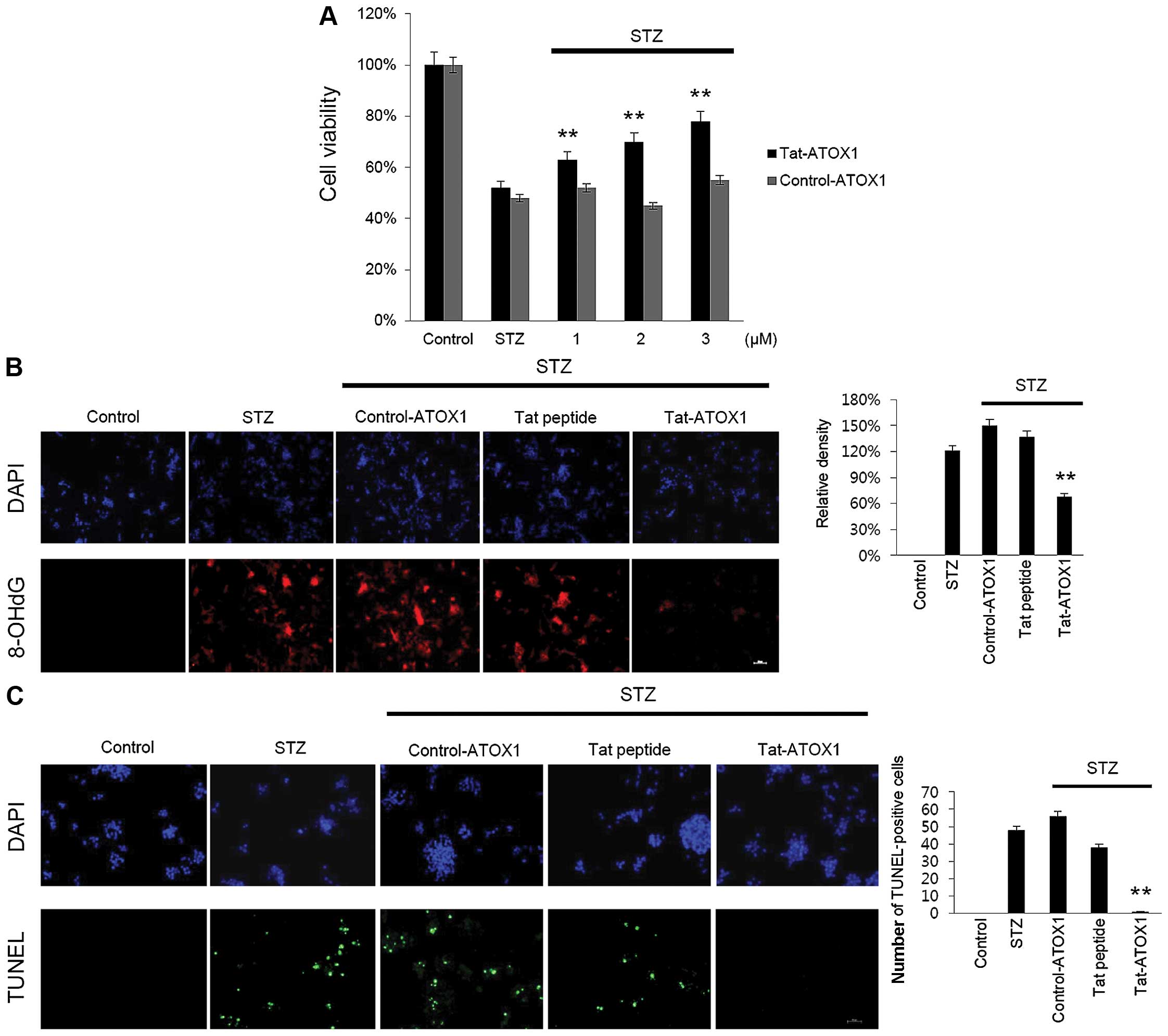
To examine the protective effect of Tat-ATOX1
protein on RINm5F cells, the cells were exposed to STZ (3 mM) for
12 h, after which cell viability was assessed using an MTT assay.
Cell viability was significantly decreased in the cells exposed to
STZ (52%) compared with the control cells, whereas in those cells
treated with Tat-ATOX1 protein cell viability was markedly
increased in a dose-dependent manner, up to 76%. However, control
ATOX1 protein exerted little or no protective effects under the
same conditions (Fig. 3A).
As STZ induces DNA damage and cell death, we
examined whether Tat-ATOX1 protein inhibits DNA fragmentation and
the production of 8-OHdG, which is one of the major byproducts of
DNA oxidation. Thus, we measured 8-OHdG in the STZ-exposed cells.
Transduced Tat-ATOX1 protein markedly reduced DNA oxidation in the
STZ-exposed cells (Fig. 3B). DNA
fragmentation was measured by TUNEL staining. As shown in Fig. 3C, the number of TUNEL-positive
stained cells significantly increased in the STZ-exposed cells
compared with that in the control cells. By contrast, the number of
TUNEL-positive stained cells significantly decreased in the
Tat-ATOX1 protein-treated cells. These results indicate that
Tat-ATOX1 protein protected against cell death by inhibiting DNA
damage.
Effects of Tat-ATOX1 protein on
STZ-induced activation of Akt and mitogen activated protein kinase
(MAPK) in RINm5F cells
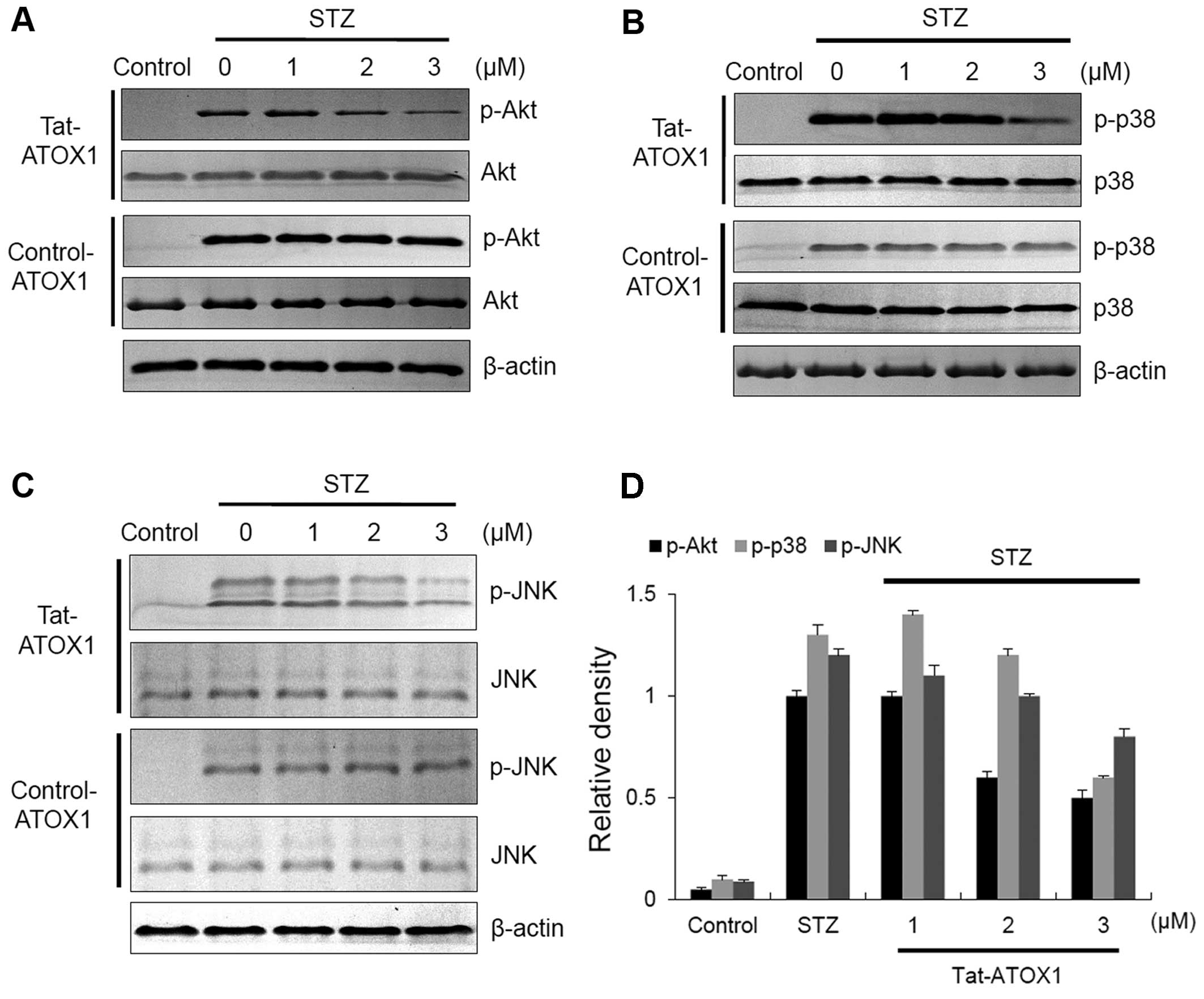
A previous study has demonstrated that STZ-induced
diabetes in animals leads to apoptotic cell death through the
activation of c-Jun N-terminal kinase (JNK), p38 and Akt (36). To examine the effects of Tat-ATOX1
protein on JNK, p38 and Akt in STZ-exposed RINm5F cells, the cells
in this study were exposed to STZ (3 mM) and evaluated by western
blot analysis (Fig. 4). In the
STZ-exposed cells, the expression of phosphorylated (p-)JNK, p-p38
and p-Akt was markedly increased. However, Tat-ATOX1 protein
significantly reduced the levels of p-JNK, p-p38 and p-Akt in the
STZ-exposed cells. By contrast, control ATOX1 protein did not seem
to have any effect on these protein expression levels. These
results indicate that Tat-ATOX1 protein protects cells by
regulating the Akt and MARK (JNK and p38) signaling pathways.
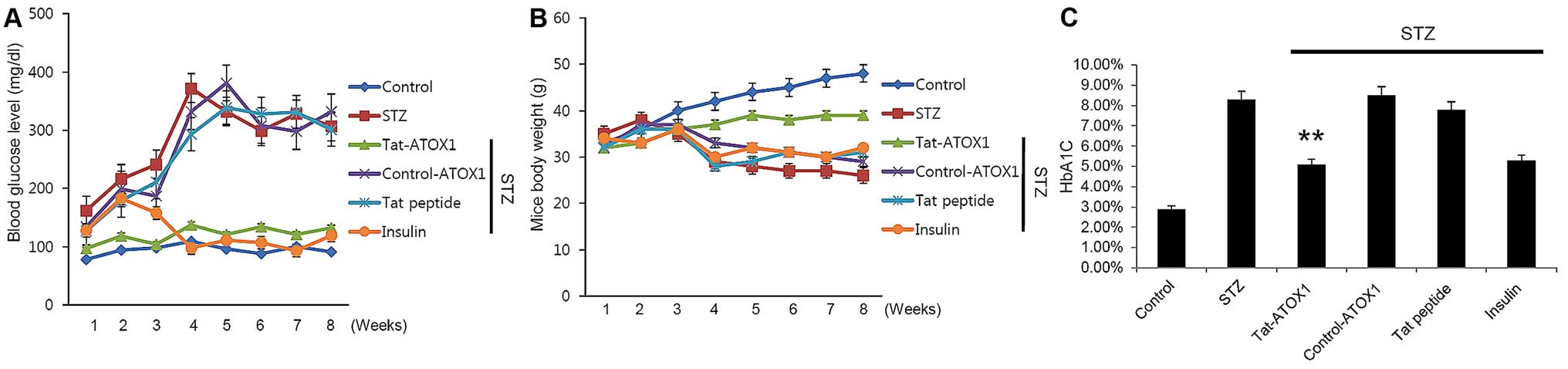
Effect of Tat-ATOX1 protein on mice with
STZ-induced diabetes
To determine the effects of Tat-ATOX1 protein on
mice with STZ-induced diabetes, we examined the changes in blood
glucose levels, body weight, and glycosylated HbA1C levels. In the
STZ-exposed mice, blood glucose levels and HbA1C levels were
markedly increased compared with those in the untreated normal
control group. By contrast, body weight was significantly reduced
in the mice with STZ-induced diabetes. However, Tat-ATOX1 protein
normalized or markedly ameliorated these effects mentioned above.
These changes were also normalized or ameliorated in the mice
treated with insulin, a reference drug which was used in order to
examine the effects of insulin in comparison with Tat-ATOX1
protein. There were no significant differences between the other
groups (control ATOX1 protein- and Tat peptide-treated groups) and
the group with STZ-induced diabetes (Fig. 5).
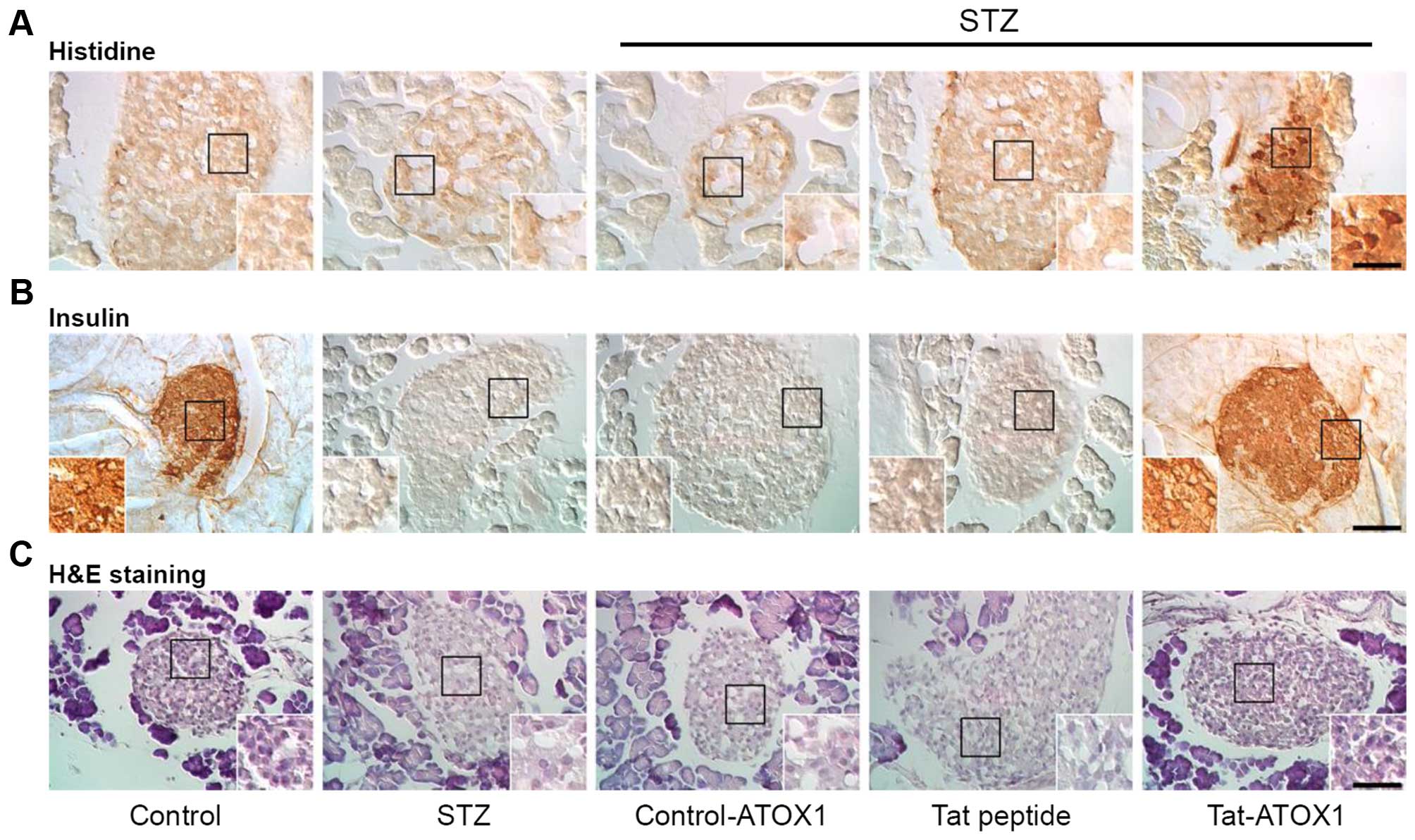
Furthermore, we also examined the effects of
Tat-ATOX1 protein on the pancreatic tissue of mice with STZ-induced
diabetes. As shown in Fig. 6,
Tat-ATOX1 protein transduced into pancreatic tissues, where it
provided significant protection against pancreatic β-cell
destruction in the mice with STZ-induced diabetes. Furthermore, we
demonstrated that Tat-ATOX1 protein markedly increased insulin
levels compared with those in the mice with STZ-induced diabetes.
However, control ATOX1 protein and Tat peptide did not exert the
same protective effects. These results indicate that transduced
Tat-ATOX1 protein prevented pancreatic β-cell destruction caused by
STZ and attenuated STZ-induced diabetes in mice.
Discussion
ATOX1 protein is a copper chaperone and plays an
important role in copper homeostasis. Copper homeostasis is
important for cellular biochemical processes, as free copper ions
cause cellular toxicity through the generation of reactive oxygen
species (ROS), resulting in cellular damage. ATOX1 is also known to
play a roles in cells, as a copper transporter and antioxidant
(8,9,11).
Other studies have demonstrated that the impairment of copper
regulation is associated with various human diseases, including
neuronal diseases, cardiomyopathy and diabetes (8,14–19).
PTDs, including Tat, are well known for their
ability to deliver proteins to cells and tissues (20–22). Previous studies have used this
ability to demonstrate the protective effects of a variety of
PTD-fusion proteins and thus, their potential for use as
therapeutic agents in the management of numerous diseases (23–30). Therefore, in the present study, we
generated a cell-permeable Tat-ATOX1 protein and examined the
protective effects of Tat-ATOX1 protein against STZ-induced RINm5F
cell death and in a mouse model of STZ-induced diabetes. As ATOX1
protein possesses a limited ability to transduce into cells, the
application of this protein was also limited. We generated
Tat-ATOX1 protein to solve this transduction problem. Purified
Tat-ATOX1 protein was confirmed by SDS-PAGE and western blot
analysis using an anti-His antibody. Moreover, we found that
Tat-ATOX1 protein was efficiently transduced into the RINm5F cells
in a time- and concentration-dependent manner. Also, fluorescence
signals of transduced Tat-ATOX1 protein were distributed in the
nucleus and cytoplasm of the cells. These results indicate that
Tat-ATOX1 protein efficiently transduced into RINm5F cells.
However, the transduction mechanism of Tat-ATOX1 protein requires
further investigation, as it is not yet fully understood.
STZ is usually used to induce DM by causing
pancreatic β-cell death through cytotoxic effects and DNA damage
(37). We determined the effects
of transduced Tat-ATOX1 protein on the cell viability of
STZ-exposed RINm5F cells. We found that transduced Tat-ATOX1
protein significantly increased cell survival in a dose-dependent
manner. Furthermore, we demonstrated that transduced Tat-ATOX1
protein prevented the DNA damage induced by STZ. Thus, we suggest
that transduced Tat-ATOX1 protein protects against STZ-induced cell
death by inhibiting DNA damage.
Both the phosphoinositide 3-kinase (PI3K)/Akt and
MAPK signaling pathways are activated in response to various
stimuli and are known to play important roles in cell survival and
thus, they also play an important role in preventing cell death
(36,38–41). In this study, we evaluated p-Akt
and MAPK (p-JNK and p-p38) levels using western blot analysis.
Tat-ATOX1 protein reduced p-Akt and MAPK (p-JNK and p-p38)
expression levels in the STZ-exposed RINm5F cells, suggesting that
transduced Tat-ATOX1 protein protects against cell death through
the regulation of the Akt and MAPK (JNK and p38) signaling
pathways.
STZ is the most frequently used agent to induce
experimental diabetes in animal models, and STZ-induced diabetes is
accompanied by destruction of pancreatic β-cells, loss of insulin
secretion and alterations in blood glucose levels (42,43). To induce diabetes in an animal
model, in this study the mice were intraperitoneally injected with
a single dose of STZ (70 mg/kg). After 8 weeks of STZ treatment, we
observed the protective effects of Tat-ATOX1 protein in the mice
with STZ-induced diabetes. We injected STZ-exposed mice six times
with Tat-ATOX1 protein at weekly intervals. In the Tat-ATOX1
protein-treated group, blood glucose and glycated HbA1C levels were
significantly decreased compared with the levels in the mice with
STZ-induced diabetes which did not receive treatment. Tat-ATOX1
protein also markedly attenuated the loss of body weight induced by
STZ. Additionally, we demonstrated that Tat-ATOX1 protein
effectively transduced into pancreatic β-cells. Moreover,
transduced Tat-ATOX1 protein protected against the destruction of
pancreatic tissues and enhanced the levels of insulin secretion.
Several studies have shown that various antioxidants prevent
STZ-induced pancreatic damage (44–46). In this study, we demonstrated that
Tat-ATOX1 protein exerted protective effects against cell damage
induced by STZ in RINm5F cells as well as in a mouse model of
STZ-induced diabetes, suggesting that Tat-ATOX1 protein has
potential applications as a treatment for oxidative stress-induced
diseases, including DM.
Acknowledgments
The present study was supported by a Priority
Research Centers Program grant (no. NRF-2009–0093812) and in part
by a grant (no. 2012R1A2A1A03006155) from the National Research
Foundation of Korea funded by the Ministry of Science, ICT and
Future Planning in the Republic of Korea, and it was also supported
by a Basic Research Program (no. 2013R1A1A4A01009050) through the
National Research Foundation of Korea funded by the Ministry of
Education (no. 2014R1A1A4A01008026).
References
|
1
|
Global report on diabetes. World Health
Organization; Geneva: 2016
|
|
2
|
Stattin P, Björ O, Ferrari P, Lukanova A,
Lenner P, Lindahl B, Hallmans G and Kaaks R: Prospective study of
hyperglycemia and cancer risk. Diabetes Care. 30:561–567. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Connolly GC, Safadjou S, Chen R, Nduaguba
A, Dunne R, Khorana AA and Hezel AF: Diabetes mellitus is
associated with the presence of metastatic spread at disease
presentation in hepatocellular carcinoma. Cancer Invest.
30:698–702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li YG, Ji DF, Zhong S, Lin TB and Lv ZQ:
Hypoglycemic effect of deoxynojirimycin-polysaccharide on high fat
diet and streptozotocin-induced diabetic mice via regulation of
hepatic glucose metabolism. Chem Biol Interact. 225:70–79. 2015.
View Article : Google Scholar
|
|
5
|
Jin L, Xue HY, Jin LJ, Li SY and Xu YP:
Antioxidant and pancreas-protective effect of aucubin on rats with
streptozotocin-induced diabetes. Eur J Pharmacol. 582:162–167.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim MJ, Kim DW, Lee BR, Shin MJ, Kim YN,
Eom SA, Park BJ, Cho YS, Han KH, Park J, et al: Transduced
Tat-glyoxalase protein attenuates streptozotocin-induced diabetes
in a mouse model. Biochem Biophys Res Commun. 430:294–300. 2013.
View Article : Google Scholar
|
|
7
|
Koroglu P, Senturk GE, Yucel D, Ozakpinar
OB, Uras F and Arbak S: The effect of exogenous oxytocin on
streptozotocin (STZ)-induced diabetic adult rat testes. Peptides.
63:47–54. 2015. View Article : Google Scholar
|
|
8
|
Montes S, Rivera-Mancia S, Diaz-Ruiz A,
Tristan-Lopez L and Rios C: Copper and copper proteins in
Parkinson's disease. Oxid Med Cell Longev. 2014:1472512014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klomp LW, Lin SJ, Yuan DS, Klausner RD,
Culotta VC and Gitlin JD: Identification and functional expression
of HAH1, a novel human gene involved in copper homeostasis. J Biol
Chem. 272:9221–9226. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu YY, Nagpure BV, Wong PT and Bian JS:
Hydrogen sulfide protects SH-SY5Y neuronal cells against
d-galactose induced cell injury by suppression of advanced
glycation end products formation and oxidative stress. Neurochem
Int. 62:603–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai H and Peng F: Knockdown of copper
chaperone antioxidant-1 by RNA interference inhibits
copper-stimulated proliferation of non-small cell lung carcinoma
cells. Oncol Rep. 30:269–275. 2013.PubMed/NCBI
|
|
12
|
Uauy R, Olivares M and Gonzalez M:
Essentiality of copper in humans. Am J Clin Nutr. 67(Suppl):
952S–959S. 1998.PubMed/NCBI
|
|
13
|
Díez M, Arroyo M, Cerdàn FJ, Muñoz M,
Martin MA and Balibrea JL: Serum and tissue trace metal levels in
lung cancer. Oncology. 46:230–234. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ozumi K, Sudhahar V, Kim HW, Chen GF,
Kohno T, Finney L, Vogt S, McKinney RD, Ushio-Fukai M and Fukai T:
Role of copper transport protein antioxidant 1 in angiotensin
II-induced hypertension: a key regulator of extracellular
superoxide dismutase. Hypertension. 60:476–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin SJ and Culotta VC: The ATX1 gene of
Saccharomyces cerevisiae encodes a small metal homeostasis factor
that protects cells against reactive oxygen toxicity. Proc Natl
Acad Sci USA. 92:3784–3788. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelner GS, Lee M, Clark ME, Maciejewski D,
McGrath D, Rabizadeh S, Lyons T, Bredesen D, Jenner P and Maki RA:
The copper transport protein Atox1 promotes neuronal survival. J
Biol Chem. 275:580–584. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Itoh S, Ozumi K, Kim HW, Nakagawa O,
McKinney RD, Folz RJ, Zelko IN, Ushio-Fukai M and Fukai T: Novel
mechanism for regulation of extracellular SOD transcription and
activity by copper: role of antioxidant-1. Free Radic Biol Med.
46:95–104. 2009. View Article : Google Scholar :
|
|
18
|
Dolgova NV, Nokhrin S, Yu CH, George GN
and Dmitriev OY: Copper chaperone Atox1 interacts with the
metal-binding domain of Wilson's disease protein in cisplatin
detoxification. Biochem J. 454:147–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Liu H, Amarsingh GV, Cheung CC,
Hogl S, Narayanan U, Zhang L, McHarg S, Xu J, Gong D, et al:
Diabetic cardiomyopathy is associated with defective myocellular
copper regulation and both defects are rectified by divalent copper
chelation. Cardiovasc Diabetol. 13:100–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wadia JS and Dowdy SF: Protein
transduction technology. Curr Opin Biotechnol. 13:52–56. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dietz GP: Cell-penetrating peptide
technology to deliver chaperones and associated factors in diseases
and basic research. Curr Pharm Biotechnol. 11:167–174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van den Berg A and Dowdy SF: Protein
transduction domain delivery of therapeutic macromolecules. Curr
Opin Biotechnol. 22:888–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kubo E, Fatma N, Akagi Y, Beier DR, Singh
SP and Singh DP: TAT-mediated PRDX6 protein transduction protects
against eye lens epithelial cell death and delays lens opacity. Am
J Physiol Cell Physiol. 294:C842–C855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwon HY, Eum WS, Jang HW, Kang JH, Ryu J,
Ryong Lee B, Jin LH, Park J and Choi SY: Transduction of
Cu,Zn-superoxide dismutase mediated by an HIV-1 Tat protein basic
domain into mammalian cells. FEBS Lett. 485:163–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Embury J, Klein D, Pileggi A, Ribeiro M,
Jayaraman S, Molano RD, Fraker C, Kenyon N, Ricordi C, Inverardi L
and Pastori RL: Proteins linked to a protein transduction domain
efficiently transduce pancreatic islets. Diabetes. 50:1706–1713.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim DW, Lee SH, Jeong MS, Sohn EJ, Kim MJ,
Jeong HJ, An JJ, Jang SH, Won MH and Hwang IK: Transduced Tat-SAG
fusion protein protects against oxidative stress and brain ischemic
insult. Free Radic Biol Med. 48:969–977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shin MJ, Kim DW, Lee YP, Ahn EH, Jo HS,
Kim DS, Kwon OS, Kang TC, Cho YJ, Park J, et al: Tat-glyoxalase
protein inhibits against ischemic neuronal cell damage and
ameliorates ischemic injury. Free Radic Biol Med. 67:195–210. 2014.
View Article : Google Scholar
|
|
28
|
Kim HR, Kim DW, Jo HS, Cho SB, Park JH,
Lee CH, Choi YJ, Yeo EJ, Park SY, Kim ST, et al: Tat-biliverdin
reductase A inhibits inflammatory response by regulation of MAPK
and NF-κB pathways in Raw 264.7 cells and edema mouse model. Mol
Immunol. 63:355–366. 2015. View Article : Google Scholar
|
|
29
|
Kim DW, Lee SH, Ku SK, Cho SH, Cho SW,
Yoon GH, Hwang HS, Park J, Eum WS, Kwon OS and Choi SY: Transduced
PEP-1-FK506BP ameliorates corneal injury in Botulinum toxin
A-induced dry eye mouse model. BMB Rep. 46:124–129. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Youn JK, Kim DW, Kim ST, Park SY, Yeo EJ,
Choi YJ, Lee HR, Kim DS, Cho SW, Han KH, et al: PEP-1-HO-1 prevents
MPTP-induced degeneration of dopaminergic neurons in a Parkinson's
disease mouse model. BMB Rep. 47:569–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon DJ, Bae YS, Ju SM, Youn GS, Choi SY
and Park J: Salicortin suppresses lipopolysaccharide-stimulated
inflammatory responses via blockade of NF-κB and JNK activation in
RAW 264.7 macrophages. BMB Rep. 47:318–323. 2014. View Article : Google Scholar :
|
|
33
|
Ninomiya Y, Cui X, Yasuda T, Wang B, Yu D,
Sekine-Suzuki E and Nenoi M: Arsenite induces premature senescence
via p53/p21 pathway as a result of DNA damage in human malignant
glioblastoma cells. BMB Rep. 47:575–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Im CN and Seo JS: Overexpression of tumor
necrosis factor receptor-associated protein 1 (TRAP1), leads to
mitochondrial aberrations in mouse fibroblast NIH/3T3 cells. BMB
Rep. 47:280–285. 2014. View Article : Google Scholar :
|
|
35
|
Kim YM, Lee SM and Chung HS: Novel AGLP-1
albumin fusion protein as a long-lasting agent for type 2 diabetes.
BMB Rep. 46:606–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Melloul D: Role of NF-kappaB in β-cell
death. Biochem Soc Trans. 36:334–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szkudelski T: The mechanism of alloxan and
streptozotocin action in B cells of the rat pancreas. Physiol Res.
50:537–546. 2001.
|
|
38
|
Mo L, Yang C, Gu M, Zheng D, Lin L, Wang
X, Lan A, Hu F and Feng J: PI3K/Akt signaling pathway-induced heme
oxygenase-1 upregulation mediates the adaptive cytoprotection of
hydrogen peroxide preconditioning against oxidative injury in PC12
cells. Int J Mol Med. 30:314–320. 2012.PubMed/NCBI
|
|
39
|
Angeloni C, Motori E, Fabbri D, Malaguti
M, Leoncini E, Lorenzini A and Hrelia S: H2O2
preconditioning modulates phase II enzymes through p38 MAPK and
PI3K/Akt activation. Am J Physiol Heart Circ Physiol.
300:H2196–H2205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nishimoto T, Matsumoto A, Kihara T, Akaike
A and Sugimoto H: Protective effect of H2O2
involves against subsequent H2O2-induced
cytotoxicity activation of the PI3K-Akt signaling pathway. Cell Mol
Biol (Noisy-le-grand). 56(Suppl): OL1447–OL1452. 2010.
|
|
41
|
Jiang X, Tang X, Zhang P, Liu G and Guo H:
Cyanidin-3-O-β-glucoside protects primary mouse hepatocytes against
high glucose-induced apoptosis by modulating mitochondrial
dysfunction and the PI3K/Akt pathway. Biochem Pharmacol.
90:135–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rakieten N, Rakieten ML and Nadkarni MV:
Studies on the diabetogenic action of streptozotocin (NSC-37917).
Cancer Chemother Rep. 29:91–98. 1963.PubMed/NCBI
|
|
43
|
Junod A, Lambert AE, Stauffacher W and
Renold AE: Diabet-ogenic action of streptozotocin: relationship of
dose to metabolic response. J Clin Invest. 48:2129–2139. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bonnefont-Rousselot D: The role of
antioxidant micronutrients in the prevention of diabetic
complications. Treat Endocrinol. 3:41–52. 2004. View Article : Google Scholar
|
|
45
|
Samarghandian S, Borji A, Delkhosh MB and
Samini F: Safranal treatment improves hyperglycemia, hyperlipidemia
and oxidative stress in streptozotocin-induced diabetic rats. J
Pharm Pharm Sci. 16:352–362. 2013.PubMed/NCBI
|
|
46
|
Pi J, Zhang Q, Fu J, Woods CG, Hou Y,
Corkey BE, Collins S and Andersen ME: ROS signaling, oxidative
stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl
Pharmacol. 244:77–83. 2010. View Article : Google Scholar :
|