Introduction
Renal ischemia-reperfusion (I/R) injury is a major
cause of acute kidney injury (AKI), and can occur in a number of
clinical settings including shock, renal transplantation (1,2),
renal artery angioplasty and contrast agent-induced nephropathy
(3–8). The prognosis for patients with I/R
injury is poor and currently there is no available effective
therapy to counteract this injury. Thus, renal I/R injury is
associated with high morbidity and mortality (9–12).
Due to its complex pathogenesis, a vast body of evidence has
suggested that inflammation plays a critical role in renal injury
following I/R. Nuclear factor-κB (NF-κB) is a pivotal pathway which
activates the inflammatory response during renal I/R injury
(13). Moreover, interleukin
(IL)-1β and tumor necrosis factor (TNF)-α are the most critical
cytokines involved in the inflammatory response in I/R injury
(14–19). Pro-inflammatory cytokines (TNF-α
and IL-1β) activate the inflammatory reaction, resulting in
inflammatory injury (16–19).
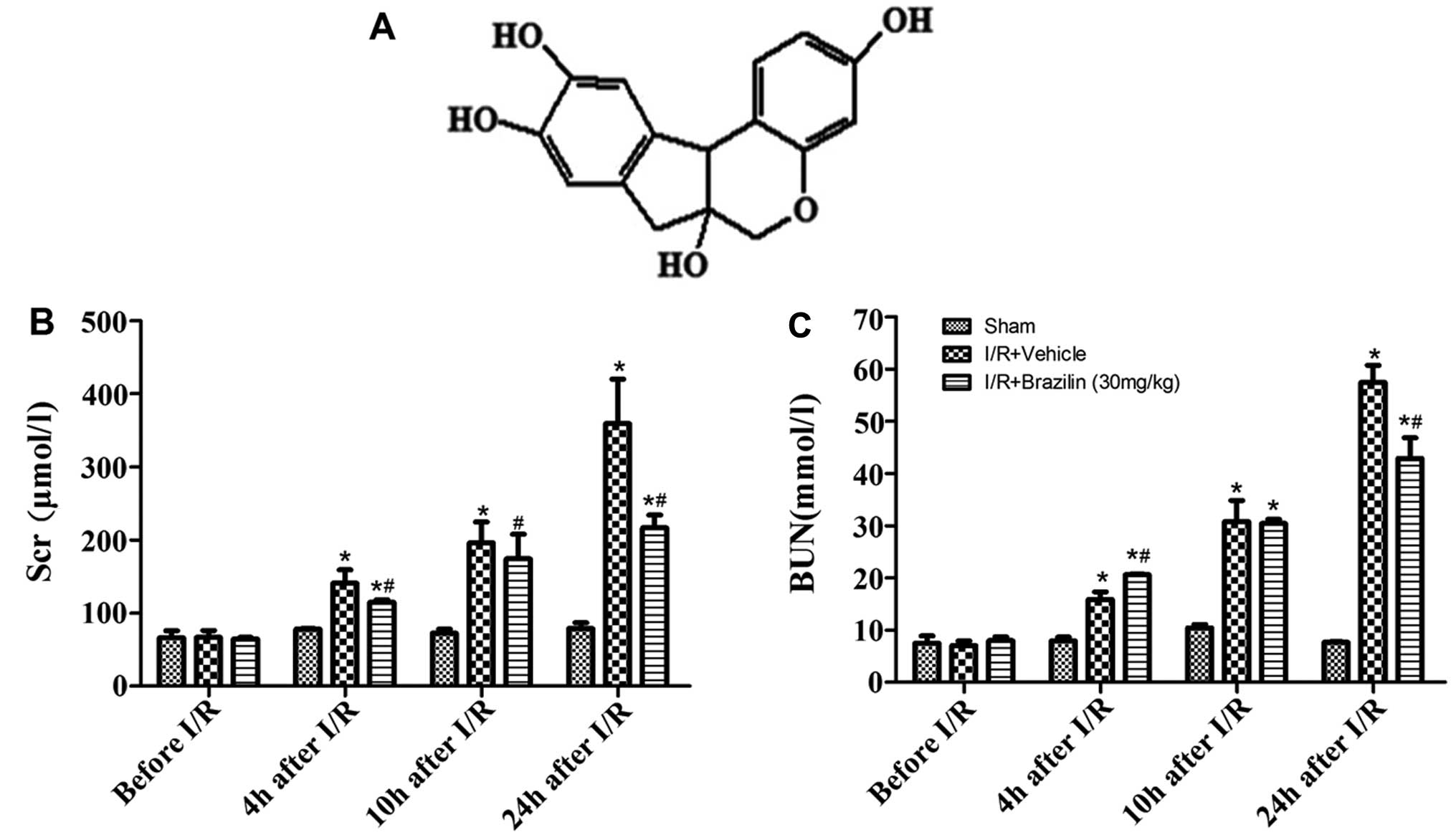
Brazilin, [7,11b-dihydrobenz(b)indeno
[1,2-d]pyran-3,6a, 9,10(6H)-tetrol] (chemical structure shown in
Fig. 1A), isolated from
Caesalpinia sappan L., has been shown to have various
biological activities, such as antioxidant and anti-inflammatory
activities, and has been shown to regulate apoptosis and cell cycle
arrest. Brazilin has been shown to protect rat hepatocytes from
BrCCl3-induced toxicity (20), and to mediate the action of HO-1
(21) and the inducible nitric
oxide synthase (iNOS) gene (22),
inhibiting inflammatory injury. Moreover, brazilin has been shown
to regulate apoptosis and cell cycle arrest by inhibiting the
activity of histone deacetylases (HDACs) (23). It has also been reported that
brazilin prevents various biological activities, such as platelet
aggregation, inflammation, vasorelaxation and apoptosis, and
inhibits vascular smooth muscle cell proliferation and migration
induced through platelet-derived growth factor (PDGF)-BB (24). However, the mechanisms responsible
for the protective effects of brazilin against renal injury remain
unexplored.
In the present study, we aimed to investigate the
potential therapeutic effects of brazilin on I/R-induced renal
injury and to elucidate the underlying mechanisms. We aimed to
determine whether brazilin plays a direct role in the protection
against inflammatory renal injury induced by I/R, as well as
whether the activation of the NF-κB pathway plays a pivotal role in
the inflammatory response during renal I/R injury.
Materials and methods
Materials
Brazilin (purity >98.0%) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (11181-201302; Beijing, China). HK-2 cells were obtained
from the European Collection of Cell Cultures (Salisbury, UK).
Dulbecco's modified Eagle's medium (DMEM) and other cell culture
supplies were purchased from Gibco (Grand Island, NY, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Sigma Chemical Co. (St. Louis, MO, USA). Rabbit
monoclonal antibodies to NF-κB (p65; 9460S), and rabbit polyclonal
antibodies specific for β-actin (4970L) and proliferating cell
nuclear antigen (PCNA; 8580S) and IκBα (4088S), were the products
of Cell Signaling Technology, Inc. (Beverly, MA, USA). All
materials for sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) were obtained from Bio-Rad Laboratories,
Inc. (Hercules, CA, USA).
Adult Sprague-Dawley rats (purchased from the
Department of Laboratory Animal Science, Fourth Military Medical
University, Xi'an, China), weighing 250–300 g, were used in the
present study. All the animal welfare and experimental procedures
were carried out strictly in accordance with the Guide for the Care
and Use of Laboratory Animals (NIH publication no. 85-23, National
Academy Press, Washington, DC, USA, revised 1996). The experimental
procedures involving animals in this study were approved by the
Animal Ethics Committee of Fourth Military Medical University. All
experimental rats were kept in an environmentally controlled
breeding room for 5 days before being used in the experiments and
were fed with standard laboratory food and water.
Rat model of renal I/R injury
Pathogen-free, male Sprague-Dawley rats were fasted
overnight. The rat model of renal I/R injury and the surgical
procedures involved wre similar to those previously described
(25,26). All rats were anesthetized with
sodium chloral hydrate (85 mg/kg intraperitoneally) (Rhone Merieux
Limited, Essex, UK) and placed in a prone position on a warming pad
at 37°C in order to perform the surgical procedures. The
sham-operated rats (group 1) were only subjected to the removal of
the right kidney, whereas the rats in groups 2 to 3 were also
subjected to acute I/R injury to the left kidney which was induced
by clamping the renal artery for 45 min using non-traumatic
vascular clips. The clamp was then removed for reperfusion. Blood
was collected from the eye socket at 4, 10 and 24 h after
reperfusion and the left kidney was removed at 24 h. The rats were
sacrificed by decapitation and exsanguination at 24 h after the I/R
procedure. The kidneys were harvested for analysis.
The rats were randomly and equally divided into 3
groups as follows: group 1 (n=8), the right kidney was extirpated
for the sham-operated animals, but neither clamping nor infusion in
the left kidney were performed; group 2 (n=8), the rats subjected
to renal I/R injury rats were treated with saline as the vehicle;
group 3 (n=8), the rats subjected to renal I/R injury were treated
with brazilin (30 mg/kg, intravenous administration) 0.5 h prior to
I/R injury.
Assessment of renal function before and
after the I/R procedure
Blood was collected using retro-orbital puncture at
4, 10 and 24 h following reperfusion. Serum was separated by
centrifugation at 2,700 × g and at 4°C, and serum creatinine (Scr)
and blood urea nitrogen (BUN) levels were determined by staff at
the Clinical Laboratory of Xijng Hospital, who were blinded to the
treatments given. Blood samples were stored at −80°C for
analysis.
Histological evaluation
Staff at the pathology department of our hospital,
who were blinded to the treatments given, performed the
morphological assessment. Transverse slices of the left kidneys
were fixed in 10% buffered formalin and embedded in paraffin. The
kidney sections were stained with hematoxylin and eosin. One
hundred intersections were examined for each kidney, and a score of
0 to 3 was given for each tubular profile. The scoring method has
been described previously (27)
and was as follows: 0, normal histology; 1, tubular cell swelling,
brush border loss, nuclear condensation, with up to one third of
the tubular profile showing nuclear loss; 2, as for score 1 but
greater than one third and less than two thirds of the tubular
profile showing nuclear loss; and 3, greater than two thirds of the
tubular profile showing nuclear loss. The total score for each
kidney was calculated by the addition of all 100 scores with a
maximum score of 300.
Determination of apoptosis
Renal cell apoptosis was detected by a terminal
deoxynucleotidyltransferase-mediated dUTP nick-end labelling
(TUNEL) assay with tissue paraffin blocks. The renal slides were
incubated with TUNEL reaction mixture in a humidified chamber for
60 min at 37°C in the dark. The renal sections were then rinsed in
phosphate-buffered saline (PBS) 3 times, and the nuclei were
mounted with 4′,6-diamidino-2-phenylindole (DAPI) at a
concentration of 300 nM. Apoptosis was quantified by calculating
the percentage of TUNEL-positive nuclei out of the total nuclei in
an average of 20 high-power fields for each section in a blinded
manner.
Immunofluorescence staining
Immunostaining of the frozen kidney sections from
the rats was performed using rabbit polyclonal anti-IL-1β (ab9722)
and anti-TNF-α (ab9755) antibodies (Abcam, Cambridge, UK). The
primary antibody was detected using Alexa Fluor® 594
donkey and anti-rabbit lgG (H+L) labeled secondary antibodies (Cat.
no. 1256153; Life Technologies, Grand Island, NY, USA), and
incubated for 120 min at room temperature in the dark. DAPI
(Sigma-Aldrich) was used for nuclear staining. Images were captured
using Nikon DS-Qi1Mc camera attached to Nikon Eclipse 90i
fluorescence microscope using an oil immersion objective 60/1.4 NA
by Nikon NIS elements AR version 3.2 software.
Oxygen-glucose deprivation (OGD) and
determination of cell viability
The cells were cultured in DMEM/F12(1:1), and
passaged every 2–3 days in 100-mm dishes supplemented with 10%
fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin and 100
μg/ml streptomycin (Life Technologies). The cells were grown
at 37°C in a humidified 5% CO2 atmosphere. In order to
mimic ischemia-like conditions in vitro, the HK-2 cells were
exposed to ischemia by replacing the medium with an 'ischemic
buffer' (5 mM HEPES, 137 mM NaCl, 4 mM KCl, 1 mM MgCl2
and 1.5 mM CaCl2, pH 7.0). The cells were then incubated
in the hypoxic/ischemic chamber (Billups-Rothenberg, Inc., Del Mar,
CA, USA) at 37°C for 60 min in a humidified atmosphere of 5%
CO2 and 95% N2. Finally, the cells were
incubated again in the culture medium in an incubator with 95% air
and 5% CO2 for an additional 24 h as previously
described (32–35). Prior to exposure to OGD, the HK-2
cells were incubated with brazilin (0.5 mM) of the vehicle for 2 h.
OGD-induced cell death was quantified by MTT assay
(Sigma-Aldrich).
Cell viability assay
Cell viability was measured using the EZ-Cytox Cell
Viability Assay kit (MTT) assay (Itsbio, Seoul, Korea). MTT assay
is based on the cleavage of the tetrazolium salt, MTT, to the
water-insoluble formazan. The formazan dye produced by viable cells
can be quantified by measuring the absorbance of the dye solution
at 460 nm. The HK-2 cells were seeded in 96-well plates
(5×103 cells/well) at 37°C in a 5% CO2
incubator in DMEM/F12. Following overnight incubation, the cells
were incubated for 24 h in the presence or absence of brazilin
(0.2, 0.5 and 1.0 mM) for 2 h prior to exposure to OGD. The final
incubation of the cells with 10 μl of kit reagent was
performed for 45 min at 37°C. The absorbance was measured at 460 nm
using a microplate reader (Bio-Rad Laboratories, Inc.). Cell
viability was calculated and averaged. The cells from the control
group were treated in the same manner without exposure to OGD, and
cell viability was expressed as a percentage of the untreated
controls.
Western blot analysis
The cells were washed with ice-cold PBS and lysed in
ice-cold modified RIPA lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM
NaCl, 50 mM NaF, 0.5% deoxycholic acid, 1% NP-40, 1 mM sodium
orthovanadate and 0.1% SDS). The insoluble material was then
removed by centrifugation at 12,000 × g for 15 min at 4°C. The
protein concentration of each sample was measured using a Bio-Rad
Protein Assay kit (Bio-Rad Laboratories, Inc.). Following
quantification of thye protein concentration, the denatured protein
was separated by SDS-PAGE and then transferred to polyvinylidene
difluoride (PVDF) membranes. After being blocked with 5% (w/v)
non-fat milk at 37°C for 30 min, the membranes were incubated
overnight at 4°C with primary antibodies to NF-κB (p65), IκBα,
β-actin and PCNA. Following 3 washes in TBS-T, the membranes were
incubated for 30 min with a horseradish peroxidase-conjugated
secondary antibody diluted in TBS-T. The blots were visualized
using the enhanced chemiluminescence method, and the bands were
scanned and quantified by densitometric analysis.
Statistical analyses
Data are expressed as the means ± SD and using SPSS
14.0 for Windows (SPSS, Inc., Chicago, IL, USA). Histopathological
scores were analyzed by Kruskal-Wallis nonparametric analysis of
variance followed by Dunn's multiple comparison test. The remaining
data were analyzed by one-way analysis of variance (ANOVA),
followed by a least square difference (LSD) multiple comparison
test. A P-value <0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using GraphPad Prism software version 5.02 (GraphPad Software,
Inc., San Diego, CA, USA).
Results
Brazilin protects against renal I/R
injury
As shown in Fig. 1B
and C, renal I/R injury led to a significant increase in the
levels of Scr and BUN in a time-dependent manner following
reperfusion. Twenty-four hours after renal reperfusion, the rats
subjected to I/R and treated with the vehicle (I/R + vehicle group)
developed significant renal dysfunction indicated by an increase in
Scr (359.25±60.69) and BUN (57.47±3.26) levels. The rats
pre-treated with brazilin rats did not exhibit such a significant
increase in Scr (216.50±17.68) and BUN (42.91±3.96) levels. The
administration of brazilin resulted in a significant decrease in
the Scr and BUN levels compared to the rats administered the
vehicle (Fig. 1B and C).
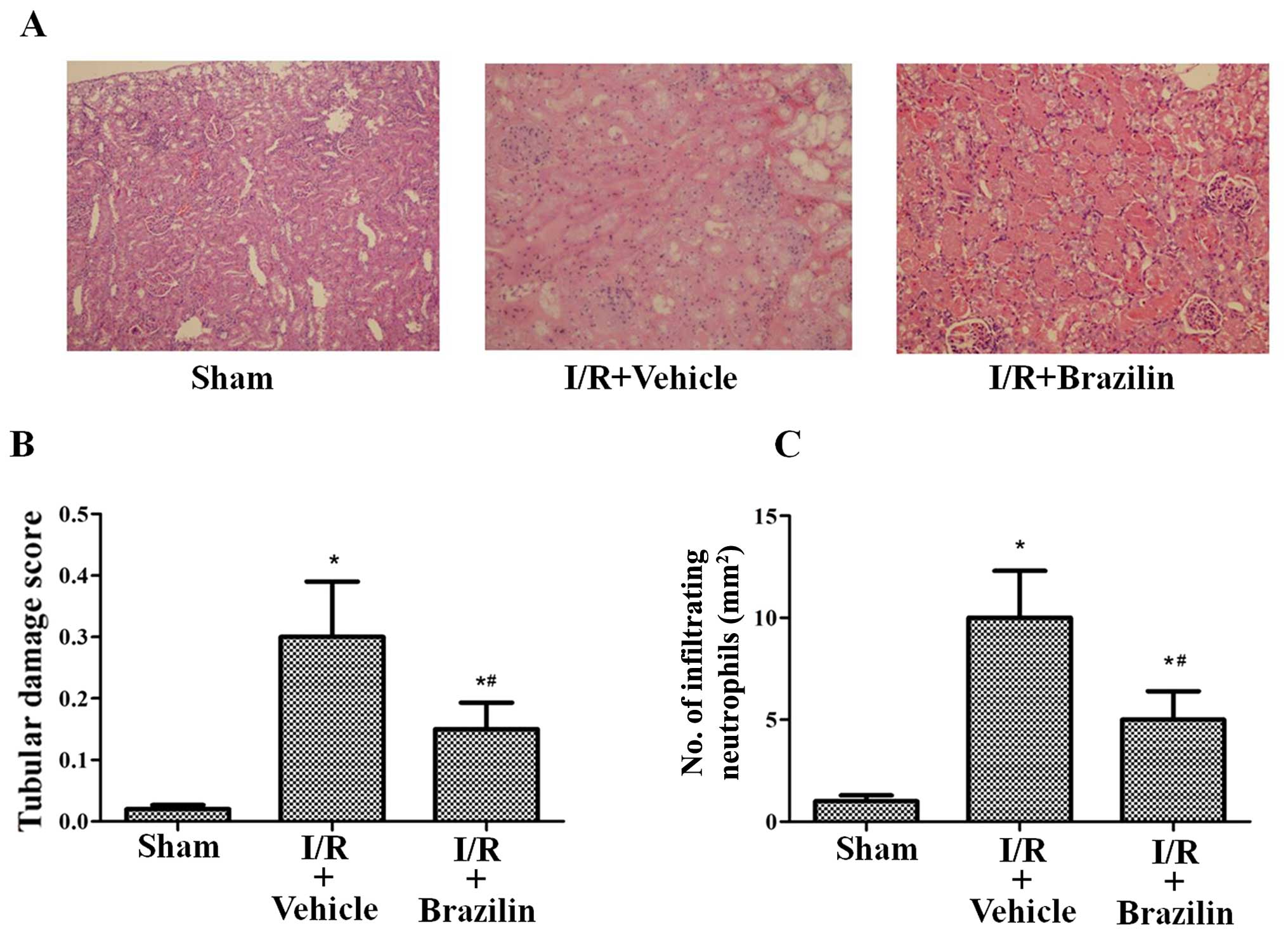
Renal tubular damage in the rats in the I/R +
vehicle group was observed in the outer medulla, as evidenced by
widespread tubular necrosis, luminal congestion and the significant
infiltration of neutrophils at 24 h following reperfusion. However,
pre-treatment with brazilin substantially reduced tubular damage
(Fig. 2A). The rats pre-treated
with brazilin exhibited a markedly lower renal tubular injury score
and neutrophil infiltration (Fig. 2B
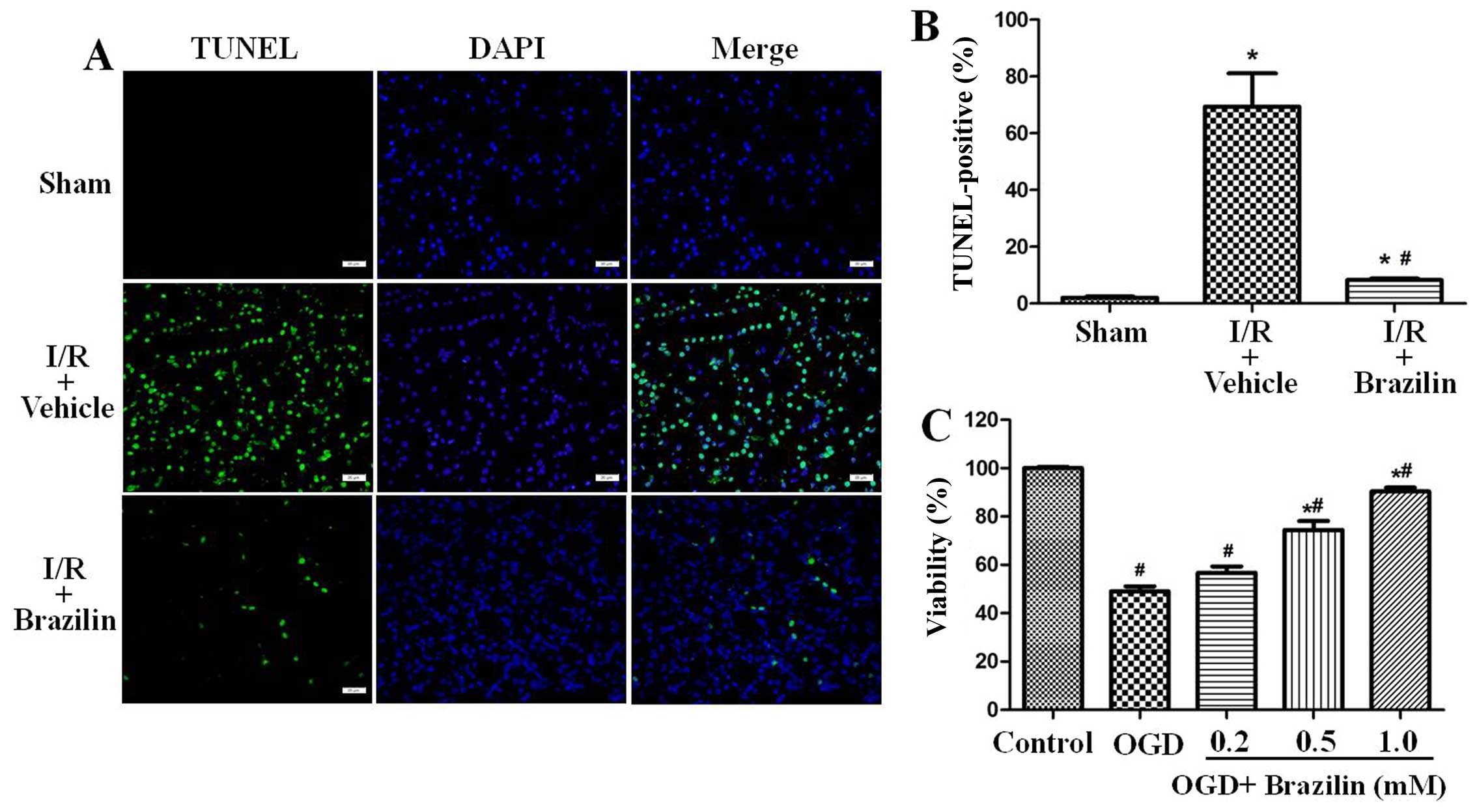
and C). TUNEL staining of the renal sections was then performed
to visualize DNA fragmentation in situ. In the I/R + vehicle
group, TUNEL-positive cells were densely distributed. Pre-treatment
with brazilin significantly reduced amount of TUNEL-positive cells
(Fig. 3A). In the rats
pre-treated with brazilin, the percentage of TUNEL-positive cells
in the renal sections decreased from 69.40±11.74 to 8.25±0.52%
(Fig. 3B).
HK-2 cells were also pre-treated with brazilin for 2
h, and were then exposed to OGD for 60 min in order to mimic
ischemic-like conditions in vitro. The cells were then
incubated again in an incubator with 95% air and 5% CO2
with the vehicle or brazilin for an additional 24 h. Exposure to
OGD resulted in 49.07±2.61 cell death. However, cell viability was
significantly increased to 90.31±1.64% in the cells pre-treated
with brazilin (1 mM) (Fig.
3C).
Brazilin supression the inflammatory
response by inhibiting the activation of NF-κB
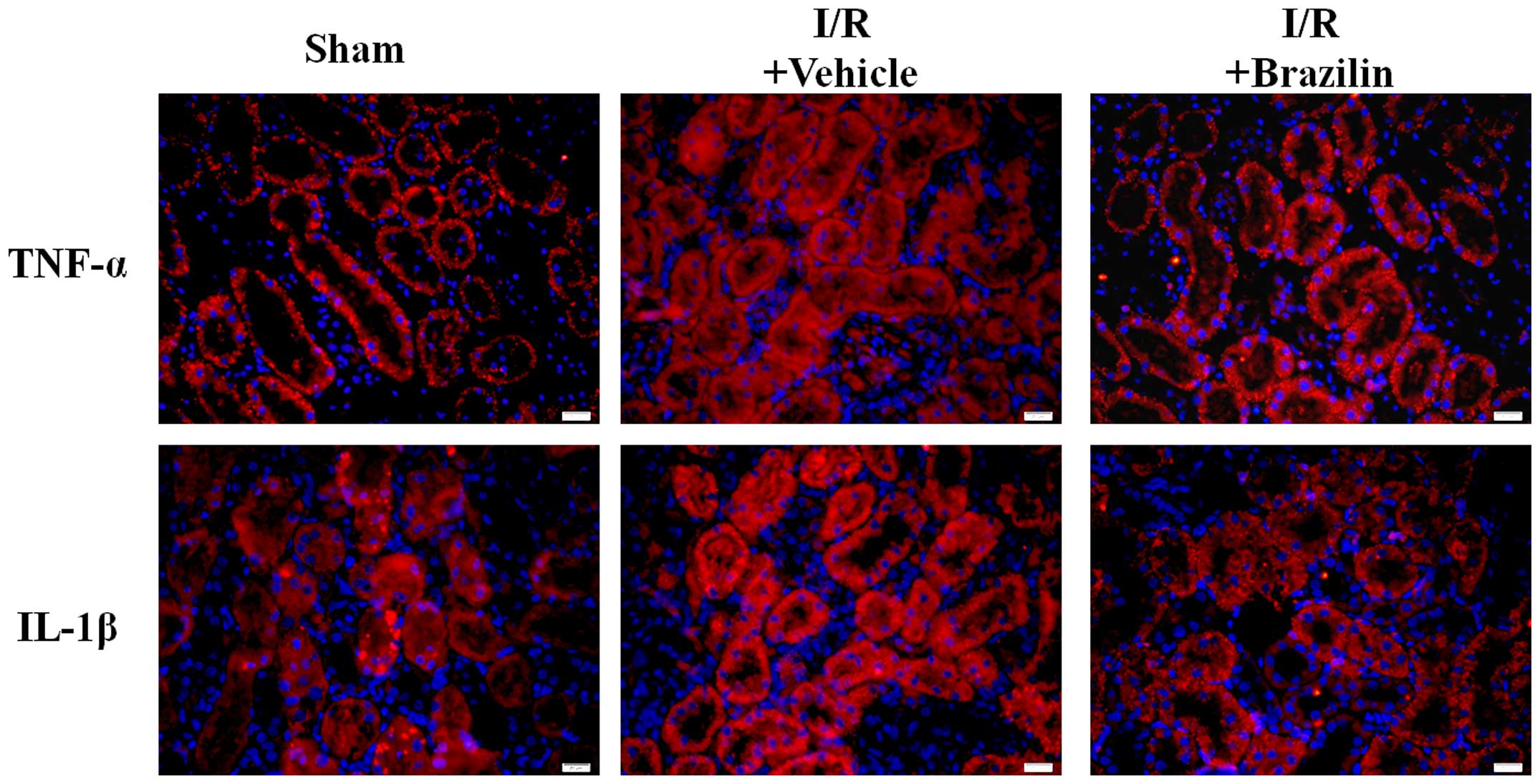
Immunofluorescence staining was performed on
histological sections in order to examine the expression of
inflammatory cytokines. The results revealed that the expression of
TNF-α and IL-1β was enhanced in the I/R + vehicle group. However,
in the renal sections from the rats pre-treated with brazilin (I/R
+ Brazilin group), we observed a marked inhibition of TNF-α and
IL-1β expression (Fig. 4).
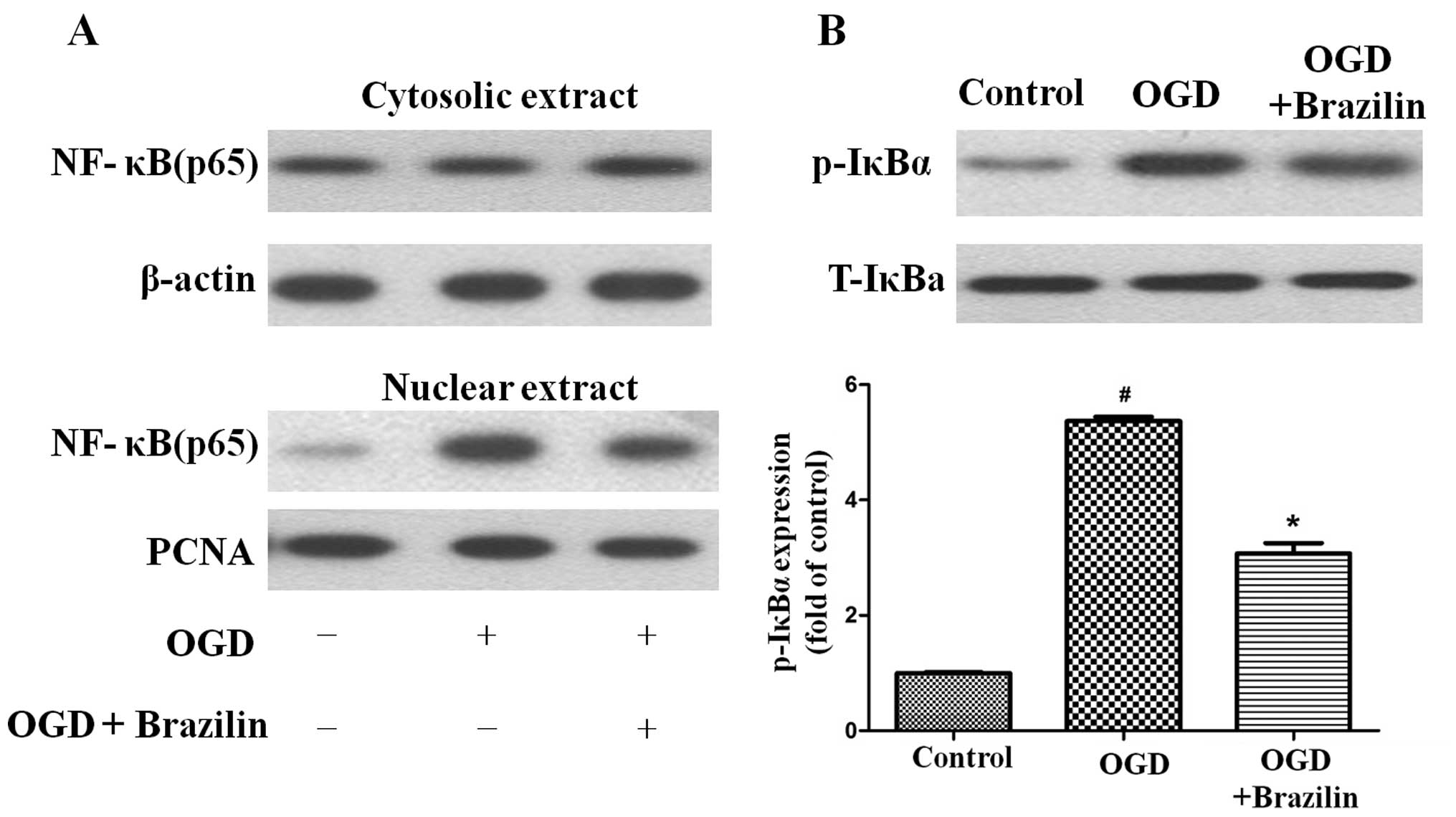
In order to confirm the NF-κB inhibitory activity of
brazilin, we examined the nuclear translocation of the NF-κB p65
subunit in the HK-2 cells. Pre-treatment with brazilin effectively
inhibited the OGD-mediated nuclear translocation of p65 (Fig. 5A). We then examined the
phosphorylation of IκBα. Upon exposure to OGD, the phosphorylation
of IκBα was increased (Fig. 5B).
Pre-treatment of the cells with brazilin significantly inhibited
the phosphorylation of IκBα. Taken together, the present data
demonstrate that brazilin suppresses inflammation by inhibiting the
activation of NF-κB following exposure to OGD.
Discussion
Previous studies have demonstrated that brazilin
protects against hepatic and brain injury (20,28,30) as an antioxidant. However, the
mechanisms responsible for the protective effects of brazilin
against renal injury remain unexplored. In the present study, we
firstly demonstrated that brazilin protected the rats against renal
I/R injury by improving morphology, reducing the levels of
inflammatory markers and preventing apoptosis. It also prevented
the activation of NF-κB. Thus, brazilin exerted anti-inflammatory
effects both in vitro and in vivo.
As already mentioned, renal I/R injury is associated
with high morbidity and mortality, due to the fact that patients
with I/R injury have a poor poor prognosis and there is currently
no effective available therapy to counteract this injury (9–12).
Thus, prevention may be strategy with which to improve the survival
following I/R injury and AKI. In this study, pre-treatment with
brazilin ameliorated renal function by decreasing the levels of Scr
and BUN, and reducing the apoptosis of renal cells. Indeed, the
pathogenesis of I/R injury involves a complex interplay between
biochemical, cellular, vascular endothelial and tissue-specific
factors.
Inflammation is one of the major factors
contributing to renal damage following I/R (29). A number of inflammatory mediators
are involved in renal I/R damage. Of these mediators, TNF-α and
IL-1β are the essential ones, and interact with each other. TNF-α
is invovled in the initial phase of the inflammatory response
induced by I/R (14). It is
capable of inducing the expression of different types of
pro-inflammatory mediators, such as IL-1β (14–19). In this study, we demonstrated that
pre-treatment with brazilin represents a feasable method with which
to reduce inflammation-related injury, as it decreased the levels
IL-1β and TNF-α in renal tissue.
In this study, the protective and anti-inflammatory
effects of brazilin on renal were examined. Furthermore, we
performed experiments in order to elucidate the possible mechanisms
involved. NF-κB is an ubiquitously expressed transcription factor
(13) and plays a significant
role in the inflammatory response (15). Our study focused on the NF-κB
signaling pathway. As is known, in its inhibited form, the NF-κB
dimer is segregated in the cytoplasm bound to an inhibitory protein
known as IκB. The inhibition of NF-κB is inactivated by various
stimuli including I/R, which firstly results in the phosphorylation
of IκB and its subsequent degradation via proteosomes.
Subsequently, NF-κB is released and translocates to the nucleus and
binds to the promoter or enhancer of specific genes, leading to the
activation of those encoding cytokines, and intercellular adhesion
molecules (31). Thus, it can be
perceived that a curb on the NF-κB signaling pathway is bound to be
implicated in the regulation of I/R-induced inflammatory reactions.
In the present study, we demonstrated that the transcription factor
NF-κB was closely involved in inflammation-related injury. This was
manifested by the increased nuclear translocation of NF-κB, and the
enhanced phosphorylation or degradation of IκBα. Taken together,
our data suggest that brazilin exerts a protective effect against
renal I/R injury, and that these effects are mediated by the
inactivation of inflammatory factors. Brazilin inhibited both the
nuclear translocation of NF-κB, and the phosphorylation or
degradation of IκBα. These results indicate that the protective and
anti-inflammatory effects of brazilin against renal I/R injury are
dependent on the inhibition of the NF-κB signaling pathway.
In conclusion, we have demonstrated that
pre-treatment with brazilin at a clinically relevant concentration
(30 mg/kg) reduces the degree of renal I/R injury, preventing
further renal damage. The protective effects of brazilin may be
mediated by its anti-inflammatory activities. The protective and
anti-inflammatory effects of brazilin against renal I/R injury are
dependent on the inhibition of the NF-κB signaling pathway. The
results of this study indicate that brazilin has potential for use
as a drug for the prevention of renal I/R injury and to improve the
outcome of patients with delayed graft function following kidney
transplantation, acute renal failure after major surgery and
trauma, or septic shock.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81201985 and 21372259),
the National Administration of Chinese Traditional Medicine of
Shannxi province (no. 113-JC047) and the China Postdoctoral Science
Foundation Grant (no. 2014M552706).
Abbreviations:
|
I/R
|
ischemia-reperfusion
|
|
Scr
|
serum creatinine
|
|
BUN
|
blood urea nitrogen
|
|
OGD
|
oxygen-glucose deprivation
|
References
|
1
|
Jang HR, Ko GJ, Wasowska BA and Rabb H:
The interaction between ischemia-reperfusion and immune responses
in the kidney. J Mol Med Berl. 87:859–864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sementilli A and Franco M: Renal acute
cellular rejection: correlation between the immunophenotype and
cytokine expression of the inflammatory cells in acute
glomerulitis, arterial intimitis, and tubulointerstitial nephritis.
Transplant Proc. 42:1671–1676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bagshaw SM, Bennett M, Haase M,
Haase-Fielitz A, Egi M, Morimatsu H, D'amico G, Goldsmith D,
Devarajan P and Bellomo R: Plasma and urine neutrophil
gelatinase-associated lipocalin in septic versus non-septic acute
kidney injury in critical illness. Intensive Care Med. 36:452–461.
2010. View Article : Google Scholar
|
|
4
|
Friedericksen DV, Van der Merwe L,
Hattingh TL, Nel DG and Moosa MR: Acute renal failure in the
medical ICU still predictive of high mortality. S Afr Med J.
99:873–875. 2009.
|
|
5
|
Parikh CR, Coca SG, Wang Y, Masoudi FA and
Krumholz HM: Long-term prognosis of acute kidney injury after acute
myocardial infarction. Arch Intern Med. 168:987–995. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mansano AM, Vianna PT, Fabris VE, Silva
LM, Braz LG and Castiglia YM: Prevention of renal
ischemia/reperfusion injury in rats using acetylcysteine after
anesthesia with isoflurane. Acta Cir Bras. 27:340–345. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morgan CJ, Gill PJ, Lam S and Joffe AR:
Peri-operative interventions, but not inflammatory mediators,
increase risk of acute kidney injury after cardiac surgery: a
prospective cohort study. Intensive Care Med. 39:934–941. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimizu S, Saito M, Kinoshita Y, Ohmasa F,
Dimitriadis F, Shomori K, Hayashi A and Satoh K: Nicorandil
ameliorates ischaemia-reperfusion injury in the rat kidney. Br J
Pharmacol. 163:272–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alsabbagh MM, Asmar A, Ejaz NI, Aiyer RK,
Kambhampati G and Ejaz AA: Update on clinical trials for the
prevention of acute kidney injury in patients undergoing cardiac
surgery. Am J Surg. 206:86–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Nardo M, Ficarella A, Ricci Z, Luciano
R, Stoppa F, Picardo S, Picca S, Muraca M and Cogo P: Impact of
severe sepsis on serum and urinary biomarkers of acute kidney
injury in critically ill children: An observational study. Blood
Purif. 35:172–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levy EM, Viscoli CM and Horwitz RI: The
effect of acute renal failure on mortality. A cohort analysis.
JAMA. 275:1489–1494. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santos WJ, Zanetta DM, Pires AC, Lobo SM,
Lima EQ and Burdmann EA: Patients with ischaemic, mixed and
nephrotoxic acute tubular necrosis in the intensive care unit - a
homogeneous population? Crit Care. 10:R682006. View Article : Google Scholar
|
|
13
|
Frantz S, Tillmanns J, Kuhlencordt PJ,
Schmidt I, Adamek A, Dienesch C, Thum T, Gerondakis S, Ertl G and
Bauersachs J: Tissue-specific effects of the nuclear factor kappaB
subunit p50 on myocardial ischemia-reperfusion injury. Am J Pathol.
171:507–512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Majid DS: Tumor necrosis factor-α and
kidney function: Experimental findings in mice. Adv Exp Med Biol.
691:471–480. 2011. View Article : Google Scholar
|
|
15
|
Shahid M, Francis J, Matrougui K and Majid
DS: Involvement of tumor necrosis factor-alpha in natriuretic
response to systemic infusion of nitric oxide synthase inhibitor in
anesthetized mice. Am J Physiol Renal Physiol. 299:F217–F224. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang P, Wu P, Siegel MI, Egan RW and
Billah MM: Interleukin (IL)-10 inhibits nuclear factor kappa B (NF
kappa B) activation in human monocytes. IL-10 and IL-4 suppress
cytokine synthesis by different mechanisms. J Biol Chem.
270:9558–9563. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bean AG, Freiberg RA, Andrade S, Menon S
and Zlotnik A: Interleukin 10 protects mice against staphylococcal
enterotoxin B-induced lethal shock. Infect Immun. 61:4937–4939.
1993.PubMed/NCBI
|
|
18
|
Gérard C, Bruyns C, Marchant A, Abramowicz
D, Vandenabeele P, Delvaux A, Fiers W, Goldman M and Velu T:
Interleukin 10 reduces the release of tumor necrosis factor and
prevents lethality in experimental endotoxemia. J Exp Med.
177:547–550. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fiorentino DF, Zlotnik A, Mosmann TR,
Howard M and O'Garra A: IL-10 inhibits cytokine production by
activated macrophages. J Immunol. 147:3815–3822. 1991.PubMed/NCBI
|
|
20
|
Moon CK, Park KS, Kim SG, Won HS and Chung
JH: Brazilin protects cultured rat hepatocytes from BrCCl3-induced
toxicity. Drug Chem Toxicol. 15:81–91. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu CM, Liu YH, Cheah KP, Li JS, Lam CS, Yu
WY and Choy CS: Heme oxygenase-1 mediates the inhibitory actions of
brazilin in RAW264.7 macrophages stimulated with
lipopolysaccharide. J Ethnopharmacol. 121:79–85. 2009. View Article : Google Scholar
|
|
22
|
Bae IK, Min HY, Han AR, Seo EK and Lee SK:
Suppression of lipopolysaccharide-induced expression of inducible
nitric oxide synthase by brazilin in RAW 264.7 macrophage cells.
Eur J Pharmacol. 513:237–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim B and Kim SH, Jeong SJ, Sohn EJ, Jung
JH, Lee MH and Kim SH: Brazilin induces apoptosis and G2/M arrest
via inactivation of histone deacetylase in multiple myeloma U266
cells. J Agric Food Chem. 60:9882–9889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo J, Li L, Wu YJ, Yan Y, Xu XN, Wang SB,
Yuan TY, Fang LH and Du GH: Inhibitory effects of Brazilin on the
vascular smooth muscle cell proliferation and migration induced by
PDGF-BB. Am J Chin Med. 41:1283–1296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chatterjee PK, Patel NS, Kvale EO,
Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H and Thiemermann C:
Inhibition of inducible nitric oxide synthase reduces renal
ischemia/reperfusion injury. Kidney Int. 61:862–871. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chatterjee PK, Zacharowski K, Cuzzocrea S,
Otto M and Thiemermann C: Inhibitors of poly (ADP-ribose)
synthetase reduce renal ischemia-reperfusion injury in the
anesthetized rat in vivo. FASEB J. 14:641–651. 2000.PubMed/NCBI
|
|
27
|
Sharples EJ, Patel N, Brown P, Stewart K,
Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery
M, et al: Erythropoietin protects the kidney against the injury and
dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol.
15:2115–2124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen J, Zhang HY, Lin H, Su H, Xing DM and
Du LJ: Brazilein protects the brain against focal cerebral ischemia
reperfusion injury correlating to inflammatory response
suppression. Eur J Pharmacol. 558:88–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonventre JV and Weinberg JM: Recent
advances in the pathophysiology of ischemic acute renal failure. J
Am Soc Nephrol. 14:2199–2210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang LT, Chen BL, Wu CT, Huang KH, Chiang
CK and Hwa Liu S: Protective role of AMP-activated protein
kinase-evoked autophagy on an in vitro model of
ischemia/reperfusion-induced renal tubular cell injury. PLoS One.
8:e798142013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nichols TC: NF-kappaB and reperfusion
injury. Drug News Perspect. 17:99–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HT, Kim M, Kim M, Kim N, Billings FT
IV, D'Agati VD and Emala CW Sr: Isoflurane protects against renal
ischemia and reperfusion injury and modulates leukocyte
infiltration in mice. Am J Physiol Renal Physiol. 293:F713–F722.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumar S, Allen DA, Kieswich JE, Patel NS,
Harwood S, Mazzon E, Cuzzocrea S, Raftery MJ, Thiemermann C and
Yaqoob MM: Dexamethasone ameliorates renal ischemia-reperfusion
injury. J Am Soc Nephrol. 20:2412–2425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HT and Emala CW: Preconditioning and
adenosine protect human proximal tubule cells in an in vitro model
of ischemic injury. J Am Soc Nephrol. 13:2753–2761. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie J and Guo Q: Apoptosis antagonizing
transcription factor protects renal tubule cells against oxidative
damage and apoptosis induced by ischemia-reperfusion. J Am Soc
Nephrol. 17:3336–3346. 2006. View Article : Google Scholar : PubMed/NCBI
|