Introduction
Glucocorticoids (GCs) are used in clinical practice
for their anti-inflammatory and immunomodulatory effects (1,2).
However, the therapeutic use of GCs in the management of
immunosuppression following organ transplantation or of
inflammatory diseases is always accompanied by substantial adverse
outcomes including diabetes and obesity as well as bone loss, which
is referred to in this case as GC-induced osteoporosis (GIOP)
(3–5). Bone loss induced by GCs occurs early
and progresses at a fast rate becoming significant within the first
6 months (6). Previous studies
have demonstrated that the long-term administration of GCs resulted
in the development of osteoporosis in approximately 50% of patients
with asthma (7). Moreover,
clinical studies have shown that low-dose GC inhalatory therapy
(8) or high-dose oral GC therapy,
particularly with dexamethasone (DXM) (9) causes bone loss in humans. Briefly,
the underlying molecular mechanisms responsible for GC-induced bone
loss involve a decrease in bone formation directly by inhibiting
osteoblasts from producing new bone and an increase in bone
resorption by increasing osteoclast activity (10,11). In vitro studies
demonstrated that GCs are capable of inducing the apoptosis of
osteoblasts and osteocytes (3,12).
Several studies have also showed that proliferation, osteogenic
differentiation and reactive activity to an osteogenic inductor
were reduced in the bone mesenchymal-derived stem cells (BMSCs) of
rats with GIOP (1,13). Moreover, prolonged GC use may
induce hypercalciuria; the 24-h urinary calcium excretion was
significantly increased at day 7 in a study where patients received
treatment with 10mg methylprednisolone daily (14). Notably, the adminstration of GCs
to patients with vitamin D insufficiency may lead to hypocalcemia
in combination with hypercalciuria and secondary
hyperparathyroidism in the absence of hypomagnesemia (15).
Pomegranate [Punica granatum L. (Punicaceae)]
is an edible fruit native to the Middle East which has been used
extensively in folk medicine for a number of therapeutic purposes
(16,17). It has been reported that
pomegranate possesses powerful antioxidant activity (18,19) and further studies are warranted
into the adjuvant therapeutic applications of pomegranate in human
breast cancer (20). Pomegranate
seeds (PSs) are known to contain the estrogenic compounds, estrone
and estradiol, which are chemically identical to those
biosynthesized in the human body (16,17). As pomegranate seed oil (PSO)
contains phytoestrogens, the hypothesis that PSO is capable of
preventing bone loss in postmenopausal women may be true (17). In vivo studies of
ovariectomized mice showed that the ovariectomy-induced decrease in
bone mineral density (BMD) was normalized by the administration of
pomegranate extract (16).
Moreover, pomegranate and its derivatives prevented bone loss in a
mouse model of postmenopausal osteoporosis through osteoblastic
stimulation and osteoclastic inhibition as well as decreasing
inflammation and oxidative stress (21). However, the effects of PSO in
other experimental animal models, such as the animal model of GIOP,
remain largely unknown.
As mentioned above, previous studies have suggested
that PS extracts exert a protective effect against bone loss.
However, there have been no reports to date in the scientific
literature, to the best of our knowledge, that PS extracts protect
against hypercalciuria. In light of these observations, we aimed to
determine whether PSO may be useful in the treatment of GIOP and
hypercalciuria, and to compare the effectiveness of this compound
with alendronate. Alendronate is a commonly prescribed
bisphosphonate and bisphosphonates are the gold standard treatment
for osteoporosis.
Materials and methods
Animal treatment
Six-week-old male C57BL/6J mice were obtained from
the Shanghai Laboratory Animals Center (SLAC; Shanghai, China) and
were allowed to acclimate to the environment for 1 week. All
experimental procedures were performed in accordance with the
Guidelines of the Department of Orthopaedics at Gongli Hospital of
Pudong New Area (Shanghai, China) on Animal Care. This study was
approved by the Ethics Committee of the Department of Orthopaedics
at Gongli Hospital of Pudong New Area. All chemicals and reagents
were purchased from Sigma-Aldrich Canada Co., (Oakville, Ontario,
Canada), except where noted otherwise.
The mice were randomly divided into four groups: i)
vehicle group mice (n=8) were injected intramuscularly with saline
instead of DXM as controls; ii) the mice in the DXM group were
injected intramuscularly with 5 mg/kg body weight DXM three times a
week for 12 weeks (n=8); iii) the mice in the ALN group received
alendronate orally at a dose of 0.1 mg/kg/day in combination with
DXM for 12 weeks (n=8); and iv) the mice in the PS group received
daily drinks of aqueous extract of pomegranate seed (AE-PS) in
combination with DXM for 12 weeks (n=8). Pomegranate seeds (100 g)
were chopped in a mortar and extracted in water (1,000 ml) by means
of agitation for 10 min at room temperature. Supernatants were
filtered out and the filtrates were given to the mice daily as
drinking water. The pomegranates were purchased from the Chinese
herbal medicine market in Nanjing (Jiangsu, China).
Chemistry of serum, urine and bone
calcium content
The concentrations of calcium (Ca) and testosterone
in the serum as well as creatinine (Cr) in the urine were measured
by ELISA using a microplate reader (BioTek, Winooski, VT, USA).
Serum was collected by cardiac exsanguination under light ether
anesthesia. Urine (24 h) was collected from the mice using
metabolic cages. The level of urine Ca was corrected by the
concentration of urine Cr. The serum levels of bone-specific
alkaline phosphatase (ALP-b), testosterone, tartrate-resistant acid
phosphatase (TRAP)-5b, C-terminal telopeptide of type I collagen
(CTX) and osteocalcin were detected using a rat bioactive
parathyroid hormone (PTH) enzyme-linked immunosorbent assay (ELISA;
Immutopics, Inc., San Clemente, CA, USA) with an ELISA reader (MD
SpectraMax M5; Molecular Devices, LLC, Sunnyvale, CA, USA).
The tibias were incinerated at 800°C for 6 h and the
ash weighed. Ten milligrams of bone ash was then dissolved in 1 ml
of 37% HCl and diluted with Millin-Q water. The Ca content was
determined using the same kits as those used for the serum and
urine calcium assays (Biosino, Beijing, China).
Bone histomorphology
Mice were sacrificed by cardiac exsanguination under
light ether anesthesia. After cardiac exsanguination, the tibias
were collected with all soft tissue removed and stored at −80°C. A
total of 8 tibias were used for histomorphological analysis. The
right tibia was used for histomorphological analysis, and the left
tibia was used for the RT-PCR and western blot analysis (n=8 in
each group). The tibias were decalcified in 0.5 M
ethylenediaminetetraacetic acid (EDTA) (pH 8.0), followed by
dehydration through a graded series of ethanol solutions and then
embedded in paraffin according to standard histological procedures
(22). Sections, 5 µm in
thickness, were cut and stained with hematoxylin and eosin
(H&E; Xinfan, Shanghai, China), and visualized under a
microscope (Leica DM 2500; Leica Microsystems GmbH, Wetzlar,
Germany).
TRAP staining was used for the identification of
osteoclasts according to the manufacturer's instructions (387-A;
Sigma-Aldrich, St. Louis, MO, USA). The number of TRAP-positive
osteoclasts was counted to quantify the number of osteoclasts below
the growth plates in the distal metaphysis of the femur. The assays
were scored by averaging the number of osteoclasts per low-power
field in at least four fields per slide, and visualized under a
microscope (Leica DM 2500; Leica Microsystems GmbH).
Reverse transcription-polymerase chain
reaction (RT-PCR)
After cardiac exsanguination, the kidneys were
collected with all soft tissue removed and stored at −80°C. The
kidneys of each animal were crushed under liquid nitrogen
conditions and RNA extraction was performed using TRIzol according
to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA).
The synthesis of cDNAs was performed by reverse transcription
reactions with 1 µg of total RNA using Moloney murine
leukemia virus reverse transcriptase (Invitrogen) with oligo(dT) 15
primers (Fermentas, Vilnius, Lithuania) as described by the
manufacturer's instructions. The first strand cDNAs served as the
template for the regular PCR performed using a Real-Time PCR System
(ABI 7300; Applied Biosystems Life Technologies, Foster City, CA,
USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as
an internal control to normalize the data to determine the relative
expression of the target genes. The reaction conditions were set
according to the kit instructions. PCR was performed with the
following primers: calcium sensing receptor (CaSR), forward,
5′-TAGGCATTCTGTAATAGCT-3′ and reverse, 5′-CTGGGCTGCTCAGTCGG-3′. The
PCR conditions were as follows: denaturation of cDNA at 95°C for 3
min, denaturation at 95°C for 15 sec; annealing 56°C for 20 sec;
extension at 72°C for 20 sec. The amplification cycle number was 40
for the target gene.
Western blot analysis
The tibias, kidney and duodenum were homogenized and
extracted in NP-40 buffer, followed by 5–10 min of boiling and
centrifugation (10,000 × g, at 4°C) in order to obtain the
supernatant. The samples containing 50 µg of protein were
separated on 10% SDS-PAGE gel, transferred to nitrocellulose
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). After
saturation with 5% (w/v) non-fat dry milk in Tris-buffered saline
(TBS) and 0.1% (w/v) Tween-20 (TBST), the membranes were incubated
with the following antibodies: transient receptor potential
vanilloid (TRPV)5 (sc-398345; 1:500), TRPV6 (sc-28763; 1:500),
osteoprotegerin (OPG; sc-8468; 1:1,000) and receptor activator of
nuclear factor-κB ligand (RANKL; sc-7628; 1:2,000), CaSR (sc-32181;
1:500), calbindin-D9k (CaBP-9k; sc-367381; 1:500), plasma membrane
Ca2+-ATPase1 (PMCA1; sc-16488; 1:500) (all from Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), overnight at 4°C.
After three washes with TBST, the membranes were incubated with
secondary immunoglobulins (Igs) conjugated to IRDye 800CW Infrared
Dye (LI-COR Biosciences, Lincoln, NE, USA), including donkey
anti-goat IgG and donkey anti-mouse IgG, at a dilution of
1:10,000–1:20,000. After a 1-h incubation at 37°C, the membranes
were washed three times with TBST. The blots were visualized using
the Odyssey Infrared Imaging system (LI-COR Biosciences). Signals
were densitometrically assessed (Odyssey Application Software
version 3.0; LI-COR Biosciences) and normalized to the GAPDH
signals to correct for unequal loading using the mouse monoclonal
anti-GAPDH antibody (AP0063; 1:20,000; Bioworld Technology, Inc.,
St. Louis Park, MN, USA).
Statistical analysis
All data are expressed as the means ± standard error
of mean (SEM). The statistical analyses were performed using the
SPSS 13.0 statistical software package (SPSS Inc., Chicago, IL,
USA). One-way analysis of variance (ANOVA) was used to perform
comparisons among the different groups, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Chemistry of serum, urine and tibias
Following DXM administration for 12 weeks, the
GC-treated animals exhibited significant decreases in serum calcium
(9.02±0.44 mg/dl), bone calcium content (5.38±0.43 mg) and
calcium/bone ash (0.38±0.04) (Fig. 1A
and C). Urine calcium levels were comparable among the four
experimental groups. At 12 weeks, when comparing the urine calcium
levels between the vehicle and the DXM groups, we observed that
calcium secretion in the urine continually increased as the
duration of DXM treatment increased (Fig. 1B). At the end of the treatment
period, the AE-PS and alendronate increased serum calcium levels,
bone calcium content and calcium/bone ash, and hypercalciuria was
markedly inhibited in the mice with GIOP (Fig. 1A–C). There was no significant
difference observed between the two treatment groups. Additionally,
the results showed that the serum testosterone level in the DXM
group was significantly decreased as compared with that of the
control group; however, AE-PS treatment alone, not alendronate, was
capable of reversing DXM-induced testosterone deficiency (Fig. 1D).
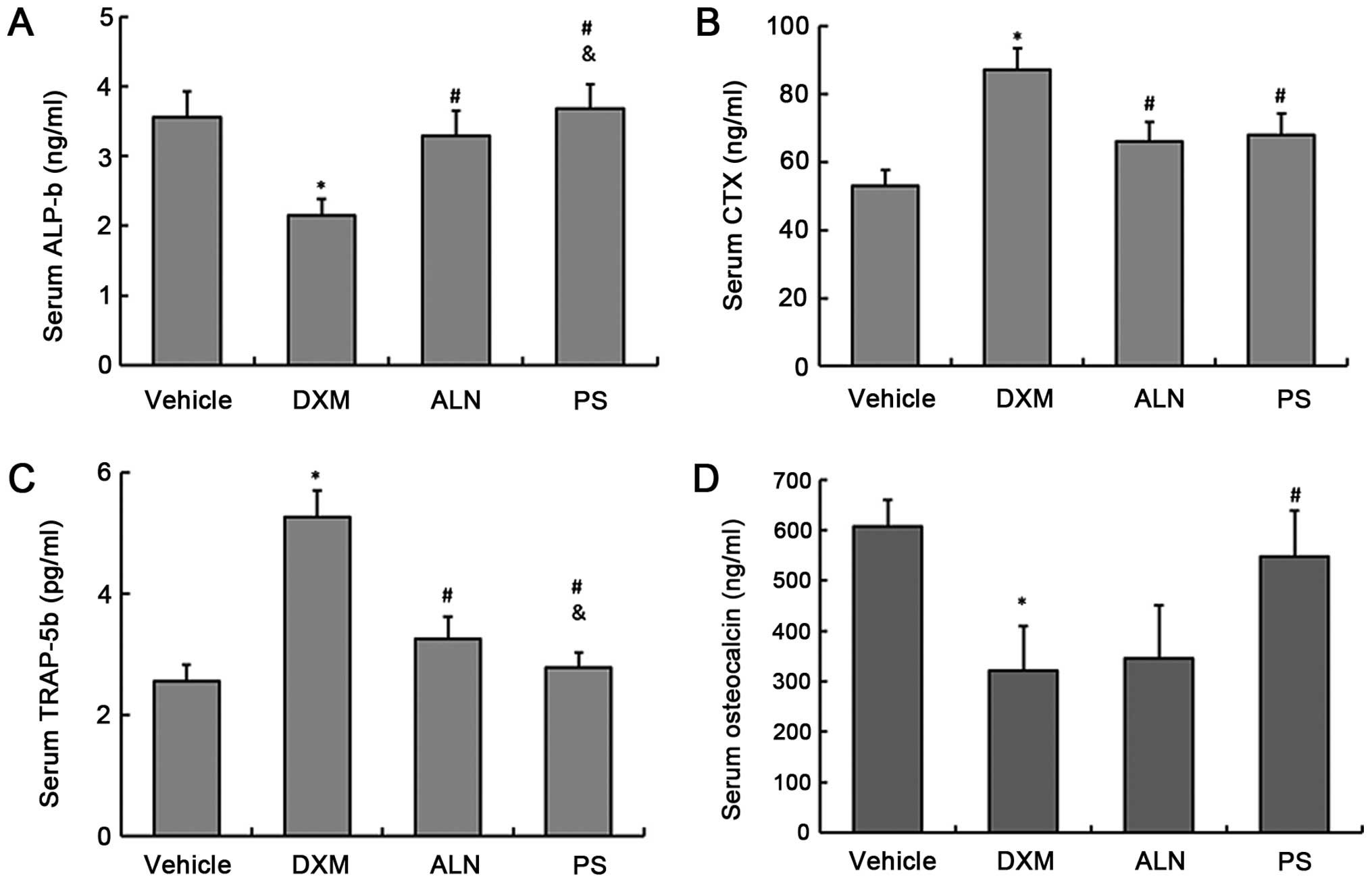
The serum concentrations of the bone turnover
markers, TRAP-5b and CTX as a bone resorption markers and ALP-b and
osteocalcin as a bone formation markers, were determined. The
results showed that the serum levels of TRAP-5b and CTX in the DXM
group were significantly increased, and the serum levels of ALP-b
and osteocalcin were significantly decreased when compared with
those of the control group (Fig.
2A–D). The AE-PS and alendronate significantly decreased the
serum levels of TRAP-5b and CTX, and increased ALP-b and
osteocalcin serum levels. However, ANOVA analysis revealed that the
AE-PS significantly reduced TRAP-5b levels and raised ALP-b levels
when compared with alendronate. There was no significant difference
in the serum CTX levels between the two treatment groups (Fig. 2A–D).
Expression of calcium transport proteins
in the kidney and in the duodenum
To examine the mechanism responsible for the
decreased serum calcium levels and the increased urine calcium
concentrations in the DXM-treated mice, we analyzed the expression
levels of calcium transport proteins in the kidney and in the
duodenum. The expression of two calcium influx channels (TRPV5 and
TRPV6), a reflux channel (PMCA1), and an intracellular calcium
buffering protein (CaBP-9k) in the duodenum and in the kidney was
examined. The protein levels of TRPV5, TRPV6, PMCA1 and CaBP-9k in
the kidney were decreased by DXM treatment; however, the decreases
in TRPV5, TRPV6, PMCA1 and CaBP-9k protein expression in the
kidneys of mice with GIOP were reversed by the AE-PS or alendronate
supplementation (Fig. 3A and B).
Moreover, the protein levels of TRPV6 and CaBP-9k were
significantly decreased by DXM in the duodenum. The AE-PS and
alendronate upregulated the protein expression of TRPV6 and CaBP-9k
in the duodenum as compared with that in the DXM-treated groups
(Fig. 4A and B). There was no
significant difference observed between the two treatment groups.
The mRNA and protein expression of renal CaSR was increased in the
mice with GIOP as compared with the expression levels in the
untreated mice. Notably, treatment with the AE-PS significantly
downregulated the mRNA expression of CaSR as well as the protein
expression of CaSR in the kidneys of mice with GIOP (Fig. 5A–C). The results of western blot
analyses revealed that the AE-PS reversed GIOP more effectively
than alendronate, although both treatments exerted marked
beneficial effects.
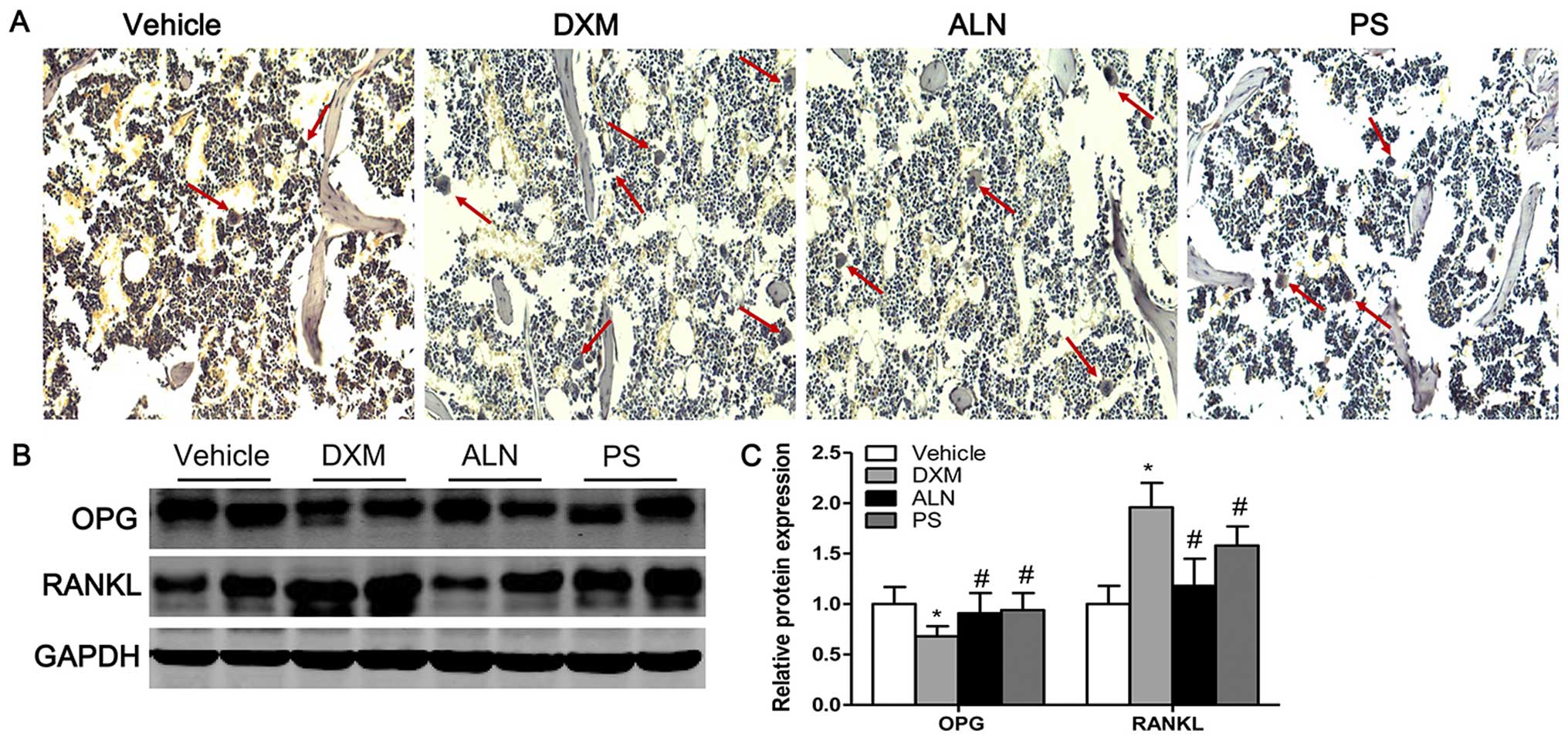
TRAP staining and protein expression of
OPG/RANKL
TRAP staining revealed that very few osteoclasts
were identified in the trabecular bone area below the growth plate
in the vehicle group, whereas the number of osteoclasts were
significantly increased in this area in the DXM group (Fig. 6A). The treatment of the mice with
GIOP with the AE-PS or alendronate significantly decreased the
number of osteoclasts (Fig. 6A).
As the maturation and formation of osteoclasts are mainly regulated
by the balance between extracellular OPG and RANKL levels, the
ratio of OPG/RANKL determines the number of matured osteoclasts.
Consistent with the TRAP staining results, the results of western
blot analysis revealed that the expression of OPG was significantly
decreased whereas the expression of RANKL was significantly
increased in the mice treated with DXM as compared with the control
groups (Fig. 6B and C). The
treatment of the DXM group with the AE-PS or alendronate
significantly elevated the expression of OPG and suppressed the
expression of RANKL (Fig. 6B and
C). There was no marked difference between the two treatment
groups.
Bone histomorphology and protein
expression of calcium transport protein in bone
Histological analysis of trabecular bone in the
proximal metaphysis of the mice was performed using the H&E
stain. The histology of trabecular bone near to the growth plate
was markedly different in the four experimental groups. H&E
staining revealed the increased disconnections and separation
between the growth plate and the trabecular bone network as well as
the reduction in trabecular bone mass of primary and secondary
spongiosa throughout the proximal metaphysis of tibia in the DXM
group. Notably, the AE-PS and alendronate reversed the deleterious
effects on the trabecular bone induced by DXM and stimulated bone
remodeling (Fig. 7A).
Histological staining showed that the AE-PS exerted greater effects
on bone structure compared with alendronate. Notably, the protein
expression of CaSR was increased in the tibias of mice with GIOP
compared with those of the untreated mice; however, AE-PS treatment
significantly downregulated the protein expression of CaSR in the
tibias of the mice with GIOP (Fig. 7B
and C). By contrast, the protein expression of CaBP-9k in the
tibias of mice with GIOP was decreased compared with those of the
untreated mice. The administration of the AE-PS to the mice with
GIOP significantly upregulated the protein expression of CaBP-9k in
the tibias (Fig. 7B and C).
Discussion
An increasing amount of evidence suggests a role for
the extracts of PS or pomegranate juice in the treatment of
postmenopausal bone loss in rodents (16,17,21,23,24). Proof of efficacy, however, in the
treatment of GIOP in comparison with currently available treatments
remains lacking. In order to clarify this issue, we examined the
effects of the AE-PS and compared them with the effects of
alendronate in an experimental mouse model of GIOP.
GCs are the mainstay of treatment of many rheumatic
diseases and are also an important cause of secondary osteoporosis
(25). Rapid loss of BMD with GC
administration is greatest in the first year of therapy and may be
as high as 30% or more in the first 3–6 months depending on the
dose (26). GCs are thought to
directly affect the differentiation and activity of osteoblasts and
osteocytes. In vitro studies have revealed that DEX induces
cytotoxicity and apoptotic cell death in osteoblastic cells and
increases the life span of osteoclastic cells, and the apoptosis of
osteoblasts is demonstrated by the decrease in the sub-G1 cell
population (27,28). In vivo studies demonstrated
that, in contrast to the non-treated rabbits or mice, the
DEX-injected subjects exhibited the typical features of GIOP as
shown by the measurement of the basic biomechanical parameters,
including reductions in BMD, and the increased disconnections and
separation of the trabecular bone network (29,30). It has been found that these
pathological changes in bone metabolism correlate with disturbances
of calcium homeostasis (31). As
expected, DEX significantly upregulated the urine calcium
concentration and downregulated the levels of serum calcium in the
mice. Consequently, this led to marked deterioration of the
trabecular bone at the proximal metaphysis of the tibia as shown by
H&E staining. By contrast, contradictory findings have been
demonstrated in premature infants, and DEX exerted no significant
effect on the urinary excretion of calcium, although DEX treatment
may increase the risk of osteopenia by enhancing phosphate
excretion (32,33).
In the present study, the administration of GC
injections successfully led to deleterious effects on the bone. The
GC-induced increase in bone resorption was confirmed by the
increased serum levels of TRAP-5b and CTX, and the decreased serum
level of ALP-b. Results from previous studies suggested that the
osteoprotective effect of PS or pomegranate juice in the treatment
of postmenopausal bone loss resulted from the suppression of bone
absorption (16,17,21). In ovariectomized mice, the bone
volume and the trabecular number were significantly increased and
the trabecular separation was decreased in the pomegranate-treated
group; some histological bone formation/resorption parameters were
significantly increased by ovariectomy and these were normalized by
administration of the pomegranate extract; these changes suggested
that the pomegranate extract inhibited ovariectomy-induced bone
loss (16).
To elucidate the underlying mechanism involved in
the regulatory effects of the AE-PS on calcium metabolism, the
expression of active calcium transport proteins in the duodenum and
the kidney were measured as it has been demonstrated previously
that the extracellular calcium concentration is regulated by the
concerted actions of the intestine (intestinal absorption), the
kidney (renal re-absorption) and the exchange of calcium to and
from bone (34,35). Active calcium transport in the
duodenum and the kidney occurs in three stages: calcium entry
through epithelial calcium channels (TRPV5 and TRPV6), buffering
and/or transporting by CaBP-9k and CaBP-28k, and extrusion through
PMCA1b (36). Thus, the
expression of the above-mentioned targets was determined in the
present study. The results showed that the protein expression of
TRPV5, TRPV6 and CaBP-9k was significantly decreased in the
duodenum and the kidney, as well as PMCA1 expression in the kidney,
in the mice with GIOP; however, the decrease in the protein
expression in the kidney and duodenum of the mice with GIOP was
reversed by the administration of the AE-PS or alendronate. The
calcium metabolic data clearly demonstrated that the AE-PS or
alendronate exerted protective effects on the maintenance of
calcium homeostasis by regulating calcium absorption in the kidney
and the duodenum of the mice with DXM-induced GIOP. Moreover, the
regulatory effect of the AE-PS on renal CaSR expression was
examined. The downregulation of CaSR in the kidney by the AE-PS may
provide a better explanation for its effective inhibition of the
elevated excretion of urinary calcium in mice with GIOP shown by
the present study. CaSR serves as a sensor of the extracellular
calcium level in different tissues and plays a key role in
regulating the of secretion of calciotropic hormones, such as PTH
and calcitonin (37).
Taken together, the findings of the present study
demonstrated that the AE-PS and alendronate succeeded in
ameliorating DXM-induced bone loss. The AE-PS and alendronate
effectively inhibited the loss of calcium from the urine and
ameliorated the histological damage caused to the bone by DXM. The
underlying molecular mechanisms, at least partially, involve the
regulation of kidney and duodenal calcitropic genes and renal CaSR
expression. According to the comparative study, we found that the
AE-PS ameliorated trabecular bone loss induced by a disrupted
calcium homeostasis due to DXM. In conclusion, these data suggest
that the AE-PS may be used as a novel alternative agent for the
management of secondary osteoporosis. However, further studies are
required in order to determine the precise effect of each
constituent of the AE-PS on bone.
References
|
1
|
Zhou DA, Zheng HX, Wang CW, Shi D and Li
JJ: Influence of glucocorticoids on the osteogenic differentiation
of rat bone marrow-derived mesenchymal stem cells. BMC
Musculoskelet Disord. 15:2392014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scudeletti M, Musselli C, Lanza L, Peirano
L, Puppo F and Indiveri F: The immunological activity of
corticosteroids. Recenti Prog Med. 87:508–515. 1996.In Italian.
PubMed/NCBI
|
|
3
|
Lin H, Wei B, Li G, Zheng J, Sun J, Chu J,
Zeng R and Niu Y: Sulforaphane reverses glucocorticoid-induced
apoptosis in osteoblastic cells through regulation of the Nrf2
pathway. Drug Des Devel Ther. 8:973–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steinbuch M, Youket TE and Cohen S: Oral
glucocorticoid use is associated with an increased risk of
fracture. Osteoporos Int. 15:323–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Staa TP, Laan RF, Barton IP, Cohen S,
Reid DM and Cooper C: Bone density threshold and other predictors
of vertebral fracture in patients receiving oral glucocorticoid
therapy. Arthritis Rheum. 48:3224–3229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bitto A, Polito F, Burnett B, Levy R, Di
Stefano V, Armbruster MA, Marini H, Minutoli L, Altavilla D and
Squadrito F: Protective effect of genistein aglycone on the
development of osteonecrosis of the femoral head and secondary
osteoporosis induced by methylprednisolone in rats. J Endocrinol.
201:321–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reid IR: Glucocorticoid osteoporosis -
mechanisms and management. Eur J Endocrinol. 137:209–217. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Capozzi A, Casa SD, Altieri B and
Pontecorvi A: Chronic low-dose glucocorticoid inhalatory therapy as
a cause of bone loss in a young man: case report. Clin Cases Miner
Bone Metab. 10:199–202. 2013.
|
|
9
|
De Vries F, Bracke M, Leufkens HG, Lammers
JW, Cooper C and Van Staa TP: Fracture risk with intermittent
high-dose oral glucocorticoid therapy. Arthritis Rheum. 56:208–214.
2007. View Article : Google Scholar
|
|
10
|
McIlwain HH: Glucocorticoid-induced
osteoporosis: Pathogenesis, diagnosis, and management. Prev Med.
36:243–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoon HY, Won YY and Chung YS: Poncirin
prevents bone loss in glucocorticoid-induced osteoporosis in vivo
and in vitro. J Bone Miner Metab. 30:509–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jahn K, Lara-Castillo N, Brotto L, Mo CL,
Johnson ML, Brotto M and Bonewald LF: Skeletal muscle secreted
factors prevent glucocorticoid-induced osteocyte apoptosis through
activation of beta-catenin. Eur Cell Mater. 24:197–210. 2012.
|
|
13
|
Ma X, Zhang X, Jia Y, Zu S, Han S, Xiao D,
Sun H and Wang Y: Dexamethasone induces osteogenesis via regulation
of hedgehog signalling molecules in rat mesenchymal stem cells. Int
Orthop. 37:1399–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duzen O, Erkoc R, Begenik H, Soyoral YU
and Aldemir MN: The course of hypercalciuria and related markers of
bone metabolism parameters associated with corticosteroid
treatment. Ren Fail. 34:338–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kinoshita Y, Masuoka K, Miyakoshi S,
Taniguchi S and Takeuchi Y: Vitamin D insufficiency underlies
unexpected hypocalcemia following high dose glucocorticoid therapy.
Bone. 42:226–228. 2008. View Article : Google Scholar
|
|
16
|
Mori-Okamoto J, Otawara-Hamamoto Y, Yamato
H and Yoshimura H: Pomegranate extract improves a depressive state
and bone properties in menopausal syndrome model ovariectomized
mice. J Ethnopharmacol. 92:93–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saravani M, Kazemi Mehrjerdi H, Mirshahi A
and Afkhami Goli A: Protective effects of pomegranate seed oil on
ovariectomized rats as a model of postmenopausal osteoporosis: a
multi-detector computed tomography evaluation. Vet Res Forum.
5:263–267. 2014.
|
|
18
|
Tzulker R, Glazer I, Bar-Ilan I, Holland
D, Aviram M and Amir R: Antioxidant activity, polyphenol content,
and related compounds in different fruit juices and homogenates
prepared from 29 different pomegranate accessions. J Agric Food
Chem. 55:9559–9570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hasnaoui N, Wathelet B and Jiménez-Araujo
A: Valorization of pomegranate peel from 12 cultivars: dietary
fibre composition, antioxidant capacity and functional properties.
Food Chem. 160:196–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim ND, Mehta R, Yu W, Neeman I, Livney T,
Amichay A, Poirier D, Nicholls P, Kirby A, Jiang W, et al:
Chemopreventive and adjuvant therapeutic potential of pomegranate
(Punica granatum) for human breast cancer. Breast Cancer Res Treat.
71:203–217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spilmont M, Léotoing L, Davicco MJ,
Lebecque P, Mercier S, Miot-Noirault E, Pilet P, Rios L, Wittrant Y
and Coxam V: Pomegranate seed oil prevents bone loss in a mice
model of osteoporosis, through osteoblastic stimulation,
osteoclastic inhibition and decreased inflammatory status. J Nutr
Biochem. 24:1840–1848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lou Q-Q, Zhang Y-F, Zhou Z, Shi YL, Ge YN,
Ren DK, Xu HM, Zhao YX, Wei WJ and Qin ZF: Effects of
perfluorooctanesulfonate and perfluorobutanesulfonate on the growth
and sexual development of Xenopus laevis. Ecotoxicology.
22:1133–1144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shuid AN and Mohamed IN: Pomegranate use
to attenuate bone loss in major musculoskeletal diseases: an
evidence-based review. Curr Drug Targets. 14:1565–1578. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spilmont M, Léotoing L, Davicco MJ,
Lebecque P, Mercier S, Miot-Noirault E, Pilet P, Rios L, Wittrant Y
and Coxam V: Pomegranate and its derivatives can improve bone
health through decreased inflammation and oxidative stress in an
animal model of postmenopausal osteoporosis. Eur J Nutr.
53:1155–1164. 2014. View Article : Google Scholar
|
|
25
|
Mok CC, Ho LY and Ma KM: Switching of oral
bisphosphonates to denosumab in chronic glucocorticoid users: a
12-month randomized controlled trial. Bone. 75:222–228. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bitto A, Burnett BP, Polito F, Levy RM,
Marini H, Di Stefano V, Irrera N, Armbruster MA, Minutoli L,
Altavilla D and Squadrito F: Genistein aglycone reverses
glucocorticoid-induced osteoporosis and increases bone breaking
strength in rats: a comparative study with alendronate. Br J
Pharmacol. 156:1287–1295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin H, Gao X, Chen G, Sun J, Chu J, Jing
K, Li P, Zeng R and Wei B: Indole-3-carbinol as inhibitors of
glucocorticoid-induced apoptosis in osteoblastic cells through
blocking ROS-mediated Nrf2 pathway. Biochem Biophys Res Commun.
460:422–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia D, O'Brien CA, Stewart SA, Manolagas
SC and Weinstein RS: Glucocorticoids act directly on osteoclasts to
increase their life span and reduce bone density. Endocrinology.
147:5592–5599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yongtao Z, Kunzheng W, Jingjing Z, Hu S,
Jianqiang K, Ruiyu L and Chunsheng W: Glucocorticoids activate the
local renin-angiotensin system in bone: possible mechanism for
glucocorticoid-induced osteoporosis. Endocrine. 47:598–608. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamura Y, Kawao N, Yano M, Okada K,
Okumoto K, Chiba Y, Matsuo O and Kaji H: Role of plasminogen
activator inhibitor-1 in glucocorticoid-induced diabetes and
osteopenia in mice. Diabetes. 64:2194–2206. 2014. View Article : Google Scholar
|
|
31
|
Zhang Y, Diao TY, Wang L, Che CT and Wong
MS: Protective effects of water fraction of Fructus Ligustri Lucidi
extract against hypercalciuria and trabecular bone deterioration in
experimentally type 1 diabetic mice. J Ethnopharmacol. 158(Pt A):
239–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sonntag J and Gaude M: Effect of
dexamethasone and spironolactone therapy in calcium and phosphate
homeostasis in premature infants with a birth weight under 1,500 g.
Klin Padiatr. 210:354–357. 1998.In German. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin YJ, Yeh TF, Lin HC, Wu JM, Lin CH and
Yu CY: Effects of early postnatal dexamethasone therapy on calcium
homeostasis and bone growth in preterm infants with respiratory
distress syndrome. Acta Paediatr. 87:1061–1065. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoenderop JG, Nilius B and Bindels RJ:
Calcium absorption across epithelia. Physiol Rev. 85:373–422. 2005.
View Article : Google Scholar
|
|
35
|
Alexander RT, Rievaj J and Dimke H:
Paracellular calcium transport across renal and intestinal
epithelia. Biochem Cell Biol. 92:467–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nijenhuis T, Hoenderop JG and Bindels RJ:
TRPV5 and TRPV6 in Ca2+ (re)absorption: regulating
Ca2+ entry at the gate. Pflugers Arch. 451:181–192.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rainone F, Arcidiacono T, Terranegra A,
Aloia A, Dogliotti E, Mingione A, Spotti D, Francucci CM, Soldati L
and Vezzoli G: Calcium sensing receptor and renal mineral ion
transport. J Endocrinol Invest. 34(Suppl): 8–12. 2011.
|