Introduction
Chronic obstructive pulmonary disease (COPD) is
mainly a disease of the lungs; however, systemic involvement is a
characteristic of the disease. The Global Strategy for the
Diagnosis, Managment and Prevention of COPD, by the Global
Initiative for Chronic Obstructive Lung Disease (GOLD) emphasizes
that COPD usually co-exists with other diseases, and that these
comorbidities have a significant effect on the disease
manifestations and prognosis of patients with COPD (1).
Osteoporosis is one of the most important
comorbidities of COPD, and patients with COPD co-presenting with
osteoporosis have a higher risk of developing fractures, and this
significantly increases both the disease burden and morbidity
associated with COPD. It has been shown that the bone mineral
density (BMD) of patients with COPD is lower than that of the
apparently normal healthy population (2,3),
and more importantly, there is a significant correlation between
osteoporosis and the severity of COPD, including emphysema
(4), which suggests that there
may be some common mechanism or combination of mechanisms involved.
Systemic inflammation in COPD is considered to play a role in the
pathogenesis of osteoporosis (5);
however, the underlying cellular and molecular mechanisms remain to
be elucidated.
The bone metabolic regulatory system of
osteoprotegerin (OPG)/receptor activator of nuclear factor-κB
(RANK)/receptor activator of nuclear factor-κB ligand (RANKL) plays
a key role in the balance of bone destruction and formation. RANK,
the receptor of RANKL, is located on the surface of the precursor
cells of osteoclasts and mature osteoclasts (6). RANKL induces the differentiation of
precursor cells into mature osteoclasts, promotes mature osteoclast
activation and suppresses osteoclast apoptosis (7). Mature osteoclasts excrete
hydrochloric acid and proteolytic enzymes, which leads to increased
bone absorption, osteopenia and osteoporosis (8).
In our previous study, we demonstrated that soluble
RANKL (sRANKL) levels and the ratio of sRANKL/OPG in the peripheral
blood of patients with COPD and osteoporosis were higher than those
from patients with COPD without osteoporosis and healthy
non-smokers. In addition, there was a negative correlation between
the level of sRANKL and BMD in patients with COPD (9). A recent study revealed that the
serum OPG level was significantly higher in patients with COPD with
a normal BMD than in those presenting with osteopenia or
osteoporosis; moreover, the serum OPG level independently predicted
hip BMD in patients with COPD (10). These results suggest that
disturbances in the OPG/RANK/RANKL system may be involved in bone
loss in COPD.
Studies on osteoimmunity have found that activated
lymphocytes express RANKL. In chronic inflammation, activated
lymphocytes produce RANKL, which binds to RANK and promotes
osteoclast differentiation and maturity (11). Previously, Kong et al
reported that activated T cells produced two types of RANKL, sRANKL
and membrane-bound RANKL (mRANKL), both of which induce osteoclast
differentiation and maturity (12). Kotake et al found that
activated RANKL-expressing T cells directly induce the
differentiation of peripheral blood mononuclear cells (PBMCs) into
osteoclasts (13). It was also
previously demonstrated that in patients with rheumatoid arthritis
(RA), peripheral CD4+ and CD8+ T cells
expressed higher levels of RANKL as compared to healthy subjects,
and the percentage of RANKL+CD4+ T cells was
higher than that of RANKL+CD8+ T cells
(14). It has also been shown
that among CD4+ T cells, Th17 cells express higher
levels of RANKL than their Th1 and Th2 counterparts (15). In vitro experiments
confirmed that Th17 cells, rather than Th1 and Th2 cells, played
the role of specific T helper cells in bone destruction (15). The study by Kikuta et al
confirmed that RANKL-expressing Th17 cells had immediate contact
with osteoclasts, and induced bone absorption by osteoclasts
(16).
Accumulating evidence has suggested that
CD4+ and CD8+ T cells, and more recently Th17
cells, play an important role in inflammation and emphysema that is
characteristic of COPD (17–19). However, whether these cells
participate in bone absorption, thus linking the disease of the
lungs and the bones in COPD, remains unexplored. Thus, in this
study, using flow cytometry, we analyzed the distribution of
RANKL-expressing T cells (CD4+ T cells, CD8+
T cells and Th17 cells) in the blood of patients with COPD, and the
correlation between RANKL-expressing T cells and the severity of
bone destruction in COPD. Finally, in an in vitro model, we
examined the effects of cigarette smoke extract (CSE) on the
proliferation and differentiation of RANKL-expressing T cells.
Materials and methods
Study subjects
A total of 57 male patients with COPD and 38 male
smokers with normal lung function were recruited in this study. In
addition, 36 male healthy non-smokers were recruited as the normal
controls. Female patients were excluded from this study in order to
avoid the influence of postmenopausal osteoporosis. Informed
consent was signed by each subject prior to enrollment, and the
local research ethics Committee approved this study (TRECKT
2008-14). The baseline characteristics of the study subjects are
summarized in Table I.
 | Table IDemographic characteristics of the
study populations. |
Table I
Demographic characteristics of the
study populations.
| Characteristic | Non-smokers
(n=36) | Smokers (n=38) | COPD patients
(n=57) | P-value among the 3
groups |
|---|
| Age (years) | 74.00±9.49 | 72.44±12.27 | 77.70±10.89 | P=0.059 |
| Smoking index | 0 | 488.33
(325–600) | 698.72
(400–900) | P=0.025 |
| FEV1/FVC% | 80.15±6.39 | 77.99±5.06 | 53.15±11.31 | P<0.001 |
| FEV1% pred | 94.24±13.80 | 90.06±12.81 | 61.93±21.11 | P<0.001 |
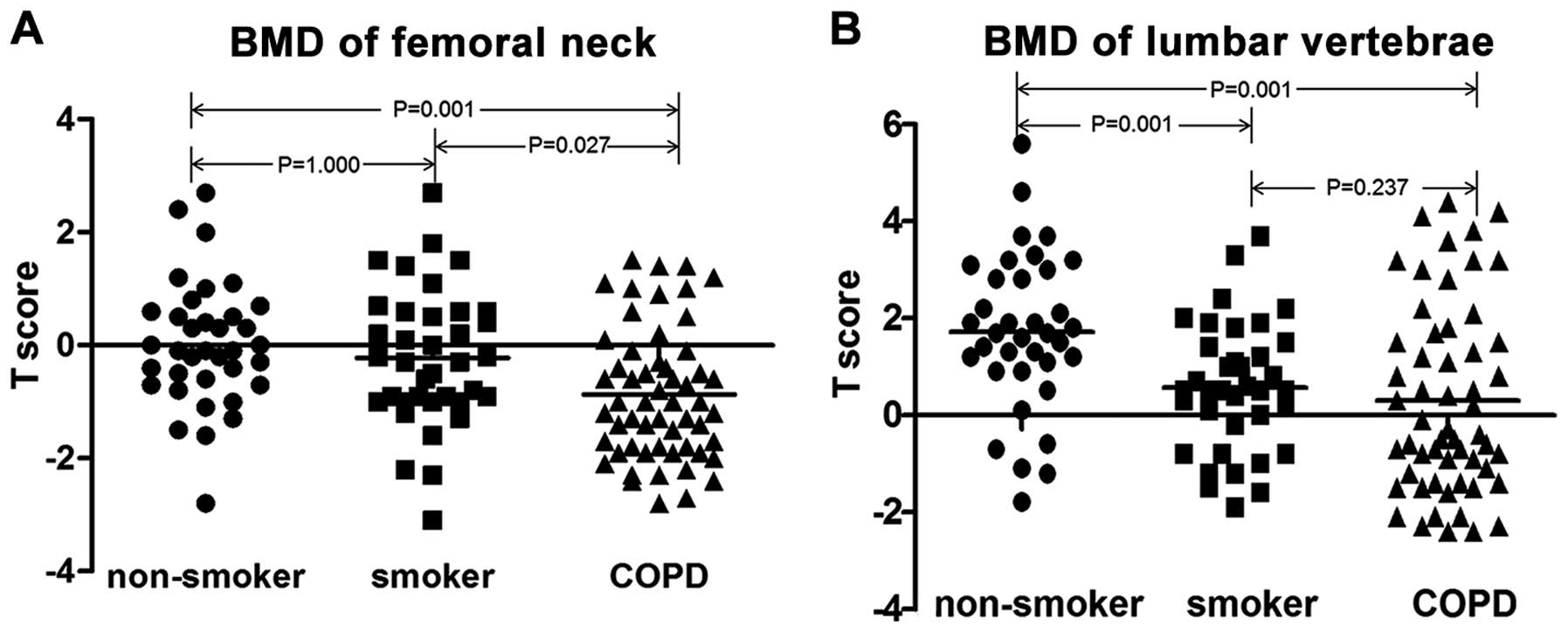
| T score of lumbar
spine | 1.72±1.62 | 0.57±1.33 | 0.30±1.95 | P<0.001 |
| T score of femoral
neck | 0.01±1.10 | −0.22±1.21 | −0.87±1.16 | P=0.001 |
The diagnosis of COPD was carried out in accordance
with the GOLD criteria (20).
Patients with COPD had a FEV1/FVC ratio <70% of the predicted
value post-bronchodilator. All patients with COPD were clinically
stable without deterioration for the preceding 3 months.
Smokers with normal lung functions had a smoking
history with a smoking index ≥200 and a FEV1/FVC ratio >70% and
an FEV1 >80% of the predicted value. The normal controls were
non-smokers with normal lung functions.
The exclusion criteria for the 3 groups were as
follows: i) other chronic pulmonary diseases such as asthma,
bronchiectasis and interstitial lung diseases, among others; ii) a
history of surgery of the lungs or the chest; iii) cancer; iv)
autoimmune diseases; v) use of systemic immunosuppressive agents or
corticosteroid; vi) acute infections such as respiratory and
urinary tract infections; vii) diseases or therapies that can
affect bone metabolism.
Measurement of BMD
BMD of the lumbar spine (L1–L4) and the bilateral
femoral neck was measured by dual X-ray absorptiometry (Lunar
Prodigy, GE Healthcare, London, UK). BMD is reported as a T score,
which represents the number of standard deviations from a young,
gender and ethnic group specific reference mean.
Cell collection
Five milliliters of peripheral blood were collected
in an EDTA-treated tube from each subject and were processed to
measure the PBMCs for flow cytometry. Fifty microliters of blood
were used to measure the absolute count and the subpopulations of T
lymphocytes. The blood samples were then layered onto Ficoll-Paque
Plus (Amersham Biosciences, Bucks, UK), centrifuged (400 × g for 20
min) and the PBMCs were harvested. The cells were washed twice in
phosphate-buffered saline (PBS) at 300 × g for 5 min. The PBMCs
were resuspended at a density of 106 cells/ml in
RPMI-1640 medium (10% fetal bovine serum, 1%
penicillin-streptomycin double-resistant), and stimulated with PMA
at 25 µg/l, and supplemented with ionomycin 1 mg/l,
brefeldin 10 mg/l and Golgi blockers 0.7 µl/ml;
(eBioscience, San Diego, CA, USA), and then cultured for 5 h in an
incubator at CO2 at 37°C.
Flow cytometry
Following incubation, the PBMCs were stained with
anti-CD4-APC (23-3153-02), anti-CD8-FITC (23-2327-04) (both from BD
Biosciences, Oxford, UK) and anti-CD254 (RANKL)-PE (12-6619-82;
e-Bioscience, San Diego, CA, USA) antibodies. Following perforation
of the cytomembrane with a 'Fix and Perm' kit (e-Bioscience),
fluorescence staining with an interleukin (IL)-17 monoclonal
antibody (anti-IL-17-percp; BD Biosciences) was performed as
previously described (21).
Corresponding isotypic controls were used as staining controls (BD
Biosciences and e-Bioscience). We analyzed the original data with a
FACSCalibur (BD Biosciences) flow cytometer using FlowJo software
(Tree Star, Inc., Ashland, OR, USA).
Effect of CSE on RANKL and IL-17
expression in T cells
CSE was prepared as previously described with some
modifications (22). Commercially
filtered cigarettes (Hongtashan; Yuxi Cigarette Factory, Yuxi,
China) which contained 13 mg of tar and 1.2 mg of
nicotine/cigarette were used. CSE was diluted in RPMI-1640 medium
to various concentrations and the final concentrations of the
solution were expressed as percentage values (CSE solution
volume/total volume) ×100. The concentrations of 1, 1.5 and 2% were
selected according to preliminary experimental results. In
addition, another 17 healthy non-smoking volunteers with normal
lung functions were recruited, who did not belong to the 36-person
control group. Thirty milliliters of peripheral blood were
collected in EDTA-treated tubes from each subject. The PBMCs were
isolated from peripheral blood and resuspended in RPMI-1640 medium.
CD4+ and CD8+ T cells were purified using
immunomagnetic beads (Miltenyi Biotech, Bergisch-Gladbach, Germany)
and cultured for 5 days.
The CD4+ and CD8+ T cells from
each volunteer were divided into 4 groups as follows: CSE 0, 1, 1.5
and 2%. CD3/28 antibody (16-0037-85, 16-0289-85; e-Bioscience) was
used in all the groups. Following culture for 5 days, both the
CD4+ and CD8+ T cells were resuspended at a
density of 106 cells/ml in RPMI-1640 medium. The
CD4+ T cells were stained with anti-CD4-APC (23-3153-02;
BD Biosciences) and anti-CD254-PE (12-6619-82; e-Bioscience), and
the CD8+ T cells were stained with anti-CD8-FITC
(23-2327-04; BD Biosciences) and anti-CD254-PE (12-6619-82;
e-Bioscience) antibodies. The expression levels of RANKL on T cells
were analyzed by FlowJo software.
To examine the effects of CSE on IL-17 expression,
CD4+ T cells from each volunteer were divided into 4
groups: CSE 0, 1, 1.5 and 2%. CD3/28 antibody was used in all
groups. Cytokines [transforming growth factor (TGF)-β 3 ng/ml,
IL-1β 10 ng/ml, IL-6 100 ng/ml and IL-23 10 ng/ml; all from
PeproTech, Rocky Hill, NJ, USA] that were required for Th17 cell
differentiation were also added. Following culture for 5 days, the
CD4+ T cells were resuspended at 106 cells/ml
in RPMI-1640 medium and were then stimulated with PMA for 5 h at
37°C in an incubator at CO2. Following incubation, the
CD4+ T cells were stained with anti-CD4-APC, and then
following perforation of the cytomembrane with a 'Fix and Perm'
kit, fluorescence staining with anti-IL-17-PerCP was performed for
each group of CD4+ T cells. The expression levels of
IL-17 in T cells were analyzed by FlowJo software.
Data analysis
Group data are expressed as the means ± SEM or as
the median and interquartile range where appropriate. For data that
was distributed normally, comparisons among 3 groups were made
using one-way ANOVA (P<0.05 was considered significant). If this
test indicated significance, the Bonferroni test was used for
post-hoc analysis for comparison between 2 groups (P<0.05 was
considered significant). For data not distributed normally,
comparisons among groups were made using one-way Kruskal-Wallis
test (P<0.05 was considered significant). If this test indicated
significance, the Mann-Whitney test was used for post-hoc analysis
for comparison between 2 groups (P<0.017 was considered
significant). Correlation analysis was assessed by calculating
Spearman's rank correlation coefficient. A value of P<0.05 was
considered to indicate a statistically significant difference. To
examine the effects of CSE on RANKL and IL-17 expression in T
cells, the differences among the 4 groups of CD4+ T cell
and CD8+ T cell cultures were analyzed using random
block design analysis of variance (an α value of P<0.05 was
considered significant). Statistical analysis was performed using
the SPSS version 16.0 statistical software package.
Results
Demographic characteristics of the study
population
The characteristics of the patients with COPD,
smokers and the healthy control subjects are summarized in Table I. All the subjects were males, and
there were no significant differences among the groups in terms of
age. The patients with COPD exhibited a markedly reduced value of
the predicted FEV1%. In addition, the patients with COPD had
significantly decreased T scores of the lumbar spine and the
femoral neck as compared to the healthy non-smokers (Fig. 1).
RANKL expression levels in T cells
(CD4+ T cells, CD8+ T cells and Th17 cells)
among non-smokers, smokers and patients with COPD
We analyzed the absolute number and the percentage
of total T lymphocytes, CD4+ and CD8+ T cells
in the peripheral blood by flow cytometry (Table II), and by doing so, determined
no significant differences among the 3 groups. Consistent with
previous findings (29), we found
a higher percentage of Th17 cells (IL-17+CD4+
T cell %) in the patients with COPD than in non-smokers
(P<0.001) (Table III).
 | Table IINumber and percentage of peripheral T
lymphocytes and their subpopulations. |
Table II
Number and percentage of peripheral T
lymphocytes and their subpopulations.
| Cell subset | Non-smokers
(n=36) | Smokers (n=38) | COPD patients
(n=57) | P-value among the 3
groups |
|---|
| CD3ab
(cells/µl) | 1105.89±381.42 | 1348.24±490.79 | 1236.41±602.68 | 0.122 |
| CD3 (%) | 68.32±8.68 | 69.68±8.13 | 70.23±10.06 | 0.137 |
| CD4ab
(cells/µl) | 653.35±242.12 | 814.08±330.63 | 723.78±428.91 | 0.139 |
| CD4 (%) | 40.46±8.43 | 41.86±7.69 | 41.46±10.59 | 0.789 |
| CD8ab
(cells/µl) | 383.76±163.08 | 478.08±203.36 | 534.00±496.83 | 0.137 |
| CD8 (%) | 23.70±6.47 | 25.14±8.10 | 25.22±6.89 | 0.588 |
 | Table IIIRANKL+ T cell subsets in
the peripheral blood of the study populations. |
Table III
RANKL+ T cell subsets in
the peripheral blood of the study populations.
| Cell subset | Non-smokers
(n=36) | Smokers (n=38) | COPD patients
(n=57) | P-value among the 3
groups |
|---|
|
RANKL+CD4+ T cell
(%) | 1.04±0.55 | 1.66±1.18 | 1.96±1.25 | 0.003 |
|
RANKL+CD8+ T cell
(%) | 0.91
(0.45–1.14) | 1.17
(0.44–1.62) | 1.55
(0.61–2.16) | 0.006 |
|
RANKL+IL-17+CD4+
T cell (%) | 0.08
(0.04–0.12) | 0.11
(0.04–0.15) | 0.15
(0.06–0.17) | 0.037 |
|
IL-17+CD4+ T cell
(%) | 0.95±0.44 | 1.17±0.53 | 1.5±0.71 | <0.001 |
|
RANKL+Th17 (%) | 8.31±5.11 | 9.61±6.82 | 10.6±7.88 | 0.508 |
We then investigated the percentages of
RANKL+ cells in different T cell subpopulations. As
shown in Figs. 2 and 3 and Table III, the patients with COPD
exhibited a higher percentage of RANKL+CD4+ T
cells (P=0.001) and a higher percentage of
RANKL+CD8+ T cells than the non-smokers
(P=0.002). Importantly, the proportion of CD4+ T cells
positive for both RANKL and IL-17
(RANKL+IL-17+CD4+ T cells)
differed among the 3 groups, and it was higher in the patients with
COPD than in the non-smokers (P=0.010).
To determine whether the increase in the number of
RANKL+IL-17+CD4+ T cells was due
to increased RANKL expression in Th17 cells, we further analyzed
the expression level of RANKL in Th17 cells. However, the
percentage of RANKL-expressing Th17 cells (RANKL+Th17%)
was similar, with no significant differences between the 3 groups
(P=0.508) as shown in Table
III.
Correlation between T cell RANKL
expression and clinical parameters
Next, we investigated whether T cell RANKL
expression correlated with clinical parameters. The percentage of
RANKL+CD4+ T cells inversely correlated with
BMD of the lumbar vertebrae (P=0.01, r=−0.229), and that of the
femoral neck (P<0.001, r=−0.350) (Fig. 4). There was no correlation between
the percentage of RANKL+CD8+ T cells and BMD
(lumbar vertebrae and femoral neck; P=0.335, P=0.057), or between
the percentage of IL-17+RANKL+CD4+
T cells and BMD (lumbar vertebrae and femoral neck; P=0.429,
P=0.188).
There were positive correlations between the smoking
index and the percentage of RANKL+CD4+ T
cells (P<0.001, r=0.483), that of
RANKL+CD8+ T cells (P=0.006, r=0.312), and
that of IL-17+RANKL+CD4+ T cells
(P=0.002, r=0.352) (Fig. 5).
There was a negative correlation between the
percentage of RANKL+CD4+ T cells and FEV1%
(P=0.006, r=−0.242), and between the
RANKL+CD8+ T cells and FEV1% (P=0.047,
r=−0.177) (Fig. 6). There was no
correlation observed between the percentage of
IL-17+RANKL+CD4+ T cells and FEV1%
(P=0.088).
Effect of CSE on RANKL expression in T
cells
We then wished to investigate whether CSE affects
RANKL expression in T cells. For this purpose, we exposed
CD4+ T cells and CD8+ T cells from healthy
volunteers in the absence of CD3/28 antibody to various
concentrations of CSE. Following culture for 5 days, we found that
CSE induced an increase in RANKL expression in the CD4+
T cells, but not in the CD8+ T cells, in a
dose-dependent manner. In line with this finding, CSE also induced
an increase in IL-17 expression in the CD4+ T cells
(Table IV and Figs. 7 and 8).
 | Table IVEffects of CSE on RANKL and IL-17
expression in T cells. |
Table IV
Effects of CSE on RANKL and IL-17
expression in T cells.
| Cell subset | CSE0% | CSE1% | CSE1.5% | CSE2% | P-value among the 4
groups |
|---|
|
RANKL+CD4+ T cell
(%) | 0.87±0.86 | 0.98±0.78 | 1.16±0.93 | 1.17±0.86 | 0.006 |
|
RANKL+CD8+ T cell
(%) | 1.20±1.04 | 1.35±0.81 | 1.80±1.46 | 1.64±1.11 | 0.106 |
|
IL-17+CD4+ T cell
(%) | 0.75±0.56 | 0.90±0.52 | 0.94±0.54 | 1.24±0.57 | <0.001 |
Discussion
Osteoporosis is one of the major comorbidities
associated with COPD. As cigarette smoking is a risk factor for
both lung injury in COPD and osteoporosis (4), it is intriguing to speculate that
the destruction of the lung parenchyma may be associated in some
way with the mechanism of the destruction of bone structure. The
link between them is most likely systemic inflammation, which in
turn results in an imbalance of the bone metabolic regulation
system RANK/RANKL/OPG, which tips the balance towards bone
destruction in COPD (5), as
evidenced in our previous study (9).
Recent studies have demonstrated that the acquired
immunity mediated by CD4+ T cells plays an important
role in COPD (23). In animal
experiments, emphysema that was caused by smoke exposure, depended
on the functional involvement of Th17 cells
(IL-17+CD4+ T cells) and IL-17 (24,25). In patients with COPD, Th17 cells
and IL-17 were shown to be increased in the lungs and
bronchoalveolar lavage fluid (26,27). Of note, Th17 cells have previously
been shown to express high levels of RANKL, and to be actively
involved in the process of bone destruction in experimental
arthritis (12–16).
In this study, we found for the first time, to the
best of our knowledge, that patients with COPD exhibited a higher
percentage of RANKL-positive CD4+ T cells, and more
notably, a higher percentage of RANKL- and IL-17 double-positive
CD4+ T cells
(RANKL+IL-17+CD4+ T cells) in
their blood, as compared to the healthy non-smoking controls.
Importantly, the percentage of RANKL+CD4+ T
cells was associated with BMD of the lumbar vertebrae and the
femoral neck.
To determine whether RANKL expression in
CD4+ T cells is associated with exposure to smoke, we
revealed that the percentage of RANKL+CD4+ T
cells and that of RANKL+IL-17+CD4+
T cells in the peripheral blood positively correlated with the
smoking indexes. In vitro experiments also revealed that
exposure to CSE increased RANKL expression in CD4+ T
cells. We also found an increase in the frequency of Th17 cells,
but not an increase in RANKL expression in these cells in the
peripheral blood of patients with COPD. These data, together with
the effect of CSE on the proliferation of Th17 cells in
vitro, suggested that the increased percentage of
RANKL+IL-17+CD4+ T cells among
CD4+ T cells from patients with COPD was due to
increased numbers of Th17 cells, which may have been as a
consequence of exposure to cigarette smoke. Taken together, these
findings indicate that by expressing RANKL, CD4+ T
cells, and Th17 cells among them, may be involved in the
pathogenesis of osteoporosis, acting as the common mechanistic link
between lung disease and its major comorbidity osteoporosis.
As mentioned above, CD4+ T cells, both
Th1 cells and Th17 cells, are found increased in the airways and
lungs of smokers with COPD. The same may be true in the peripheral
blood in patients with COPD, though only a few studies have
examined circulatory CD4+ T cells. Majori et al
reported an increased percentage of
CD4+IFN-γ+ T cells in the circulation of
patients with COPD (28). In a
recent study, an increase in the frequency of circulatory Th17
cells was also observed in patients with COPD, as in Th1 and
regulatory T cell subsets (29).
Inverse correlations were also found between Th17 cells and FEV1%.
In addition, increases in Th17 cells predicted the presence and
severity of airflow limitations (29). The implications of increased
CD4+ T cells, including Th17 cells in the circulation
still need further study, but it is logical to speculate that these
cells may play an important role in the persistence of systemic
inflammation. The results of the present study provide evidence of
the potential role of these cells in COPD, linking the disease of
the distal lung to systemic comorbidities.
CD8+ T cells have long been believed to
play a central role in both airway inflammation and lung
destruction by producing mediators and enzymes (23). Although the expression of RANKL in
CD8+ T cells has been previously confirmed (30), its implication in bone destruction
remains controversial. This study demonstrated that the percentage
of RANKL+CD8+ T cells was increased in
patients with COPD as compared to healthy non-smokers; however, it
did not correlate with BMD. Although the percentage of
RANKL+CD8+ T cells weakly correlated with the
smoking index, our in vitro experiments failed to show an
effect of CSE exposure on RANKL expression in CD8+ T
cells. This suggests that although CD8+ T cells are key
players in lung disease, they may not be involved in bone disease
in COPD. An earlier study by Choi et al showed that, while
CD4+ T cells induced osteoclast differentiation in the
presence of M-CSF, CD8+ T cells did not. On the
contrary, in the presence of M-CSF and sRANKL, CD8+ T
cells suppressed osteoclastogenesis (30). Apparently, the implication of
peripheral RANKL+CD8+ T cells in patients
with COPD warrants further investigation.
The present study has several limitations. Although
a correlation between RANKL expression in CD4+ T cells
and BMD was observed, the r-values (i.e., correlation index) were
small (−0.229 and −0.35), suggesting that
RANKL+CD4+ T cells may only have a partial
effect on BMD decrease in COPD, and other important mechanisms are
thus likely to be involved. In addition, we failed to reveal a
correlation between an increased number
RANKL+IL-17+CD4+ T cells and BMD,
which may be explained by the relatively small increase in the
number of Th17 cells in COPD, and the very low percentage of
RANKL+IL-17+CD4+ T cells in the
peripheral blood, although this lack of correlation does not
necessarily exclude the potential role of these cells in bone
destruction. Further studies using animal models of COPD with
exposure to cigarette smoke are required in order to formally
demonstrate a role for Th17 cells in bone loss.
In conclusion, this study demonstrated that the
percentages of RANKL+CD4+ T cells and RANKL-
and IL-17 double-positive CD4+ T cells were increased in
the peripheral blood of patients with COPD. The percentage of
RANKL+CD4+ T cells was associated with bone
loss, which provided evidence of the potential role of these
adaptive immune cells in osteoporosis. Further studies on the
common pathways that may lead to lung destruction and bone loss in
both patients and animal models that are exposed to environmental
smoke would perhaps provide more informative insight into the
pathogenesis of COPD and its associated comorbidities.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81170039) and the Natural Science
Foundation of Beijing (no. 7152037).
References
|
1
|
Committee GE: Global strategy for the
diagnosis, management, and prevention of chronic obstructive
pulmonary disease. Revised. 2011.
|
|
2
|
Incalzi RA, Caradonna P, Ranieri P, Basso
S, Fuso L, Pagano F, Ciappi G and Pistelli R: Correlates of
osteoporosis in chronic obstructive pulmonary disease. Respir Med.
94:1079–1084. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katsura H and Kida K: A comparison of bone
mineral density in elderly female patients with COPD and bronchial
asthma. Chest. 122:1949–1955. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bon J, Fuhrman CR, Weissfeld JL, Duncan
SR, Branch RA, Chang CC, Zhang Y, Leader JK, Gur D, Greenspan SL
and Sciurba FC: Radiographic emphysema predicts low bone mineral
density in a tobacco-exposed cohort. Am J Respir Crit Care Med.
183:885–890. 2011. View Article : Google Scholar :
|
|
5
|
Lehouck A, Boonen S, Decramer M and
Janssens W: COPD, bone metabolism, and osteoporosis. Chest.
139:648–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boyce BF and Xing L: The RANKL/RANK/OPG
pathway. Curr Osteoporos Rep. 5:98–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Väänänen HK and Laitala-Leinonen T:
Osteoclast lineage and function. Arch Biochem Biophys. 473:132–138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bai P, Sun Y, Jin J, Hou J, Li R, Zhang Q
and Wang Y: Disturbance of the OPG/RANK/RANKL pathway and systemic
inflammation in COPD patients with emphysema and osteoporosis.
Respir Res. 12:1572011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pobeha P, Petrasova D, Tkacova R and Joppa
P: Circulatory osteoprotegerin is related to osteoporosis of the
hip in patients with COPD. Respir Med. 108:621–627. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rifas L, Arackal S and Weitzmann MN:
Inflammatory T cells rapidly induce differentiation of human bone
marrow stromal cells into mature osteoblasts. J Cell Biochem.
88:650–659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong YY, Feige U, Sarosi I, Bolon B,
Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, et al:
Activated T cells regulate bone loss and joint destruction in
adjuvant arthritis through osteoprotegerin ligand. Nature.
402:304–309. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kotake S, Udagawa N, Hakoda M, Mogi M,
Yano K, Tsuda E, Takahashi K, Furuya T, Ishiyama S, Kim KJ, et al:
Activated human T cells directly induce osteoclastogenesis from
human monocytes: Possible role of T cells in bone destruction in
rheumatoid arthritis patients. Arthritis Rheum. 44:1003–1012. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miranda-Carús ME, Benito-Miguel M, Balsa
A, Cobo-Ibáñez T, Pérez de Ayala C, Pascual-Salcedo D and
Martín-Mola E: Peripheral blood T lymphocytes from patients with
early rheumatoid arthritis express RANKL and interleukin-15 on the
cell surface and promote osteoclastogenesis in autologous
monocytes. Arthritis Rheum. 54:1151–1164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato K, Suematsu A, Okamoto K, Yamaguchi
A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y,
et al: Th17 functions as an osteoclastogenic helper T cell subset
that links T cell activation and bone destruction. J Exp Med.
203:2673–2682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kikuta J, Wada Y, Kowada T, Wang Z,
Sun-Wada GH, Nishiyama I, Mizukami S, Maiya N, Yasuda H, Kumanogoh
A, et al: Dynamic visualization of RANKL and Th17-mediated
osteoclast function. J Clin Invest. 123:866–873. 2013.PubMed/NCBI
|
|
17
|
Sullivan AK, Simonian PL, Falta MT,
Mitchell JD, Cosgrove GP, Brown KK, Kotzin BL, Voelkel NF and
Fontenot AP: Oligoclonal CD4+ T cells in the lungs of
patients with severe emphysema. Am J Respir Crit Care Med.
172:590–596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maeno T, Houghton AM, Quintero PA,
Grumelli S, Owen CA and Shapiro SD: CD8+ T cells are
required for inflammation and destruction in cigarette
smoke-induced emphysema in mice. J Immunol. 178:8090–8096. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doe C, Bafadhel M, Siddiqui S, Desai D,
Mistry V, Rugman P, McCormick M, Woods J, May R, Sleeman MA, et al:
Expression of the T helper 17-associated cytokines IL-17A and
IL-17F in asthma and COPD. Chest. 138:1140–1147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar
|
|
21
|
Hou J, Sun Y, Hao Y, Zhuo J, Liu X, Bai P,
Han J, Zheng X and Zeng H: Imbalance between subpopulations of
regulatory T cells in COPD. Thorax. 68:1131–1139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oltmanns U, Chung KF, Walters M, John M
and Mitchell JA: Cigarette smoke induces IL-8, but inhibits eotaxin
and RANTES release from airway smooth muscle. Respir Res. 6:742005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brusselle GG, Joos GF and Bracke KR: New
insights into the immunology of chronic obstructive pulmonary
disease. Lancet. 378:1015–1026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen K, Pociask DA, McAleer JP, Chan YR,
Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls
JK and Zheng M: IL-17RA is required for CCL2 expression, macrophage
recruitment, and emphysema in response to cigarette smoke. PLoS
One. 6:e203332011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shan M, Yuan X, Song LZ, Roberts L,
Zarinkamar N, Seryshev A, Zhang Y, Hilsenbeck S, Chang SH, Dong C,
et al: Cigarette smoke induction of osteopontin (SPP1) mediates
T(H)17 inflammation in human and experimental emphysema. Sci Transl
Med. 4:117ra92012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eustace A, Smyth LJ, Mitchell L,
Williamson K, Plumb J and Singh D: Identification of cells
expressing IL-17A and IL-17F in the lungs of patients with COPD.
Chest. 139:1089–1100. 2011. View Article : Google Scholar
|
|
27
|
Zhang J, Chu S, Zhong X, Lao Q, He Z and
Liang Y: Increased expression of CD4+IL-17+
cells in the lung tissue of patients with stable chronic
obstructive pulmonary disease (COPD) and smokers. Int
Immunopharmacol. 15:58–66. 2013. View Article : Google Scholar
|
|
28
|
Majori M, Corradi M, Caminati A, Cacciani
G, Bertacco S and Pesci A: Predominant TH1 cytokine pattern in
peripheral blood from subjects with chronic obstructive pulmonary
disease. J Allergy Clin Immunol. 103:458–462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vargas-Rojas MI, Ramírez-Venegas A,
Limón-Camacho L, Ochoa L, Hernández-Zenteno R and Sansores RH:
Increase of Th17 cells in peripheral blood of patients with chronic
obstructive pulmonary disease. Respir Med. 105:1648–1654. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi Y, Woo KM, Ko SH, Lee YJ, Park SJ,
Kim HM and Kwon BS: Osteoclastogenesis is enhanced by activated B
cells but suppressed by activated CD8(+) T cells. Eur J Immunol.
31:2179–2188. 2001. View Article : Google Scholar : PubMed/NCBI
|