Introduction
Glaucoma is the second leading cause of blindness
worldwide, disproportionately affecting women and Asians (1). It is a neurodegenerative disease
characterized by elevated intraocular pressure (IOP), which causes
damage to visual function and optic nerves. Glaucoma filtration
surgery is the most frequent technique applied to reduce IOP in
cases of uncontrolled glaucoma. However, the long-term success is
often impaired by the post-operative wound healing process.
Fibroblasts from Tenon's capsule are involved in the process that
finally leads to the obstruction of the created fistula and
subconjunctival filtration area (2). In recent years, 5-fluorouracil
(5-FU) and mitomycin C (MMC) have been found to reduce scar
formation; however, their applications are limited by severe
side-effects (3). Therefore,
great attention has been paid to the therapy of post-operative scar
formation and it is exigent to find safe and effective therapeutic
strategies.
Transforming growth factor-β (TGF-β) is believed to
be a pivotal mediator, driving both normal wound healing and tissue
fibrosis. There are three TGF-β isoforms in humans: TGF-β1, TGF-β2
and TGF-β3, of which TGF-β2 has been found to be the predominant
isoform in ocular scarring-related diseases, such as conjunctival
scarring and proliferative vitreoretinopathy (4). Protein and mRNA of TGF-β receptors
type I, II and III are expressed in cultured human Tenon's capsule
fibroblasts (HTFs). A significant increase in proliferation has
been detected following exogenous stimulation with TGF-β1 and
TGF-β2 (5).
To reduce the abnormal proliferation of fibroblasts,
previous studies have mostly focused on the inhibition of Smads
(6,7). However, Smads are located upstream
of the TGF-β signaling pathway which contains multitudinous target
genes (8). The complete
inhibition of Smads may lead to poor wound healing (9,10).
Therefore, it has become a critical issue to search for safer and
more effective inhibition targets downstream of the TGF-β signaling
pathway. Recent studies have indicated that integrin-linked kinase
(ILK) is a serine/threonine protein kinase located in focal
adhesions, which regulates cell growth, proliferation, survival,
differentiation, migration and invasion (11,12). In earlier experiments, ILK was
shown to play an important role in mediating epithelial-mesenchymal
transition (EMT) induced by TGF-β1. TGF-β1 induced ILK expression
in renal tubule epithelial cells in a dose-dependent manner, which
was dependent on intracellular Smad signaling. ILK also induced
matrix metalloproteinase-2 (MMP-2) expression and promoted cell
migration and invasion in Matrigel (13). Simultaneously, ILK-deficient
fibroblasts treated with TGF-β1 have been shown to exhibit
decreased Smad2 and 3 phosphorylation, accompanied by the impaired
transcriptional activation of Smad targets, such as α-smooth muscle
actin (α-SMA). ILK-deficient fibroblasts also exhibited
abnormalities in the actin cytoskeleton, indicating a severe
impairment in their capacity to differentiate into myofibroblasts
(14). On the other hand, in the
study of tumors, ILK overexpression, the downregulation of
E-cadherin and the activation of Akt have been observed in primary
colon carcinoma (15). The
overexpression of ILK has also been shown to promote glioma cell
invasion and migration, and to downregulate E-cadherin (16). At the same time, the inhibition of
ILK has been show to induce G1 phase cell cycle arrest and promote
apoptosis in PTEN-negative prostate cancer cells (17). The levels of ILK expression
correlate strongly with tumor invasion and cancer cell
proliferation. Experiments on the effects of ILK in HTFs, however,
have not been reported to date, at least to the best of our
knowledge. ILK may meet the requirements for a novel therapeutic
target and may thus be an important factor in scarring following
glaucoma filtration surgery. RNA interference (RNAi) has been
proven to be one of the most potent, robust, and easy-to-use tools
for the inhibition of gene expression. Short interfering RNAs
(siRNAs) are now used routinely to assess the role of selected
genes in cell-based assays (18).
In this study, HTFs were cultured in vitro
following the delivery of ILK-targeted siRNA by lentiviral vectors.
The proliferation and migration of HTFs induced by TGF-β2 were
investigated. The effects of the silencing of ILK in vitro
on cell cycle progression, and on α-SMA and E-cadherin expression
were examined simultaneously. ILK was shown to have the potential
to influence cell-signaling events linked to fibroblast activation
and differentiation, which provides the basis for further
study.
Materials and methods
Primary cell culture
HTFs were obtained from excised Tenon's capsule
specimens during glaucoma surgery. Written informed consent was
obtained prior to operative excision and this study was approved by
the Research Ethics Committee of Xi'an Jiaotong University, Xi'an,
China. Tenon's capsule tissues (1×5×3 mm) were resected during
surgery and placed in a 60-mm culture dish containing Dulbecco's
modified Eagle's medium (DMEM) supplemented with 15% fetal bovine
serum, 100 U/ml penicillin and streptomycin (HyClone, Logan, UT,
USA). Tissues were cut into small sections (1 mm3) and
were then incubated in 25 cm2 culture bottles at 37°C in
a 5% CO2 environment. The cells were allowed to migrate
from the explant tissue and observed under an inverted phase
contrast microscope (COIC IBE1000; Chongqing COIC Industrial Co.,
Ltd, Chongqing, China). The medium was changed every 2–3 days and
the cells were allowed to reach 80% confluence. Subsequently, the
cells were disaggregated with 0.25% trypsin and 0.02% EDTA at 37°C
for 3 min and were passaged every 3–5 days. Cells that maintained
their proliferative potential and acquired a fibroblast-like
elongated morphology between the third and fifth passage were used
in this study (data not shown). To examine the purity of HTF
cultures, immunofluorescence was staining was performed with
vimentin and keratin. The stained cells were observed under an
immunofluorescence microscope (Leica DMI3000B; Leica Microsystems
GmbH, Wetzlar, Germany).
Construction of ILK-siRNA lentiviral
vectors
The lentivirus expressing siRNA targeting ILK
(GeneChem, Shanghai, China) was constructed to inhibit ILK gene
expression in order to examine the effects of ILK on the
proliferation and migration of HTFs. Green fluorescent protein
(GFP) was used as a reporter gene transferred into the HTFs and the
siRNA (5′-GCC GTAGTGTAATGATTGA-3′) sequence for ILK was designed
using the manufacturer's RNAi designer program, and the negative
control construct (control siRNA) was created using a scrambled
sequence (5′-TTCTCCGAACGTGTCACGT-3′). DNA oligos were chemically
synthesized (GeneChem), annealed and inserted into the expression
vector by double digestion with AgeI and EcoRI (New
England Biolabs, Ipswich, MA, USA), and ligated with T4 DNA ligase
(Takara Biotechnology Co., Ltd., Dalian, China) in accordance with
the manufacturer's instructions. The ligation was transformed into
competent E. coli cells and then confirmed by restriction
enzyme analysis and DNA sequencing. These quences were then cloned
into pGCSIL-GFP to generate lentiviral vectors. The expression
vectors and package vectors were then transfected into 293T cells
(ATCC, Manassas, VA, USA) using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA). Following 48 h of culture, the supernatants
containing the lentiviruses, such as ILK-siRNA-LV and NC-GFP-LV
(negative control) were harvested. Purification was then performed
using ultracentrifugation and the lentiviral titer was
determined.
Lentivirus-mediated RNAi silencing of
ILK
The cells (5×104 cells/well) were seeded
in 6-well cell culture plates the day before transfection. The
lentivirus (1×108 TU/ml, 50 µl) was mixed with
the cells (1×105 cells/well) in serum-free medium, and
the medium was replaced by fresh cell medium containing 15% FBS at
24 h post-transfection. The positive cells were selected by
puromycin (2.5 µg/ml; Sigma-Aldrich, St. Louis, MO, USA),
and then further cultured for 5 days. The cells were divided into 3
groups as follows: i) the normal control group (no LV
transfection); ii) the negative control LV-transfected group; and
iii) the group transfected with ILK-siRNA-LV. The cells were
observed under a fluorescence microscope (Leica DMI3000B; Leica
Microsystems GmbH).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using an RNA
extraction kit (Fastagen Biotech., Shanghai, China) and reverse
transcribed into complementary DNA (cDNA) using the PrimeScript 1st
Strand cDNA Synthesis kit (Takara) following the manufacturer's
instructions. The quality of the RNA samples was controlled by
measuring the absorbance at A260/280; absorptions between 1.8 and
2.1 indicate good quality. qPCR was used to evaluate the knockdown
efficiency of ILK, using SYBR Premix Ex Taq™ II (Tli RNaseH Plus)
following the manufacturer's instructions (Takara Biotechnology
Co., Ltd.). The primers used for PCR were as follows: ILK forward,
5′-CCCAACACAAACACTTCTCTCCTG-3′ and reverse,
5′-AAGCCTGAGGACTGTGGAGTGAT-3′; and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-ATGGTGGTGAAGACGCCAGT-3′.
The program was initially run for 30 sec at 95°C, followed by 40
cycles of 5 sec at 95°C, 20 sec at 60°C and 20 sec at 72°C. The
qPCR analysis used a Bio-Rad IQ5 multicolor detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). A comparative
cycle threshold method was used to determine the relative
quantification of RNA expression. Gene expression levels were
normalized to the GAPDH endogenous control and fold changes were
calculated using the ΔΔCt method. All PCR reactions were performed
at least in triplicate.
MTT assay
The HTFs in the 3 groups were seeded in 96-well cell
culture plates. The cells were then cultured for 48 h in the
presence or absence of TGF-β2 (3 ng/ml). MTT (20 µl) (5
mg/ml in PBS) was added to each well, and the cells were then
incubated at 37°C for 4 h. MTT solution was discarded by gently
inverting the plates, and the wells were filled with 200 µl
DMSO. After the plates were shaken vigorously for 20 min, the
absorbance of each well was read using a plate reader (Epoch™;
Bio-Tek Instruments Inc., Winooski, VT, USA) at 490 nm. Each data
point was obtained as an average of five values from five
wells.
Wound healing assay
The HTFs were seeded in 6-well cell culture plates
at a concentration of 5×105 cells/well and allowed to
grow to 80% confluence. The cells were starved overnight and wounds
were gently made in the center of the cell mono-layer using a
sterile 200 µl pipette tip. To remove the cell debris, the
wells were washed twice with PBS, and then incubated with or
without TGF-β2 (3 ng/ml). Images of marked regions along the wound
area were obtained using an inverted phase contrast microscope
(Leica DMI3000B; Leica Microsystems GmbH) immediately after
creating the wound. The width of the scratch was determined by
images taken every 24 h, employing Adobe Photoshop software.
Cell cycle analysis
The HTFs in the 3 groups were seeded in 25
cm2 culture bottles at a density of 1×106
cells/bottle. The cells were starved overnight and were cultured in
the presence or absence of TGF-β2 (3 ng/ml). Following incubation
at 37°C for 48 h, the cells were collected and the cell suspension
was added into 75% ice-cold ethanol and fixed overnight. The cells
were then washed twice with PBS and re-suspended in 400 µl
of solution containing PI (100 µg/ml) and RNase (0.1 mg/ml)
and then incubated in the dark for 20 to 40 min. Finally, the cell
cycle distribution was detected by flow cytometry, and the cells
were analyzed using a FACSCalibur and FACSort CellQuest software
(BD Biosciences, Franklin Lakes, NJ, USA).
Immunofluorescence staining
The cells (1×105 cells/well) were seeded
on an 8×8 mm cover slip in 24-well plates, starved overnight and
then treated with 3 ng/ml TGF-β2. Following 48 h of treatment, the
cells were fixed with 4% paraformaldehyde for 20 min and washed 3
times with PBS. The cells were permeabilized in PBS with 0.3%
Triton X-100 for 5 min. The cells were then blocked with 5% normal
goat serum for 1 h. Primary rabbit anti-human α-SMA antibody
(1:500, ab5694) and rabbit anti-human E-cadherin antibody (1:1,000,
ab53226) (both from Abcam, Cambridge, MA, USA) were incubated with
the cells overnight at 4°C. After being rinsed extensively, the
cells were exposed to the fluorescent secondary antibody (goat
anti-rabbit Cy3, 1:10,000, CW0114S; goat anti-rabbit FITC,
1:10,000, CW0159S; both from CWBIO, Beijing, China) in the dark for
30 min. The coverslips were rinsed again with PBS 3 times and
mounted with DAPI for 5 min, as a nuclear counterstain. Finally,
the cells were observed under a fluorescence microscope (DP71;
Olympus, Tokyo, Japan).
Western blot analysis
Proteins were separated using SDS-PAGE and
transferred onto a PVDF membrane. The membranes were blocked in
TBST containing 5% skim milk at room temperature for 3 h. Following
this, the membrane was exposed to the primary antibodies (rabbit
anti-human anti-cyclin D1 antibody, 1:1,000; ab134175),
(anti-E-cadherin antibody, 1:1000), (anti-integrin-linked ILK
antibody, 1:1000; ab52480), and (anti-α-SMA antibody, 1:500) (all
from Abcam) in a sealed bag overnight at 4°C and then washed 3
times for 15 min each in TBST. The membranes were then incubated
with secondary antibody (goat polyclonal secondary antibody to
rabbit lgG-HRP, ab6721), diluted at 1:10,0000 in TBST for 2 h at
room temperature. The membranes were washed 3 times in TBST for 20
min each time. A Genshare ECL substrate solution (Genshare,
Shaanxi, China) was placed on the membranes for 2 min.
Subsequently, the membranes were placed in a LAS-3000 FujiFilm
intelligent dark box. The illuminated bands were detected and the
image captured using Image reader LAS-3000 software. Western blot
analysis was performed at least on 3 separate occasions.
Statistical analysis
All the data are presented as the means ± standard
deviation (SD). Statistical analyses were conducted using SPSS
version 18.0 for Windows (SPSS, Inc., Chicago, IL, USA). The
statistical comparisons were performed using a Student's t-test or
ANOVA followed by planned comparisons of multiple conditions. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell morphological observation and
identification of HTFs by immunofluorescence staining
The cells migrated out from the explant tissue after
7 to 10 days of adherent culture. Microscopically, the cells
exhibited a fusiform appearance with clear outlines, and the
cellular plasma was abundant and bright, and the size uniform
(Fig. 1A). The cells were
passaged every 3–5 days. Following subculture, the cells had spread
much more and were arranged in fasciculus or swirling patterns
(Fig. 1B). There were no
differences observed between the recovered cells and those before
cryopreservation as regards morphology and growth characteristics.
The results revealed that the cells were positive for the
expression of vimentin, and the specific fluorescence could be
observed within the cytoplasm (Fig.
1C and D). The cells were negative for the expression of
keratin (Fig. 1E and F).
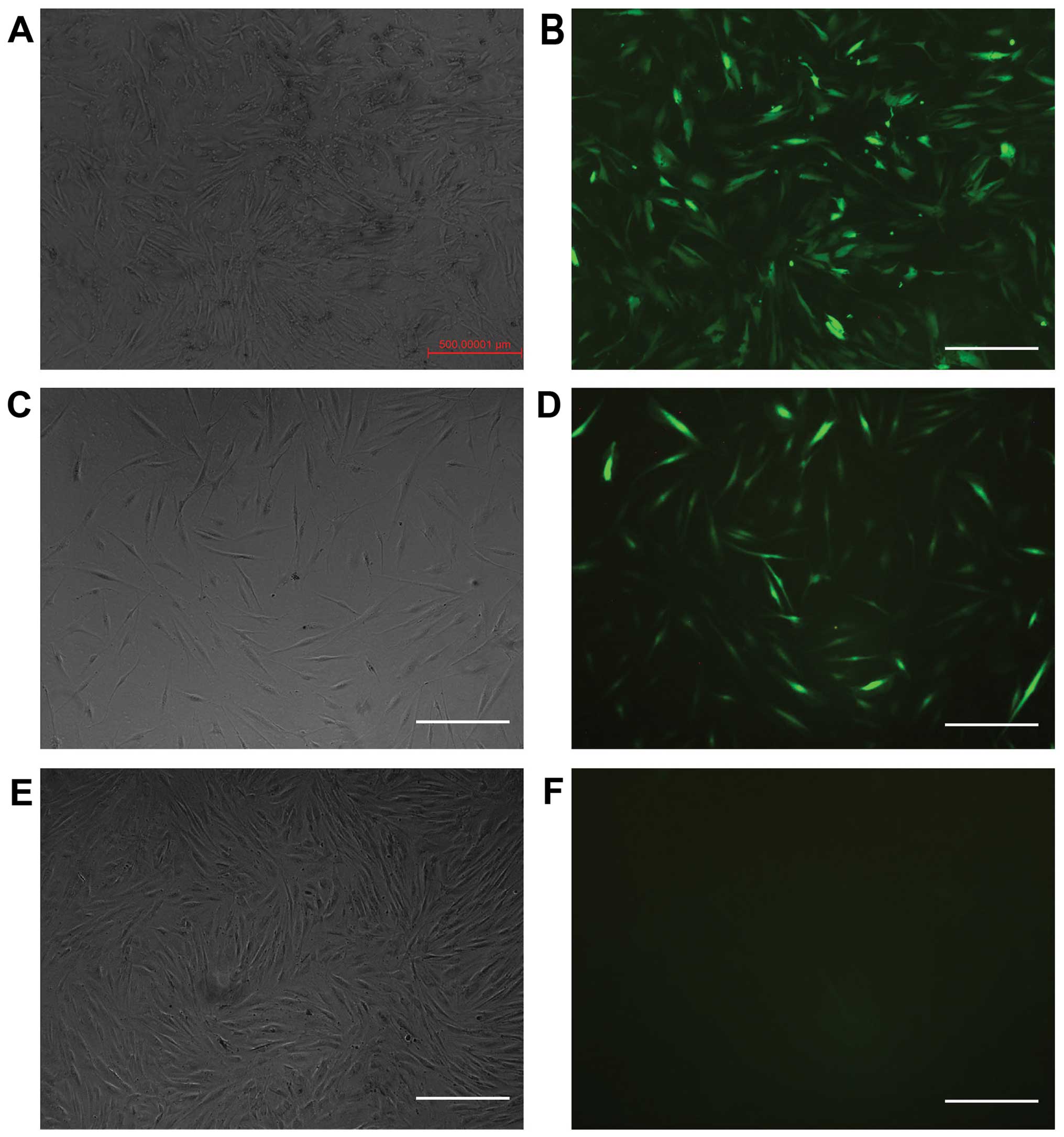
RNAi can be used to inhibit ILK
expression in HTFs
The cells were transfected with different levels of
viral titer. When the multiplicity of infection (MOI) was 50, the
transfection efficiency was the highest. The positive rate was 95%
following selection with puromycin. The expression of GFP was
positive in the ILK-siRNA-LV-transfected group (Fig. 2A and B) and in the negative
control LV-transfected group (Fig. 2C
and D) on the 4th day after transfection, but it was negative
in the normal control group (Fig. 2E
and F). The results of RT-qPCR revealed that ILK mRNA
expression was significantly decreased in the
ILK-siRNA-LV-transfected group (Fig.
3).
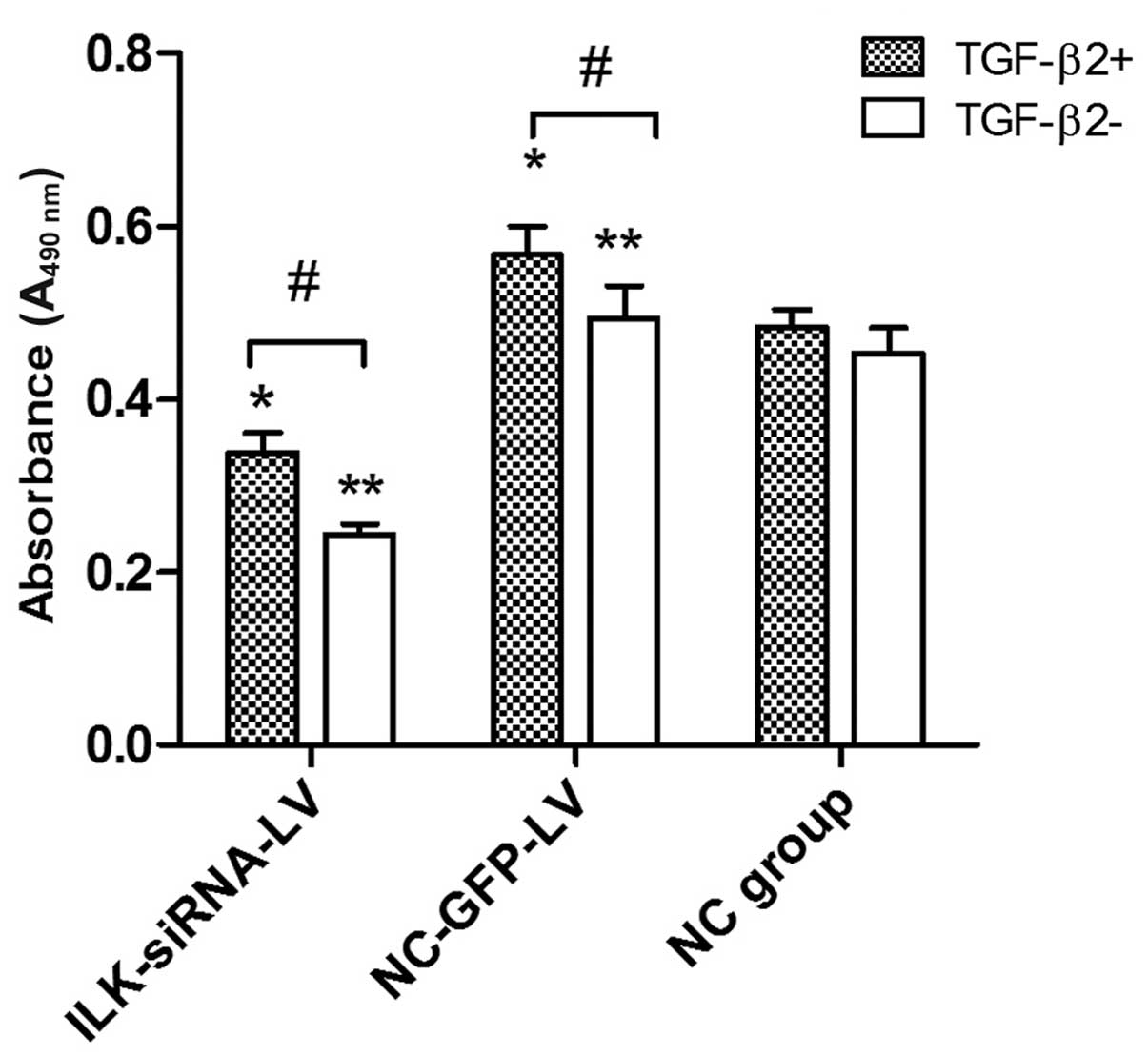
Silencing of ILK attenuates the abnormal
proliferation of HTFs induced by TGF-β2
Based on cell culture model in vitro, MTT
assay was used to examine the role of ILK in the proliferative
activity of HTFs exposed to TGF-β2. At concentrations of 3 ng/ml,
TGF-β2 induced an increase in proliferation in the
lentivirus-transfected group. However, we found that TGF-β2 had no
obvious promoting effect on the proliferation of HTFs in the normal
control group. Furthermore, in the presence or absence of TGF-β2 (3
ng/ml), the cells transfected with ILK-siRNA-LV exhibited a lower
proliferative activity (Fig.
4).
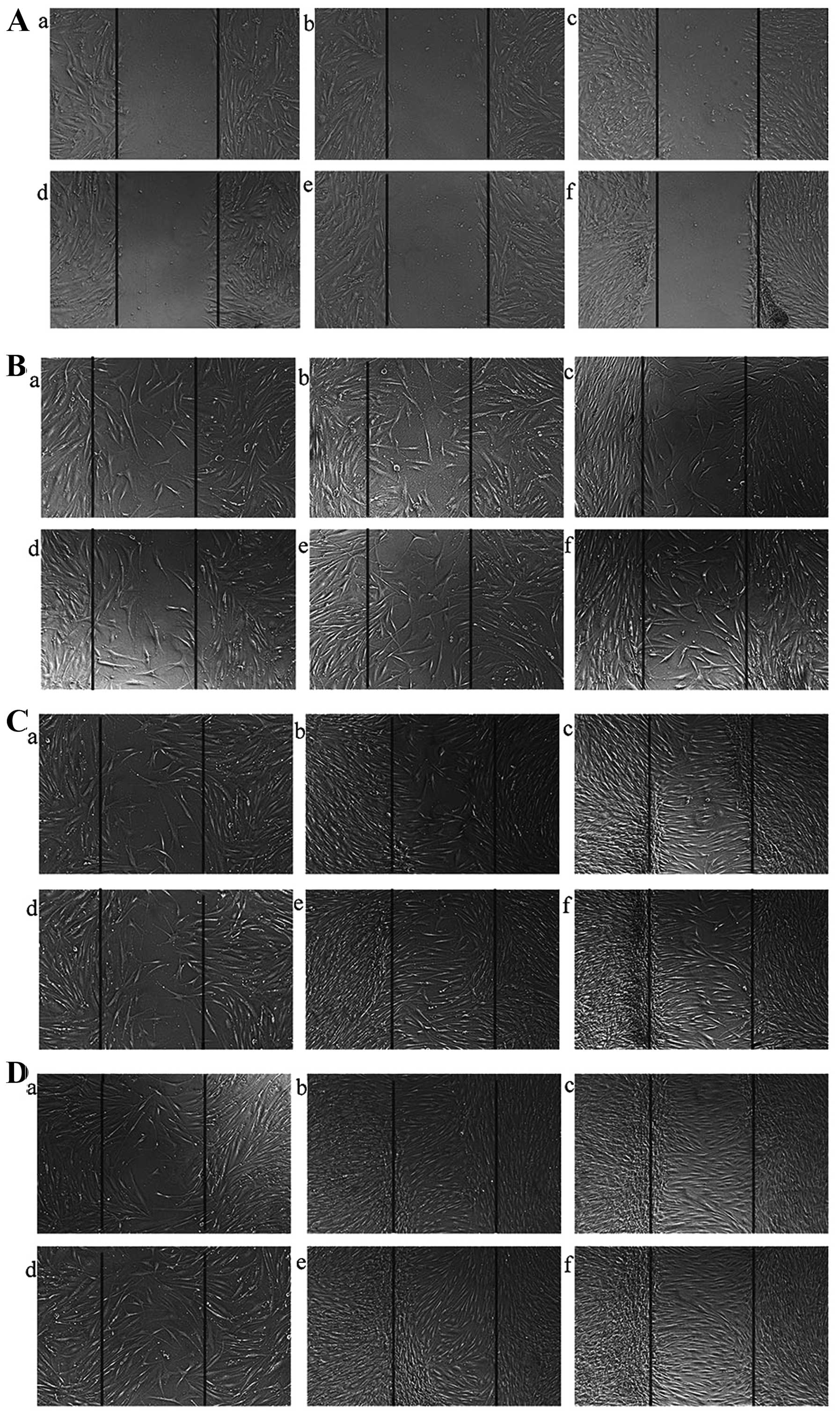
HTFs have a potent migratory ability;
this migratory ability is decreased following the silencing of the
ILK gene
Since we found that transfection with ILK-siRNA-LV
suppressed the proliferation of HTFs induced by TGF-β2, we examined
the hypothesis that the silencing of the ILK gene has the potential
to suppress the migration of HTFs. Wounds were made in the center
of cell monolayers (Fig. 5A) and
the migration distance in each group was similar at 24 h (Fig. 5B). The distance covered by cells
in the ILK-siRNA-LV-transfected group (Fig. 5C, panels a and d) was shorter than
that covered by the cells in the negative control LV-transfected
group (Fig. 5C, panels b and e)
and the normal control group (Fig.
5C, panels c and f) at 48 h (Fig.
5C). The wound had healed in the control group at 72 h;
however, there were still gaps observed in the
ILK-siRNA-LV-transfected group (Fig.
5D). These data indicated that the migratory ability of the
HTFs decreased after ILK gene silencing, although there was no
significant differences observed between the same groups
(transfected groups) of cells exposed to TGF-β2 (Fig. 5 panels d-f) and those that were
not exposed to TGF-β2 (Fig. 5
panels a–c).
Silencing of ILK induces G1 phase cell
cycle arrest in HTFs
The percentages of cells in the G1/G0 phase in the
NC-GFP-LV-transfected group and the normal control group exposed to
TGF-β2 decreased, while the percentages of cells in the S and G2/M
phases increased compared with the control cells not exposed to
TGF-β2 (Fig. 6B). In the presence
of TGF-β2, the percentages of cells in the G1/G0 phase in the
ILK-siRNA-LV-transfected group significantly increased compared to
the control groups (NC-GFP-LV group and NC group). These results
indicate that in the presence of TGF-β2, the silencing of ILK
induces G1 phase cell cycle arrest in HTFs (Fig. 6A and B).
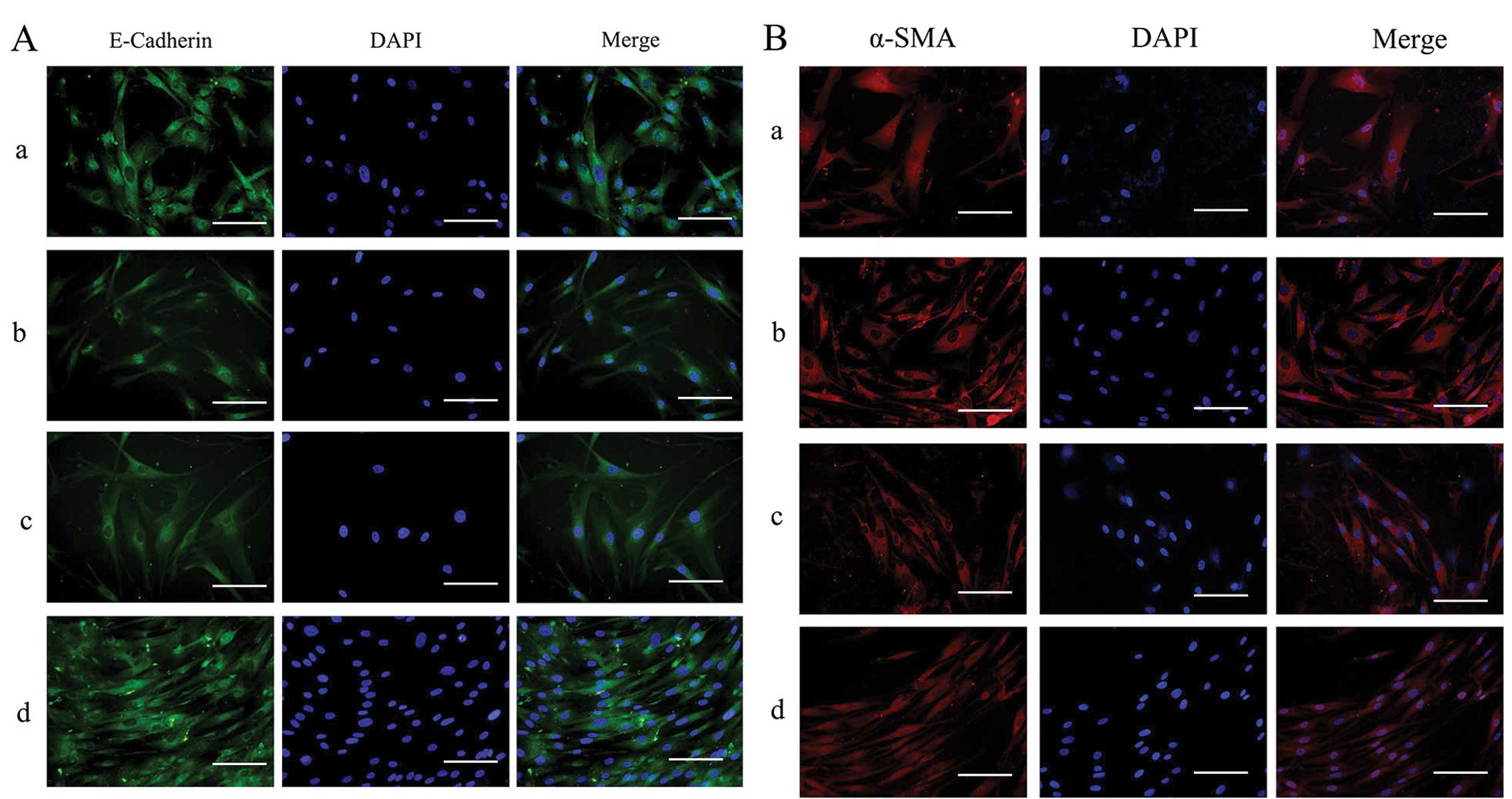
Effects of silencing of ILK on the
protein expression of α-SMA, cyclin D1 and E-cadherin in HTFs
exposed to TGF-β2
The expression and distribution of E-cadherin and
α-SMA in the HTFs were detected by immunofluorescence staining.
Under physiological conditions, E-cadherin was expressed at low
levels throughout the cytoplasm (Fig.
7A, panel d) and α-SMA was also localized in the cytoplasm in
the HTFs (Fig. 7B, panel d). In
the presence of TGF-β2, in the ILK-siRNA-LV-transfected group, the
staining of E-Cadherin was stronger than that in the
NC-GFP-LV-transfected group and the NC group (Fig. 7A, panels a and c). Staining for
α-SMA was present diffusely and stronger in the
NC-GFP-LV-transfected group and NC group (Fig. 7B, panels b and c) than in the
ILK-siRNA-LV-transfected group (Fig.
7B, panel a).
The results of western blot analysis revealed that
ILK was silenced at the protein level. Simultaneously, compared
with the normal control group not exposed to TGF-β2, the
significant upregulation of cyclin D1 and α-SMA expression, and the
downregulation of E-cadherin expression were observed following
exposure to TGF-β2 for 48 h (Fig.
8). These results indicated that TGF-β2 stimulated the
differentiation capacity of HTFs. This capacity was suppressed when
ILK was silenced. The expression of α-SMA and cyclin D1 decreased
in the ILK-siRNA-LV-transfected group compared with the
NC-GFP-LV-transfected group, while E-Cadherin expression increased
(Fig. 8).
Discussion
Glaucoma filtration surgery involves the surgical
formation of an artificial drainage pathway from the anterior
chamber to the subconjunctival space. The rate of fluid drainage
from the subconjunctival space is actually the determining factor
in the reduction of IOP (19).
Though there are many new devices for glaucoma filtration surgery,
scarring is the main cause of surgical failure (20,21). HTFs play a major role in this
process. The culture, isolation and expansion of HTFs has been
demonstrated (22). The tissue
explant technique is the most common method used. In this study, we
successfully cultured HTFs at a low cost, which is a good basis for
studying post-operative scarring. The cultured cells were in a
fibrocyte-like form and had a spindle-shape, and were arranged in a
fasciculus or whirlpool pattern after using the tissue explants
adherence method. The cells were identified by cytoplasmic
proteins. Vimentin, a type of intermediate filament, is a specific
marker protein of mesenchymal cells, while keratin makes up the
largest subgroup of intermediate filament proteins and represents
the most abundant protein in epithelial cells (23). As HTFs are a type of mesenchymal
cells, the results of immunofluorescence staining, and those of the
morphology and growth of the cells proved that the cells we
cultured were fibroblasts.
TGF-β governs developmental processes and regulates
homeostasis by controlling cellular proliferation, survival,
differentiation and migration (24). TGF-β is produced by platelets, the
vascular endothelium and macrophages in the wound healing process.
It is a hot spot of research in cancer, and in cardiovascular,
musculoskeletal or fibrotic diseases (25,26). Due to its significant effect on
fibrosis, TGF-β has become an anti-scarring target in ocular
disease research (27). For
example, in an animal experiments, in a mouse corneoscleral wound
model, fibroblasts developed within the first 2 days of surgery,
the collagen component increased over time and TGF-β2 appeared
early in the process at high levels (28). In our study, TGF-β2 was
reconfirmed to stimulate the proliferation of HTFs by increasing
the percentages of cells in the G2/M and S phases of the cell
cycle. However, we did not observe any obvious promoting effects of
TGF-β2 on cell proliferation and migration. This may be due to the
fact that HTFs have a strong self-renewal ability,
multi-differentiation potential, and a strong proliferative and
migration ability in vitro. During the process of
cultivating, cells trigger the stress response to external
environments, which even causes differentiation into myofibroblasts
to some degree.
ILK is overexpressed in many types of human diseases
downstream of the TGF-β signaling pathway. At present, research on
ILK is mainly focused on invasion and metastasis of epithelial
malignancies or on epithelial to mesenchymal transition (EMT) in
tissue fibrosis. For example, in a study on lung fibrosis, TGF-β1
was used to induce fibrotic characteristics in alveolar epithelial
cells. ILK was found to be involved in the upregulation of vimentin
(29). In a study on peritoneal
fibrosis, a low vimentin expression and a high E-cadherin
expression was observed when ILK gene expression was silenced by
siRNA, suggesting that EMT, which is a process for fully
differentiated epithelial cells to undergo a phenotypic change into
fibroblasts via diverse intracellular signaling pathways, was
inhibited (30). Another study
also demonstrated that the migratory and invasive ability of SW480
cells was significantly enhanced following the enforced
overexpression of ILK. SW480 cells stably overexpressing ILK
underwent EMT, with a decreased expression of E-cadherin, and an
increased expression of vimentin, Snail and Slug (16). In addition, ILK overexpression is
associated with tumor progression. In a previous study, both ILK
mRNA and protein expression levels were significantly upregulated
in primary colorectal cancer samples compared with their
corresponding normal tissues (31). Another study demonstrated that
when TGF-β1 was used to promote EMT and migration in mammary
epithelial cells, the TGF-β1-induced processes were significantly
suppressed by inhibiting ILK activity. ILK was essential in
TGF-β1-induced EMT in mammary epithelial cells (32). ILK silencing by siRNA has been
shown to inhibit EMT, and the metastasis and growth of bladder
cancer cells and tongue cancer cells (33–35). Moreover, as previously
demonstrated, the phosphorylation of ILK can lead to the
phosphorylation of Akt and the inactivation of ILK reverses the
α-parvin (PARVA)-induced invasion of lung cancer cells (36). In ocular disease research, ILK is
required for the complete activation of MAPK and PI3K/Akt
signaling, downstream of the FGF receptor in lens epithelial cells
during development, and is involved in epithelial proliferation,
survival and subsequent fibre differentiation (37). In addition to the studies at the
cellular level, some experiments on animals have suggested that
cardiac-specific ILK knockout mice spontaneously developed lethal
dilated cardiomyopathy and heart failure with an early increase in
apoptosis, fibrosis, and cardiac inflammation (38). Nevertheless, the effect of ILK in
HTFs which is mesenchymal origin cell has not been reported. In
this study, we found that the silencing of ILK suppressed the
proliferation and migration of HTFs induced by TGF-β2, along with
G1 phase cell cycle arrest. The downregulation of cyclin D1
expression was consistent with this phenomenon.
Under physiological conditions, fibroblasts exist in
the interstitium, maintain the dynamic balance of the interstitium
and adjacent tissue, which plays an important role on cell
proliferation and extracellular matrix production. Many factors can
stimulate fibroblast activation and differentiation into
myofibroblasts that exhibit a stronger ability for proliferation
and secrete much more extracellular matrix. Myofibroblasts are the
major source of extracellular matrix during fibrosis. More
importantly, myofibroblasts have the ability to contract as smooth
muscle cells (39–41). In this study, the expression of
α-SMA, as a marker of myofibroblasts, was decreased in the cells in
which ILK was silenced, which indicated that the cell
differentiation ability was decreased. However, α-SMA was observed
in both the TGF-β2-exposed group and the normal control group in
our study. We hypothesized that this may be due to the fact that
some HTFs had already differentiated into myofibroblasts prior to
exposure to TGF-β2. E-cadherin is a subclass of the cadherin family
that plays a major role in cell-cell adhesion in the normal
epithelium. E-cadherin is located on cell-cell boundaries in the
normal epithelium (42). It has
been reported that the reduction or loss of E-cadherin expression
is associated with invasion and metastasis in cancer. Changes in
E-cadherin immunoreactivity and cellular localization occur in the
premalignant metaplastic epithelium of the oesophagus (43). Cadherins also play an integral
role in neuronal morphogenesis (44). The roles of E-cadherin in
different types of cells remain to be fully elucidated. In this
study, we found that E-cadherin was expressed in the cytoplasm in
HTFs, which is not localized in the cell membrane as in epithelial
cells. This result indicates that E-cadherin may not act as an
intercellular adhesion molecule, but as a regulatory factor of cell
activity in HTFs.
In conclusion, by transfecting HTFs with
ILK-siRNA-LV to silence ILK expression, we found that the silencing
of ILK attenuated the abnormal proliferation of fibroblasts induced
by TGF-β2 and the migration ability also decreased after ILK gene
silencing. ILK may be an important factor in scarring following
glaucoma filtration surgery and the downstream factor of the TGF-β
signaling pathway. Our study demonstrated that ILK silencing also
suppressed the differentiation ability of HTFs induced by TGF-β2.
However, several limitations to our study need to be pointed out.
We only observed the biological behavior of HTFs, following ILK
gene silencing, and the molecular mechanisms responsible for this
process were not revealed. In further studies, we aim to to examine
the effects of the silencing of ILK on other signal transduction
pathways (Smads, Rock, PI3K), in order to fully explore the complex
interaction among these pathways. This may provide a new direction
for improving the success rate of glaucoma filtration surgery.
Acknowledgments
This study was supported by National Natural Science
Foundation of China (NSFC; no. 81300765).
References
|
1
|
Quigley HA and Broman AT: The number of
people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol.
90:262–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grisanti S, Szurman P, Warga M, Kaczmarek
R, Ziemssen F, Tatar O and Bartz-Schmidt KU: Decorin modulates
wound healing in experimental glaucoma filtration surgery: a pilot
study. Invest Ophthalmol Vis Sci. 46:191–196. 2005. View Article : Google Scholar
|
|
3
|
Ashaye AO and Komolafe OO: Post-operative
complication of trabeculectomy in Ibadan, Nigeria: outcome of
1-year follow-up. Eye (Lond). 23:448–452. 2009. View Article : Google Scholar
|
|
4
|
Cordeiro MF: Role of transforming growth
factor beta in conjunctival scarring. Clin Sci (Lond). 104:181–187.
2013. View Article : Google Scholar
|
|
5
|
Denk PO, Hoppe J, Hoppe V and Knorr M:
Effect of growth factors on the activation of human Tenon's capsule
fibroblasts. Curr Eye Res. 27:35–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyer-Ter-Vehn T, Katzenberger B, Han H,
Grehn F and Schlunck G: Lovastatin inhibits TGF-beta-induced
myofibroblast transdifferentiation in human tenon fibroblasts.
Invest Ophthalmol Vis Sci. 49:3955–3960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyazono K: TGF-beta signaling by Smad
proteins. Cytokine Growth Factor Rev. 11:15–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piek E, Ju WJ, Heyer J, Escalante-Alcalde
D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP
and Roberts AB: Functional characterization of transforming growth
factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J
Biol Chem. 276:19945–19953. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grotendorst GR, Rahmanie H and Duncan MR:
Combinatorial signaling pathways determine fibroblast proliferation
and myofibroblast differentiation. FASEB J. 18:469–479. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schiller M, Javelaud D and Mauviel A:
TGF-beta-induced SMAD signaling and gene regulation: consequences
for extracellular matrix remodeling and wound healing. J Dermatol
Sci. 35:83–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu C and Dedhar S: Integrin-linked kinase
(ILK) and its inter-actors: a new paradigm for the coupling of
extracellular matrix to actin cytoskeleton and signaling complexes.
J Cell Biol. 155:505–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Yang ZL, Ren X, Zou Q, Yuan Y, Liang
L, Chen M and Chen S: ILK and PRDX1 are prognostic markers in
squamous cell/adenosquamous carcinomas and adenocarcinoma of
gallbladder. Tumour Biol. 34:359–68. 2013. View Article : Google Scholar
|
|
13
|
Li Y, Yang J, Dai C, Wu C and Liu Y: Role
for integrin-linked kinase in mediating tubular epithelial to
mesenchymal transition and renal interstitial fibrogenesis. J Clin
Invest. 112:503–516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vi L, de Lasa C, DiGuglielmo GM and
Dagnino L: Integrin-linked kinase is required for TGF-β1 induction
of dermal myofibroblast differentiation. J Invest Dermatol.
131:586–593. 2011. View Article : Google Scholar
|
|
15
|
Bravou V, Klironomos G, Papadaki E,
Taraviras S and Varakis J: ILK over-expression in human colon
cancer progression correlates with activation of beta-catenin,
down-regulation of E-cadherin and activation of the Akt-FKHR
pathway. J Pathol. 208:91–99. 2006. View Article : Google Scholar
|
|
16
|
Liang F, Zhang S, Wang B, Qiu J and Wang
Y: Overexpression of integrin-linked kinase (ILK) promotes glioma
cell invasion and migration and down-regulates E-cadherin via the
NF-κB pathway. J Mol Histol. 45:141–151. 2014. View Article : Google Scholar
|
|
17
|
Persad S, Attwell S, Gray V, Delcommenne
M, Troussard A, Sanghera J and Dedhar S: Inhibition of
integrin-linked kinase (ILK) suppresses activation of protein
kinase B/Akt and induces cell cycle arrest and apoptosis of
PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A.
97:3207–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verreault M and Bally MB: siRNA-mediated
integrin-linked kinase suppression: nonspecific effects of
siRNA/cationic liposome complexes trigger changes in the expression
of phosphorylated-AKT and mTOR independently of ILK silencing.
Oligonucleotides. 19:129–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu DY, Morgan WH, Sun X, Su EN, Cringle
SJ, Yu PK, House P, Guo W and Yu X: The critical role of the
conjunctiva in glaucoma filtration surgery. Prog Retin Eye Res.
28:303–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holló G: Wound healing and glaucoma
surgery: modulating the scarring process with conventional
antimetabolites and new molecules. Dev Ophthalmol. 50:79–89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan ZC, Bai YJ, Tian Z, Hu HY, You XH, Lin
JX, Liu SR, Zhuo YH and Luo RJ: Anti-proliferation effects of
Sirolimus sustained delivery film in rabbit glaucoma filtration
surgery. Mol Vis. 17:2495–2506. 2011.PubMed/NCBI
|
|
22
|
De Falco E, Scafetta G, Napoletano C, Puca
R, Vingolo EM, Ragona G, Iorio O and Frati G: A standardized
laboratory and surgical method for in vitro culture isolation and
expansion of primary human Tenon's fibroblasts. Cell Tissue Bank.
14:277–287. 2013. View Article : Google Scholar
|
|
23
|
Stahnke T, Löbler M, Kastner C, Stachs O,
Wree A, Sternberg K, Schmitz KP and Guthoff R: Different fibroblast
subpopulations of the eye: a therapeutic target to prevent
postoperative fibrosis in glaucoma therapy. Exp Eye Res. 100:88–97.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horbelt D, Denkis A and Knaus P: A
portrait of transforming growth factor β superfamily signalling:
background matters. Int J Biochem Cell Biol. 44:469–474. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karoń P, Olejek A and Olszak-Wasik K:
TGF-β expression in vulvar cancer. Ginekol Pol. 85:847–851. 2014.
View Article : Google Scholar
|
|
26
|
Bentley-Hewitt KL, De Guzman CE, Ansell J,
Mandimika T, Narbad A and Lund EK: Polyunsaturated fatty acids
modify expression of TGF-β in a co-culture model ultilising human
colorectal cells and human peripheral blood mononuclear cells
exposed to Lactobacillus gasseri, Escherichia coli and
Staphylococcus aureus. Eur J Lipid Sci Technol. 116:505–513. 2014.
View Article : Google Scholar
|
|
27
|
Zhu X, Li L, Zou L, Zhu X, Xian G, Li H,
Tan Y and Xie L: A novel aptamer targeting TGF-β receptor II
inhibits transdifferentiation of human tenon's fibroblasts into
myofibroblast. Invest Ophthalmol Vis Sci. 53:6897–6903. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mietz H, Chévez-Barrios P and Lieberman
MW: A mouse model to study the wound healing response following
filtration surgery. Graefes Arch Clin Exp Ophthalmol. 236:467–475.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kavvadas P, Kypreou KP, Protopapadakis E,
Prodromidi E, Sideras P and Charonis AS: Integrin-linked kinase
(ILK) in pulmonary fibrosis. Virchows Archiv. 457:563–75. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo L, Liu H, Dong Z, Sun L, Peng Y and
Liu F: Small interfering RNA targeting ILK inhibits EMT in human
peritoneal mesothelial cells through phosphorylation of GSK 3β. Mol
Med Rep. 10:137–144. 2014.PubMed/NCBI
|
|
31
|
Li R, Liu B, Yin H, Sun W, Yin J and Su Q:
Overexpression of integrin-linked kinase (ILK) is associated with
tumor progression and an unfavorable prognosis in patients with
colorectal cancer. J Mol Histol. 44:183–189. 2013. View Article : Google Scholar
|
|
32
|
Serrano I, McDonald PC, Lock FE and Dedhar
S: Role of the integrin-linked kinase (ILK)/Rictor complex in
TGFβ-1-induced epithelial-mesenchymal transition (EMT). Oncogene.
32:50–60. 2013. View Article : Google Scholar
|
|
33
|
Yao X, Li D, Xiong DM, Li L, Jiang R and
Chen JX: A novel role of ribonuclease inhibitor in regulation of
epithelial-to-mesenchymal transition and ILK signaling pathway in
bladder cancer cells. Cell Tissue Res. 353:409–423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li L, Pan XY, Shu J, Jiang R, Zhou YJ and
Chen JX: Ribonuclease inhibitor up-regulation inhibits the growth
and induces apoptosis in murine melanoma cells through repression
of angiogenin and ILK/PI3K/AKT signaling pathway. Biochimie.
103:89–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xing Y, Qi J, Deng S, Wang C, Zhang L and
Chen J: Small interfering RNA targeting ILK inhibits metastasis in
human tongue cancer cells through repression of
epithelial-to-mesenchymal transition. Exp Cell Res. 319:2058–2072.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang AH, Pan SH, Chang WH, Hong QS, Chen
JJ and Yu SL: PARVA promotes metastasis by modulating ILK
signalling pathway in lung adenocarcinoma. PLoS One.
10:e01185302015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Teo ZL, McQueen-Miscamble L, Turner K,
Martinez G, Madakashira B, Dedhar S, Robinson ML and de Iongh RU:
Integrin linked kinase (ILK) is required for lens epithelial cell
survival, proliferation and differentiation. Exp Eye Res.
121:130–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dai J, Matsui T, Abel ED, Dedhar S,
Gerszten RE, Seidman CE, Seidman JG and Rosenzweig A: Deep sequence
analysis of gene expression identifies osteopontin as a downstream
effector of integrin-linked kinase (ILK) in cardiac-specific ILK
knockout mice. Circ Heart Fail. 7:184–193. 2014. View Article : Google Scholar
|
|
39
|
Buntrock P: Ultrastructural
characteristics of fibroblasts, myofibroblasts, and fibroclasts in
the process of wound healing (author's transl). Zentralbl Allg
Pathol. 124:48–59. 1980.In German.
|
|
40
|
Ohtani H and Sasano N: Stromal cell
changes in human colorectal adenomas and carcinomas. An
ultrastructural study of fibroblasts, myofibroblasts, and smooth
muscle cells. Virchows Arch A Pathol Anat Histopathol. 401:209–222.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Desmoulière A, Geinoz A, Gabbiani F and
Gabbiani G: Transforming growth factor-beta 1 induces alpha-smooth
muscle actin expression in granulation tissue myofibroblasts and in
quiescent and growing cultured fibroblasts. J Cell Biol.
122:103–111. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oka H, Shiozaki H, Kobayashi K, Inoue M,
Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S,
Takeichi M, et al: Expression of E-cadherin cell adhesion molecules
in human breast cancer tissues and its relationship to metastasis.
Cancer Res. 53:1696–16701. 1993.PubMed/NCBI
|
|
43
|
Jankowski J, Newham P, Kandemir O, Hirano
S, Takeichi M and Pignatelli M: Differential expression of
e-cadherin in normal, metaplastic and dysplastic esophageal mucosa
- a putative biomarker. Int J Oncol. 4:441–448. 1994.PubMed/NCBI
|
|
44
|
Suzuki SC and Takeichi M: Cadherins in
neuronal morphogenesis and function. Dev Growth Differ. 50(Suppl
1): S119–S130. 2008. View Article : Google Scholar : PubMed/NCBI
|