Introduction
A pterygium is an ocular mass that forms over the
perilimbal conjunctiva and extends onto the corneal surface. There
are no specific eye drops available to prevent a pterygium from
invading the cornea, which may cause subsequent visual impairment.
Pathologically, pterygium tissues are characterized by a
proliferative epithelium, an invasive nature and are highly
vascularized tissues (1). We
previously demonstrated that there was a high level of
proliferation in the pterygium epithelium compared with that in
normal conjunctiva (2,3). Vascular endothelial growth factor
(VEGF)-A, basic fibroblast growth factor (FGF2) (4) and erythropoietin receptor (5) have been reported to exist at higher
levels in pterygium tissues and lead to a pterygium extension
through angiogenesis. Previous findings have shown that not only
blood vessels but also lymphatic vessels may be clearly observed in
human pterygium tissues; histological analyses proved that the
lymphatic microvessel density (LMVD) was significantly higher in
pterygia compared with that in normal conjunctiva (6,7).
Lin et al demonstrated that the LMVD was an independent risk
factor and a valuable predictive factor for the recurrence of
pterygia (8). We recently
demonstrated that VEGF-C and the VEGF receptor-3-signaling pathway
led to lymphangiogenesis, which was associated with the
pathogenesis and development of pterygia (7). However, the molecular mechanisms
underlying elevated VEGF-C expression have yet to be elucidated in
the conjunctiva and pterygium.
According to previous findings, chronic stimulation
by the inflammatory cytokines tumor necrosis factor (TNF)-α and
interleukin-1β (IL-1β) may be responsible for the increased
expression of matrix metalloproteinases (MMPs) in cultured primary
pterygium body fibroblasts (9).
These data have clinical implications for the progression of
pterygia and recurrence associated with the incomplete excision of
primary fibroblasts under the influence of ocular surface
inflammation (9). On the other
hand, there was a highly significant correlation between VEGF-C and
the levels of inflammatory cytokines such as TNF-α and IL-1β in the
synovial fluid of patients with rheumatoid arthritis (RA). As a
result, VEGF-C and the cytokines cause synovial inflammation and
hyperplasia in RA by contributing to local lymphangiogenesis
(10). The high expression of
VEGF-C stimulated by TNF-α induces many human diseases such as
chronic progressive kidney diseases (11), gallbladder carcinoma (12), melanoma lymph node metastasis
(13) and breast cancer (14). Based on previous findings, we
hypothesized that such inflammatory cytokines provoke VEGF-C
expression in the human conjunctiva and pterygium.
The aim of this study was to analyze the changes in
VEGF-C expression induced by TNF-α or IL-1β stimulation and to
examine the relationship between VEGF-C and TNF receptor 1 (TNFR1)
in the pterygium and normal conjunctival tissues of humans.
Materials and methods
Cell culture and chemicals
Cultured human conjunctival epithelial cells (clone
1-5c-4 HeLa derivative) were purchased from the European Collection
of Authenticated Cell Cultures (Salisbury, UK). The cell line was
cultured under a humidified atmosphere containing 5% CO2
at 37°C in Medium 199 (Sigma-Aldrich, St. Louis, MO, USA)
containing 10% fetal bovine serum.
After serum starvation, human conjunctival
epithelial cells were treated with recombinant human TNF-α (0, 4
and 20 ng/ml; R&D Systems, Minneapolis, MN, USA) or recombinant
human IL-1β (0, 0.2, 2.0 and 20 ng/ml; Thermo Scientific
Biomarkers, Hennigsdorf, Germany) for 24 and/or 48 h and processed
for analysis to detect mRNA and protein expression levels.
Phosphate-buffered saline (PBS) was added to the serum-free medium
for the controls.
For the TNF-α neutralization bioassay, recombinant
human TNF-α at 20 ng/ml (R&D Systems) was pre-incubated with
200 ng/ml of rabbit anti-TNF-α neutralizing antibody (D1B4; Cell
Signaling Technology, Danvers, MA, USA) for 1 h at 37°C. After
pre-incubation, the cells were treated for 48 h and processed for
analysis to detect VEGF-C protein expression levels. Normal rabbit
IgG (R&D Systems) was used as the control for the anti-TNF-α
neutralizing antibody.
Quantitative PCR (qPCR) and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells using the TRIzol
method (Life Technologies, Carlsbad, CA, USA). Reverse
transcription to synthesize 1.0 µg of total RNA to cDNA was
performed using GoScript Reverse Transcriptase (Promega, Madison,
WI, USA) according to the manufacturer's instructions.
qPCR was performed using 0.1 µl cDNA from
each sample, GoTaq qPCR Master Mix (Promega), 0.25 µM
primers and 0.2 µl CXR reference dye (Promega), in a final
volume of 20 µl for the SYBR assay. For the TaqMan assay, we
used 0.1 µl cDNA from each sample, THUNDERBIRD Probe qPCR
Mix, 0.2 µl ROX reference dye (both from Toyobo, Tokyo,
Japan), 0.9 µM of primers and 0.25 µM of FAM-dye
labeled TaqMan MGB probe in a final volume of 20 µl. After
an initial denaturation at 95°C for 2 min, the samples were
subjected to 40 cycles of amplification comprised of denaturation
at 95°C for 15 sec and annealing/extension at 60°C for 1 min. All
data were calculated using the ΔΔCq method (15) using glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) for normalization.
RT-PCR was performed using 0.1 µl cDNA from
each sample, 0.5 units of GoTaq DNA polymerase (Promega), 0.4
µM of primers, 0.2 mM of dNTP mix and 1X GoTaq reaction
buffer in a final volume of 20 µl. After an initial
denaturation at 95°C for 2 min, the samples were subjected to 35
cycles of amplification consisting of 95°C for 30 sec, 60°C for 30
sec and 72°C for 15 sec. The products were electrophoresed on 2%
agarose gels, stained with SYBR Safe (Invitrogen, Carlsbad, CA,
USA) and visualized using RAS-4000 image analyzer (FujiFilm, Tokyo,
Japan).
The following primers for genes were used: human
GAPDH (NM_002046.5) sense, 5′-CCT GGC CAA GGT CAT CCA TG-3′
and antisense, 5′-GGA AGG CCA TGC CAG TGA GC-3′ (224 bp); and TNF
receptor superfamily, member 1A (TNFRSF1A; NM_001065.3)
sense, 5′-CTG CCA GGA GA AAC AGA ACA C-3′ and antisense, 5′-CTC AAT
CTG GGG TAG GCA CAA-3′ (130 bp). The TaqMan assay primer and probe
set for VEGFC (NM_005429.3; TaqMan assay ID: Hs00153458_m1)
was purchased from Life Technologies.
Enzyme-linked immunosorbent assay
(ELISA)
The concentration of VEGF-C in cell cultured media
was measured using the human VEGF-C ELISA kit (R&D Systems)
according to the manufacturer's instructions.
Preparation of human tissues
Nasal pterygia were surgically removed from 10
patients (mean age, 73.6±5.6 years) who were enrolled in this
study. Age-matched normal bulbar conjunctival tissues were obtained
from patients (mean age, 62.3±4.2 years) during glaucoma surgery.
The tissues were then fixed in 4% paraformaldehyde. After fixation,
the slides were washed in PBS and processed for paraffin
sectioning. This study was conducted in accordance with the World
Medical Association Declaration of Helsinki. Written informed
consent was obtained from all patients after receiving approval
from the institutional review board of Hokkaido University Hospital
(Sapporo, Japan) (IRB #014-0295).
Immunofluorescence microscopy
After hematoxylin and eosin staining
(Sigma-Aldrich), formalin-fixed, paraffin-embedded serial tissue
sections were deparaffinized and hydrated through exposure to
xylene and graded alcohols followed by water. As a pre-treatment,
microwave-based antigen retrieval was performed in a 10 mM citrate
buffer (pH 6.0). The cultured human conjunctival epithelial cells
were fixed in 4% paraformaldehyde and then washed in PBS. Ten
percent serum depending on the secondary antibody source was used
to block non-specific binding. The sections were incubated with the
following primary antibodies: rabbit polyclonal anti-TNFR1
(ab19139; 1:100 dilution; Abcam, Cambridge, MA, USA) and goat
polyclonal anti-VEGF-C (AF752; 1:10 dilution; R&D Systems).
Secondary antibodies for fluorescence detection were Alexa Fluor
488 and 546 (Life Technologies). 4′,6-Diamidino-2-phenylindole
(DAPI) was used for nuclear staining and sections were mounted with
a fluorescent mounting medium (Dako, Glostrup, Denmark). The
sections were visualized under a Keyence BZ-9000 fluorescence
microscope (Keyence, Tokyo, Japan).
Immunohistochemistry
Following the microwave-based antigen retrieval
treatment, the sections were incubated in 0.3% hydrogen peroxide in
order to block endogenous peroxidase activity and 10% normal goat
serum (Life Technologies) to block non-specific binding. The
sections were then incubated with rabbit polyclonal anti-TNFR1
antibody (ab19139; 1:100 dilution; Abcam). We replaced anti-TNFR1
antibody with PBS as a negative control. Color was developed using
3,3′-diaminobenzidine tetrahydrochloride (Dako) and counter-stained
with hematoxylin. The sections were examined under a Keyence
BZ-9000 fluorescence microscope (Keyence).
Statistical analysis
All the results are expressed as the means ± SED as
indicated. The student's t-test was used for statistical comparison
of the concentration of VEGF-C protein. A p-value <0.05 was
considered to indicate a statistically significant difference
between the means.
Results
Increased expression and secretion of
VEGF-C in cultured human conjunctival epithelial cells following
TNF-α stimulation
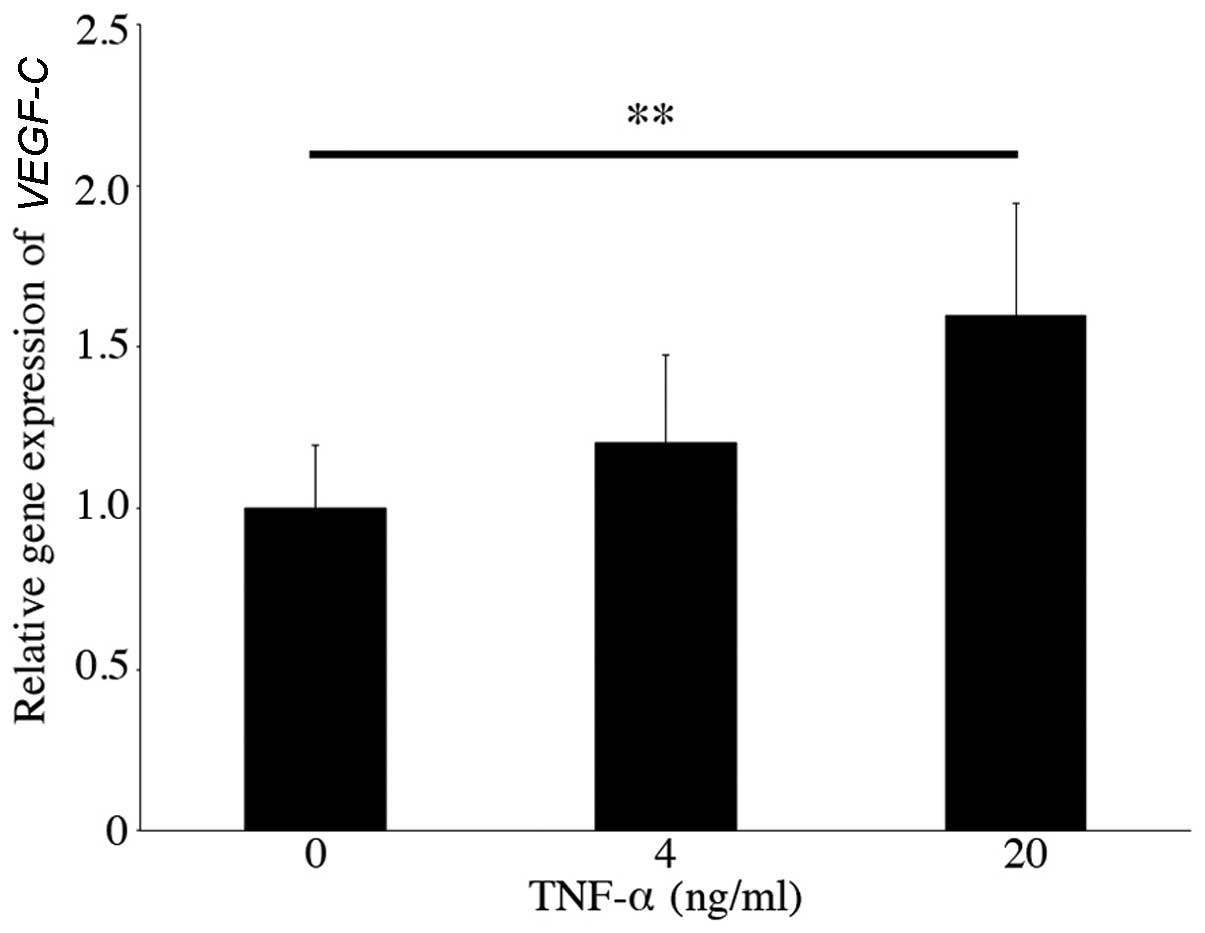
Firstly, we examined the gene expression of
VEGFC in cultured human conjunctival epithelial cells
stimulated with TNF-α or IL-1β for 24 h. The VEGFC
expression level increased (fold-change, 1.60±0.35, n=6, p<0.01)
following stimulation with 20 ng/ml TNF-α (Fig. 1). By contrast, the gene expression
of VEGFC mildly increased (fold-change, 1.23±0.17, n=6,
p<0.05) with the addition of 0.2 ng/ml IL-1β (Fig. 2).
To determine whether stimulation with TNF-α or IL-1β
increases VEGF-C secretion from cultured human conjunctival
epithelial cells, we measured the VEGF-C protein concentrations in
the supernatants 24 and 48 h after the addition of TNF-α or IL-1β.
Forty-eight hours after the addition of 20 ng/ml TNF-α, the protein
concentration of VEGF-C increased (195.92±33.41 pg/mg, n=6,
p<0.05), which was significantly higher when compared with the
controls (Fig. 3). By contrast,
there was no significant difference in VEGF-C concentrations with
or without IL-1β stimulation (data not shown).
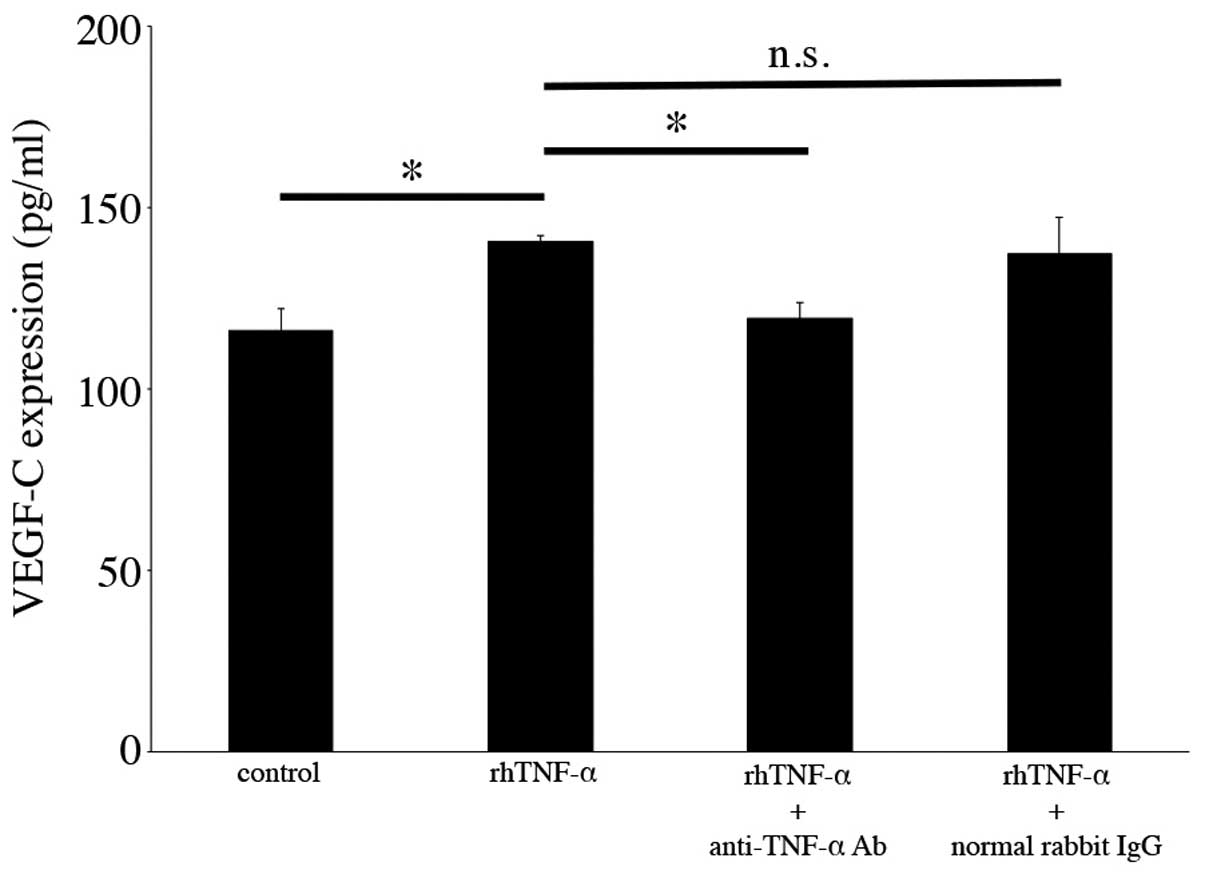
Neutralization of TNF-α in VEGF-C
secretion from cultured human conjunctival epithelial cells
To verify that TNF-α stimulation increases VEGF-C
secretion from cultured human conjunctival epithelial cells, we
treated the cultured cells with TNF-α and anti-TNF-α neutralizing
antibody, and examined the secretion levels of VEGF-C. As shown in
Fig. 4, increased VEGF-C protein
secretion following TNF-α addition was significantly decreased by
anti-TNF-α neutralizing antibody treatment (140.44±1.92 to
119.33±4.41 pg/ml, n=3, p<0.05), whereas there were no specific
changes following treatment with normal rabbit IgG (200 ng/ml)
(Fig. 4).
Expression of TNFR1 in cultured human
conjunctival cells and pterygium tissues
TNF-α signaling occurs through two types of
receptor, TNFR1 and TNFR2. TNFR1 is expressed in almost all
mammalian cell types whereas TNFR2 is typically found in immune
endothelial cells. Among numerous cell types, TNFR1 exists as the
key mediator of TNF signaling except in the lymphoid system
(16,17), as well as in other human
conjunctival epithelial cell lines (18). In this study, we explored the
existence of TNFR1 and the gene expression of TNFR1 (also
known as TNFRSF1A) in cultured human conjunctival epithelial
cells and pterygium tissues. TNFRSF1A was detected in
cultured human conjunctival epithelial cells (Fig. 5). TNFR1 was also immunolocalized
in cultured human conjunctival cells (Fig. 6); the TNFR1 signal was similar to
that observed in the cultured dorsal root ganglion cell body
(19). We then
immunohistochemically examined TNFR1 expression in human tissues,
in either pterygia or normal conjunctiva (Fig. 7). TNFR1 immunoreactivity was
detected in the pterygium epithelial cells (Fig. 7B arrows), whereas the reactivity
was less marked in normal conjunctival epithelia (Fig. 7E). Moreover, TNFR1 signals
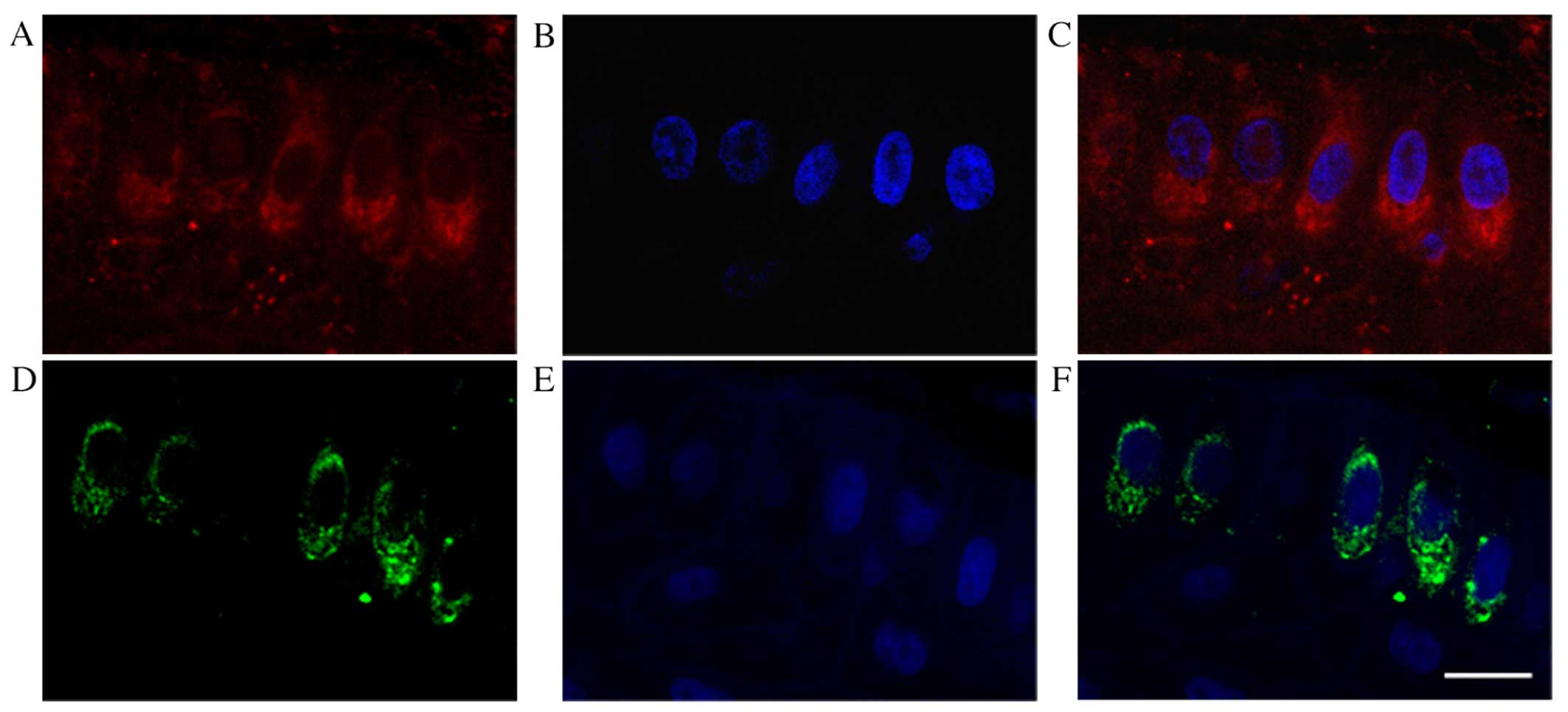
(Fig. 8D and F) were observed in
VEGF-C-positive epithelial cells from human pterygia (Fig. 8A and C) using serial sections.
Discussion
In this study we demonstrated for the first time to
the best of our knowledge, that stimulation by TNF-α, but not
IL-1β, enhanced both the gene expression and the protein secretion
of VEGF-C in cultured human conjunctival epithelial cells. It is
known that TNF-α leads to cell proliferation in the pterygium by
increasing the expression of MMPs (9). In addition, high levels of VEGF-C
secretion from epithelial cells induces lymphangiogenesis, which
play a critical role in the pathogenesis and development of
pterygia (7). It has also been
reported that immunoreactivity for TNF-α was marked in pterygium
tissues (20). Therefore, our
data suggest that TNF-α-mediated VEGF-C expression may be an
important trigger of lymphangiogenesis during the development of
pterygia.
It has been shown that TNFR1 is expressed by a
variety of cell types (16,18,21). In this study, TNFRSF1A and
TNFR1 were detected in cultured human conjunctival epithelial
cells. Moreover, TNFR1 immunoreactivity was strongly detected in
the pterygium tissues. In addition, we further demonstrated that
TNFR1 immunoreactivity was detected in VEGF-C-positive epithelial
cells from human pterygia. According to the immunoreactivity
results, we hypothesized that VEGF-C was secreted from the
TNFR1-positive cells. These results suggest that TNF-α possibly
binds to TNFR1, which results in increased VEGF-C secretion and its
gene expression.
TNFR1 immunoreactivity was found not only in
epithelial cells from human pterygia but also in normal
conjunctival epithelia. This result suggests that the increased
expression of TNF-α rather than the constitutive presence of TNFR1
may stimulate the ligand-receptor system, which causes VEGF-C
induction leading subsequently to a higher lymphatic vessel number
in the pterygium than in the normal conjunctiva. According to
previous findings, acute ultraviolet irradiation exposure results
in the induction of cornea-derived proinflammatory cytokines such
as IL-1, IL-6, IL-8 and TNF-α (22). It is generally known that pterygia
are closely associated with ultraviolet light (23). Indeed, ultraviolet exposure may
lead to an increase in the production of TNF-α in stromal cells of
the human cornea (22). Exposure
to ultraviolet light may be one of the regulators of TNF-α
expression. Further studies are needed to clarify the regulation of
TNF-α in the ocular surface.
Surgical resection of the proliferative tissues and
subsequent reconstruction are the only conventional treatments for
human pterygia. However, postoperative recurrence has been reported
in >50% of cases (24).
Therefore, the development of pharmacotherapy may contribute to the
further prevention of pterygium invasion and subsequent recurrence.
In this study, we demonstrated that TNF-α mediates VEGF-C
expression, suggesting that blockage of TNF-α may be a novel
therapeutic target for the treatment of pterygia in the future.
In conclusion, TNF-α induced VEGF-C expression in
cultured human conjunctival epithelial cells. This pathway may
involve the upstream regulation of VEGF-C expression and secretion
in the pathogenesis and development of lymphangiogenesis in
pterygia.
Acknowledgments
The present study was supported by the Japan Society
for the Promotion of Science (JSPS) Grant-in-Aid for Young
Scientists (B) (grant no. 24791824); and the Ministry of Education,
Culture, Sports, Science and Technology (MEXT). We thank Ikuyo
Hirose and Shiho Yoshida (Hokkaido University) for their skilled
technical assistance.
References
|
1
|
Gebhardt M, Mentlein R, Schaudig U, Pufe
T, Recker K, Nölle B, Al-Samir K, Geerling G and Paulsen FP:
Differential expression of vascular endothelial growth factor
implies the limbal origin of pterygia. Ophthalmology.
112:1023–1030. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kase S, Takahashi S, Sato I, Nakanishi K,
Yoshida K and Ohno S: Expression of p27(KIP1) and cyclin D1, and
cell proliferation in human pterygium. Br J Ophthalmol. 91:958–961.
2007. View Article : Google Scholar
|
|
3
|
Kase S, Osaki M, Sato I, Takahashi S,
Nakanishi K, Yoshida K, Ito H and Ohno S: Immunolocalisation of
E-cadherin and beta-catenin in human pterygium. Br J Ophthalmol.
91:1209–1212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Detorakis ET, Zaravinos A and Spandidos
DA: Growth factor expression in ophthalmic pterygia and normal
conjunctiva. Int J Mol Med. 25:513–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kase S, Osaki M, Jin XH, Ohgami K, Yoshida
K, Saito W, Takahashi S, Nakanishi K, Ito H and Ohno S: Increased
expression of erythropoietin receptor in human pterygial tissues.
Int J Mol Med. 20:699–702. 2007.PubMed/NCBI
|
|
6
|
Ling S, Liang L, Lin H, Li W and Xu J:
Increasing lymphatic microvessel density in primary pterygia. Arch
Ophthalmol. 130:735–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukuhara J, Kase S, Ohashi T, Ando R, Dong
Z, Noda K, Ohguchi T, Kanda A and Ishida S: Expression of vascular
endothelial growth factor C in human pterygium. Histochem Cell
Biol. 139:381–389. 2013. View Article : Google Scholar
|
|
8
|
Lin H, Luo L, Ling S, Chen W, Liu Z, Zhong
X, Wu C, Chen W and Liu Y: Lymphatic microvessel density as a
predictive marker for the recurrence time of pterygium: a
three-year follow-up study. Mol Vis. 19:166–173. 2013.PubMed/NCBI
|
|
9
|
Solomon A, Li DQ, Lee SB and Tseng SC:
Regulation of collagenase, stromelysin, and urokinase-type
plasminogen activator in primary pterygium body fibroblasts by
inflammatory cytokines. Invest Ophthalmol Vis Sci. 41:2154–2163.
2000.PubMed/NCBI
|
|
10
|
Cha HS, Bae EK, Koh JH, Chai JY, Jeon CH,
Ahn KS, Kim J and Koh EM: Tumor necrosis factor-alpha induces
vascular endothelial growth factor-C expression in rheumatoid
synoviocytes. J Rheumatol. 34:16–19. 2007.PubMed/NCBI
|
|
11
|
Kimura H, Mikami D, Kamiyama K, Sugimoto
H, Kasuno K, Takahashi N, Yoshida H and Iwano M: Telmisartan, a
possible PPAR-δ agonist, reduces TNF-α-stimulated VEGF-C production
by inhibiting the p38MAPK/HSP27 pathway in human proximal renal
tubular cells. Biochem Biophys Res Commun. 454:320–327. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du Q, Jiang L, Wang X, Wang M, She F and
Chen Y: Tumor necrosis factor-α promotes the lymphangiogenesis of
gallbladder carcinoma through nuclear factor-κB-mediated
upregulation of vascular endothelial growth factor-C. Cancer Sci.
105:1261–1271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peppicelli S, Bianchini F and Calorini L:
Inflammatory cytokines induce vascular endothelial growth factor-C
expression in melanoma-associated macrophages and stimulate
melanoma lymph node metastasis. Oncol Lett. 8:1133–1138.
2014.PubMed/NCBI
|
|
14
|
Wen Y, Wang M, Yang J, Wang Y, Sun H, Zhao
J, Liu W, Zhou Z, Deng H, Castillo-Pedraza C, et al: A comparison
of fentanyl and flurbiprofen axetil on serum VEGF-C, TNF-α, and
IL-1β concentrations in women undergoing surgery for breast cancer.
Pain Pract. 15:530–537. 2015. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Method Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Brenner D, Blaser H and Mak TW: Regulation
of tumour necrosis factor signalling: live or let die. Nat Rev
Immunol. 15:362–374. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wajant H, Pfizenmaier K and Scheurich P:
Tumor necrosis factor signaling. Cell Death Differ. 10:45–65. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cook EB, Stahl JL, Graziano FM and Barney
NP: Regulation of the receptor for TNFalpha, TNFR1, in human
conjunctival epithelial cells. Invest Ophthalmol Vis Sci.
49:3992–3998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Richards N, Batty T and Dilley A: CCL2 has
similar excitatory effects to TNF-α in a subgroup of inflamed
C-fiber axons. J Neurophysiol. 106:2838–2848. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kria L, Ohira A and Amemiya T:
Immunohistochemical localization of basic fibroblast growth factor,
platelet derived growth factor, transforming growth factor-beta and
tumor necrosis factor-alpha in the pterygium. Acta Histochem.
98:195–201. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakimoto T, Yamada A and Sawa M: Release
of soluble tumor necrosis factor receptor 1 from corneal epithelium
by TNF-alpha-converting enzyme-dependent ectodomain shedding.
Invest Ophthalmol Vis Sci. 50:4618–4621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kennedy M, Kim KH, Harten B, Brown J,
Planck S, Meshul C, Edelhauser H, Rosenbaum JT, Armstrong CA and
Ansel JC: Ultraviolet irradiation induces the production of
multiple cytokines by human corneal cells. Invest Ophthalmol Vis
Sci. 38:2483–2491. 1997.PubMed/NCBI
|
|
23
|
Yam JC and Kwok AK: Ultraviolet light and
ocular diseases. Int Ophthalmol. 34:383–400. 2014. View Article : Google Scholar
|
|
24
|
Díaz L, Villegas VM, Emanuelli A and
Izquierdo NJ: Efficacy and safety of intraoperative mitomycin C as
adjunct therapy for pterygium surgery. Cornea. 27:1119–1121. 2008.
View Article : Google Scholar : PubMed/NCBI
|