Introduction
Osteosarcoma (OS), which commonly occurs in the
developing bones of adolescents and children, is ranked as the most
frequent primary bone malignancy (1). At present, adjuvant chemotherapy,
radiotherapy and surgery are the principal treatments for OS
(2). The overall survival rate
and 5 year survival rate are approximately 60% despite the fact
that remarkable advances have been achieved with chemotherapy
(2,3). Furthermore, drug resistance
frequently develops in OS and the use of chemotherapeutic drugs is
restricted by dose-limiting toxicity (3). Despite the administration of
combination therapy (surgery combined with chemotherapy regimens
using multiple agents), nearly one third of patients with OS
characterized by localized lesions at the time of diagnosis
suffered relapse (4) and lung
metastases contributed to 90% of relapses. Sixty to seventy percent
of patients with non-metastatic OS receiving conventional therapy
have achieved 5-year survival and some individuals have reached two
decades (5) whereas the long-term
survival rate of patients with recurrent or metastatic malignancy
is <20% (6).
MicroRNAs (miRNAs) are a class of small, non-coding
RNAs, which bind to the 3′-untranslated region (3′-UTR) of target
mRNAs in order to suppress the expression of genes. Alterations in
miRNA expression have been associated with the metastasis,
progression and initiation of malignancy. Previous findings have
demonstrated that miRNAs may serve as oncogenes or tumor
suppressors during the development of cancer (7,8).
Previous findings have indicated that miRNAs play an essential role
in the progression and pathogenesis of OS (9).
Non-coding polymorphisms associated with treatment
response, prognosis and cancer risk which involve common
dysregulated signaling pathways, such as the phosphoinositide
3-kinase (PI3K)/AKT pathways or the mitogen-activated protein
kinase (MAPK) pathway, may serve as critical starting points for
the functional validation and assessment of such polymorphisms in
understanding the initiation, progression and metastasis of various
malignancies (10). Researchers
have explored the involvement of a silent polymorphism rs61764370,
which is situated in the 3′-UTR of Kirsten rat sarcoma viral
oncogene homolog (KRAS), one of the most commonly mutated oncogenes
in the development of malignancy (11). Tentative bioinformatic and
biochemical analysis indicated that the miRNA let-7 binding site
was weakened by rs61764370 T>G variant, which led to the
increased expression of KRAS (12). Previous studies have reported that
let-7g, another member of the let-7 family, is functionally
involved in controlling the metastasis of OS (13); moreover, the dysregulation of the
KRAS signaling pathway was believed to be responsible for the
metastasis of OS (14). By
contrast, KRAS has been identified as a direct target gene of
let-7a in lung cancer cells and colon cancer cells (15,16,17). Based on computational analysis
(http://mirtarbase.mbc.nctu.edu.tw/),
the rs61764370 polymorphism was found to be located close to the
putative binding site of let-7a in the 3′-UTR of KRAS, and we
therefore hypothesized that the rs61764370 polymorphism may be
associated with an increased risk of metastatic disease in OS by
disrupting the interaction between let-7a and KRAS mRNA.
Materials and methods
Study population and tissue samples
A total of 36 surgically resected OS tissue
specimens were obtained (prior to the administration of neoadjuvant
chemotherapy) from the Department of Orthopedic Surgery at the
First Affiliated Hospital of Harbin Medical University (Harbin,
China). Written informed consent was obtained from all patients,
and the present study was approved by the Ethics Committee of
Harbin Medical University (Harbin, China).
DNA extraction and sequence analysis
Genomic DNA was extracted using a DNA extraction kit
(Promega, Fitchburg, WI, USA). DNA quality and quantity was
assessed using the Nanodrop 1000 (Thermo Fisher Scientific,
Waltham, MA, USA). The T>G variant at the single nucleotide
polymorphism (SNP) rs61764370 was verified by the purification of
the PCR product amplified with forward primer,
5′-TTTTGGGGTGGTGGTGTGCCAA-3′ and reverse primer,
5′-AGTCACCACACAAGGCACTG-3′ and sequenced using the forward primer.
Sequencing and mutational analysis was performed.
Cell culture and transfection
Two OS cell cultures, which were genotyped as
rs61764370 TT (named as OSTT) and TG (named as OSTG), were derived
from the biopsies obtained from the Department of Orthopedic
Surgery at the First Affiliated Hospital of Harbin Medical
University (Harbin, China) in accordance with the protocol approved
by the Ethics Committee of Harbin Medical University. The MG-63
cell line was purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and maintained in Dulbecco's modified
Eagle's medium (DMEM)/F12 (HyClone, Logan, UT, USA) supplemented
with 10% fetal bovine serum (Gibco, Grand Island, NY, USA). We
obtained scramble RNA, let-7a mimics (5′-AGUGGGGAACCCUUCCAUGAGG-3′)
and KRAS siRNA (5′-AGTACTGGCGATTCTCTGA GGC-3′) from GenePharma
(Shanghai, China). The transfection of cells with blank control (no
RNA was transfected), scramble control, let-7a mimics and KRAS
siRNA was performed using Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA).
Wound-healing assay
A 6-well plate was used to culture OSTT and OSGG
cells. After 24 h, when the cells reached 80–90% confluence, we
removed the attached cells with a sterile plastic pipette tip to
create a single wound in the center of the well. Serum-free medium
was used to remove the debris. An inverted microscope (IX83;
Olympus, Tokyo, Japan) was employed to visualize and capture images
of the cells which had migrated into the wounded area after 24 h of
culturing. Each experiment was performed independently at least
three times.
Dual luciferase assay
The full length of the KRAS 3′-UTR was subcloned
into the pmirGLO vector (Promega, Madison, WI, USA) to generate the
luciferase reporter vector. The MG-63 cells were seeded in 48-well
plates at a density of 1×104 cells/well. Let-7a mimics
and luciferase reporter vectors were co-transfected using
Lipofectamine 2000 (Invitrogen Life Technologies). After 48 h, the
cells were harvested and assayed for luciferase activity using the
Dual-Luciferase Reporter Assay system (Promega) according to the
manufacturer's instructions. The firefly luciferase activities were
normalized to Renilla luciferase activity. Each treatment
was performed in triplicate in three independent experiments.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The viability of the cells was measured using an MTT
assay according to the manufacturer's instructions (Sigma, St.
Louis, MO, USA). Briefly, the cells were seeded at a density of
2,000 cells/well in 96-well plates containing 100 µl culture
medium and incubated overnight. To each well, 20 µl of 5
mg/ml MTT was added and the plates were incubated for an additional
4 h at 37°C. After removing the medium, the formazan complex was
solubilized with 100 µl dimethyl sulfoxide (DMSO; Sigma).
Optical density (OD) was determined by measuring the absorbance at
a test wavelength of 490 nm and a reference wavelength of 630 nm.
Wells without cells (DMSO alone) were used as blanks. Each group
contained six wells; experiments were repeated three times
independently and the results are expressed as the means ± standard
deviation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Forty-eight hours after transfection, the total RNA
(including miRs) was extracted using an miRNANeasy Mini kit
(Qiagen, Hilden, Germany) according to the manufacturer's
instructions. The concentration of RNA was measured using a Beckman
DU-640 spectrophotometer (Beckman Coulter, Inc., Fullerton, CA,
USA). The quality of RNA was determined by 1% formaldehyde-agarose
gel electrophoresis. PCR amplification for the quantification of
let-7a and KRAS mRNA was performed using a TaqMan® miRNA
reverse transcription kit and TaqMan miRNA assay kits (both from
Applied Biosystems, Foster City, CA, USA). The following PCR
protocol was used: 40 cycles at 95°C for 15 sec, 60°C for 15 sec
and 72°C for 30 sec on a real-time PCR system (Applied Biosystems).
RNU48 (Applied Biosystems) was used as the endogenous control. The
relative gene expression levels were then normalized to RNU48 and
calculated using the 2−ΔΔCT method.
Western blot analysis
Proteins were extracted from the transfected cells
or the tissue samples using radioimmunoprecipitation assay (RIPA)
lysis buffer (Upstate Biotechnology, Inc., Lake Placid, NY, USA).
The protein level was determined using protein assay reagents
(Bio-Rad Laboratories, Hercules, CA, USA) according to standard
protocols. Briefly, 25 µg of total protein was loaded onto a
12% PAGE gel (NuPAGE; Invitrogen Life Technologies) and subjected
to electrophoresis. After blotting onto pure nitrocellulose
membranes (Bio-Rad Laboratories) the proteins were blocked with
Odyssey® Blocking Buffer (LI-COR Biosciences, Lincoln,
NE, USA) for 2 h. Subsequently, the membranes were incubated in
primary antibodies buffer (primary antibodies, Odyssey Blocking
Buffer, 0.1% Tween®-20) overnight at 4°C. The primary
antibodies, KRAS and β-actin were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). The following day, the
membranes were washed four times for 5 min in Tris-buffered saline
and Tween-20 (TBST). Finally, the membranes were incubated with
secondary antibodies, goat anti-rabbit lgG (Cell Signaling
Technology, Inc.). The blots were then treated with an ECL kit and
the relative density of the target bands was calculated using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Transwell migration assay
After transfection, the OSTT or OSGG cells
(5×103) were suspended in serum-free medium and seeded
onto the upper part of the chamber for the cell migration assay.
Complete medium with 20% serum was added to the lower chamber as a
chemoattractant. After 48 h, the Transwell membrane was fixed with
methanol, and the cells that had migrated to the lower surface of
the filters were stained with 0.1% crystal violet stain solution
(Sigma). Migration was determined by counting the cell number under
a microscope (Olympus). The average number of migrating cells in
five random fields was taken as the cell migration number of the
group.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Comparison of datasets for
each cell group was performed using the independent t-test or
one-way ANOVA, applying Bonferonni's multiple comparison test. A
P-value <0.05 was considered to indicate a statistically
significant difference. The values reported correspond to the means
± standard deviation.
Results
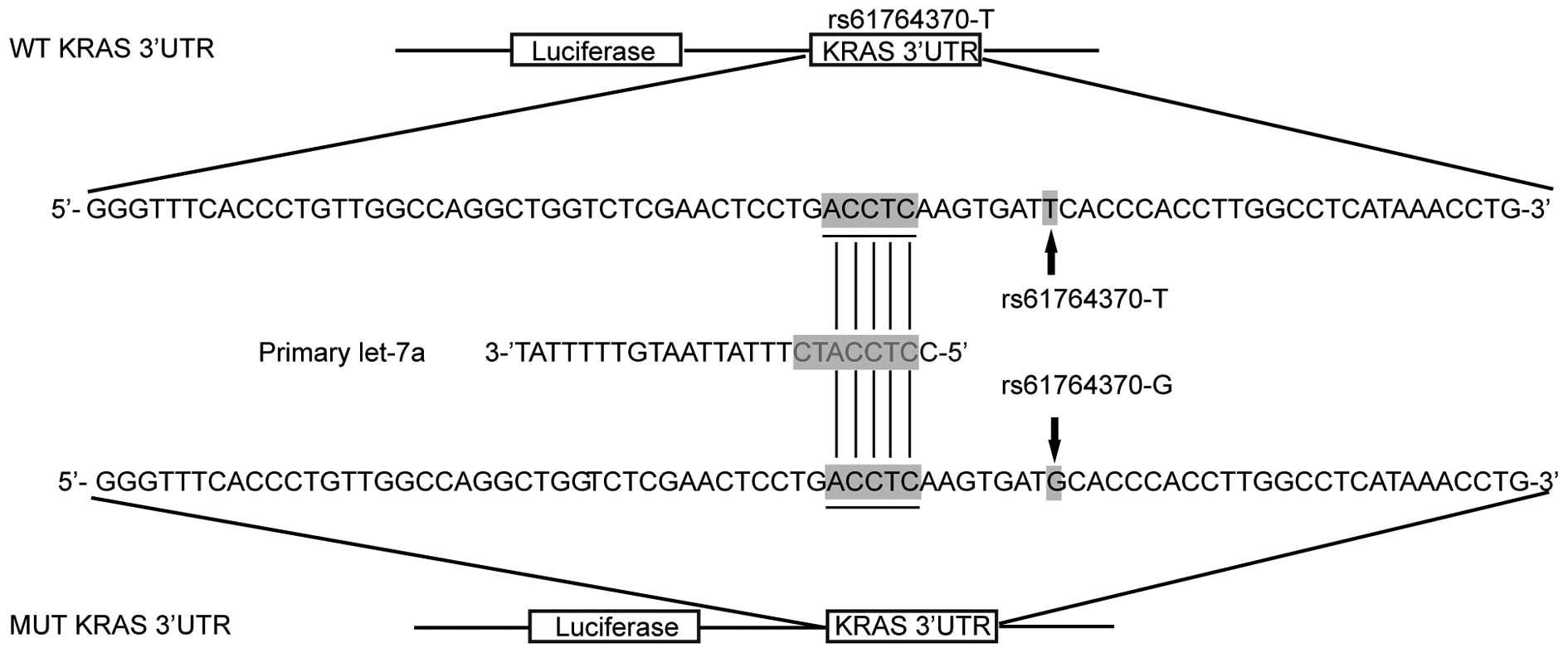
Effect of rs61764370 polymorphism on the
interaction between the KRAS 3′-UTR and let-7a in OS cells
It has been previously shown that KRAS was a target
of let-7a in human cells (15–17). Based on computational analysis,
the KRAS 3′-UTR rs61764370 polymorphism was found to be located in
the flanking sequence close to the predicted 'binding site' for
let-7a (Fig. 1). To determine
whether let-7a targets KRAS 3′-UTR in OS cells, we constructed
reporter vectors carrying wild-type or mutant KRAS 3′-UTR, as
described in Fig. 1.
Subsequently, we used them for transient transfection of the OS
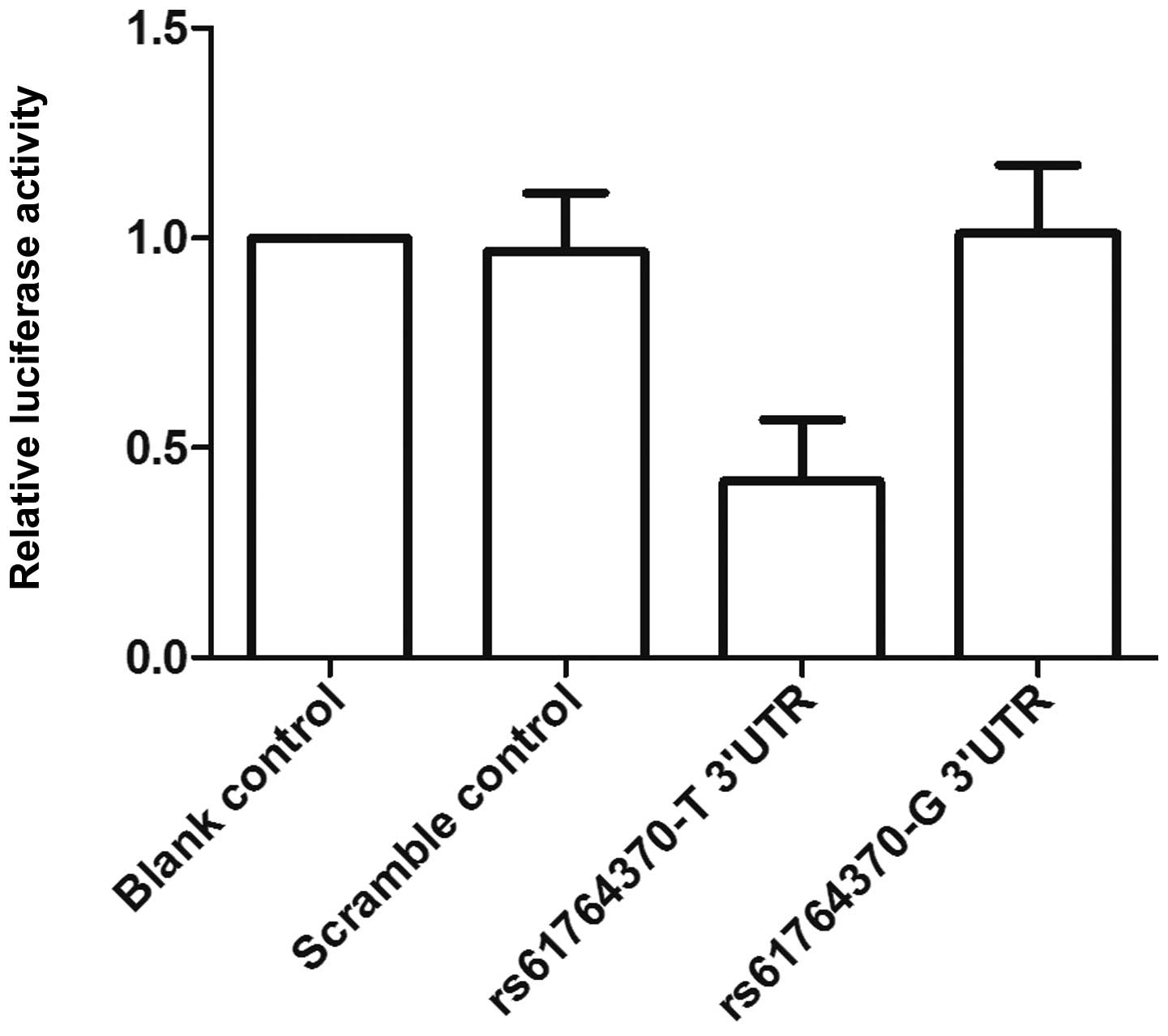
cells with let-7a mimics or scrambled controls. As shown in
Fig. 2, only the luciferase
activity from the cells cotransfected with wild-type KRAS 3′-UTR
and let-7a mimics decreased by 50% compared with the control, and
all other groups were comparable. The results confirmed that KRAS
is a validated target of let-7a in OS cells, and the rs61764370
polymorphism compromised the interaction between let-7a and KRAS
3′-UTR, and the nucleotide substitution completely abrogated the
miRNA/mRNA interaction in OS cells.
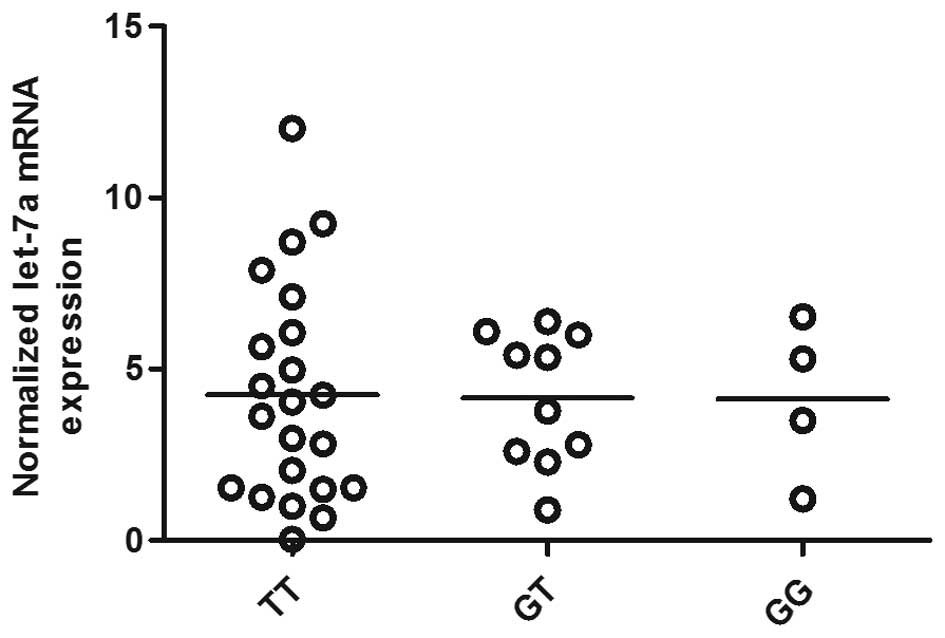
Determination of let-7a and KRAS
expression in OS tissues of different genotypes
The OS tissues of three different genotypes (TT,
n=22, GT, n=10, GG, n=4) were used to further explore the impact of
the polymorphism on the interaction between let-7a and KRAS 3′-UTR.
Using RT-qPCR, we found that let-7a expression was comparable among
the TT, GT and GG groups (Fig.
3). Additionally, to characterize the effect of KRAS 3′-UTR
rs61764370 polymorphism on the miRNA/mRNA interaction in OS cells,
which plays a central role in the development of OS, we quantified
the expression of KRAS using RT-qPCR as well as western blot
analysis. As shown in Fig. 3, the
expression of let-7a was similarly distributed among each genotype
group, whereas in Fig. 4 the mRNA
and protein expression of KRAS in the TT genotype group was
significantly lower than that in the GT and GG groups. We then
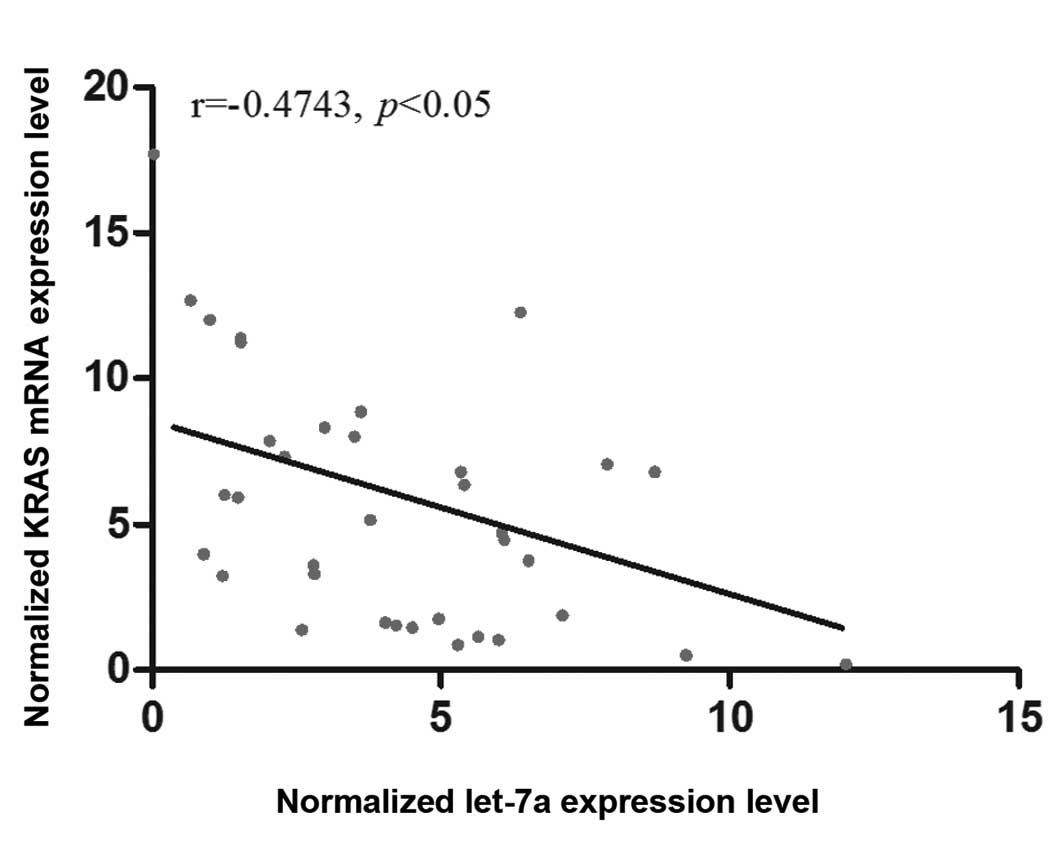
analyzed the correlation between the mRNA expression of let-7a and
KRAS among the OS tissues (n=36), and found a negative correlation
between them (Fig. 5).
To further validate the hypothesis of the negative
regulatory relationship between let-7a and KRAS and to explore the
effect of the polymorphism in such a relationship, we examined the
mRNA/protein expression of KRAS in two primary OS cell cultures
which were genotyped as TT (OSTT) and TG (OSTG). We transfected the
two OS cell groups with either scrambled control, let-7a mimics or
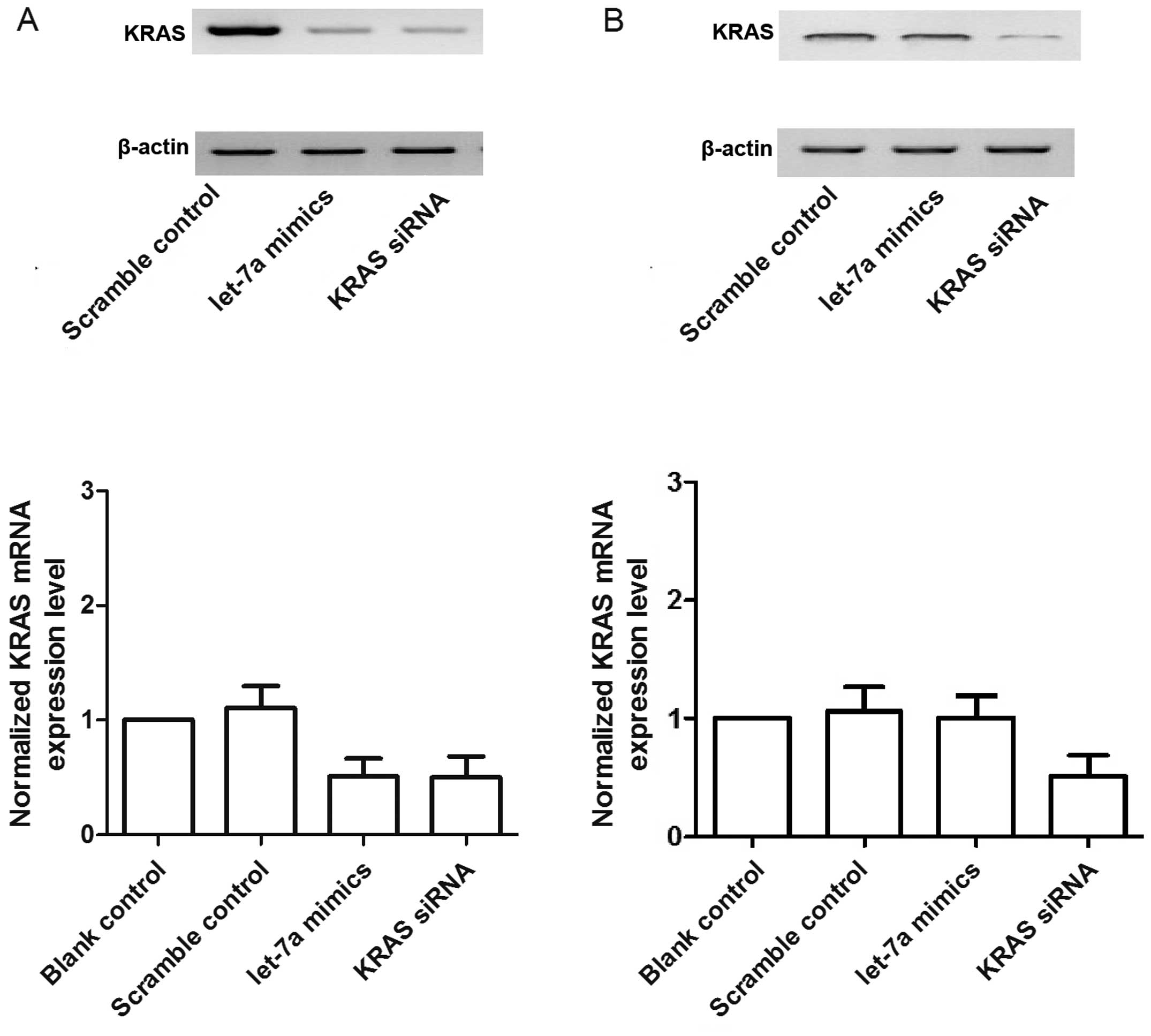
KRAS siRNA. As shown in Fig. 6A and
C, the mRNA and protein expression of KRAS in the OSTT cells
treated with let-7a mimics and KRAS siRNA was lower than that in
the scrambled control. The OSTG cells treated with KRAS siRNA
showed substantial downregulation of the mRNA and protein
expression of KRAS whereas let-7a mimics exerted minimal effects on
the expression of KRAS compared with the control (Fig. 6B).
Let-7a and KRAS affect the
viability of OS cells
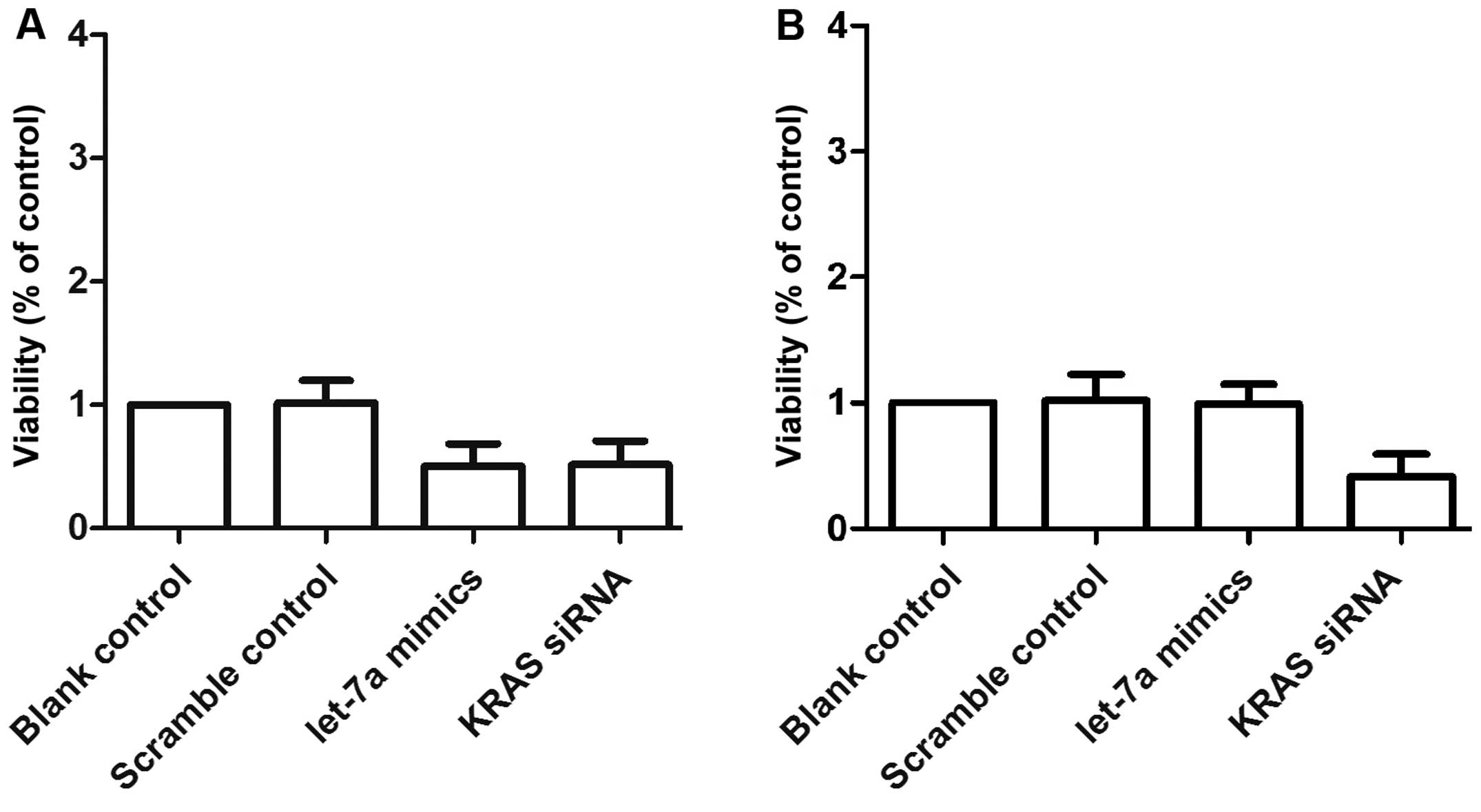
We also examined the relative viability of OS cells
following transfection with either scrambled control, let-7a mimics
or KRAS siRNA. The OSTT cells transfected with let-7a mimics and
KRAS siRNA showed comparably lower viability compared with the
scrambled control group. The viability of the OSTG cells was
comparable between the scrambled control and the let-7a mimic
group, and the viability of both groups was notably higher than
that of KRAS siRNA group (Fig. 7A and
B).
Let-7a and KRAS interfer with the
invasiveness and migratory ability of OS cells
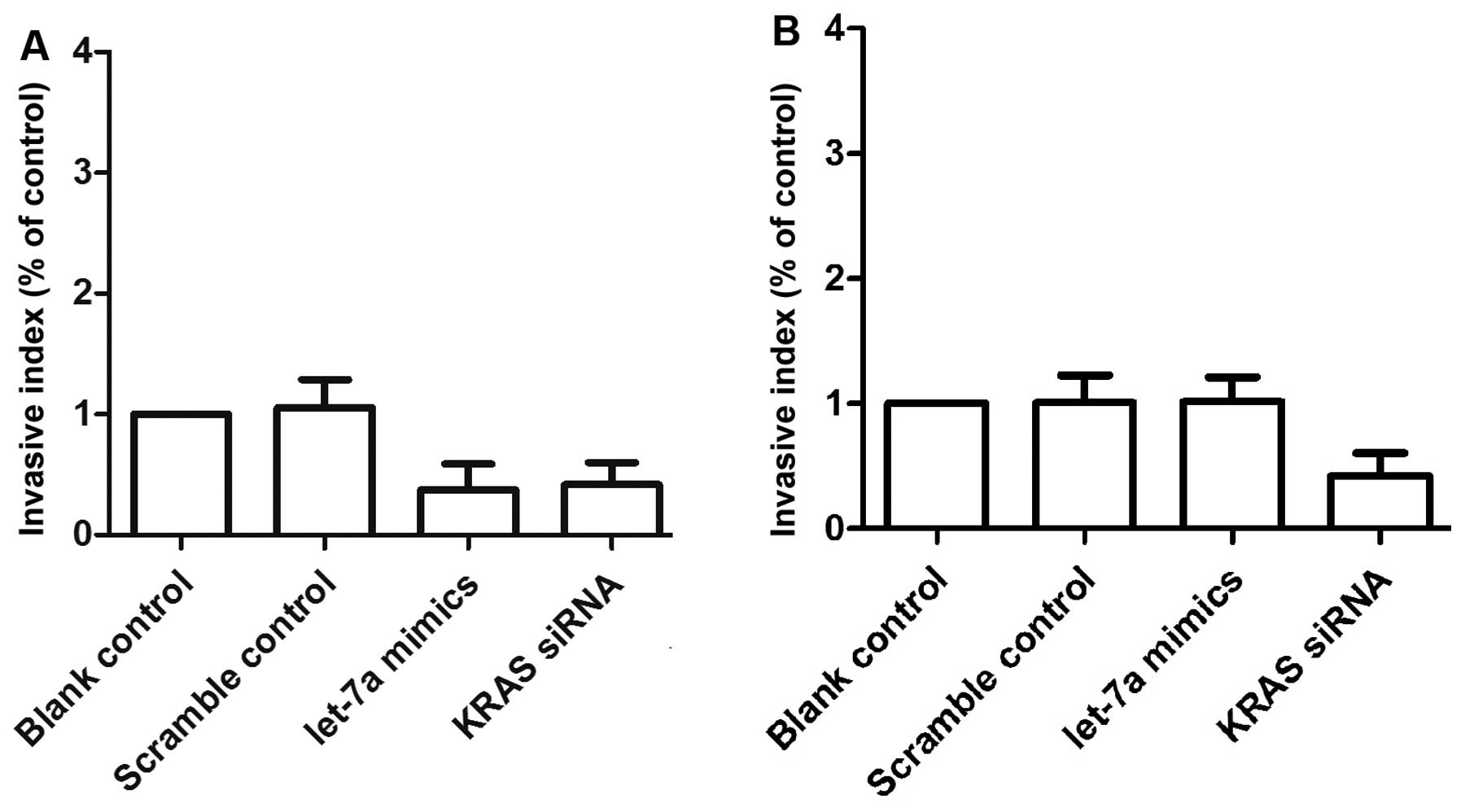
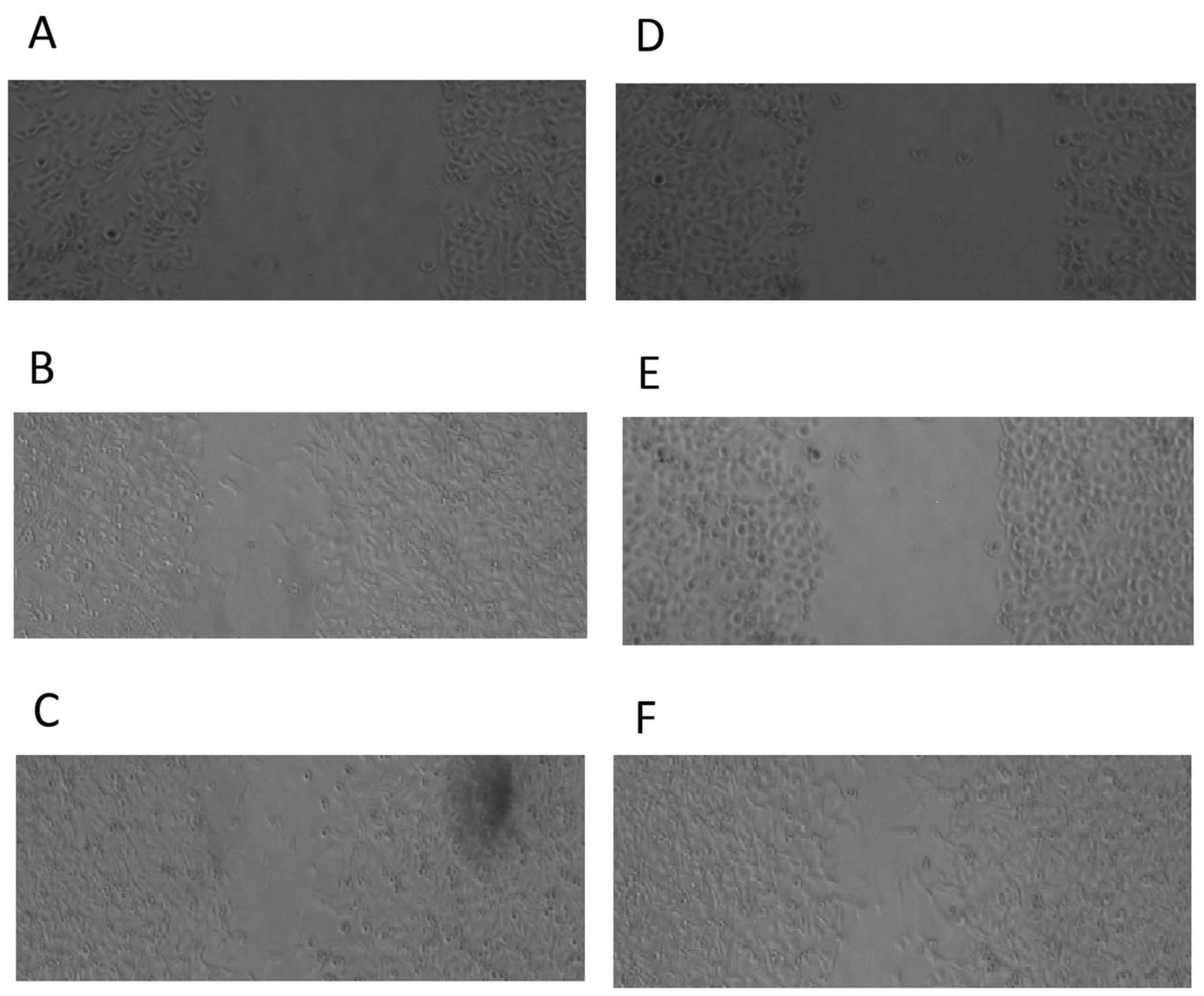
We also examined the relative invasion (Fig. 8) and migration (Fig. 9) of the two primary OS cell
cultures following transfection with either scrambled control,
let-7a mimics or KRAS siRNA. The invasiveness (Fig. 8A) and migratory ability (Fig. 9A–C) of the OSTT cells transfected
with KRAS siRNA and let-7a mimics was notably downregulated when
compared with the scrambled controls. The OSTG cells transfected
with let-7a mimics and scramble controls showed comparable
invasiveness and migratory abilities which were markedly higher
compared with the OSTG cells treated with KRAS siRNA (Fig. 8B and Fig. 9D–F).
Discussion
As the first member of the miRNA family to be
identified, let-7a has been reported to negatively regulate RAS
expression (18). Currently,
numerous in vitro and in vivo studies have identified
members of the let-7 family to be suppressors of tumor cell
proliferation (19). There are 12
miRNAs in the let-7 family in humans, including let-7a which has
been confirmed to be closely associated with the prognosis of
patients with lung cancer; low let-7a miRNA levels usually
correlate with low survival rates (20) implying that let-7a may inhibit the
metastasis, invasion and proliferation of lung cancer cells. Target
genes of let-7a include c-Myc, the high-mobility group AT-Hook 2
(HMGA2) which takes part in cell differentiation and proliferation,
the RAS family, cyclin D2, cyclin-dependent kinase 6 (CDK6) and
cell division cycle 25A (CDC25A) (21). In the present study, we confirmed
that KRAS is a target of let-7a in OS cells, and the introduction
of rs61764370 minor allele into the 3′-UTR of KRAS significantly
compromised the miRNA/mRNA interaction using a luciferase reporter
system.
The major RAS signaling pathway consists of the
mTOR/Akt/PI3K/Ras, p38-MAPK/MEK/Raf/Ras, and ERK/MEK/Raf/Ras
pathways. Overactivation of the ERK/MEK/Raf/Ras pathway has been
reported to account for the lung metastasis of OS in a mouse model
(22). Some researchers believed
that the three Ras signaling pathways function in different ways.
Cancer cell differentiation may be promoted by the MEK/p38 pathway.
The Ras/PI3K/Akt pathway may protect cells against apoptosis
(23). By contrast, the
ERK/MEK/Raf/Ras pathway plays roles in metastasis, tumourigenesis,
and the epithelial-mesenchymal transition (EMT) (24), and it is essential in many stages
of cancer metastasis such as the regulation of
angiogenesis/endothelial apoptosis (25), the EMT, the production of
proteases such as matrix metallopeptidase 9 (MMP-9) and the
expression of integrin receptors binding extracellular matrix
proteins (26). The
ERK/MEK/Raf/Ras pathway has been shown to be upregulated in
approximately 50% of metastatic tumours (27). Significant correlations between
the presence of lymph node metastases and the expression of
activated MEK/ERK were demonstrated by cohort studies of breast
carcinomas (28). In this study,
we analyzed the correlation between the mRNA expression of let-7a
and KRAS among the OS tissues (n=35), and found a negative
correlation between them. In addition, we also showed that let-7a
expression was similar among each genotype group whereas the mRNA
and protein expression of KRAS in the TT genotype group was
significantly lower than that in the GT and GG groups.
Previous studies have noted that the polymorphism
acts as a marker for an increased risk of developing various types
of malignancies such as ovarian carcinoma (29), non-small cell lung cancer (NSCLC)
(30) and triple-negative breast
cancer (12). Furthermore, some
variants have also been identified as adverse prognostic indicators
in some types of cancer (31).
Variants that are associated with miRNA have been reported in both
pre-miRNA and 3′-UTR of the target gene. The one located in the
pre- or pri-miRNA may compromise the generation of mature miRNA by
interfering with the mature processing, undermining its secondary
structure, or alternating the arm selection during the production
of the miRNA (32). However, the
variants in the UTR of the target genes may abolish or attenuate
the inhibitory effect of miRNA on mRNA of the target by disrupting
the binding or interaction between the miRNA and mRNA (33). KRAS 3′-UTR rs61764370 has been
shown to be associated with some cancer phenotypes, such as
prognosis in rectal cancer (34),
and response to chemotherapy in colorectal cancer (35). Its involvement in some other types
of cancer has been ruled out (36). In the present study, we reported
that the mRNA and protein expression of KRAS in the OSTT cells
treated with let-7a mimics and KRAS siRNA was lower than that in
the scrambled control. The OSTG cells treated with KRAS siRNA
showed substantial downregulation of the mRNA and protein
expression of KRAS whereas let-7a mimics exerted minimal effects on
KRAS expression compared with the control. Moreover, the OSTT cells
transfected with KRAS siRNA and let-7a mimics exibited a notable
decrease in invasiveness (fig.
8A) and migratory ability (fig.
9A–C) when compared with the scrambled controls. The OSTG cells
transfected with let-7a mimics and scrambled controls exhibited
comparable invasiveness and migratory abilitiy, which were markedly
higher than that of the OSTG cells treated with KRAS siRNA.
Taken together, these findings suggest that the
protein expression of KRAS is markedly affected by variant
rs61764370 which may explain its role in controlling the metastasis
of OS. Therefore, the rs61764370 polymorphism may function as a
predictive biomarker or therapeutic target in the management of
OS.
References
|
1
|
Arndt CA and Crist WM: Common
musculoskeletal tumors of childhood and adolescence. N Engl J Med.
341:342–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bielack SS, Marina N, Ferrari S, Helman
LJ, Smeland S, Whelan JS and Reaman GH: Osteosarcoma: the same old
drugs or more? J Clin Oncol. 26:3102–3103; author reply 3104–3105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gill J, Ahluwalia MK, Geller D and Gorlick
R: New targets and approaches in osteosarcoma. Pharmacol Ther.
137:89–99. 2013. View Article : Google Scholar
|
|
4
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: state
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrari S and Palmerini E: Adjuvant and
neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr
Opin Oncol. 19:341–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21(Suppl 7): vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bang YJ: Advances in the management of
HER2-positive advanced gastric and gastroesophageal junction
cancer. J Clin Gastroenterol. 46:637–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs - the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu JQ, Liu P, Si MJ and Ding XY:
MicroRNA-126 inhibits osteosarcoma cells proliferation by targeting
Sirt1. Tumour Biol. 34:3871–3877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Yuan X, Chen Y, Du XJ, Yu S and
Yang M: Role of EGFR SNPs in survival of advanced lung
adenocarcinoma patients treated with gefitinib. Gene. 517:60–64.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prior IA, Lewis PD and Mattos C: A
comprehensive survey of Ras mutations in cancer. Cancer Res.
72:2457–2467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paranjape T, Heneghan H, Lindner R, Keane
FK, Hoffman A, Hollestelle A, Dorairaj J, Geyda K, Pelletier C,
Nallur S, et al: A 3′-untranslated region KRAS variant and
triple-negative breast cancer: a case-control and genetic analysis.
Lancet Oncol. 12:377–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu JM, Long XH, Zhang GM, Zhou Y, Chen
XY, Huang SH, Liu ZL and Zhang ZH: Let-7g reverses malignant
phenotype of osteosarcoma cells by targeting Aurora-B. Int J Clin
Exp Pathol. 7:4596–4606. 2014.PubMed/NCBI
|
|
14
|
Pignochino Y, Grignani G, Cavalloni G,
Motta M, Tapparo M, Bruno S, Bottos A, Gammaitoni L, Migliardi G,
Camussi G, et al: Sorafenib blocks tumour growth, angiogenesis and
metastatic potential in preclinical models of osteosarcoma through
a mechanism potentially involving the inhibition of ERK1/2, MCL-1
and ezrin pathways. Mol Cancer. 8:1182009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He XY, Chen JX, Zhang Z, Li CL, Peng QL
and Peng HM: The let-7a microRNA protects from growth of lung
carcinoma by suppression of k-Ras and c-Myc in nude mice. J Cancer
Res Clin Oncol. 136:1023–1028. 2010. View Article : Google Scholar
|
|
16
|
Wang YY, Ren T, Cai YY and He XY: MicroRNA
let-7a inhibits the proliferation and invasion of nonsmall cell
lung cancer cell line 95D by regulating K-Ras and HMGA2 gene
expression. Cancer Biother Radiopharm. 28:131–137. 2013. View Article : Google Scholar
|
|
17
|
Luu C, Heinrich EL, Duldulao M, Arrington
AK, Fakih M, Garcia-Aguilar J and Kim J: TP53 and let-7a micro-RNA
regulate K-Ras activity in HCT116 colorectal cancer cells. PLoS
One. 8:e706042013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar MS, Erkeland SJ, Pester RE, Chen CY,
Ebert MS, Sharp PA and Jacks T: Suppression of non-small cell lung
tumor development by the let-7 microRNA family. Proc Natl Acad Sci
USA. 105:3903–3908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee YS and Dutta A: The tumor suppressor
microRNA let-7 represses the HMGA2 oncogene. Genes Dev.
21:1025–1030. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu Y, Luk F, Yang JL and Walsh WR:
Ras/Raf/MEK/ERK pathway is associated with lung metastasis of
osteosarcoma in an orthotopic mouse model. Anticancer Res.
31:1147–1152. 2011.PubMed/NCBI
|
|
23
|
Song L, Xiong H, Li J, Liao W, Wang L, Wu
J and Li M: Sphingosine kinase-1 enhances resistance to apoptosis
through activation of PI3K/Akt/NF-κB pathway in human non-small
cell lung cancer. Clin Cancer Res. 17:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shirakihara T, Horiguchi K, Miyazawa K,
Ehata S, Shibata T, Morita I, Miyazono K and Saitoh M: TGF-β
regulates isoform switching of FGF receptors and
epithelial-mesenchymal transition. EMBO J. 30:783–795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakabayashi H and Shimizu K: HA1077, a Rho
kinase inhibitor, suppresses glioma-induced angiogenesis by
targeting the Rho-ROCK and the mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase (MEK/ERK) signal
pathways. Cancer Sci. 102:393–399. 2011. View Article : Google Scholar
|
|
26
|
Halaban R, Zhang W, Bacchiocchi A, Cheng
E, Parisi F, Ariyan S, Krauthammer M, McCusker JP, Kluger Y and
Sznol M: PLX4032, a selective BRAF (V600E) kinase inhibitor,
activates the ERK pathway and enhances cell migration and
proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res.
23:190–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wellbrock C, Karasarides M and Marais R:
The RAF proteins take centre stage. Nat Rev Mol Cell Biol.
5:875–885. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sivaraman VS, Wang H, Nuovo GJ and Malbon
CC: Hyperexpression of mitogen-activated protein kinase in human
breast cancer. J Clin Invest. 99:1478–1483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pharoah PD, Palmieri RT, Ramus SJ, Gayther
SA, Andrulis IL, Anton-Culver H, Antonenkova N, Antoniou AC,
Goldgar D, Beattie MS, et al: The role of KRAS rs61764370 in
invasive epithelial ovarian cancer: implications for clinical
testing. Clin Cancer Res. 17:3742–3750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chin LJ, Ratner E, Leng S, Zhai R, Nallur
S, Babar I, Muller RU, Straka E, Su L, Burki EA, et al: A SNP in a
let-7 microRNA complementary site in the KRAS 3′ untranslated
region increases non-small cell lung cancer risk. Cancer Res.
68:8535–8540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nelson HH, Christensen BC, Plaza SL,
Wiencke JK, Marsit CJ and Kelsey KT: KRAS mutation, KRAS-LCS6
polymorphism, and non-small cell lung cancer. Lung Cancer.
69:51–53. 2010. View Article : Google Scholar :
|
|
32
|
Jia Y, Zang A, Shang Y, Yang H, Song Z,
Wang Z, Ren L, Wei Y, Hu L, Shi H and Li H: MicroRNA-146a rs2910164
polymorphism is associated with susceptibility to non-small cell
lung cancer in the Chinese population. Med Oncol. 31:1942014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kong Y, Wu JB, Wang X, Zhao JF, Song H and
Yuan LD: Polymorphism of the OLR1 3′ UTR potential microRNA binding
site and risk of Alzheimer's disease: a meta-analysis. Genet Mol
Res. 13:10162–10172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sclafani F, Chau I, Cunningham D, Peckitt
C, Lampis A, Hahne JC, Braconi C, Tabernero J, Glimelius B,
Cervantes A, et al: Prognostic role of the LCS6 KRAS variant in
locally advanced rectal cancer: results of the EXPERT-C trial. Ann
Oncol. 26:1936–1941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ying HQ, Wang F, He BS, Pan YQ, Gao TY, Xu
YQ, Li R, Deng QW, Sun HL and Wang SK: The involvement of Kras gene
3′ UTR polymorphisms in risk of cancer and influence on patient
response to anti-EGFR therapy in metastatic colorectal cancer: a
meta-analysis. Onco Targets Ther. 7:1487–1496. 2014.
|
|
36
|
Ovarian Cancer Association Consortium;
Breast Cancer Association Consortium; Consortium of Modifiers of
BRCA1 and BRCA2; Hollestelle A, van der Baan FH, Berchuck A,
Johnatty SE, Aben KK, Agnarsson BA, Aittomäki K, Alducci E,
Andrulis IL, Anton-Culver H, et al: No clinical utility of KRAS
variant rs61764370 for ovarian or breast cancer. Gynecol Oncol.
141:386–401. 2016. View Article : Google Scholar
|