Introduction
Retinoblastoma (RB) is the most common intraocular
malignancy in children with an incidence of 1 in 15,000 to 1 in
20,000 births, accounting for approximately 4% of all pediatric
malignancies (1,2). Most cases of unilateral RB are
caused by sporadic somatic mutations in the RB1 gene, representing
approximately 60% of all RB cases whereas about 40% of cases occur
in infants with germline mutations (3). Treatment strategies for RB include
intravenous chemoreduction, enucleation, transpupillary
thermotherapy, cryotherapy, thermotherapy, laser photocoagulation,
brachytherapy, plaque radiotherapy, orbital exenteration, external
beam radiotherapy, and chemotherapy, depending on the stage of
tumor development and the location and size of the primary tumor
(4,5). Despite progress in the treatment of
RB, a number of these treatments have possible side effects, such
as blindness, infection, fever, gastrointestinal toxicity and
neurotoxicity (6). Therefore,
there is an urgent need for the development of novel therapeutic
agents for use in the management of RB.
Curcumin [also known as diferuloylmethane and
1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], is a
naturally occurring polyphenolic compound present in turmeric
(Curcuma longa) which has been employed to treat a number of
diseases including asthma, bronchial hyperactivity, allergy,
anorexia, coryza, cough, sinusitis and hepatic disease in Asian
countries for thousands of years (7). Recently, accumulating evidence has
demonstrated that curcumin exerts antitumor effects on various
types of cancer cells as well as being non-cytotoxic to normal
cells (8–13). However, the precise mechanisms
responsible for the effects of curcumin on RB cells have not been
fully explored. Several studies have reported that curcumin exerts
antitumor effects through inducing apoptosis in a variety of types
of cancer cells which involves the activation of caspases (14), mitochondrial dysfunction triggered
by enhanced Bax levels (15), and
pro-apoptotic endoplasmic reticulum stress (16). Furthermore, a number of studies
have revealed that curcumin exerts antitumor effects through
mediating various cellular signaling pathways including nuclear
factor κB (NF-κB) (17), signal
transducer and activator of transcription 3 (STAT3) (18), protein kinase B (PKB/Akt)
(19), mitogen-activated protein
kinase (MAPK) (20), and other
pathways. In this study, we examined the molecular mechanism
responsible for curcumin-induced cytotoxicity in human RB
cells.
Materials and methods
Cell culture
The human RB cell line Y79 was obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
cells were cultured in RPMI-1640 medium containing 10% fetal bovine
serum, 1% penicillin, and streptomycin (Gibco, Grand Island, NY,
USA) at 37°C in 95% air and 5% CO2.
Reagents and antibodies
Curcumin and ZVAD-FMK were purchased from
Sigma-Aldrich (San Diego, CA, USA). SP600125 was obtained from AG
Scientific, Inc. (San Diego, CA, USA). SB203580 was purchased from
Calbiochem (San Diego, CA, USA). Antibodies against cyclin D3
(ab63535), p21 (ab109520), p27 KIP1 (ab32034), cytochrome c
(ab53056), caspase-3 (ab90437), caspase-9 (ab25758), and GAPDH
(ab37168) were purchased from Abcam (Cambridge, UK). Antibodies
against cyclin-dependent kinase (CDK)2 (#2546), CDK6 (#13331),
c-Jun N-terminal kinase (JNK; #9252), p38 MAPK (#9212), phospho-JNK
(Thr183/Tyr185; #9251), and phosphor-p38 MAPK (Thr180/Tyr182;
#9211) were obtained from Cell Signaling Technology (Danvers, MA,
USA).
Cell treatment
Curcumin was dissolved in dimethylsulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) and diluted immediately prior to
each experiment. Final curcumin concentrations of 10–80 µM
were obtained by dilution in culture media. Controls containing
0.07% DMSO were included in all experiments. In order to inhibit
caspase activity, Y79 cells were treated with ZVAD-FMK at a
concentration of 50 µM for 1 h prior to curcumin treatment.
Y79 cells were treated in the absence or presence of 20 µM
JNK inhibitor (SP600125) or p38 MAPK inhibitor (SB203580) for 1 h,
then treated with 80 µM curcumin for 24 h before examining
the phosphorylation levels of JNK and p38 MAPK.
Measurement of cell viability
The Cell Counting Kit-8 (CCK-8) (Dojindo Molecular
Technologies, Inc., Beijing, China) was used to determine cell
viability. Briefly, cells (5×103/well) were incubated
with curcumin-containing RPMI-1640 in 96-well plates for 24 h, and
then the culture medium was replaced with fresh medium containing
10 ml CCK-8 solution. The cells were further incubated for 2 h at
37°C, and the optical density (OD) at 450 nm was measured.
Cell cycle analysis by flow
cytometry
Y79 cells were seeded at a density of
4×105 in 6-well culture plates, grown overnight in
medium containing 10% FBS, and treated with or without various
concentrations of curcumin (0–80 µM) for 24 h. Cell cycle
analysis was then performed. Briefly, the cells were suspended in
0.5 ml propidium iodide (PI) solution, and incubated for 30 min in
the dark according to the manufacturer's instructions. The cell
cycle distribution was analyzed by flow cytometry
[fluorescence-activated cell sorting (FACS) analysis; BD
Biosciences, San Jose, CA, USA].
Analysis of apoptosis by flow
cytometry
Apoptosis was examined using the Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(BioVision Inc., Mountain View, CA, USA), according to the
manufacturer's instructions.
Western blot analysis
Protein concentrations in the cell extracts were
determined (Bio-Rad, Richmond, CA, USA). Briefly, equal amounts of
each sample were resolved in SDS-PAGE gels and then transferred to
a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica,
MA, USA), and probed with the primary antibodies described above in
Reagents and antibodies. Protein band intensities were quantified
by densitometric analysis using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Determination of mitochondrial membrane
potential (ΔΨm)
Briefly, Y79 cells were exposed to various
concentrations of curcumin (0–80 µM) for 24 h. The cells
were then harvested and incubated with 40 nmol/l DiOC6
(Abcam) at 37°C in the dark for 20 min. Finally, the mean
fluorescence intensity (MFI) was determined by peforming flow
cytometric analysis.
Statistical analysis
The data are expressed as the means ± standard
deviation (SD). Comparisons were made using a one-way ANOVA
followed by Dunnett's test with SPSS software (version 17.0; SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Curcumin significantly inhibits cell
viability in Y79 cells
To explore the effect of curcumin on the cell
viability of RB cells in vitro, human RB (Y79) cells were
treated with varying concentrations of curcumin (0–80 µM)
for 24 h and changes in cell viability were assessed by the CCK-8
assay. As shown in Fig. 1, the
viability of the Y79 cells exposed to curcumin was significantly
lower compared with that of the control cells. A sharp decrease in
cell viability was present at a curcumin concentration of 40
µM.
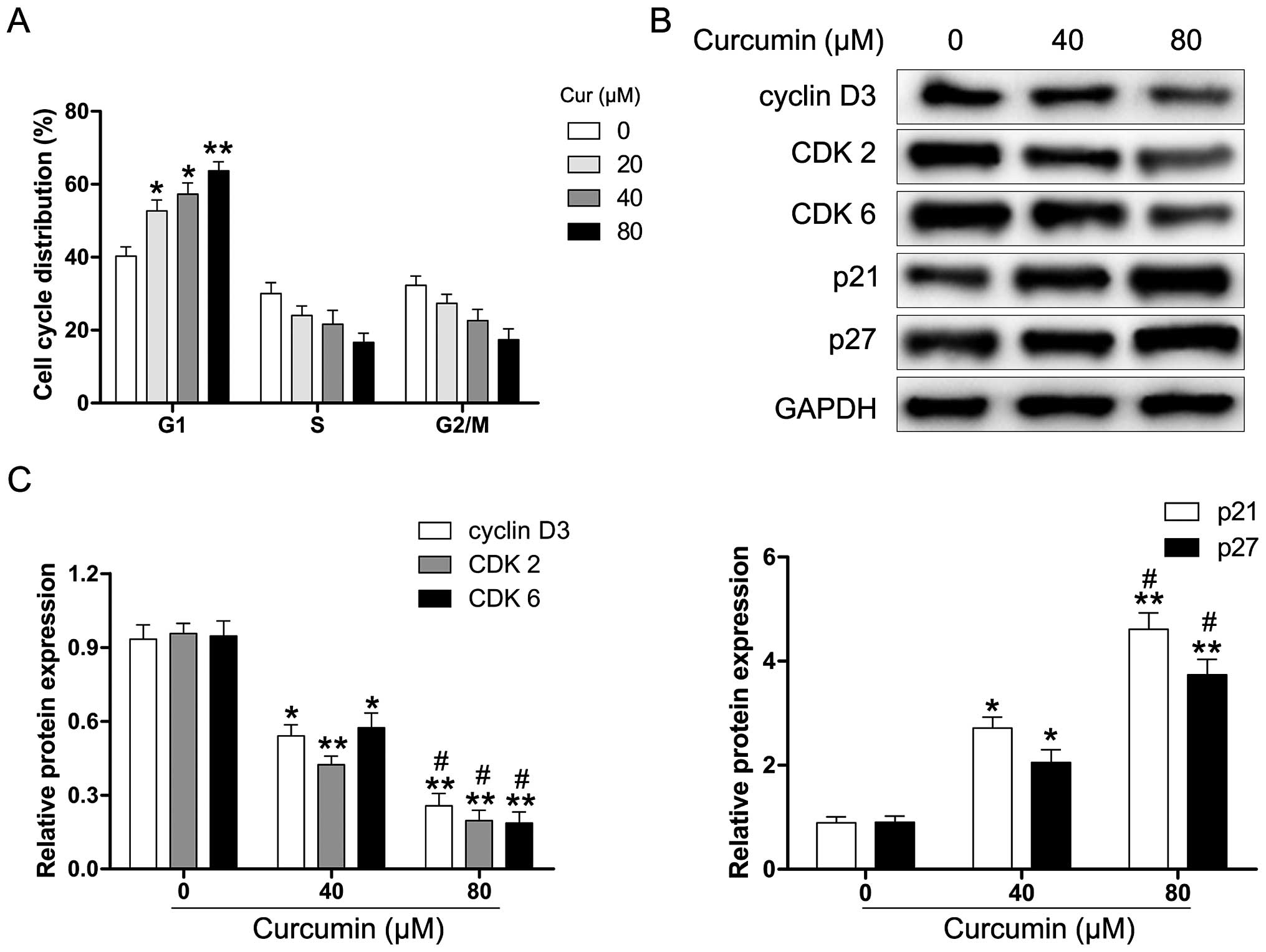
Curcumin induces cell cycle arrest of Y79
cells
We explored whether curcumin induces cell cycle
arrest in human RB cells. The Y79 cells were exposed to various
concentrations of curcumin for 24 h and then analyzed for
alterations in the cell cycle by flow cytometry. As shown in
Fig. 2A, curcumin-treated Y79
cells were inhibited in the G1 phase after 24 h of treatment. To
further examine the molecular mechanisms underlying
curcumin-induced G1 phase arrest, the cells were treated with
various concentrations of curcumin (0–80 µM) for 24 h, and
harvested for protein extraction and western blot analysis. As
shown in Fig. 2B and C, the
protein expression of cyclin D3, CDK2 and CDK6 was markedly reduced
in the curcumin-treated Y79 cells. Moreover, the levels of the CDK
inhibitor proteins p21 and p27 were significantly upregulated
following the exposure of Y79 cells to curcumin. These results
suggest that curcumin-induced G1 phase cell cycle arrest in human
RB cells may be regulated through the cyclin-CDK checkpoint.
Curcumin induces the apoptosis of Y79
cells
To determine whether the effect of curcumin on cell
viability was caused by apoptotic cell death, Y79 cells were
exposed to various concentrations of curcumin (0–80 µM) for
24 h, and the extent of apoptosis was evaulated using the Annexin
V/PI assay. As shown in Fig. 3A and
B, the percentage of apoptotic cells (PI-negative/Annexin
V-positive and PI-positive/Annexin V-positive) increased from 4.13
to 19.59, 29.41, and 40.95%, respectively, after the Y79 cells were
either untreated (control) or treated with 20, 40, and 80 µM
curcumin. DiOC6, a lipophilic cationic dye, has been
reported to specifically accumulate in the mitochondrial matrix
depending on the ΔΨm which is decreased in apoptotic cells
(21,22). To further ascertain the effects of
curcumin on ΔΨm in human RB cells, Y79 cells were exposed to
various concentrations of curcumin (0–80 µM) for 24 h, and
then analyzed in order to determine the MFI of DiOC6 by
flow cytometry. We found that treatment with curcumin significantly
decreased the MFI of DiOC6 (Fig. 3C). The data suggest that
curcumin-induced apoptosis may occur through ΔΨm dissipation in Y79
cells.
Curcumin induces the apoptosis of Y79
cells through intrinsic pathways
Accumulating evidence has revealed that ΔΨm plays an
important role in regulating cellular functions. Disturbances of
ΔΨm may change the membrane dynamics of mitochondria and result in
the release of cytochrome c, which triggers the formation of
the apoptosome complex, and the subsequent activation of caspase-9.
To determine whether curcumin induces apoptosis through the release
of cytochrome c and the activation of caspase-9 in human RB,
Y79 cells were exposed to various concentrations of curcumin (0–80
µM) for 24 h. As shown in Fig.
4A and B, curcumin significantly enhanced cytochrome c
levels in a dose-dependent manner. We also observed that the
released cytochrome c triggered the activation of caspase-9
and caspase-3 (Fig. 4B).
Moreover, we used the pan-caspase inhibitor (ZVAD-FMK) to evaluate
the effect of curcumin on apoptotic cell death in Y79 cells. As
depicted in Fig. 4C,
pre-treatment with ZVAD-FMK attenuated the curcumin-induced
reduction of viability in the Y79 cells. We also found that
ZVAD-FMK attenuated the apoptotic effect of curcumin in the Y79
cells, which suggested that the activation of caspases is involved
in curcumin-regulated apoptosis of human RB cells (Fig. 4D).
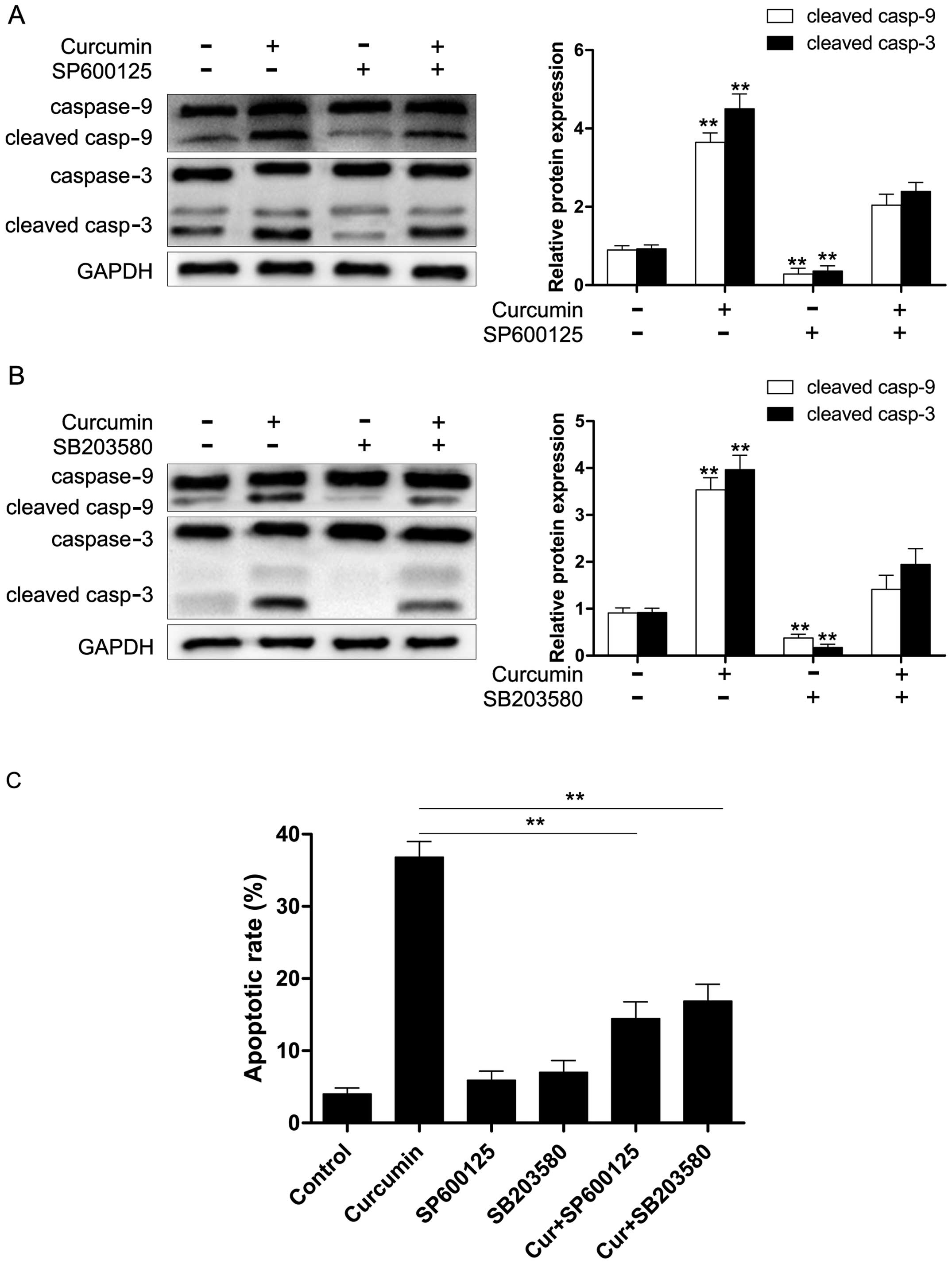
JNK and p38 MAPK signaling play essential
roles in caspase-9/-3 activation induced by curcumin
Previous studies have demonstrated that JNK and p38
MAPK are involved in the effects exerted by curcumin in tumor cells
(10,23). However, the role of MAPKs in
curcumin-induced apoptosis of RB cells was not examined. In this
study, we explored whether JNK and p38 MAPK were activated in
curcumin-treated Y79 cells. As shown in Fig. 5, curcumin induced an increase in
the phosphorylation of JNK and p38 MAPK in the Y79 cells. To
determine whether JNK and p38 MAPK were necessary for
curcumin-induced apoptosis, we examined the relationships among
caspases-9/-3 and JNK, and p38 MAPK in the presence of curcumin.
The Y79 cells were treated in the absence or presence of 20
µM JNK inhibitor (SP600125) or p38 MAPK inhibitor (SB203580)
for 1 h and then treated with 80 µM curcumin for 24 h.
Protein expression was evaluated by western blot analysis. As shown
in Fig. 6, SP600125 and SB203580
markedly suppressed the activation of JNK and p38 MAPK induced by
curcumin. Moreover, we also found that both SP600125 and SB203580
significantly reduced curcumin-induced caspase-9/-3 activation
(Fig. 7A and B) and apoptosis
(Fig. 7C). These results revealed
that the activation of caspase-9/-3 in the presence of curcumin may
occur through the activation of JNK and p38 MAPK in Y79 cells.
Discussion
RB is the most common pediatric eye cancer. It is
second only to uveal melanoma in terms of the frequency of
occurrence of malignant intraocular tumors (24). Although chemotherapy has become an
important part of the present management of RB, it causes
noteworthy complications including secondary malignancies and
results in long-term survival rates that remain low in developing
countries (25,26). Thus, the search for novel
treatment modalities is imperative. Curcumin, a natural
polyphenolic compound, exerts powerful growth inhibitory and
apoptosis-inducing effects on cancer cells through the regulation
of various signaling pathways (27–29). Although the potent anticancer
effects of curcumin have been demonstrated in many types of cancer,
the precise mechanism responsible for the effects of curcumin in
human RB has not been fully explored. In the present study, we
examined whether curcumin may potentially be used in the treatment
of human RB and explored the potential mechanisms responsible for
the anticancer effects of curcumin in Y79 cells.
Our results showed that curcumin reduced cell
viability in a dose-dependent manner in Y79 cells. Cell
proliferation is regulated by the cell cycle, which is a complex
and stepwise process. The activity of CDKs is mediated by cyclin
regulatory subunits. These form a complex with the catalytic
subunit of CDKs and are controlled at a specific phase of the cell
cycle (30,31). In the present study, we found that
curcumin treatment induced an accumulation of Y79 cells in the G1
phase of the cell cycle. We also observed that curcumin reduced the
protein expression of cyclin D3, CDK2 and CDK6, and enhanced the
expression of CDK inhibitor proteins p21 and p27 which suggested
that changes in these protein levels appear to make a major
contribution to curcumin-induced G1 arrest in Y79 cells.
Apoptosis is a major biological process that leads
to specific cell death via an intrinsic 'suicide' mechanism
(32). The loss of ΔΨm has been
reported to induce cytochrome c release which is essential
for the activation of caspase-9 (33). A number of studies have
demonstrated that caspases-9 and -3 play key roles in the apoptotic
cascade (34,35). Our data revealed that curcumin
induced ΔΨm dissipation and activated the caspase-dependent
apoptotic pathway in mitochondria. Moreover, the pan-caspase
inhibitor ZVAD-FMK significantly reduced the curcumin-induced
apoptosis of Y79 cells suggesting that activation of caspase-9/-3
is involved in the curcumin-regulated apoptosis of Y79 cells.
MAPKs consists of several subfamilies, such as
ERK1/2, JNKs and p38. JNK and p38 MAPK are involved in a variety of
cellular responses including cell proliferation, differentiation,
and apoptosis (36–39). Our previous study also revealed
that advanced glycation end products induce the apoptosis of human
corneal epithelial cells through the generation of reactive oxygen
species and the activation of JNK and p38 MAPK pathways (40), and it has also been demonstrated
that the activation of JNK signaling mediates connective tissue
growth factor expression and scar formation in corneal wound
healing (41). However, the role
of JNK and p38 MAPK signaling pathways in curcumin-induced
apoptosis of Y79 cells was not investigated. In this study, we
found that curcumin induced the activation of JNK and p38 MAPK in
Y79 cells. The JNK-specific inhibitor, SP600125, and the p38
MAPK-specific inhibitor, SB203580, suppressed the activation of
caspases-9 and -3, and inhibited the apoptosis of Y79 cells induced
by curcumin. These results suggest that the activation of JNK and
p38 MAPK signaling pathways plays a crucial role in
curcumin-induced apoptosis of Y79 cells by regulating the activity
of caspase-9 and -3.
In conclusion, the present study showed that
curcumin exerts an antitumor effect on human RB cells by inducing
cell cycle arrest and apoptosis. These findings suggest a novel
therapeutic strategy for the management of RB which warrants
further investigation.
Acknowledgments
The authors thank Dr Edward C. Mignot, Shandong
University, for linguistic advice.
References
|
1
|
Villegas VM, Hess DJ, Wildner A, Gold AS
and Murray TG: Retinoblastoma. Curr Opin Ophthalmol. 24:581–588.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Moura LR, Marshall JC, Di Cesare S,
Fernandes BF, Antecka E and Burnier MN: The effect of imatinib
mesylate on the proliferation, invasive ability, and
radiosensitivity of retinoblastoma cell lines. Eye (Lond).
27:92–99. 2013. View Article : Google Scholar
|
|
3
|
Melamud A, Palekar R and Singh A:
Retinoblastoma. Am Fam Physician. 73:1039–1044. 2006.PubMed/NCBI
|
|
4
|
Eagle RC Jr: The pathology of ocular
cancer. Eye (Lond). 27:128–136. 2013. View Article : Google Scholar
|
|
5
|
Dimaras H, Kimani K, Dimba EA, Gronsdahl
P, White A, Chan HS and Gallie BL: Retinoblastoma. Lancet.
379:1436–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsiao WT, Tsai MD, Jow GM, Tien LT and Lee
YJ: Involvement of Smac, p53, and caspase pathways in induction of
apoptosis by gossypol in human retinoblastoma cells. Mol Vis.
18:2033–2042. 2012.PubMed/NCBI
|
|
7
|
He Y, Yue Y, Zheng X, Zhang K, Chen S and
Du Z: Curcumin, inflammation, and chronic diseases: how are they
linked? Molecules. 20:9183–9213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu L, Guo L, Liang Y, Liu X, Jiang L and
Wang L: Curcumin suppresses stem-like traits of lung cancer cells
via inhibiting the JAK2/STAT3 signaling pathway. Oncol Rep.
34:3311–3317. 2015.PubMed/NCBI
|
|
9
|
Jin H, Qiao F, Wang Y, Xu Y and Shang Y:
Curcumin inhibits cell proliferation and induces apoptosis of human
non-small cell lung cancer cells through the upregulation of
miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol
Rep. 34:2782–2789. 2015.PubMed/NCBI
|
|
10
|
Yao Q, Lin M, Wang Y, Lai Y, Hu J, Fu T,
Wang L, Lin S, Chen L and Guo Y: Curcumin induces the apoptosis of
A549 cells via oxidative stress and MAPK signaling pathways. Int J
Mol Med. 36:1118–1126. 2015.PubMed/NCBI
|
|
11
|
Xu X, Chen D, Ye B, Zhong F and Chen G:
Curcumin induces the apoptosis of non-small cell lung cancer cells
through a calcium signaling pathway. Int J Mol Med. 35:1610–1616.
2015.PubMed/NCBI
|
|
12
|
Wu H, Liu Q, Cai T, Chen YD and Wang ZF:
Induction of microRNA-146a is involved in curcumin-mediated
enhancement of temozolomide cytotoxicity against human
glioblastoma. Mol Med Rep. 12:5461–5466. 2015.PubMed/NCBI
|
|
13
|
Zhao Z, Li C, Xi H, Gao Y and Xu D:
Curcumin induces apoptosis in pancreatic cancer cells through the
induction of forkhead box O1 and inhibition of the PI3K/Akt
pathway. Mol Med Rep. 12:5415–5422. 2015.PubMed/NCBI
|
|
14
|
Zhu L, Han MB, Gao Y, Wang H, Dai L, Wen Y
and Na LX: Curcumin triggers apoptosis via upregulation of
Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes.
Mol Med Rep. 12:1151–1156. 2015.PubMed/NCBI
|
|
15
|
Yang SJ, Lee SA, Park MG, Kim JS, Yu SK,
Kim CS, Kim JS, Kim SG, Oh JS, Kim HJ, et al: Induction of
apoptosis by diphenyldifluoroketone in osteogenic sarcoma cells is
associated with activation of caspases. Oncol Rep. 31:2286–2292.
2014.PubMed/NCBI
|
|
16
|
Mathur A, Abd Elmageed ZY, Liu X,
Kostochka ML, Zhang H, Abdel-Mageed AB and Mondal D: Subverting
ER-stress towards apoptosis by nelfinavir and curcumin coexposure
augments docetaxel efficacy in castration resistant prostate cancer
cells. PLoS One. 9:e1031092014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo JJ, Chang HH, Tsai TH and Lee TY:
Curcumin ameliorates mitochondrial dysfunction associated with
inhibition of gluconeogenesis in free fatty acid-mediated hepatic
lipoapoptosis. Int J Mol Med. 30:643–649. 2012.PubMed/NCBI
|
|
18
|
Hu A, Huang JJ, Jin XJ, Li JP, Tang YJ,
Huang XF, Cui HJ, Xu WH and Sun GB: Curcumin suppresses
invasiveness and vasculogenic mimicry of squamous cell carcinoma of
the larynx through the inhibition of JAK-2/STAT-3 signaling
pathway. Am J Cancer Res. 5:278–288. 2014.
|
|
19
|
Peng SF, Lee CY, Hour MJ, Tsai SC, Kuo DH,
Chen FA, Shieh PC and Yang JS: Curcumin-loaded nanoparticles
enhance apoptotic cell death of U2OS human osteosarcoma cells
through the Akt-Bad signaling pathway. Int J Oncol. 44:238–246.
2014.
|
|
20
|
Zhu GH, Dai HP, Shen Q, Ji O, Zhang Q and
Zhai YL: Curcumin induces apoptosis and suppresses invasion through
MAPK and MMP signaling in human monocytic leukemia SHI-1 cells.
Pharm Biol. July 1–2015.Epub ahead of print. PubMed/NCBI
|
|
21
|
Guo Y, Zhang W, Yan YY, Ma CG, Wang X,
Wang C and Zhao JL: Triterpenoid pristimerin induced HepG2 cells
apoptosis through ROS-mediated mitochondrial dysfunction. J BUON.
18:477–485. 2013.PubMed/NCBI
|
|
22
|
Shao Q, Zhao X and Yao L: Matrine inhibits
the growth of retinoblastoma cells (SO-Rb50) by decreasing
proliferation and inducing apoptosis in a mitochondrial pathway.
Mol Biol Rep. 41:3475–3480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao F, Liu T, Xu Y, Xu D and Feng S:
Curcumin inhibits cell proliferation and promotes apoptosis in
human osteoclastoma cell through MMP-9, NF-κB and JNK signaling
pathways. Int J Clin Exp Pathol. 8:6037–6045. 2015.
|
|
24
|
Pandey AN: Retinoblastoma: An overview.
Saudi J Ophthalmol. 28:310–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sarici A, Kizilkilic O, Celkan T and Gode
S: Blue toe syndrome as a complication of intra-arterial
chemotherapy for retinoblastoma. JAMA Ophthalmol. 131:801–802.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suesskind D, Schrader M, Foerster MH,
Ernemann U and Aisenbrey S: Cataract formation: a possible
complication of intra-arterial chemotherapy for retinoblastoma. Eur
J Ophthalmol. 24:449–453. 2014. View Article : Google Scholar
|
|
27
|
Wu J, Tang Q, Zhao S, Zheng F, Wu Y, Tang
G and Hahn SS: Extracellular signal-regulated kinase
signaling-mediated induction and interaction of FOXO3a and p53
contribute to the inhibition of nasopharyngeal carcinoma cell
growth by curcumin. Int J Oncol. 45:95–103. 2014.PubMed/NCBI
|
|
28
|
Fan Z, Duan X, Cai H, Wang L, Li M, Qu J,
Li W, Wang Y and Wang J: Curcumin inhibits the invasion of lung
cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling
pathway. Oncol Rep. 34:691–698. 2015.PubMed/NCBI
|
|
29
|
Kim HJ, Park SY, Park OJ and Kim YM:
Curcumin suppresses migration and proliferation of Hep3B
hepatocarcinoma cells through inhibition of the Wnt signaling
pathway. Mol Med Rep. 8:282–286. 2013.PubMed/NCBI
|
|
30
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jacks T and Weinberg RA: Cell-cycle
control and its watchman. Nature. 381:643–644. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cao A, Li Q, Yin P, Dong Y, Shi H, Wang L,
Ji G, Xie J and Wu D: Curcumin induces apoptosis in human gastric
carcinoma AGS cells and colon carcinoma HT-29 cells through
mitochondrial dysfunction and endoplasmic reticulum stress.
Apoptosis. 18:1391–1402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gupta S, Kass GE, Szegezdi E and Joseph B:
The mitochondrial death pathway: a promising therapeutic target in
diseases. J Cell Mol Med. 13:1004–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang YC, Kuo CL, Lu KW, Lin JJ, Yang JL,
Wu RS, Wu PP and Chung JG: 18α-glycyrrhetinic acid induces
apoptosis of HL-60 human leukemia cells through caspases - and
mitochondria-dependent signaling pathways. Molecules. 21:pii: E872.
2016. View Article : Google Scholar
|
|
35
|
Jiang YQ, Chang GL, Wang Y, Zhang DY, Cao
L and Liu J: Geniposide prevents hypoxia/reoxygenation-induced
apoptosis in H9c2 cells: Improvement of mitochondrial dysfunction
and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell
Physiol Biochem. 39:407–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yiang GT, Yu YL, Lin KT, Chen JN, Chang WJ
and Wei CW: Acetaminophen induces JNK/p38 signaling and activates
the caspase-9-3-dependent cell death pathway in human mesenchymal
stem cells. Int J Mol Med. 36:485–492. 2015.PubMed/NCBI
|
|
37
|
Yu D, Mu S, Zhao D, Wang G, Chen Z, Ren H
and Fu Q: Puerarin attenuates glucocorticoid-induced apoptosis of
hFOB1.19 cells through the JNK- and Akt-mediated mitochondrial
apoptotic pathways. Int J Mol Med. 36:345–354. 2015.PubMed/NCBI
|
|
38
|
Zhen Y, Zhang W, Liu C, He J, Lu Y, Guo R,
Feng J, Zhang Y and Chen J: Exogenous hydrogen sulfide promotes C6
glioma cell growth through activation of the p38 MAPK/ERK1/2-COX-2
pathways. Oncol Rep. 34:2413–2422. 2015.PubMed/NCBI
|
|
39
|
Liu ZG, Jiao XY, Chen ZG, Feng K and Luo
HH: Estrogen receptorβ2 regulates interlukin-12 receptorβ2
expression via p38 mitogen-activated protein kinase signaling and
inhibits non-small-cell lung cancer proliferation and invasion. Mol
Med Rep. 12:248–254. 2015.PubMed/NCBI
|
|
40
|
Shi L, Yu X, Yang H and Wu X: Advanced
glycation end products induce human corneal epithelial cells
apoptosis through generation of reactive oxygen species and
activation of JNK and p38 MAPK pathways. PLoS One. 8:e667812013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi L, Chang Y, Yang Y, Zhang Y, Yu FS and
Wu X: Activation of JNK signaling mediates connective tissue growth
factor expression and scar formation in corneal wound healing. PLoS
One. 7:e321282012. View Article : Google Scholar : PubMed/NCBI
|