Introduction
Diabetes mellitus (DM) has gained increasing
attention globally. Diabetic nephropathy (DN) is one of the most
seriousdiabetic microvascular complications in DM, which leads to
end-stage renal failure, seriously threatening the lives of
patients (1). Recent studies have
shown that oxidative stress plays an important role in the
progression of DN (2,3). Ischemic injury often occurs in
patients with diabetes during the peri-operative period. Diabetic
nephropathy with ischemia/reperfusion (I/R) injury is also closely
related to oxidative stress (4,5).
However, the underlying mechanisms responsible for the adverse
effects caused by oxidative stress on renal injury induced by
hyperglycemia with I/R insults have not yet been completely
elucidated.
Currently, several hypotheses have emerged regarding
DJ-1, which is also known as Park 7. DJ-1 was originally described
as an oncogene (6). It is a
multifunctional protein related to Parkinson's disease,
neurodegeneration and oxidative stress (7,8).
Several lines of evidence have demonstrated the antioxidative
function of DJ-1 in various disease models both in vitro and
in vivo (9-12). In a study on hypertensive
nephropathy, the physiological role of DJ-1 was shown to be
associated with reactive oxygen species (ROS) in primary renal
tubular epithelial cells (13).
Another study demonstrated a compensatory increase in DJ-1
expression in the renal cortex against increased oxidative stress
of the hyperglycemic milieu (14). Thus, it is suggested that DJ-1 may
be effective against oxidative stress and it is increasingly
considered as an important target for DN therapy (15–17).
The protective role of DJ-1 has been indicated in
many organs and tissues, such as the heart, brain, liver, kidneys
and pancreas. It has been shown that DJ-1 exerts neuroprotective
effects against ischemic damage to the spinal cord through its
antioxidant functions (18). The
overexpression of DJ-1 may participate in a protective strategy
against I/R injury-induced oxidative stress in rat heart-derived
H9c2 cells (19). We previously
demonstrated that the hyperglycemia-induced inhibition of DJ-1
expression was implicated in the severity of myocardial I/R injury
(20). However, the potential
mechanisms of action of DJ-1 in renal cells exposed to high glucose
(HG) and hypoxia/reoxygenation (H/R) injury have not yet been fully
clarified. Moreover, DJ-1 can regulate the expression of various
antioxidant genes, including nuclear factor (erythroid-derived
2)-like 2 (Nrf2) (21,22) and heme oxygenase-1 (HO-1),
enhancing the antioxidant ability of cells (23).
In the present study, we hypothesized that the
overexpression of DJ-1 reduced oxidative stress and attenuated H/R
injury in rat proximal tubular epithelial (NRK-52E) cells exposed
to HG. As the antioxidant, N-acetylcysteine (NAC), has been shown
to protect the kidneys against I/R injury by regulating the Nrf2
signaling pathway (24), we
therefore, also examined the effects of NAC and compared them to
those of DJ-1.
Materials and methods
Materials
The following materials were used: NRK-52E cells
(American Tissue Type Culture Collection, Manassas, VA, USA);
Dulbecco's modified Eagle's medium (DMEM; HyClone, Logan, UT, USA);
phosphate-buffered saline (PBS; Gino Biological Medical Technology
Co., Ltd., Hangzhou, China); fetal bovine serum (FBS; Gibco,
Carlsbad, CA, USA); penicillin and streptomycin (Beyotime Institute
of Biotechnology, Haimen, China); 0.25% Trypsin with 0.02%
ethylenediaminetetraacetic acid (EDTA) (Gino Biological Medical
Technology Co., Ltd.); D-Glucose (Sinopharm Chemical Reagent Co.,
Ltd., Shanghai, China); NAC (Sigma, St. Louis, MO, USA); the empty
vector plasmid and pEX-2-EGFP-DJ-1 (GenePharma, Suzhou, China);
Attractene transfection reagent (Qiagen, Valencia, CA, USA). The
following kits were also used: the cell counting kit-8 (CCK-8;
Dojindo, Kumamoto, Japan); lactate dehydrogenase (LDH) kit;
superoxide dismutase (SOD) and malondialdehyde (MDA) kit (both from
Nanjing Jiancheng Bioengineering Institute, Nanjing, China); the
BCA protein assay kit; nuclear and cytoplasmic protein extraction
kit (both from Beyotime Institute of Biotechnology). The antibodies
used are listed as follows: rabbit anti-rat DJ-1 monoclonal
antibody (#5933; Cell Signaling Technology, Danvers, MA, USA);
rabbit anti-rat Nrf2 (sc-722) and HO-1 (sc-10789) polyclonal
antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
rabbit anti-rat β-actin polyclonal antibody (GB13001; Wuhan Goodbio
Technology Co., Ltd., Wuhan, China) and rabbit anti-rat Lamin B1
polyclonal antibody (BA1228; Boster, Wuhan, China). All other
chemicals were obtained from commercial sources and were of highest
grade available.
Cell culture
Rat proximal tubular epithelial (NRK-52E) cells were
maintained in low-glucose DMEM medium (the concentration of glucose
was 5.5 mM), which was supplemented with 10% FBS, 100 U/ml
penicillin and 0.1 mg/ml streptomycin, and the medium was replaced
every 24 h. The cells were subcultured using 0.25% trypsin with
0.02% EDTA after being washed with PBS twice and allowed to grow up
to 70–80% confluency.
The NRK-52E cells were inoculated in 6-well plates
with low-glucose DMEM medium synchronously. When the cells grew to
the appropriate density, they were incubated in low-glucose DMEM
medium without FBS for 24 h, pre-treated for 2 h with NAC (1 mM),
incubated with low glucose concentrations (LG; final concentration,
5.5 mM) and HG (final concentration, 30 mM) for various periods of
time, srespectively, and then exposed to hypoxia (5%
CO2, 1% O2 and 94% N2) for 4 h,
then to reoxygenation (5% CO2, 21% O2 and 74%
N2) for 2 h.
Plasmid transfection
The day prior to transfection, the cells were seeded
in 6-well plates at 30–50% confluence containing 1% serum, 100 U/ml
penicillin and 0.1 mg/ml streptomycin. The cells were 40–80%
confluent on the day of transfection and were transfected with the
plasmids according to the manufacturer's instructions. The empty
vector plasmid and pEX-2-EGFP-DJ-1 (4 µg) were transfected
into the cells without FBS and removed after 12 h with fresh
low-glucose and HG medium added respectively, and then exposed to
hypoxia for 4 h, then to reoxygenation for 2 h.
Determination of cell viability and LDH
assay
The cell suspension (100 µl) was inoculated
in 96-well plates, and the supernatant was collected for the LDH
toxicity emitting experiment. According to the instructions of the
manufacturer of the LDH kit, the absorbance was measured at 450 nm
using a microplate reader (Victor3 1420-050; Perkin Elmer, Waltham,
MA, USA). The medium was removed and the cells were washed twice
with PBS. The fresh medium and 10 µl CCK-8 solution were
added to each well followed by incubation for 2 h in 37°C and 5%
CO2. The absorbance was then measured at 450 nm using a
microplate reader (Victor3 1420-050; Perkin Elmer) and the cell
viability was calculated.
Detection of oxidative stress
The NRK-52E cells were washed 3 times with PBS and
sonicated using an ultrasonic crusher (FB120220; Thermo Fisher
Scientific, Waltham, MA, USA). The cells were examined to determine
the contents of SOD and MDA using the respective kits (from Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
instructions. Cellular protein was measured using the BCA protein
assay kit. The MDA content in the NRK-52E cells was measured by the
colorimetric method (MDA kit; Nanjing Jiancheng Bioengineering
Institute). The absorbance (530 nm) was measured using a microplate
reader (Victor3 1420-050; Perkin Elmer) and the results were
expressed in nmol of MDA/mg protein. SOD activity in the NRK-52E
cells was measured at an optical density at 450 nm according to the
WST-1 method (SOD kit; Nanjing Jiancheng Bioengineering Institute).
The results were expressed as U/mg protein and 1 unit of enzyme is
defined as the enzyme activity that inhibits the autoxidation of
pyrogallol by 50%.
Western blot analysis
The cells were washed 3 times with PBS and
trypsinized after almost completely covering the bottom of the
wells. Cell suspensions were centrifuged for 5 min at 1,000 rpm,
after which the supernatant was discarded. Total cellular proteins
were extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Nuclear and cytoplasmic proteins were extracted
according to the manufacturer's instructions (nuclear and
cytoplasmic protein extraction kit; Beyotime Institute of
Biotechnology). An equal amount of protein was loaded onto sodium
dodecyl sulfate (SDS) polyacrylamide gels, electrophoresed and
transferred to PVDF membranes. To prevent non-specific background
binding of the antibody, the membranes were blocked by using 5% BSA
and incubated for 2 h at room temperature under agitation. The
membranes were then incubated with the specific rabbit anti-rat
DJ-1 (1:1,000), Nrf2 (1:200), HO-1 (1:200), β-actin (1:2,000) and
Lamin B1 (1:200) antibodies overnight at 4°C. After repeated
washing, the membranes were incubated with the corresponding goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(1:10,000; IRDye 800CW, LI-COR Biosciences, Lincoln, NE, USA) for 1
h at room temperature. The intensity of the identified bands
accomplished with chemiluminescence was detected on an Odyssey
two-color infrared laser imaging system (Li-Cor, Lincoln, NE USA)
and densitometry was carried out using Odyssey software.
Statistical analysis
All data are presented as the means ± SEM. All
statistical analyses were performed using GraphPad Prism 5.0
software (GraphPad Software, La Jolla, CA, USA). Comparisons
between multiple groups were evaluated by one-way analysis of
variance (ANOVA) and comparisons between 2 groups by the Student's
unpaired t-test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of HG on cell viability,
oxidative stress and DJ-1 expression in NRK-52E cells over
time
We first examined the effects of HG on cell
viability and oxidative injury in NRK-52E cells over time. Both
cell viability measured by CCK-8 assay and the antioxidant activity
measured by SOD activity assay in the NRK-52E cells were
significantly decreased following 48 h of exposure to HG, with a
decreasing tendency continuing 72–96 h after initial the HG
exposure (Fig. 1A and B). By
contrast, the MDA content was significantly increased at 48 h, and
further increased 72–96 h following exposure to HG (Fig. 1C).
To investigate the role of DJ-1 in NRK-52E cells
exposed to HG, we also measured the protein expression of DJ-1.
DJ-1 expression did not significantly increase until 48 h, a peak
increase occurred at 72 h, and DJ-1 expression was reduced to basal
levels within 96 h following exposure to HG (Fig. 1D). Thus, we selected the duration
of exposure to HG to be 48 h for our subsequent experiments. The
osmotic control, mannitol, exerted no effects on cell viability,
injury and DJ-1 expression (data not shown).
Effects of H/R on cell viability,
oxidative stress and DJ-1 expression in NRK-52E cells exposed to
HG
As the diabetic kidneys are more vulnerable to I/R
injury (25,26), we thus wished to examine the
effects of H/R on NRK-52E cells following exposure to HG. H/R
significantly decreased the levels of CCK-8 and SOD activity as
compared to the LG control or HG control groups not subjected to
H/R (Fig. 2A and B). Following
exposure to HG for 48 h, H/R further decreased the levels of CCK-8
and SOD activity (P<0.05, LG + H/R vs. HG + H/R). By contrast,
the MDA content in the HG control group was much higher than that
in the LG control group. H/R further increased the MDA content as
compared with that in the LG control or HG control groups not
subjected to H/R (Fig. 2C). Of
note, H/R significantly increased DJ-1 protein expression in the LG
control group, but decreased DJ-1 protein expression in the NRK-52E
cells following exposure to HG for 48 h (Fig. 2D). Thus, the decreased expression
of DJ-1 may be involved in the vulnerability to renal I/R injury in
diabetes.
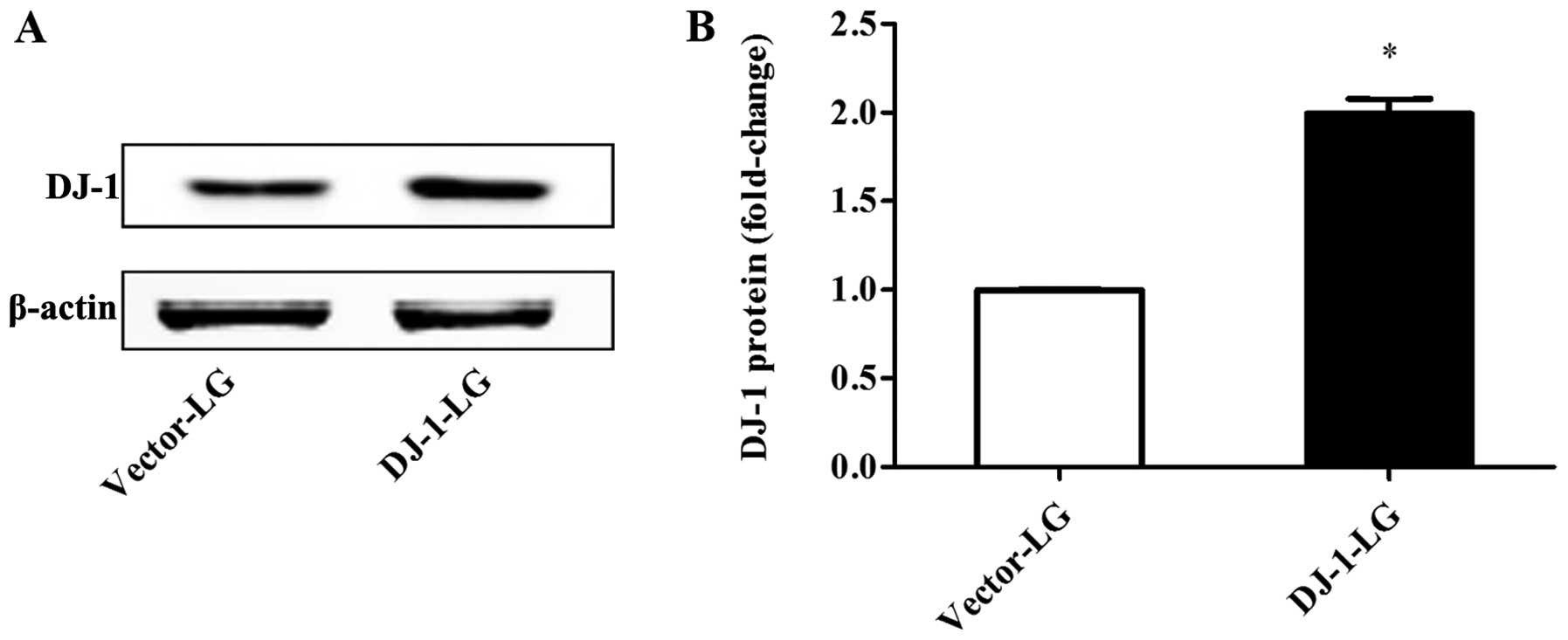
Overexpression of DJ-1 in NRK-52E
cells
To confirm the role of DJ-1 in H/R injury in renal
cells, we transfected the NRK-52E cells with a DJ-1 overexpression
vector (pEX-2-EGFP-DJ-1; DJ-1 group) or an empty vector plasmid as
the negative control group (vector group). The protein expression
of DJ-1 was significantly increased following transfection with the
overexpressio plasmid, as compared with that in the vector group
(Fig. 3).
Effects of DJ-1 overexpression and NAC on
H/R injury in NRK-52E cells following exposure to HG
To examine the molecular mechanisms of action of
DJ-1 under HG and H/R conditions, we also treated the cells with
the antioxidant, NAC. Both DJ-1 overexpression and NAC had no
significant effects on CCK-8, LDH release, MDA content and SOD
activity in the NRK-52 cells under LG conditions (Fig. 4). Exposure to HG significantly
decreased the levels of CCK-8 and SOD activity, but significantly
increased LDH release and the MDA content as compared with that in
the LG group. All these changes were further intensified by H/R.
However, DJ-1 overexpression and treatment with the antioxidant,
NAC, significantly attenuated or reversed the increase in LDH
release and the MDA content, and elevated the levels of CCK-8 and
SOD activity.
Effects of DJ-1 overexpression and NAC on
the protein expression of Nrf2 and HO-1
After the NRK-52E cells were transfected with
pEX-2-EGFP-DJ-1, the protein expression levels of DJ-1, Nrf2 and
HO-1 were significantly increased as compared with the
corresponding control group transfected with the empty vector
(Fig. 5A, C and E). In addition,
treatment with NAC attenuated the HG-induced increase in DJ-1, Nrf2
and HO-1 expression, and attenuated the decrease in the protein
expression of DJ-1 and Nrf2 following exposure to HG and H/R
(Fig. 1B, D and F).
Discussion
The present observations provide several pieces of
important evidence supporting the protective effects of DJ-1
against oxidative stress in response to HG and H/R injury in renal
cells. Firstly, it was demonstrated that HG decreased cell
viability and SOD activity, but increased the MDA content, with
DJ-1 protein expression increasing compensatively within 72 h of
exposure to HG. Furthermore, H/R injury markedly decreased cell
viability and increased oxidative stress in the cells exposed to
HG, but decreased DJ-1 protein expressoin. Finally, the
overexpression of DJ-1 exerted antioxidant effects against HG and
H/R injury, which were similar to the effects of NAC, promoting the
protein expression of Nrf2 and HO-1. In short, the overexpression
of DJ-1 reduced oxidative stress and attenuated H/R injury in
NRK-52E cells exposed to HG.
It has been suggested that increased oxidative
stress is recognized as the key factor in the pathogenesis and
progression of DN (27–29). It has been indicated that HG
enhances oxidative stress in renal cell injury (30). As an end-product of lipid
peroxidation, MDA is widely used to detect the influence of
oxidative stress on the mitochondria respiratory chain (31), while SOD is an antioxidant,
protecting cells against oxidative stress. In the present study,
the viability of NRK-2E cells was significantly decreased in a
time-dependent manner following exposure to HG. A significant
increase in MDA content and a decrease in SOD activity induced by
HG were also observed. These results demonstrate that the
antioxidant activity was weakened in parallel with the severity of
NRK-52E cell injury. The results mentioned above indicate that the
model of renal injury in oxidative stress was successfully
established, which may be considered as an early-stage cell model
of DN.
DJ-1 is an ubiquitous cytoprotective protein, and
acts as an antioxidant to scavenge ROS in various cells. It has
been shown that DJ-1 protects the morphology and function of the
mitochondria and protects against cell injury (32). With the increase in blood glucose
levels, the content of DJ-1 has been shown to increase in
pancreatic β-cells, to inhibit the production of ROS (33). The antioxidant function of DJ-1
maintains the integrity and physiological characteristics of the
mitochondria, which is a prerequisite for glucose-stimulated
insulin secretion. However, the mechanisms responsible for the
protective effects of DJ-1 on renal tubular epithelial cells under
HG conditions remain unclear. Our data demonstrated that the
protein expression of DJ-1 was compensatively increased in a
time-dependent manner and was significantly enhanced until the 72-h
time period, but was decreased at the later time period of 96 h in
the NRK-52E cells exposed to HG. Based on our results of the
examination of cell injury and oxidative stress, we hypothesized
that DJ-1 protein expression may be compensatively increased due to
its antioxidant function in renal cells during the earlier time
periods, but it is decreased following its depletion over a longer
periods of time, and may thus be insufficient to protect against
more severe oxidative stress. It can be considered that DJ-1 plays
an important role in oxidative stress induced by HG in renal
cells.
In the clinical peri-operative period, I/R injury
occurs quite frequently, producing redundant ROS, leading to a
multi-organ oxidative stress, including that in the kidneys
(34). It has been shown that in
rat renal I/R injury, the disrusption of mitochondrial metabolism
and oxidative stress, results in ventricular function disorder, and
leads to cardiac and renal injury (35). High glucose levels have been shown
to highly associated with a a lower tolerance to ischemia, and an
increased severity of renal I/R injury (36,37). The present study demonstrated that
the viability of renal cells in response to H/R decreased, and the
oxidative stress level increased, while the cells exposed to HG and
H/R exhibited more severe oxidative stress. It is inferred that in
the peri-operative period, non-diabetic patients may suffer from
oxidative stress injury induced by I/R; however, patients with
diabetes may suffer more severely, and this matter requires more
attention. We have already reported that cardiac DJ-1 expression
was downregulated in hyperglycemia-induced I/R injury (20). However, there is little evidence
of the association between I/R injury and DJ-1 in DN. In the
present study, compared with the LG group, the NRK-52E cells in the
LG + H/R group exhibited a much higher protein expression of DJ-1.
However, compared with the HG group, the HG + H/R group exhibited a
much lower protein expression of DJ-1. All the above-mentioned data
indicated that HG or H/R led to oxidative stress injury, which
caused a compensatory increase in the protein expression of DJ-1
during the early stage. However, in the HG + H/R group, DJ-1
expression could not be maintained at higher, compensatory levels
but was decreased, leading to more severe oxidative stress. This
evidence proves DJ-1 to be vitally important for diabetic renal I/R
injury in the peri-operative period.
Oxidative stress is involved in cellular injury in
kidney, and overexpression of antioxidant proteins and treatment
with antioxidants prevents renal damage. To further assess the
cytoprotective function of DJ-1, we examined the overexpression of
DJ-1 in H/R exposed to HG compared with the antioxidant NAC.
Plasmid-derived overexpression of DJ-1 showed the anti-oxidative
effect on high glucose and H/R injury. Interestingly, NAC played
the similar role of DJ-1 overexpression in protecting cells against
oxidative stress and increased DJ-1 expression during H/R injury
exposed to HG. These results suggest that overexpression of DJ-1
can be partially explained by its inhibitory effects on oxidative
stress induced by HG and H/R, yet weaker than NAC treatment.
Several enzymes and signaling pathways are related
the antioxidative functions of DJ-1. It has been shown that renal
I/R leads to increased apoptosis and oxidative stress in renal
tubular epithelial cells, accompanied by the increased protein
expression of Nrf2 and HO-1 (38). The activation of the DJ-1/Nrf2
pathway was found to exert a significant protective effect against
oxidative stress (21,39). The overexpression of DJ-1 reduces
the ubiquitination of Nrf2 to stabilize the Nrf2 protein level,
whereas the knockdown of DJ-1 decreases the stability of Nrf2
protein (40). Our results
revealed that the overexpression of DJ-1 promoted the expression of
Nrf2 and HO-1 following exposure to HG and H/R injury. To gain
insight into the potential mechanisms responsible for the
protective effects of DJ-1 against H/R injury, we further evaluated
the role of the DJ-1/Nrf2 pathway by treating the cells with NAC.
We observed a significant increase in the protein expression levels
of DJ-1, Nrf2 and HO-1 in the cells in the HG + H/R group treated
with NAC. All the above-mentioned findings indicate that the
upregulation of DJ-1 may attenuate the progression of diabetic
renal I/R injury, and is associated with a significant increase in
the expression levels of Nrf2 and HO-1.
In conclusion, the findings of the present study
demonstrates that the overexpression of DJ-1 reduces oxidative
stress and attenuates H/R injury in NRK-52E rat proximal tubular
epithelial cells exposed to HG.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81170768, 81300674 and
81501648). We would like to thank the Central Laboratory, Renmin
Hospital of Wuhan University (Wuhan, Hubei, China) for their
support of our study.
References
|
1
|
Radcliffe NJ, Seah JM, Clarke M, MacIsaac
RJ, Jerums G and Ekinci EI: Clinical predictive factors in diabetic
kidney disease progression. J Diabetes Investig. April 25–2016.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verma AK, Chandra S, Singh RG, Singh TB,
Srivastava S and Srivastava R: Serum prolidase activity and
oxidative stress in diabetic nephropathy and end stage renal
disease: A correlative study with glucose and creatinine. Biochem
Res Int. 2014:2914582014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li B, Liu S, Miao L and Cai L: Prevention
of diabetic complications by activation of Nrf2: Diabetic
cardiomyopathy and nephropathy. Exp Diabetes Res. 2012:2165122012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
László E, Kiss P, Horváth G, Szakály P,
Tamás A and Reglődi D: The effects of pituitary adenylate cyclase
activating polypeptide in renal ischemia/reperfusion. Acta Biol
Hung. 65:369–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tawfik MK: Renoprotective activity of
telmisartan versus pioglitazone on ischemia/reperfusion induced
renal damage in diabetic rats. Eur Rev Med Pharmacol Sci.
16:600–609. 2012.PubMed/NCBI
|
|
6
|
Nagakubo D, Taira T, Kitaura H, Ikeda M,
Tamai K, Iguchi-Ariga SM and Ariga H: DJ-1, a novel oncogene which
transforms mouse NIH3T3 cells in cooperation with ras. Biochem
Biophys Res Commun. 231:509–513. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aleyasin H, Rousseaux MW, Marcogliese PC,
Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P,
Callaghan SM, et al: DJ-1 protects the nigrostriatal axis from the
neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad
Sci USA. 107:3186–3191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim RH, Peters M, Jang Y, Shi W, Pintilie
M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, et al: DJ-1, a
novel regulator of the tumor suppressor PTEN. Cancer Cell.
7:263–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilson MA: The role of cysteine oxidation
in DJ-1 function and dysfunction. Antioxid Redox Signal.
15:111–122. 2011. View Article : Google Scholar :
|
|
10
|
Billia F, Hauck L, Grothe D, Konecny F,
Rao V, Kim RH and Mak TW: Parkinson-susceptibility gene DJ-1/PARK7
protects the murine heart from oxidative damage in vivo. Proc Natl
Acad Sci USA. 110:6085–6090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taira T, Saito Y, Niki T, Iguchi-Ariga SM,
Takahashi K and Ariga H: DJ-1 has a role in antioxidative stress to
prevent cell death. EMBO Rep. 5:213–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cuevas S, Yang Y, Konkalmatt P, Asico LD,
Feranil J, Jones J, Villar VA, Armando I and Jose PA: Role of
nuclear factor erythroid 2-related factor 2 in the oxidative
stress-dependent hypertension associated with the depletion of
DJ-1. Hypertension. 65:1251–1257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cuevas S, Zhang Y, Yang Y, Escano C, Asico
L, Jones JE, Armando I and Jose PA: Role of renal DJ-1 in the
pathogenesis of hypertension associated with increased reactive
oxygen species production. Hypertension. 59:446–452. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moraes R, Valente RH, Leon IR, Trugilho
MR, Pacheco AG, Nobrega AC, Gomes MB, Perales J and Tibirica E:
Alterations of the kidney cortex proteome in response to exercise
training in normoglycemic and hyperglycemic conditions. Curr Top
Med Chem. 14:450–461. 2014. View Article : Google Scholar
|
|
15
|
Eltoweissy M, Dihazi GH, Müller GA, Asif
AR and Dihazi H: Protein DJ-1 and its anti-oxidative stress
function play an important role in renal cell mediated response to
profibrotic agents. Mol Biosyst. 12:1842–1859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Q, Shen ZY, Meng QT, Liu HZ, Duan WN
and Xia ZY: The role of DJ-1/Nrf2 pathway in the pathogenesis of
diabetic nephropathy in rats. Ren Fail. 38:294–304. 2016.
View Article : Google Scholar
|
|
17
|
Merikallio H, Pääkkö P, Kinnula VL, Harju
T and Soini Y: Nuclear factor erythroid-derived 2-like 2 (Nrf2) and
DJ1 are prognostic factors in lung cancer. Hum Pathol. 43:577–584.
2012. View Article : Google Scholar
|
|
18
|
Kim W, Kim DW, Jeong HJ, Yoo DY, Jung HY,
Nam SM, Kim JH, Choi JH, Won MH, Yoon YS, et al: Tat-DJ-1 protects
neurons from ischemic damage in the ventral horn of rabbit spinal
cord via increasing antioxidant levels. Neurochem Res. 39:187–193.
2014. View Article : Google Scholar
|
|
19
|
Yu HH, Xu Q, Chen HP, Wang S, Huang XS,
Huang QR and He M: Stable overexpression of DJ-1 protects H9c2
cells against oxidative stress under a hypoxia condition. Cell
Biochem Funct. 31:643–651. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M, Zhou B, Xia ZY, Zhao B, Lei SQ,
Yang QJ, Xue R, Leng Y, Xu JJ and Xia Z: Hyperglycemia-induced
inhibition of DJ-1 expression compromised the effectiveness of
ischemic postcon-ditioning cardioprotection in rats. Oxid Med Cell
Longev. 2013:5649022013. View Article : Google Scholar
|
|
21
|
Clements CM, McNally RS, Conti BJ, Mak TW
and Ting JP: DJ-1, a cancer- and Parkinson's disease-associated
protein, stabilizes the antioxidant transcriptional master
regulator Nrf2. Proc Natl Acad Sci USA. 103:15091–15096. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jung KA, Choi BH, Nam CW, Song M, Kim ST,
Lee JY and Kwak MK: Identification of aldoketo reductases as
NRF2-target marker genes in human cells. Toxicol Lett. 218:39–49.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lev N, Barhum Y, Ben-Zur T, Melamed E,
Steiner I and Offen D: Knocking out DJ-1 attenuates astrocytes
neuroprotection against 6-hydroxydopamine toxicity. J Mol Neurosci.
50:542–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Zhu Z, Liu J, Zhu Z and Hu Z:
Protective effect of N-acetylcysteine (NAC) on renal
ischemia/reperfusion injury through Nrf2 signaling pathway. J
Recept Signal Transduct Res. 34:396–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fouad AA, Al-Mulhim AS, Jresat I and Morsy
MA: Protective effects of captopril in diabetic rats exposed to
ischemia/reperfusion renal injury. J Pharm Pharmacol. 65:243–252.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou SP, Liao WT, Yang LK and Sun L:
Effects of sevoflurane pretreatment on renal Src and FAK expression
in diabetic rats after renal ischemia/reperfusion injury. Mol Cell
Biochem. 384:203–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Forbes JM, Coughlan MT and Cooper ME:
Oxidative stress as a major culprit in kidney disease in diabetes.
Diabetes. 57:1446–1454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamagishi S and Matsui T: Advanced
glycation end products, oxidative stress and diabetic nephropathy.
Oxid Med Cell Longev. 3:101–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Zhao Y, Chu Q, Wang ZY, Li H and
Chi ZH: Zinc modulates high glucose-induced apoptosis by
suppressing oxidative stress in renal tubular epithelial cells.
Biol Trace Elem Res. 158:259–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gutteridge JM and Halliwell B: The
measurement and mechanism of lipid peroxidation in biological
systems. Trends Biochem Sci. 15:129–135. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin TK, Liou CW, Chen SD, Chuang YC, Tiao
MM, Wang PW, Chen JB and Chuang JH: Mitochondrial dysfunction and
biogenesis in the pathogenesis of Parkinson's disease. Chang Gung
Med J. 32:589–599. 2009.PubMed/NCBI
|
|
33
|
Jain D, Jain R, Eberhard D, Eglinger J,
Bugliani M, Piemonti L, Marchetti P and Lammert E: Age- and
diet-dependent requirement of DJ-1 for glucose homeostasis in mice
with implications for human type 2 diabetes. J Mol Cell Biol.
4:221–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szeto HH: Mitochondria-targeted
cytoprotective peptides for ischemia-reperfusion injury. Antioxid
Redox Signal. 10:601–619. 2008. View Article : Google Scholar
|
|
35
|
Tai ST, Fu YH, Yang YC and Wang JJ: Niacin
ameliorates kidney warm ischemia and reperfusion injury-induced
ventricular dysfunction and oxidative stress and disturbance in
mitochondrial metabolism in rats. Transplant Proc. 47:1079–1082.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Caetano AM, Vianna Filho PT, Castiglia YM,
Golim MA, de Souza AV, de Carvalho LR, Deffune E, de Oliveira C and
Vianna PT: Erythropoietin attenuates apoptosis after
ischemia-reperfusion-induced renal injury in transiently
hyperglycemic Wister rats. Transplant Proc. 43:3618–3621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Souza AV, Golim MA, Deffune E,
Domingues MA, de Carvalho LR, Vianna IG, Castiglia YM and Vianna
PT: Evaluation of renal protection from high doses of melatonin in
an experimental model of renal ischemia and reperfusion in
hyperglycemic rats. Transplant Proc. 46:1591–1593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bahmed K, Messier EM, Zhou W, Tuder RM,
Freed CR, Chu HW, Kelsen SG, Bowler RP, Mason RJ and Kosmider B:
DJ-1 modulates Nrf2-mediated protection in human primary alveolar
type II cells in smokers. Am J Respir Cell Mol Biol. April
19–2016.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gan L, Johnson DA and Johnson JA:
Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J
Neurosci. 31:967–977. 2010. View Article : Google Scholar : PubMed/NCBI
|