Introduction
Articular cartilage and the synovial membrane (SM)
are the main components of synovial joints. The SM produces
hyaluronic acid and lubricin, which are important for articular
cartilage lubrication (1,2). Synoviocytes also secrete factors
which induce the synthesis of metalloproteinases by chondrocytes
(3). Moreover, normal synovial
fluid stimulates the synthesis of collagen type II and
glycosaminoglycans in articular cartilage (4). The question remains, whether
cartilage is a passive beneficiary of SM activity or whether
chondrocytes can also influence the metabolism of cartilage by
cytokines or other factors. Chondrocyte survival and
differentiation require their interaction with extracellular matrix
(5). The secretory activity of
chondrocytes is usually studied in vitro, after their
release from the matrix, but such an approach has some limitations.
The yield of isolated cells is low in comparison with their content
in cartilage, possibly resulting in the uncontrolled selection of
certain chondrocyte subpopulations (6). Furthermore, the application of
enzymes for the purpose of chondrocyte isolation changes their gene
expression (7). Chondrocytes
cultured as a monolayer downregulate the expression of cartilage
matrix molecules such as collagen type II and aggrecan as well as
increase the expression of collagen type I and versican, which is
typical of fibroblast-like cells (8–10).
They also undergo other changes in phenotype expression with the
upregulation of markers regarded as distinctive for mesenchymal
stem cells (11).
Cultured in vitro articular chondrocytes,
particularly after stimulation by proinflammatory agents, secrete
numerous cytokines, matrix metalloproteinases (MMPs), tissue
inhibitors of metalloproteinases (TIMPs) and other factors
(12). These experiments,
however, were performed on cells released from the cartilage and
thus, it is difficult to estimate the type of cytokine and the rate
of production by chondrocytes in their natural environment, without
modifications imposed by enzymatic baths or culture conditions.
In this study, we aimed to establish which cytokines
are produced by chondrocytes in the cartilage and also, to evaluate
the influence of these cytokines on the SM as a possible target
organ. The concept of the study emerged from the McCutchen
(13) theory of 'weeping'
lubrication in synovial joints. According to this study and others
(14,15) cartilage matrix contains a fluid
phase, representing about 70% of its volume. During joint loading,
about 10% of this liquid is squeezed from the cartilage surface
(which, in a molecular sense, is porous) into the intraarticular
cavity, and is responsible for hydrostatic lubrication. It is,
therefore, plausible that cartilage interstitial fluid (CIF)
squeezed from cartilage during joint loading contains cytokines
produced by chondrocytes.
We have previously demonstrated that rat SM
dissected from the knee joint and incubated in vitro
responded to stimulation with cytokines and lipopolysaccharide by
increasing the production of hyaluronic acid and changing the mRNA
expression of hyaluronan synthases (HASs), cytokines, MMPs and
TIMPs (16,17). These findings suggested that the
SM in this experimental model would also respond to factors present
in the CIF. CIF was obtained by rinsing out the interstitial fluid
from the dissected articular-epiphyseal cartilage complexes of
newborn rats at a pressure of three bar and the cytokine content of
the CIF was evaluated using an enzyme-linked immunosorbent assay
(ELISA). SM exposed to CIF exhibited changes in the mRNA expression
of cytokines, MMPs, TIMPs and components of the extracellular
matrix. Incubating the SM with a cocktail of all factors found in
CIF (CIF-like cocktail) demonstrated that this set of cytokines, to
a considerable degree, imitates the effects of CIF.
Materials and methods
Animals
SMs were removed from the knee joints of specific
pathogen-free, inbred, male Lewis rats (n=24; 3 months old)
purchased from the Animal Unit of the Mossakowski Medical Research
Centre at the Polish Academy of Sciences (Warsaw, Poland).
Three-to-five day-old inbred Lewis rats (n=20) of both genders
served as cartilage donors. The present study and the methods were
approved by the Animal Ethics Committee of the Medical University
of Warsaw (Warsaw, Poland).
Preparation of rat SMs
Rats were euthanized by inhalation of halothane.
After opening the knee joint cavity, the SM was excised together
with the patella, the patellar ligament and the joint capsule. The
SM with the infrapatellar fat pad was then separated from the other
tissues according to the method described previously (18).
Preparation of CIF
Newborn rats were euthanized by decapitation. CIF
was rinsed out from the articular-epiphyseal cartilage complexes
dissected from the newborn rats, with the exclusion of calcified
fragments of the growth plate, which could be recognized and
separated during dissection. After clearing from the surrounding
tissues, cartilages from several animals were weighed. The mean
weight of cartilage obtained from one animal was 110 mg. For CIF
preparation, cartilage from 2 animals were placed in 2 ml
phosphate-buffered saline (PBS; Gibco-BRL, Paisley, UK) and cut
into small fragments. Since cutting involves the exertion of
pressure on the cartilage, some CIF was probably already squeezed
into PBS. The fluid together with the cartilage fragments was
transferred into a 50 ml Luer Lock syringe closed with the PTFE
Body Two-Way Valve from Hamilton (Sigma-Aldrich Chemie, Steinheim,
Germany) and the plunger was pressed to compress the air in the
syringe so as to increase the pressure to three bar. Then, the
plunger was slowly released. This procedure was repeated 20 times.
Cutting the dissected cartilage and rinsing out the CIF lasted
about 15–20 min. The fluid was separated from the cartilage
fragments by centrifugation, and desalting was performed on PD-10
columns (Amersham Biosciences, Uppsala, Sweden) and lyophilized.
CIF from 10–20 rats was pooled to obtain more uniform material. The
lyophilisate was dissolved in RPMI-1640 (Gibco-BRL) medium and the
protein content was determined. The total amount of protein in the
CIF squeezed from the cartilage obtained from one animal varied
from 0.87 to 1.1 mg. A working solution of CIF was standardized to
contain 1 mg/ml protein. The presence and concentration of factors
supposedly occurring in CIF [tumor necrosis factor (TNF),
transforming growth factor β1 (TGFβ1), basic fibroblast growth
factor (bFGF), platelet-derived growth factor (PDGF), epidermal
growth factor (EGF), interleukin (IL)-1β, IL-6, IL-7, IL-10,
granulocyte-macrophage (GM)-colony-stimulating factor (CSF)
granulocyte-macrophage (GM)-CSF, macrophage (M)-CSF, granulocyte
(G)-CSF, insulin-like growth factor (IGF)-1, leukemia inhibitory
factor (LIF), bone morphogenetic protein (BMP)2, BMP7, lubricin and
hyaluronic acid (HA)] were estimated using an ELISA.
Chondrocyte culture
Cartilage fragments used for harvesting CIF were
digested with constant stirring in an enzymatic solution containing
0.25% collagenase (type I), 0.05% DNase, 17.5 µM
Nα-p-tosyl-L-lysine chloromethyl ketone (TLCK) and 1%
antibiotic-antimycotic solution (all from Sigma, St Louis, MO, USA)
in RPMI-1640 medium (Gibco-BRL) for 3 h at 37°C. The viability of
chondrocytes was checked using the trypan blue test (Sigma).
Chondrocytes were seeded into 24-well plates (Corning, Inc.,
Corning, NY, USA) at a density of 5×105 cells/well in 1
ml of culture medium and observed for 7 days (10).
Incubation of SM
As a standard procedure, dissected SMs were
incubated in RPMI-1640 (Gibco-BRL) medium in flat-bottomed 24-well
plates (Corning, Inc.) in a humidified atmosphere of 5%
CO2 in air at 37°C with constant, slow motion, for 4 h.
The SM from one knee joint served as the control to the SM from the
opposite knee. The control medium was enriched by 0.1% bovine
albumin (Sigma). Experimental SMs were incubated either in CIF or
in the CIF-like cocktail with commercial cytokines identical in
concentration with that present in CIF. The following cytokines
were used: G-CSF, M-CSF, LIF, BMP7 and bFGF (PromoKine; PromoCell
GmbH, Heidelberg, Germany), TGFβ1 (Sigma) and IGF1 (R&D Systems
Inc., Minneapolis, MN, USA). After culture, total RNA from SM cells
was isolated and the expression of genes encoding HAS1, HAS2,
lubricin, collagen type I, aggrecan, versican, MMP2, MMP3, TIMP1,
TIMP2, TIMP3, IL-1β, IL-6, TNF and TGFβ1 was examined.
Protein determination
Ten microliters of CIF dissolved in the medium
(without serum) or medium alone (blank test) was placed in a
flat-bottomed 96-well plate (Corning, Inc.) and 0.2 ml BCA protein
assay reagent (Pierce, Rockford, IL, USA) was added to each well.
The plate was incubated at 37°C for 30 min. Protein concentrations
were determined spectrophotometrically at 550 nm in a microplate
reader (model 550; SLT Spectra Labinstruments, Crailsheim,
Germany).
Analysis of CIF by ELISA
Cytokine and extracellular matrix protein levels
were evaluated using rat immunoassay kits for IL-6, IL-10, TNF,
TGFβ1, IGF1, GM-CSF and BMP2 from R&D Systems, Inc., for IL-7,
bFGF, M-CSF, G-CSF, LIF, BMP7, EGF and PDGF from Biotang, Inc.
(Waltham, MA, USA), for HA and lubricin from Cusabio Biotech Co.,
Ltd. (Hubei, China), and for IL-1β from Life Technology (Frederick,
MD, USA) according to the manufacturers' instructions.
Total RNA isolation from SM samples
RNA was isolated using a NucleoSpin® RNA
II kit (Macherey-Nagel, Duren, Germany), according to
manufacturer's instructions. The quantity and quality of the
isolated total RNA was evaluated spectrophotometrically using a
NanoDrop 2000 spectrophotometer (ND-2000) with software for the
analysis of nucleic acids (both from Thermo Fisher Scientific,
Wilmington, DE, USA).
Reverse transcription
Reverse transcription was performed using a High
Capacity cDNA Reverse Transcription kit (Applied Biosystems,
Warrington, UK) according to the manufacturer's instructions in an
Eppendorf gradient Mastercycler (Eppendorf AG, Hamburg, Germany).
cDNA samples were stored at −20°C.
Real-time polymerase chain reaction
(PCR)
Real-time PCR was performed in an ABI PRISM 7500
(Applied Biosystems) using 96-well optical plates. Each sample was
run in triplicate and was supplied with an endogenous control [rat
GAPDH endogenous control (VIC®/MGB Probe)]. For gene
expression analysis, the appropriate TaqMan expression assays was
used. All probes were stained with FAM (Applied Biosystems). The
reaction was run in 25 µl mix of TaqMan Universal Master
Mix, appropriate primer set, MGB probe and 50 ng cDNA template.
Universal thermal conditions (10 min at 95°C, 40 cycles of 15 sec
at 95°C and 1 min at 60°C) were used. Data analysis was performed
using sequence detection software ver. 1.2 (Applied Biosystems).
The amount of RNA transcript in SMs maintained in the control
medium was estimated by ΔCt.
Statistical analysis
Data were analyzed by the Wilcoxon matched-pair test
or by the Mann-Whitney U test (Statistica software) (19). A p-value <0.05 was considered
to indicate a statistically significant difference.
Results
More than 90% of chondrocytes isolated from
cartilage fragments used for CIF production were viable and
achieved 2 population doublings within 7 days of culture (data not
shown), which were similar to the findings of a study examining
chondrocytes isolated from intact cartilage (10).
Seven cytokines were detected in the CIF following
ELISA analysis (Table I). bFGF,
and IGF1 predominated with the value >2,000 pg/ml, TGFβ1 reached
500 pg/ml whereas BMP7, M-CSF, G-CSF and LIF were <100 pg/ml.
Nine cytokines (IL-1β, IL-7, IL-6, IL-10, PDGF, EGF, TNF, GM-CSF
and BMP2) were either absent or below the sensitivity level of the
assay. The matrix proteins lubricin and HA were not detected.
Cytokine concentrations present in the CIF and calculated per mg of
wet weight of cartilage from which CIF was obtained are listed in
Table I.
 | Table IConcentration of cytokines in CIF. |
Table I
Concentration of cytokines in CIF.
| Cytokine | Mean
concentration
(pg/ml) ± SD | Cytokine
concentration
(pg/1 mg of cartilage) |
|---|
| bFGF | 2320±210 | 21.1 |
| IGF1 | 2054±246 | 18.7 |
| TGFβ1 | 517±96 | 4.7 |
| BMP7 | 80.5±29 | 0.73 |
| M-CSF | 61±15 | 0.55 |
| LIF | 24±3 | 0.22 |
| G-CSF | 23±5 | 0.21 |
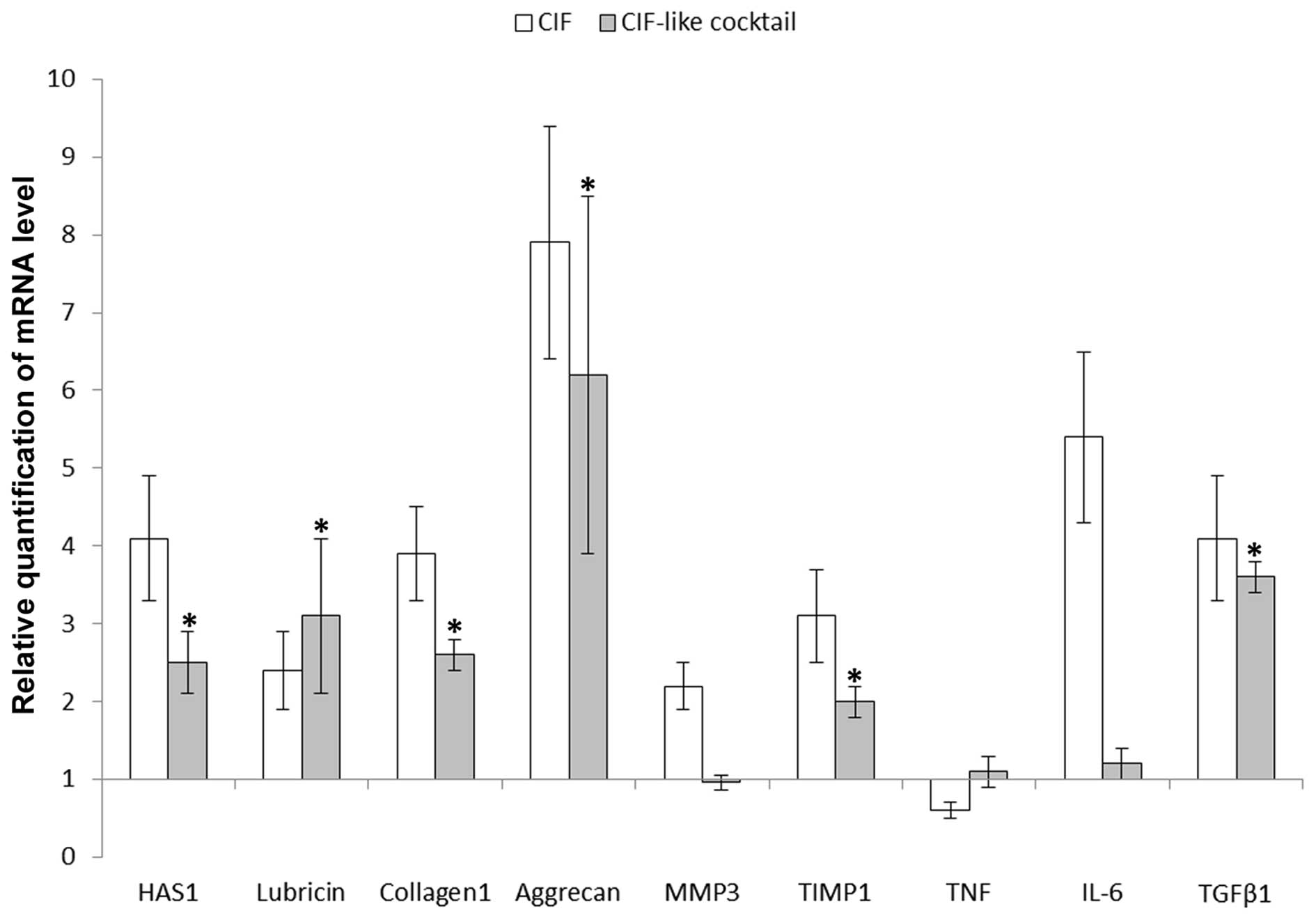
CIF stimulated the mRNA expression of HAS1
(p<0.005), HAS2 (p<0.005), lubricin (p<0.003), collagen
type I (p<0.005), versican (p<0.007), aggrecan (p<0.005),
MMP2 (p<0.003), MMP3 (p<0.008), TIMP1(p<0.008),
TIMP2(p<0.008), TIMP3 (p<0.008), IL-6 (p<0.03) and TGFβ1
(p<0.005), whereas the expression of TNF (p<0.002) and IL-1β
(p<0.03) was inhibited (Fig.
1). Observations regarding the influence of factors present in
CIF (CIF-like cocktail) on the SM were limited to nine selected
genes (Fig. 2). CIF-like cocktail
stimulated the mRNA expression of HAS1 (p<0.01), lubricin
(p<0.01), collagen type I (p<0.02), aggrecan (p<0.02),
TIMP1 (p<0.02) and TGFβ1 (p<0.01) genes. There was no
statistical difference between the expression levels of these genes
after CIF and CIF-like cocktail treatment (for HAS1 p>0.05, for
the rest of genes p>0.1). Contrary to CIF, CIF-like cocktail did
not change the expression of MMP-3 (p>0.5), IL-6 (p>0.25) and
TNF (p>0.35) and therefore the mRNA levels of these genes were
different from the mRNA levels following CIF treatment (for MMP3
and IL-6 p<0.01, for TNF p<0.05).
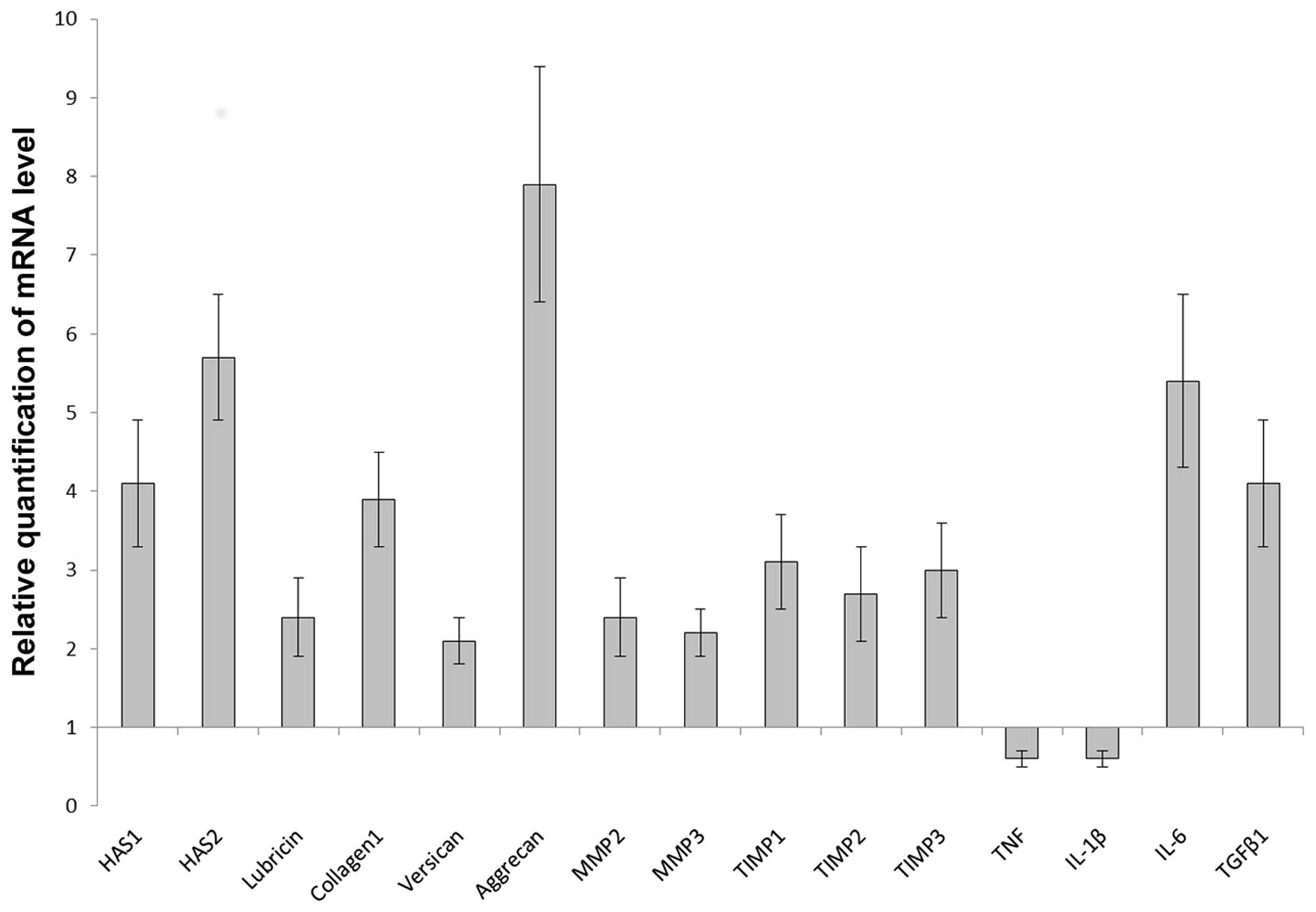 | Figure 1mRNA expression of hyaluronan synthase
(HAS)1 and HAS2, extracellular matrix proteins, matrix
metalloproteinases (MMPs), tissue inhibitors of metalloproteinase
(TIMPs) and cytokines in the synovial membrane after 4 h of
incubation with 1 mg/ml cartilage interstitial fluid (CIF) measured
by real-time PCR. Values are expressed as the means ± SE. In each
group, n=12. Relative expression was calculated against the
reference gene, GAPDH. Analysis was conducted as a relative
quantification study, using control synovial membrane gene
expression as a calibrator (value, 1). Differences in the
expression of all genes were significant, according to the Wilcoxon
matched-pair test at p<0.05. TNF, tumor necrosis factor; IL-1β,
interleukin-1β; IL-6, interleukin-6; TGFβ1, transforming growth
factor β1. |
Discussion
While presenting the first study detecting cytokines
in CIF and its effects on the SM, to the best of our knowledge, we
have to consider several limitations of this approach. The amount
of available cartilage in newborn rats is low, and it is necessary
to collect articular cartilage from many joints together with
non-calcified fragments of growth plates which cannot be separated
during dissection. It is, however, important that CIF is prepared
without damage to the chondrocytes, which after CIF harvesting,
survived enzymatic isolation and grew in culture (data not shown).
The concentrations of various factors in CIF probably represent
their average value in the whole cartilage, without taking into
consideration zonal chondrocyte distribution (20) and gradients between chondrocytes
and territorial or interterritorial matrix. Cytokines are probably
released, as we expected, following McCutchen's (13) theory of 'weeping' lubrication,
during each loading of cartilage. Thus, their concentrations in the
synovial fluid may vary depending on physical activity. Agents
present in CIF (Table I) may act
on chondrocytes in an auto- or paracrine fashion, or after being
squeezed from the cartilage, during loading of the synovial cells.
They may influence both the formation of cartilage matrix
components and the production of cytokines.
The SM is formed from four main types of cells,
namely synoviocytes (fibroblast-like cells), macrophages,
adipocytes and epitheliocytes. Each of these cell types produces a
panel of cytokines (17); thus,
CIF may stimulate their secretion and they, in turn, may affect
expression of particular genes.
Numerous studies describe the effects of particular
factors detected in CIF on the expression of connective tissue
matrix components. Thus, IGF1 stimulates the synthesis of cartilage
matrix proteins (21,22) and collagen type I (23). IGFs are also presumably the major
regulatory factors of cartilage proteoglycan synthesis present in
human synovial fluid (24). TGFβ1
is involved in the control of differentiation and dedifferentiation
of chondrocytes, the synthesis of collagen type II and
proteoglycans, and maintaining the homeostasis of cartilage
(25). It also enhances the mRNA
expression of type I collagen (26).
CIF and CIF-like cocktail stimulated the mRNA
expression of collagen type I and aggrecan, a proteoglycan specific
for cartilage (Fig. 2) in the SM.
The presence in CIF of both IGF1 and TGFβ1 may be important for
keeping chondrocytes in the differentiated state, since these
factors, acting jointly, reexpressed aggrecan and type II collagen
genes in dedifferentiated articular chondrocytes (27). A similar synergistic action of
both factors was observed by Seifarth et al (28), who found that chondrocytes
dedifferentiated by IL-1 regained a chondrocyte-like phenotype
after treatment with IGF1 and/or TGFβ1 alone, but co-treatment with
IGF1 and TGFβ1 exerted additive anabolic effects.
TGFβ1 stimulated HAS1 expression in fibroblasts
(29) and hyaluronan synthesis in
rat SM (16). The expression of
lubricin and HAS1 was also stimulated by CIF and CIF-like cocktail
(Fig. 2) suggesting that the
factors present in CIF may influence joint lubrication.
TGFβ1 inhibited MMP3 synthesis and stimulated TIMP1
production in various tissues (30). CIF, however, increased the mRNA
expression of MMP3 and TIMP1 whereas CIF-like cocktail had no
effect on MMP3 but stimulated the expression of TIMP1.
Since TGFβ1 can induce its own gene expression
(31), CIF and CIF-like cocktail
could stimulate TGFβ1 expression through a similar mechanism
(Fig. 2).
bFGF is synthesized by chondrocytes and functions as
an autocrine/paracrine mitogen via its deposition into the
cartilage extracellular matrix and subsequent release depending on
the biological activity of cartilage (32). It may, depending on the dose and
age of cartilage, stimulate or inhibit the synthesis of matrix
proteins and accelerate proteoglycan degradation (21,33). bFGF was present in CIF at a
relatively high concentration but in view of the above-mentioned
reports, its effect on the SM is difficult to estimate.
The low content in CIF of G-CSF and M-CSF is in
agreement with observations that they were absent or present at low
levels in unstimulated cultures of articular cartilage or
chondrocytes (34,35).
LIF, a member of the IL-6 family of cytokines,
displays pleiotropic effects on various cell types and organs
(36). It was not detected in
non-stimulated, short-term chondrocyte cultures, but appeared after
stimulation with IL-1 or TNF (37). The small amount of LIF detected in
CIF is in agreement with these observations (Table I).
Comparing the effects of CIF with those evoked by a
CIF-like cocktail indicates that in the latter some factors were
missing. Particularly, CIF-like cocktail did not contain factors
responsible for the stimulation of IL-6 gene expression and
inhibition of TNF gene expression (Fig. 2).
IL-6 is a multifunctional cytokine with well-defined
pro- and anti-inflammatory properties (38). It is produced by articular
chondrocytes (39), and by the
four main cell types in the SM (17). TGFβ1 increased IL-6 production by
chondrocytes (39) and human
fibroblasts (40) but IGF1 had no
significant effect (39). In the
present study, CIF strongly increased the mRNA expression of IL-6,
but CIF-like cocktail had no effect.
TNF is a major proinflammatory mediator with a
marked functional duality, being strongly engaged both in tissue
regeneration/expansion and destruction (41). TNF is expressed in macrophages
(42) which are presumably its
main source in the SM (17). The
administration of CIF inhibited TNF expression, whereas CIF-like
cocktail had no statistically valid effect (Fig. 2). The reason for differences in
the expression of TNF in the SM under the influence of CIF and
CIF-like cocktail remains unclear, since exposing macrophages to
IGF1 at a dose similar to that present in CIF and CIF-like cocktail
enhanced TNF release and its mRNA level (43).
To sum up, the stimulatory effect of CIF on collagen
type I and aggrecan expression observed in this study is in accord
with previously published data demonstrating increased expression
under the influence of IGF1 and TGFβ1. It is also interesting that
in the case of MMP3, TIMP1 and IL-6, CIF exerted stimulatory
effects whereas CIF-like cocktail stimulated only the expression of
TIMP1. On the other hand, CIF inhibited TNF expression and CIF-like
cocktail had no effect. It suggests a need for further, more
thorough studies on the content of CIF, and also indicates that the
influence of CIF on the SM may not only depend on the activity of
particular factors but also on their interactions.
Harvesting CIF from the cartilage of larger animals
and humans in order to determine its contents may require more
sophisticated equipment than a syringe. Once, however, technical
problems have been resolved, studies on CIF may provide valuable
information regarding the relationship between cartilage and the SM
in physiological and pathological states.
Abbreviations:
|
CIF
|
cartilage interstitial fluid
|
Acknowledgments
The study was supported by the National Science
Centre (Poland) on the basis of decision number:
DEC-2012/05/B/NZ4/02646.
References
|
1
|
Swann DA: Structure and function of
lubricin, the glycoprotein responsible for the boundary lubrication
of articular cartilage. Articular Synovium. Franchimont P and
Karger S: Basel: pp. 45–58. 1982
|
|
2
|
Hui AY, McCarty WJ, Masuda K, Firestein GS
and Sah RL: A systems biology approach to synovial joint
lubrication in health, injury, and disease. Wiley Interdiscip Rev
Syst Biol Med. 4:15–37. 2012. View
Article : Google Scholar
|
|
3
|
Bandara G, Georgescu HI, Lin CW and Evans
CH: Synovial activation of chondrocytes: evidence for complex
cytokine interactions. Agents Actions. 34:285–288. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee DA, Salih V, Stockton EF, Stanton JS
and Bentley G: Effect of normal synovial fluid on the metabolism of
articular chondrocytes in vitro. Clin Orthop Relat Res.
342:228–238. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirsch MS, Lunsford LE, Trinkaus-Randall V
and Svoboda KK: Chondrocyte survival and differentiation in situ
are integrin mediated. Dev Dyn. 210:249–263. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jakob M, Démarteau O, Schäfer D, Stumm M,
Heberer M and Martin I: Enzymatic digestion of adult human
articular cartilage yields a small fraction of the total available
cells. Connect Tissue Res. 44:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayman DM, Blumberg TJ, Scott CC and
Athanasiou KA: The effects of isolation on chondrocyte gene
expression. Tissue Eng. 12:2573–2581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schulze-Tanzil G, de Souza P, Villegas
Castrejon H, John T, Merker HJ, Scheid A and Shakibaei M:
Redifferentiation of dedifferentiated human chondrocytes in
high-density cultures. Cell Tissue Res. 308:371–379. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marlovits S, Hombauer M, Tamandl D, Vècsei
V and Schlegel W: Quantitative analysis of gene expression in human
articular chondrocytes in monolayer culture. Int J Mol Med.
13:281–287. 2004.PubMed/NCBI
|
|
10
|
Osiecka-Iwan A, Hyc A, Niderla-Bielińska J
and Moskalewski S: Chondrocyte-associated antigen and matrix
components in a 2-and 3-dimensional culture of rat chondrocytes.
Mol Med Rep. 1:881–887. 2008.PubMed/NCBI
|
|
11
|
Polacek M, Bruun J-A, Elvenes J,
Figenschau Y and Martinez I: The secretory profiles of cultured
human articular chondrocytes and mesenchymal stem cells:
implications for autologous cell transplantation strategies. Cell
Transplant. 20:1381–1393. 2011. View Article : Google Scholar
|
|
12
|
Melas IN, Chairakaki AD, Chatzopoulou EI,
Messinis DE, Katopodi T, Pliaka V, Samara S, Mitsos A, Dailiana Z,
Kollia P and Alexopoulos LG: Modeling of signaling pathways in
chondrocytes based on phosphoproteomic and cytokine release data.
Osteoarthritis Cartilage. 22:509–518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCutchen CW: Sponge-hydrostatic and
weeping bearings. Nature. 184:1284–1285. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morrell KC, Hodge WA, Krebs DE and Mann
RW: Corroboration of in vivo cartilage pressures with implications
for synovial joint tribology and osteoarthritis causation. Proc
Natl Acad Sci USA. 102:14819–14824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caligaris M and Ateshian GA: Effects of
sustained interstitial fluid pressurization under migrating contact
area, and boundary lubrication by synovial fluid, on cartilage
friction. Osteoarthritis Cartilage. 16:1220–1227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hyc A, Osiecka-Iwan A, Niderla-Bielińska
J, Jankowska-Steifer E and Moskalewski S: Pro- and
anti-inflammatory cytokines increase hyaluronan production by rat
synovial membrane in vitro. Int J Mol Med. 24:579–585.
2009.PubMed/NCBI
|
|
17
|
Hyc A, Osiecka-Iwan A, Niderla-Bielińska J
and Moskalewski S: Influence of LPS, TNF, TGF-β1 and IL-4 on the
expression of MMPs, TIMPs and selected cytokines in rat synovial
membranes incubated in vitro. Int J Mol Med. 27:127–137. 2011.
|
|
18
|
Hyc A, Osiecka-Iwan A, Dziunycz P and
Moskalewski S: Preparation of rat synovial membrane for studies of
cytokine secretion. Folia Histochem Cytobiol. 45:57–60.
2007.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Darling EM and Athanasiou KA: Growth
factor impact on articular cartilage subpopulations. Cell Tissue
Res. 322:463–473. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sah RL, Chen AC, Grodzinsky AJ and Trippel
SB: Differential effects of bFGF and IGF-I on matrix metabolism in
calf and adult bovine cartilage explants. Arch Biochem Biophys.
308:137–147. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trippel SB: Growth factor actions on
articular cartilage. J Rheumatol Suppl. 43:129–132. 1995.PubMed/NCBI
|
|
23
|
Blackstock CD, Higashi Y, Sukhanov S, Shai
SY, Stefanovic B, Tabony AM, Yoshida T and Delafontaine P:
Insulin-like growth factor-1 increases synthesis of collagen type I
via induction of the mRNA-binding protein LARP6 expression and
binding to the 5′ stem-loop of COL1a1 and COL1a2 mRNA. J Biol Chem.
289:7264–7274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schalkwijk J, Joosten LA, van den Berg WB,
van Wyk JJ and van de Putte LB: Insulin-like growth factor
stimulation of chondrocyte proteoglycan synthesis by human synovial
fluid. Arthritis Rheum. 32:66–71. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patil AS, Sable RB and Kothari RM: An
update on transforming growth factor-β (TGF-β): sources, types,
functions and clinical applicability for cartilage/bone healing. J
Cell Physiol. 226:3094–3103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Verrecchia F and Mauviel A: TGF-β and
TNF-α: Antagonistic cytokines controlling type I collagen gene
expression. Cell Signal. 16:873–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yaeger PC, Masi TL, de Ortiz JL, Binette
F, Tubo R and McPherson JM: Synergistic action of transforming
growth factor-β and insulin-like growth factor-I induces expression
of type II collagen and aggrecan genes in adult human articular
chondrocytes. Exp Cell Res. 237:318–325. 1997. View Article : Google Scholar
|
|
28
|
Seifarth C, Csaki C and Shakibaei M:
Anabolic actions of IGF-I and TGF-β1 on interleukin-1β-treated
human articular chondrocytes: Evaluation in two and three
dimensional cultures. Histol Histopathol. 24:1245–1262.
2009.PubMed/NCBI
|
|
29
|
Campo GM, Avenoso A, Campo S, D'Ascola A,
Traina P and Calatroni A: Effects of cytokines on hyaluronan
synthase activity and response to oxidative stress by fibroblasts.
Br J Biomed Sci. 66:28–36. 2009. View Article : Google Scholar
|
|
30
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar
|
|
31
|
Piek E, Ju WJ, Heyer J, Escalante-Alcalde
D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP
and Roberts AB: Functional characterization of transforming growth
factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J
Biol Chem. 276:19945–19953. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luan Y, Praul CA, Gay CV and Leach RM Jr:
Basic fibroblast growth factor: an autocrine growth factor for
epiphyseal growth plate chondrocytes. J Cell Biochem. 62:372–382.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Loeser RF, Chubinskaya S, Pacione C and Im
HJ: Basic fibroblast growth factor inhibits the anabolic activity
of insulin-like growth factor 1 and osteogenic protein 1 in adult
human articular chondrocytes. Arthritis Rheum. 52:3910–3917. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alsalameh S, Firestein GS, Oez S, Kurrle
R, Kalden JR and Burmester GR: Regulation of granulocyte macrophage
colony stimulating factor production by human articular
chondrocytes. Induction by both tumor necrosis factor-alpha and
interleukin 1, downregulation by transforming growth factor β and
upregulation by fibroblast growth factor. J Rheumatol. 21:993–1002.
1994.PubMed/NCBI
|
|
35
|
Campbell IK, Ianches G and Hamilton JA:
Production of macrophage colony-stimulating factor (M-CSF) by human
articular cartilage and chondrocytes. Modulation by interleukin-1
and tumor necrosis factor alpha. Biochim Biophys Acta. 1182:57–63.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mathieu ME, Saucourt C, Mournetas V,
Gauthereau X, Thézé N, Praloran V, Thiébaud P and Bœuf H:
LIF-dependent signaling: new pieces in the Lego. Stem Cell Rev.
8:1–15. 2012. View Article : Google Scholar :
|
|
37
|
Henrotin YE, De Groote DD, Labasse AH,
Gaspar SE, Zheng SX, Geenen VG and Reginster JY: Effects of
exogenous IL-1β, TNF alpha, IL-6, IL-8 and LIF on cytokine
production by human articular chondrocytes. Osteoarthritis
Cartilage. 4:163–173. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wolf J, Rose-John S and Garbers C:
Interleukin-6 and its receptors: a highly regulated and dynamic
system. Cytokine. 70:11–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guerne PA, Carson DA and Lotz M: IL-6
production by human articular chondrocytes. Modulation of its
synthesis by cytokines, growth factors, and hormones in vitro. J
Immunol. 144:499–505. 1990.PubMed/NCBI
|
|
40
|
Seong GJ, Hong S, Jung SA, Lee JJ, Lim E,
Kim SJ and Lee JH: TGF-β-induced interleukin-6 participates in
transdifferentiation of human Tenon's fibroblasts to
myofibroblasts. Mol Vis. 15:2123–2128. 2009.
|
|
41
|
Wajant H, Pfizenmaier K and Scheurich P:
Tumor necrosis factor signaling. Cell Death Differ. 10:45–65. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nathan CF: Secretory products of
macrophages. J Clin Invest. 79:319–326. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Renier G, Clément I, Desfaits AC and
Lambert A: Direct stimulatory effect of insulin-like growth
factor-I on monocyte and macrophage tumor necrosis factor-alpha
production. Endocrinology. 137:4611–4618. 1996.PubMed/NCBI
|