Introduction
The normal epidermal barrier results from an
equilibrated differentiation process, in which proliferative
undifferentiated keratinocytes move from the basal to the granular
layer, turning to a differentiated state of the cornified envelope
(CE) (1). Epidermal
differentiation involves the expression switch from basal keratin 5
(KRT5) and KRT14 to suprabasal KRT1 and KRT10 (2,3).
In addition, structural proteins, such as involucrin (IVL) and
periplakin (PPL) are crosslinked with other proteins and serve as
substrates for lipids in the CE (4–6).
Psoriasis is a common chronic inflammatory skin disease with or
without joint involvement. Psoriatic skin lesions are characterized
by a thickened epidermis due to increased keratinocyte
proliferation, abnormal differentiation and the infiltration of
inflammatory cells into the dermis and epidermis. KRT6 and KRT16,
markers of abnormal hyperproliferation, are upregulated in
psoriatic lesions, whereas KRT1 and KRT10, markers of terminal
differentiation, are downregulated (7). Calcium is a major regulator of
keratinocyte differentiation. Alterations in calcium levels from
0.03 to 0.1 mM trigger keratinocyte differentiation in vitro
(8). In the epidermis, calcium
gradients from low levels in the proliferative basal layer to high
levels in the differentiated granular layer, have been reported
(9). This gradient disappeares
after barrier disruption (10) or
in psoriasis (11).
In vitro data have demonstrated that the
extracellular Ca2+ concentration ([Ca2+]o)
initiates keratinocyte differentiation by increasing the
intracellular Ca2+ concentration ([Ca2+]i).
This process is regulated by proteins of the plasma membrane and
endoplasmic reticulum, such as the calcium-sensing receptor (CASR)
(12,13) and store-operated Ca2+
entry (SOCE) proteins (14). It
has recently been demonstrated that the two major SOCE components,
stromal interaction molecule (STIM) and calcium release-activated
calcium modulator (CRAC or ORAI) (15–17) are involved in keratinocyte
proliferation and differentiation (18). Moreover, the epidermis is the
major source of the calcium homeostasis regulator, vitamin D.
Keratinocytes metabolize vitamin D to the active
1,25-dihydroxyvitamin D3
[1,25(OH)2D3] by several cytochrome P450
enzymes (19). This metabolite
regulates epidermal proliferation in the basal layer and promotes
differentiation in the upper layers after binding to the vitamin D
receptor (VDR) (20). In addition
to CASR (21), other
calcium-regulating proteins are modulated by
1,25(OH)2D3; e.g., the calcium-binding
calbindin 1 (CALB1) is involved in early keratinocyte
differentiation (22).
Additionally, transient receptor potential subfamily V member 6
(TRPV6) is a highly selective Ca2+ channel upregulated
by 1,25(OH)2D3 (23) and is involved in
Ca2+/1,25(OH)2D3-induced
keratinocyte differentiation (24). On the other hand, antimicrobial
peptides found in the epidermis, such as S100 calcium-binding
protein A7 (S100A7) (25), which
bind calcium and zinc (26), are
inducible in keratinocytes by differentiation (27), and are highly overexpressed in
psoriasis (28).
Currently, the knowledge about the expression of
calcium-regulating proteins in the epidermal plaques of psoriasis
vulgaris is limited. In this study, we thus aimed to investigate
the gene expression of calcium-regulating proteins in the plaques
of patients with psoriasis vulgaris with joint inflammation (PVPsA)
and without joint inflammation (PV).
Subjects and methods
Subject characteristics
This study, approved by the Ethics Committee at the
Medical Faculty of the Friedrich-Schiller University Jena, Jena,
Germany (project 1940-01/07), was conducted according to the
principles of the Declaration of Helsinki. Written consent was
obtained from all participants prior to enrollment. Patients were
diagnosed by dermatologists at the Department of Dermatology of the
Jena University Hospital. The presence of joint manifestations was
confirmed by power doppler ultrasonography (Esaote, Genoa, Italy)
and Rheumascan Xeralite (Mivenion GmbH, Berlin, Germany). Eighteen
patients with psoriasis vulgaris and six healthy controls (HC) were
included. Seven patients with psoriasis vulgaris had no clinical
signs of joint inflammation (PV) and eleven were diagnosed with
psoriatic arthritis (PVPsA). The mean age ranges of the patients
were as follows: PV group, 45.1±22.7; PVPsA group, 51.9±14.9; and
HC group, 48.7±9.9. Patients with other types of psoriasis, skin
diseases, allergy, autoimmune diseases, any topical or systemic
treatment, including vitamin D supplementation or phototherapy 5
months before or at the time of recruitment, were excluded.
Skin biopsies
One centimeter biopsies were obtained from patient
lesional and control skin after local anesthesia. Biopsies were
divided into two 5-mm pieces: one was preserved frozen in 'RNA
Later' solution for quantitative polymerase chain reaction (PCR)
assessment, and the other embedded in paraffin for posterior 5
µm thicknesses sectioning and processing with Alizarin Red S
staining and immunohistochemistry.
Alizarin Red S staining for calcium
deposition
Dewaxed skin section slides were incubated for 2 min
with Alizarin Red S solution (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The slides were mounted in 'DPX mounting medium'
(Sigma-Aldrich, St. Louis, MO, USA) and evaluated under a Zeiss
Axio Imager M1 microscope (Carl Zeiss, Jena, Germany).
Quantitative PCR analysis of skin
differentiation markers and calcium-regulating proteins
Total RNA was isolated from the skin biopsies using
an RNeasy Fibrous Tissue kit (Qiagen, Hilden, Germany) and then
reverse transcribed into cDNA using the high capacity RNA-to-cDNA
kit (Applied Biosystems, Foster City, CA, USA) according to the
manufacturer's instructions. The KRT10, KRT16, IVL, PPL, CASR,
ORAI1, ORAI3, STIM1, VDR, CALB1, TRPV6 and S100A7 mRNA levels were
quantified using a 7500 TaqMan Real-Time PCR system with human
KRT10 (Hs01043114_g1), KRT16 (Hs00955088_g1), IVL (Hs00846307_s1),
PPL (Hs00160312_m1), CASR (Hs01047793_m1), ORAI1 (Hs03046013_m1),
ORAI3 (Hs00743683_s1), STIM1 (Hs00162394_m1), VDR (Hs00172113_m1),
CALB1 (Hs01077197_m1), TRPV6 (Hs01114089_g1), S100A7
(Hs01923188_u1) and GADPH (endogeneous control) TaqMan Gene
Expression assays (all from Applied Biosystems). According to the
manufacturer's instructions, 50 ng cDNA/50 µl final volume
PCR reaction mix were used under the following conditions: an
initial AmpErase Uracil N-glycosylase activation step at 50°C for 2
min followed by 10 min denaturation at 95°C, and 40 cycles as
follows: 15 sec at 95°C and 1 min at 60°C. Relative quantification
was performed using ΔΔCt method, as previously described (29).
Immunohistochemistry of skin
biopsies
Dewaxed and rehydrated slides were processed for
antigen unmasking. Briefly, the slides were boiled in Tris-EDTA
buffer (10 mM Tris Base, 1 mM EDTA, 0.05% Tween-20, pH 9.0) for 20
min and then cooled with tap water. The slides were permeabilized
for 15 min and blocked for 30 min at room temperature. The samples
were incubated overnight at 4°C with the diluted 1:200 primary
antibodies [anti-human KRT10 (sc-51581), KRT16 (sc-53255), IVL
(sc-15225), PPL (sc-16754), CASR (sc-32182), ORAI1 (sc-377281),
ORAI3 (sc-292104), STIM1 (sc-68897), VDR (sc-1009), CALB1
(sc-365360), TRPV6 (sc-28763), S100A7 (sc-67047), sterol
27-hydroxylase (CYP27A1) (sc-390974), 25-hydroxyvitamin
D3 1-α-hydroxylase (CYP27B1) (sc-49643) and
1,25-dihydroxyvitamin D3 24-hydroxylase (CYP24A1)
(sc-66851); all from Santa Cruz Biotechnology, Inc.]. After
washing, the slides were incubated 2 h at room temperature with the
1:400 diluted secondary antibodies [Alexa Fluor 488 (A-21441)-, 594
(A-11058)- and 647 (A-31571)-conjugated; Molecular Probes, Eugene,
OR, USA]. Additionally, the slides were incubated for 5 min with 2
µg/ml DAPI (Sigma-Aldrich) and observed under a Zeiss Axio
Imager M1 microscope (Carl Zeiss).
Statistical analyses
Statistical analyses were performed using GraphPad
Prism software (GraphPad Software, La Jolla, CA, USA). Differences
between groups were analyzed by the Mann-Whitney or t-test. A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
Low calcium levels in plaques of patients
with psoriasis
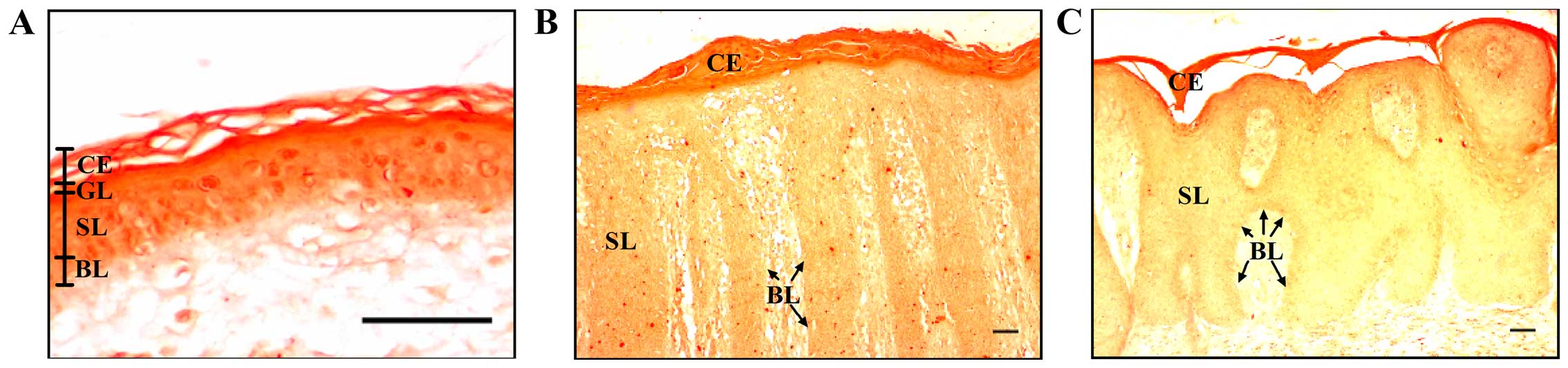
The normal epidermis is characterized by a calcium
gradient which ranges from low levels in the basal layer to high
levels in the spinous and granular layers (30). In this study, we performed
Alizarin Red S staining, which is usually used to identify calcium
in bone cells and tissue sections, to detect calcium in the skin
biopsies. This dye forms an orange-red complex with calcium. We
observed lower levels of calcium in the epidermis from the biopsies
of patients with psoriasis, as evidenced by the light orange
staining in the basal and spinous layers, compared with the dark
orange observed in the same layers in the control epidermis
(Fig. 1). There were no
differences in the staining of the epidermis of the patients with
psoriasis vulgaris with or without joint inflammation. Our results
confirmed the already reported altered calcium gradient in the
epidermis of patients with psoriasis (11).
Keratinocyte hyperproliferation and
altered epidermal differentiation in patients with psoriasis
Subsequently, we assessed the keratinocyte
differentiation state by measuring the mRNA expression of
keratinocyte differentiation markers in the epidermis from biopsies
of patients with PV and PVPsA. as well as in the controls. Our
results revealed that the mRNA levels of KRT10, one of the first
keratins expressed by keratinocyte differentiation during
cornification (31), displayed no
statistically significant differences between the PV, PVPsA and
control groups (P>0.05; Fig.
2A). By contrast, KRT16, a marker for keratinocyte
hyperproliferation (7), exhibited
highly increased mRNA levels in the plaques of patients with
psoriasis compared with the controls (98-fold, P=0.004, and
107-fold, P=0.001, respectively; Fig.
2B). The early and late keratinocyte differentiation markers,
IVL and PPL, respectively, play important roles in the crosslinking
of CE proteins (32,33). We did not find any statistically
significant differences in the mRNA levels of IVL between the PV,
PVPsA and control groups (P>0.05; Fig. 2C). However, the PPL mRNA levels
were lower in the plaques of patients with PV and PVPsA compared
with the controls (2.1-fold, P=0.009, and 1.9-fold, P=0.006,
respectively; Fig. 2D). At the
protein level, we observed a similar expression pattern of KRT10 in
the upper spinous layer in the biopsy specimens of patients in the
PV, PVPsA and control groups. By contrast, KRT16 was expressed
mainly in the spinous layer in the plaques of patients with PV and
PVPsA, and was almost absent in the control epidermis (Fig. 2E). Furthermore, IVL was expressed
in a similar pattern in the upper spinous layer in biopsy specimens
of PV, PVPsA and controls, while PPL was expressed in the spinous
and granular layers of the control epidermis, and was almost absent
in the plaques of patients with PV and PVPsA (Fig. 2F). These results confirmed altered
keratinocyte differentiation in the plaques of patients with
psoriasis vulgaris.
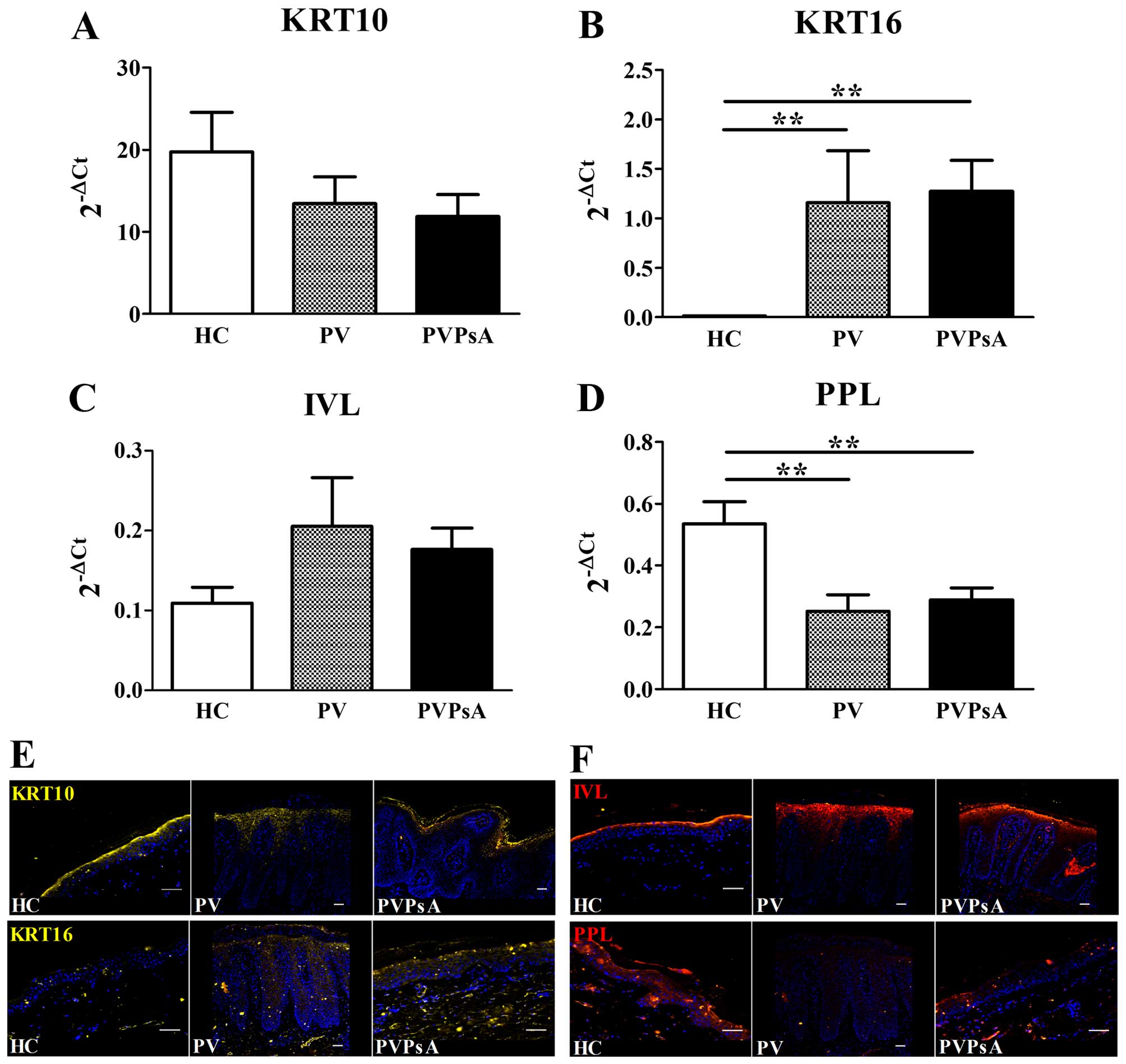 | Figure 2Keratinocyte hyperproliferation and
epidermal differentiation markers in patients with psoriasis
vulgaris with or without joint inflammation and healthy controls.
Quantitative PCR analysis of skin biopsies from healthy controls
(HC), and patients with psoriasis vulgaris without joint
inflammation (PV) and with joint inflammation (PVPsA). Columns
represent the means ± SEM of 2−ΔCt (x-axes) of (A)
keratin 10 (KRT10), (B) KRT16, (C) involucrin (IVL) and (D)
periplakin (PPL). HC, n=6; PV, n=7; PVPsA, n=11.
**P<0.01. (E and F) Microscopic images representative
of immunofluorescence staining of (E) KRT10 and KRT16, and (F) IVL
and PPL, in 5-µm-thick skin sections from the HC, PV and
PVPsA groups. Scale bar, 100 µm. |
Low expression of calcium-regulating
proteins and S100A7 overexpression differences in the plaques of
patients with psoriasis
[Ca2+]o plays a critical role in
keratinocyte differentiation (34) and [Ca2+]i is regulated
by proteins of the plasma membrane and endoplasmic reticulum
(14). Thus, we then evaluated
the gene expression of calcium-regulating proteins in the plaques
of patients with PV and PVPsA. Our results revealed lower mRNA
levels of CASR (4.6-fold, P=0.012; 3.6-fold, P=0.004,
respectively), ORAI1 (4.7-fold, P<0.0001; 3.6-fold, P<0.0001,
respectively), ORAI3 (3.5-fold, P<0.0001; 2.8-fold, P<0.0001,
respectively) and STIM1 (3.1-fold, P<0.0001; 2.5-fold,
P<0.0001, respectively) in the plaques of patients with PV and
PVPsA compared with the control epidermis (Fig. 3A–D, respectively).
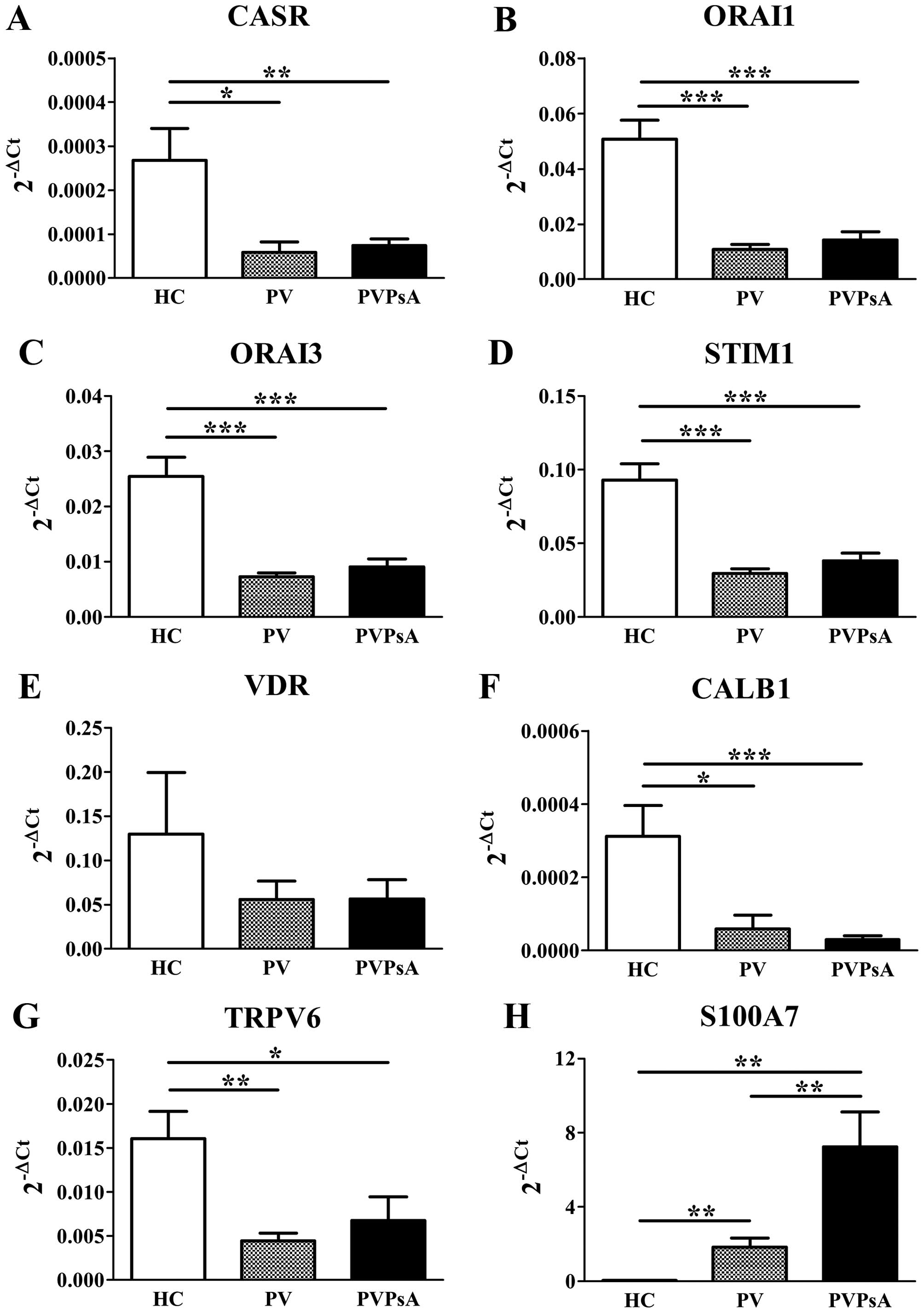 | Figure 3Gene expression of the
calcium-modulating proteins, vitamin D receptor (VDR) and S100
calcium-binding protein A7 (S100A7) in plaques of patients with
psoriasis vulgaris with or without joint inflammation, and the
control epidermis. Quantitative PCR analysis of skin biopsies from
healthy controls (HC), and patients with psoriasis vulgaris without
joint inflammation (PV) and with joint inflammation (PVPsA).
Columns represent the means ± SEM of 2−ΔCt (x-axes) of
(A) calcium-sensing receptor (CASR), (B) calcium release-activated
channel modulator 1 (ORAI1), (C) ORAI3, (D) stromal interaction
molecule 1 (STIM1), (E) VDR, (F) calbindin 1 (CALB1), (G) transient
receptor potential cation channel 6 (TRPV6) and (H) S100A7. HC,
n=6; PV, n=7; PVPsA, n=11. *P<0.05;
**P<0.01; ***P<0.001. |
In addition, 1,25(OH)2D3 is
involved in the regulation of keratinocyte differentiation by
calcium through VDR (35). Our
results revealed no statistically significant differences in the
VDR mRNA levels (Fig. 3E). CALB1
is regulated by 1,25(OH)2D3 and is involved
in intracellular Ca2+ translocation (36). Our results revealed lower CALB1
mRNA levels in the plaques of patients with PV and PVPsA compared
with the control epidermis (5.3-fold, P=0.014; 10.3-fold,
P<0.0001, respectively; Fig.
3F). In addition, TRPV6 is another protein regulated by
1,25(OH)2D3 and is a highly selective
Ca2+ channel (23)
involved in keratinocyte differentiation (24). Again, our results revealed lower
TRPV6 mRNA levels in the plaques of patients with psoriasis
compared with the control epidermis (3.6-fold, P=0.003; 2.4-fold,
P=0.014, respectively; Fig. 3G).
By contrast, S100A7, another calcium-binding protein, inducible in
keratinocytes by differentiation (27) and involved in the epidermal
barrier formation (26), is
highly overexpressed in psoriasis (28). Our results confirmed those of
published studies by showing a much higher S100A7 gene expression
in the plaques of patients with PV and PVPsA compared with the
controls (61-fold, P=0.003, and 240-fold, P=0.003, respectively).
Surprisingly, the S100A7 mRNA levels were even greater in the
plaques of patients also suffering from joint inflammation (PVPsA)
compared those of patients without joint inflammation (PV)
(3.9-fold, P<0.01; Fig.
3H).
Immunohistochemical staining also revealed that the
protein levels of CASR, ORAI1, ORAI3 and STIM were lower in the
plaques of patients with PV and PVPsA compared with the control
epidermis (Fig. 4A–D).
Principally, no differences were observed in the VDR expression
pattern in the plaques of patients with psoriasis patients and the
control epidermis (Fig. 4E).
CALB1 and TRPV6 also displayed a lower protein expression in the
plaques of patients with psoriasis patients compared with the
control epidermis; e.g., CALB1 localized mainly in the granular
layer of control epidermis and was almost absent in psoriatic
plaques (Fig. 4F); and TRPV6 was
expressed in the spinous and granular layers of the control
epidermis and in very low levels in the plaques of patients with PV
and PVPsA (Fig. 4G). By contrast,
S100A7 expression was higher in the psoriatic plaques compared with
the control epidermis. S100A7 localized in the whole epidermis of
patients with PV and PVPsA compared with the granular layer of the
control epidermis (Fig. 4H).
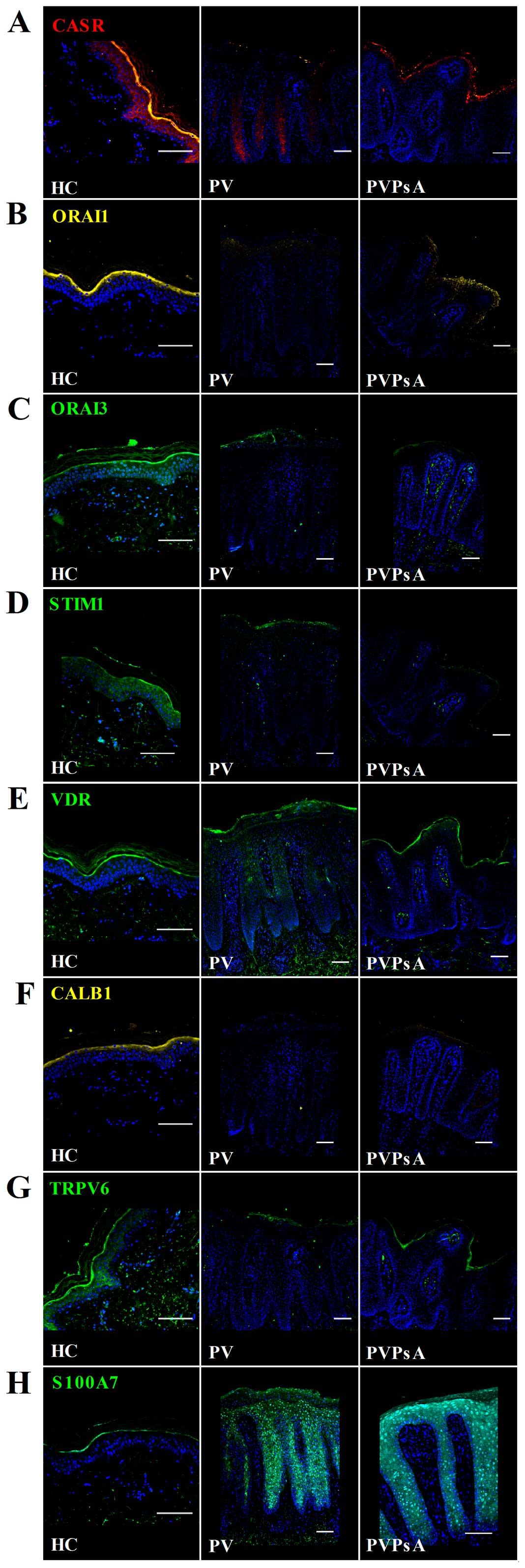 | Figure 4Protein levels of the
calcium-modulating proteins, vitamin D receptor (VDR) and S100
calcium-binding protein A7 (S100A7) in plaques of patients with
psoriasis vulgaris with or without joint inflammation, and control
epidermis. Immunofluorescent microscopic images of skin sections
from healthy controls (HC), and patients with psoriasis vulgaris
without joint inflammation (PV) and with joint inflammation
(PVPsA). Images represent staining of (A) calcium-sensing receptor
(CASR), (B) calcium release-activated channel modulator 1 (ORAI1),
(C) ORAI3, (D) stromal interaction molecule 1 (STIM1), (E) VDR, (F)
calbindin 1 (CALB1), (G) transient receptor potential cation
channel 6 (TRPV6) and (H) S100A7. Scale bar, 100 µm. |
These results suggest an altered keratinocyte
response to [Ca2+]o and the regulation of
[Ca2+]i in the epidermis of patients with psoriasis.
Moreover, the data with S100A7 indicated a dependence on
comorbidity revealed by the mRNA and protein expression differences
in the skin of patients with psoriasis vulgaris with or without
joint inflammation.
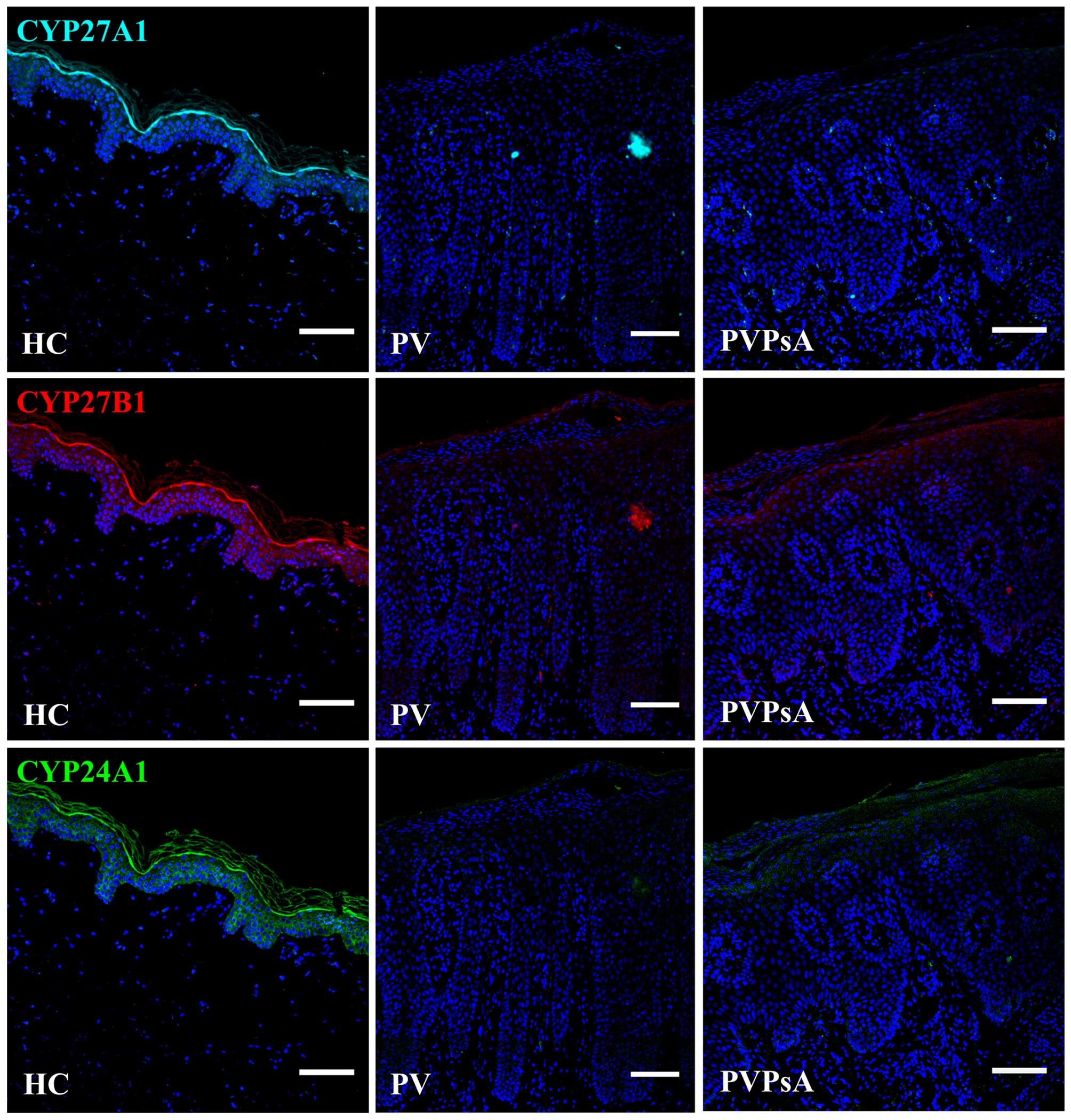
Low protein levels of CYP27A1, CYP27B1
and CYP24A1 in the plaques of patients with psoriasis
The vitamin D active form
1,25(OH)2D3 is synthesized by cytochrome P450
enzymes. First, vitamin D3 is hydroxylated to
25(OH)D3 by CYP27A1 in the liver (37), then CYP27B1 converts 25(OH)
D3 to 1,25(OH)2D3 in the kidneys
(38), and CYP24A1 can
hydroxylate 1,25(OH)2D3, as well as
25(OH)D3, generating metabolically inactive products
(39). Additionally,
keratinocytes contain these enzymes (12,40). As these enzymes can be considered
markers of vitamin D metabolism, and the expression of the
calcium-regulating proteins examined in this study is modulated by
1,25(OH)2D3, we then examined their protein
levels in the plaques of patients with PV and PVPsA. Our results
revealed lower protein levels of CYP27A1, CYP27B1 and CYP24A1 in
the plaques of patients with PV and PVPsA compared with the control
epidermis (Fig. 5).
Discussion
The beneficial effects of vitamin D induced by
exposure to sunlight in the treatment of psoriasis vulgaris have
been known for decades. Moreover, the topical application of
vitamin D analogs has been used successfully as the first-line
treatment for psoriasis vulgaris (41). In this study, we analyzed vitamin
D-dependent, as well as calcium-regulating proteins in the plaques
of patients with psoriasis vulgaris. The data presented,
schematically summarized in Table
I, show an altered expression of differentiation markers in the
plaques of patients with psoriasis vulgaris. Although no
differences in VDR expression were found, the expression of the
calcium-regulating proteins, CASR, ORAI1, ORAI3, STIM1, CALB1 and
TRPV6 was reduced, and by contrast, S100A7 was overexpressed in the
plaques of patients with psoriasis vulgaris. In addition, the
protein levels of CYP27A1, CYP27B1 and CYP24A1 were reduced in the
plaques of these patients. Despite the limitation of the use of
whole skin gene expression, the results from immunohistochemical
analysis confirmed the epidermal expression of these proteins.
 | Table IExpression overview of proteins
involved in keratinocyte proliferation and differentiation, vitamin
D-modulated calcium regulators and metabolical enzymes in plaques
of patients with psoriasis vulgaris with or without joint
inflammation compared with control epidermis. |
Table I
Expression overview of proteins
involved in keratinocyte proliferation and differentiation, vitamin
D-modulated calcium regulators and metabolical enzymes in plaques
of patients with psoriasis vulgaris with or without joint
inflammation compared with control epidermis.
| Protein | ↓ | ↔ | ↑ |
|---|
| Keratinocyte
proliferation and differentiation | PPL | KRT10
IVL | KRT16 |
| Calcium regulation
and vitamin D | CASR
ORAI1
ORAI3
STIM1
CALB1
TRPV6 | | S100A7 |
| Vitamin D | CYP27A1
CYP27B1
CYP24A1 | VDR | |
Calcium and vitamin D play important roles in
keratinocyte differentiation (42,43). In the normal epidermis, calcium
gradients have been reported (9).
An increase in [Ca2+]o results in the expression of
early differentiation markers (44). Low calcium levels confirmed in the
plaques of patients with psoriatic correspond to the high
expression of the hyperproliferation marker, KRT16. However, no
differences in KRT10 expression, a marker of keratinocyte
differentiation, were observed between patients with psoriasis and
the controls. The integrity of the epidermal barrier is crucial for
the maintenance of the epidermal calcium gradient (45). According to de Koning et al
(46) our results demonstrated
IVL expressed in the granular layer of control epidermis, but
extended into the spinous layer in psoriatic plaques, suggesting a
disrupted barrier. In addition, we observed reduced PPL levels in
plaques of patients with psoriasis corresponding with an impaired
epidermal barrier observed in PPL-deficient mice (47).
Intracellular calcium is regulated by an increase in
Ca2+ influx through CASR and Ca2+ release
from intracellular stores, followed by Ca2+ re-uptake
through SOCE proteins (14,48). CASR is required for normal
keratinocyte differentiation (49). The overexpression of CASR
accelerates epidermal differentiation, hair follicle formation and
permeability (50). while its
inactivation or deletion inhibits calcium-induced keratinocyte
differentiation by reducing Ca2+ intracellular stores,
and disrupts epidermal Ca2+ gradient and permeability
(48,51,52,53). Our results revealed a low CASR
expression in the plaques of patients with psoriasis vulgaris,
suggesting an altered capacity to regulate [Ca2+]o
influx. ORAI and STIM form clusters and co-localize with each other
to enable Ca2+ influx and release from intracellular
stores (54). The knockdown or
inhibition of ORAI1 and/or STIM1 alters Ca2+ storage and
decreases the differentiation and migration of undifferentiated
keratinocytes (18). ORAI3 forms
heteromultimeric channel complexes with ORAI1 and STIM1, and
mutated ORAI1 is sufficient to exert a negative effect on the other
CRAC members (55). In this
study, we observed a low expression of ORAI1, ORAI3 and STIM1 in
the plaques of patients with psoriasis, suggesting that
keratinocytes in these patients have an altered capacity to
regulate [Ca2+]i levels.
1,25(OH)2D3 increases
keratinocyte differentiation by increasing [Ca2+]i
levels (35). The loss of VDR or
the loss of the capacity to produce
1,25(OH)2D3 disrupts epidermal
differentiation, resulting in keratinocyte hyperproliferation
(56,57). In this study, we did not observe
any differences in the expression of VDR. However, the levels of
CASR, CALB1 and TRPV6 vitamin D-regulated proteins (58,59), essential in
Ca2+/1,25(OH)2D3-induced
differentiation of human keratinocytes (22,24), were reduced in the plaques of
patients with psoriasis vulgaris. Other TRP family channels have
been shown to be involved in keratinocyte differentiation (60) and complexes with ORAI and STIM
have been implicated in the regulation of [Ca2+]i
(61). However, only TRPC
subfamily members have been investigated in altered Ca2+
influx in psoriatic keratinocytes in response to high
[Ca2+]o (62).
The binding from 1,25(OH)2D3
to VDR and heterodimerization with retinoid X receptors affects the
expression of genes that have vitamin D responsive elements in
their promoters (63). The
expression of genes such as CASR, CALB1, TRPV6 and STIM1 is
regulated by 1,25(OH)2D3. In addition,
1,25(OH)2D3 is a potent regulator of the
NF-κB transcription factor (64),
which controls ORAI1 and STIM1 expression (65), and modulates SOCE (66). In accordance with the study by
Ala-Houhala et al (67),
our results revealed lower protein levels of CYP27A1, CYP27B1 and
additionally of CYP24A1 in the plaques of patients with psoriasis
compared with the control epidermis. The expression of CYP27A1 and
CYP27B1 is downregulated by 1,25(OH)2D3
(12,68), and the expression of CYP24A1 is
induced by 1,25(OH)2D3 (69,70), suggesting that low levels of
1,25(OH)2D3 are possibly associated with the
low calcium-regulating protein levels observed. However, upstream
alterations in vitamin D metabolism, e.g., cholesterol metabolism
cannot be ruled out. In addition to vitamin D3, CYP27A1
can hydroxylate cholesterol (71). Elevated cholesterol levels in
psoriatic lesioned skin is essential for IL-17A signaling and
results in the suppression of genes of cholesterol and fatty acid
biosynthesis (72).
1,25(OH)2D3 has been shown to
exert anti-proliferative effects on keratinocytes (73). Moreover,
1,25(OH)2D3 and analogs reduce S100A7 levels
in the reconstituted human epidermis stimulated by IL-22 (74), in IL-17-stimulated keratinocytes
and in skin of patients with psoriasis (75). Apart from its chemotactic and
immunomodulatory functions (76),
S100A7, a calcium-binding protein, crosslinks with CE proteins
during the terminal stages of keratinocyte differentiation mediated
by calcium (77), and is
upregulated after epidermal barrier disruption (78) and in psoriatic plaques (28,79). Our results confirmed S100A7
overexpression in the plaques of patients with psoriasis vulgaris,
and provide interesting evidence of a higher S100A7 expression in
the plaques of patients with PVPsA compared with PV. Bone
homeostasis depends on a balance between osteoclasts and
osteoblasts. Disordered circulating mediators of bone remodelling
(80), and an increased number of
circulating osteoclast precursors have been reported in patients
with psoriatic arthritis (81).
Serum levels of S100A7 are increased in patients with psoriasis
(28). S100A7 has been shown to
enhance osteoclast formation in vitro (82). Moreover, a S100A7 single
nucleotide polymorphism has been shown to be associated with
psoriatic arthritis (83).
In conclusion, the altered balance between
keratinocyte proliferation and differentiation, together with the
altered epidermal barrier observed in psoriatic plaques may be
associated with an altered capacity to respond to
[Ca2+]o and to regulate [Ca2+]i, related with
a reduced expression of vitamin D-dependent and calcium-regulating
proteins, such as CASR, ORAI1, ORAI3, STIM1, CALB1 and TRPV6, as
well as with a decreased 1,25(OH)2D3
synthesis. However, further studies are required to assess the
mechanisms involved. In addition, we demonstrated S100A7
overexpression in the plaques of patients with PVPsA compared with
PV, suggesting a dependence on the presence of joint inflammation.
These data provide new insight into vitamin D-dependent calcium
regulation in psoriasis and also reinforce the importance of
vitamin D and light therapy in patients with psoriasis with joint
inflammation.
Abbreviations:
|
HC
|
healthy controls
|
|
PV
|
psoriasis vulgaris
|
|
PVPsA
|
psoriasis vulgaris with joint
inflammation
|
|
CE
|
cornified envelope
|
Acknowledgments
We would like to thank the Departments of
Dermatology and Women from the Jena University Hospital for their
help in collecting skin biopsies and data from patients with
psoriasis and healthy controls. We would also like to thank the
Experimental Dermatology III and Histopathology groups and the
Institute of Anatomy II. This study was supported by the University
Hospital of Jena.
References
|
1
|
Candi E, Schmidt R and Melino G: The
cornified envelope: a model of cell death in the skin. Nat Rev Mol
Cell Biol. 6:328–340. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eichner R, Sun TT and Aebi U: The role of
keratin subfamilies and keratin pairs in the formation of human
epidermal intermediate filaments. J Cell Biol. 102:1767–1777. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuchs E and Green H: Changes in keratin
gene expression during terminal differentiation of the
keratinocyte. Cell. 19:1033–1042. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marekov LN and Steinert PM: Ceramides are
bound to structural proteins of the human foreskin epidermal
cornified cell envelope. J Biol Chem. 273:17763–17770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steinert PM and Marekov LN: The proteins
elafin, filaggrin, keratin intermediate filaments, loricrin, and
small proline-rich proteins 1 and 2 are isodipeptide cross-linked
components of the human epidermal cornified cell envelope. J Biol
Chem. 270:17702–17711. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steven AC and Steinert PM: Protein
composition of cornified cell envelopes of epidermal keratinocytes.
J Cell Sci. 107:693–700. 1994.PubMed/NCBI
|
|
7
|
McKay IA and Leigh IM: Altered
keratinocyte growth and differentiation in psoriasis. Clin
Dermatol. 13:105–114. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hennings H, Michael D, Cheng C, Steinert
P, Holbrook K and Yuspa SH: Calcium regulation of growth and
differentiation of mouse epidermal cells in culture. Cell.
19:245–254. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Menon GK, Grayson S and Elias PM: Ionic
calcium reservoirs in mammalian epidermis: ultrastructural
localization by ion-capture cytochemistry. J Invest Dermatol.
84:508–512. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mauro T, Bench G, Sidderas-Haddad E,
Feingold K, Elias P and Cullander C: Acute barrier perturbation
abolishes the Ca2+ and K+ gradients in murine
epidermis: quantitative measurement using PIXE. J Invest Dermatol.
111:1198–1201. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Menon GK and Elias PM: Ultrastructural
localization of calcium in psoriatic and normal human epidermis.
Arch Dermatol. 127:57–63. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bikle DD, Nemanic MK, Gee E and Elias P:
1,25-Dihydroxyvitamin D3 production by human
keratinocytes. Kinetics and regulation. J Clin Invest. 78:557–566.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu CL, Oda Y and Bikle DD: Effects of a
calcium receptor activator on the cellular response to calcium in
human keratinocytes. J Invest Dermatol. 113:340–345. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lewis RS: The molecular choreography of a
store-operated calcium channel. Nature. 446:284–287. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liou J, Kim ML, Heo WD, Jones JT, Myers
JW, Ferrell JE Jr and Meyer T: STIM is a Ca2+ sensor
essential for Ca2+-store-depletion-triggered
Ca2+ influx. Curr Biol. 15:1235–1241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roos J, DiGregorio PJ, Yeromin AV, Ohlsen
K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD,
et al: STIM1, an essential and conserved component of
store-operated Ca2+ channel function. J Cell Biol.
169:435–445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vig M, Peinelt C, Beck A, Koomoa DL, Rabah
D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R and
Kinet JP: CRACM1 is a plasma membrane protein essential for
store-operated Ca2+ entry. Science. 312:1220–1223. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Numaga-Tomita T and Putney JW: Role of
STIM1- and Orai1-mediated Ca2+ entry in
Ca2+-induced epidermal keratinocyte differentiation. J
Cell Sci. 126:605–612. 2013. View Article : Google Scholar :
|
|
19
|
Jones G, Prosser DE and Kaufmann M:
Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res.
55:13–31. 2014. View Article : Google Scholar :
|
|
20
|
Bikle DD, Tu CL, Xie Z and Oda Y: Vitamin
D regulated keratinocyte differentiation: role of coactivators. J
Cell Biochem. 88:290–295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ratnam AV, Bikle DD and Cho JK: 1,25
Dihydroxyvitamin D3 enhances the calcium response of
keratinocytes. J Cell Physiol. 178:188–196. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rizk-Rabin M and Pavlovitch JH: Epidermal
calcium-binding protein: a marker of early differentiation of basal
layer keratinocytes of rats. Cell Tissue Res. 272:161–168. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoenderop JG, van der Kemp AW, Urben CM,
Strugnell SA and Bindels RJ: Effects of vitamin D compounds on
renal and intestinal Ca2+ transport proteins in
25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice.
Kidney Int. 66:1082–1089. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lehen'kyi V, Beck B, Polakowska R,
Charveron M, Bordat P, Skryma R and Prevarskaya N: TRPV6 is a
Ca2+ entry channel essential for Ca2+-induced
differentiation of human keratinocytes. J Biol Chem.
282:22582–22591. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schröder JM and Harder J: Antimicrobial
skin peptides and proteins. Cell Mol Life Sci. 63:469–486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eckert RL, Broome AM, Ruse M, Robinson N,
Ryan D and Lee K: S100 proteins in the epidermis. J Invest
Dermatol. 123:23–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martinsson H, Yhr M and Enerbäck C:
Expression patterns of S100A7 (psoriasin) and S100A9
(calgranulin-B) in keratinocyte differentiation. Exp Dermatol.
14:161–168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Madsen P, Rasmussen HH, Leffers H, Honoré
B, Dejgaard K, Olsen E, Kiil J, Walbum E, Andersen AH, Basse B, et
al: Molecular cloning, occurrence, and expression of a novel
partially secreted protein 'psoriasin' that is highly up-regulated
in psoriatic skin. J Invest Dermatol. 97:701–712. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Pillai S, Menon GK, Bikle DD and Elias PM:
Localization and quantitation of calcium pools and calcium binding
sites in cultured human keratinocytes. J Cell Physiol. 154:101–112.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weiss RA, Eichner R and Sun TT: Monoclonal
antibody analysis of keratin expression in epidermal diseases: a
48- and 56-kdalton keratin as molecular markers for
hyperproliferative keratinocytes. J Cell Biol. 98:1397–1406. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Robinson NA, LaCelle PT and Eckert RL:
Involucrin is a covalently crosslinked constituent of highly
purified epidermal corneocytes: evidence for a common pattern of
involucrin crosslinking in vivo and in vitro. J Invest Dermatol.
107:101–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruhrberg C, Hajibagheri MA, Parry DA and
Watt FM: Periplakin, a novel component of cornified envelopes and
desmosomes that belongs to the plakin family and forms complexes
with envoplakin. J Cell Biol. 139:1835–1849. 1997. View Article : Google Scholar
|
|
34
|
Eckert RL: Structure, function, and
differentiation of the keratinocyte. Physiol Rev. 69:1316–1346.
1989.PubMed/NCBI
|
|
35
|
Bikle DD, Gee E and Pillai S: Regulation
of keratinocyte growth, differentiation, and vitamin D metabolism
by analogs of 1,25-dihydroxyvitamin D. J Invest Dermatol.
101:713–718. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feher JJ: Facilitated calcium diffusion by
intestinal calcium-binding protein. Am J Physiol. 244:C303–C307.
1983.PubMed/NCBI
|
|
37
|
Cali JJ and Russell DW: Characterization
of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450
that catalyzes multiple oxidation reaction in bile acid
biosynthesis. J Biol Chem. 266:7774–7778. 1991.PubMed/NCBI
|
|
38
|
Gray RW, Omdahl JL, Ghazarian JG and
DeLuca HF: 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular
location and properties. J Biol Chem. 247:7528–7532.
1972.PubMed/NCBI
|
|
39
|
Ebert R, Schütze N, Adamski J and Jakob F:
Vitamin D signaling is modulated on multiple levels in health and
disease. Mol Cell Endocrinol. 248:149–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lehmann B: The vitamin D3
pathway in human skin and its role for regulation of biological
processes. Photochem Photobiol. 81:1246–1251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Soleymani T, Hung T and Soung J: The role
of vitamin D in psoriasis: a review. Int J Dermatol. 54:383–392.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jones KT and Sharpe GR: Intracellular free
calcium and growth changes in single human keratinocytes in
response to vitamin D and five 20-epi-analogues. Arch Dermatol Res.
286:123–129. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Su MJ, Bikle DD, Mancianti ML and Pillai
S: 1,25-Dihydroxyvitamin D3 potentiates the keratinocyte
response to calcium. J Biol Chem. 269:14723–14729. 1994.PubMed/NCBI
|
|
44
|
Bikle DD, Ratnam A, Mauro T, Harris J and
Pillai S: Changes in calcium responsiveness and handling during
keratinocyte differentiation. Potential role of the calcium
receptor. J Clin Invest. 97:1085–1093. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Menon GK, Elias PM and Feingold KR:
Integrity of the permeability barrier is crucial for maintenance of
the epidermal calcium gradient. Br J Dermatol. 130:139–147. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
de Koning HD, van den Bogaard EH, Bergboer
JG, Kamsteeg M, van Vlijmen-Willems IM, Hitomi K, Henry J, Simon M,
Takashita N, Ishida-Yamamoto A, et al: Expression profile of
cornified envelope structural proteins and keratinocyte
differentiation-regulating proteins during skin barrier repair. Br
J Dermatol. 166:1245–1254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sevilla LM, Nachat R, Groot KR, Klement
JF, Uitto J, Djian P, Määttä A and Watt FM: Mice deficient in
involucrin, envoplakin, and periplakin have a defective epidermal
barrier. J Cell Biol. 179:1599–1612. 2007. View Article : Google Scholar
|
|
48
|
Tu CL, Chang W and Bikle DD: The role of
the calcium sensing receptor in regulating intracellular calcium
handling in human epidermal keratinocytes. J Invest Dermatol.
127:1074–1083. 2007. View Article : Google Scholar
|
|
49
|
Komuves L, Oda Y, Tu CL, Chang WH, Ho-Pao
CL, Mauro T and Bikle DD: Epidermal expression of the full-length
extracellular calcium-sensing receptor is required for normal
keratinocyte differentiation. J Cell Physiol. 192:45–54. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Turksen K and Troy TC: Overexpression of
the calcium sensing receptor accelerates epidermal differentiation
and permeability barrier formation in vivo. Mech Dev. 120:733–744.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tu CL, Oda Y, Komuves L and Bikle DD: The
role of the calcium-sensing receptor in epidermal differentiation.
Cell Calcium. 35:265–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tu CL, Chang W, Xie Z and Bikle DD:
Inactivation of the calcium sensing receptor inhibits
E-cadherin-mediated cell-cell adhesion and calcium-induced
differentiation in human epidermal keratinocytes. J Biol Chem.
283:3519–3528. 2008. View Article : Google Scholar
|
|
53
|
Tu CL, Crumrine DA, Man MQ, Chang W,
Elalieh H, You M, Elias PM and Bikle DD: Ablation of the
calcium-sensing receptor in keratinocytes impairs epidermal
differentiation and barrier function. J Invest Dermatol.
132:2350–2359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Luik RM, Wu MM, Buchanan J and Lewis RS:
The elementary unit of store-operated Ca2+ entry: local
activation of CRAC channels by STIM1 at ER-plasma membrane
junctions. J Cell Biol. 174:815–825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lis A, Peinelt C, Beck A, Parvez S,
Monteilh-Zoller M, Fleig A and Penner R: CRACM1, CRACM2, and CRACM3
are store-operated Ca2+ channels with distinct
functional properties. Curr Biol. 17:794–800. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bikle DD, Chang S, Crumrine D, Elalieh H,
Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K and Elias
PM: 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal
epidermal differentiation and permeability barrier homeostasis. J
Invest Dermatol. 122:984–992. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC,
Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S and Bikle DD:
Lack of the vitamin D receptor is associated with reduced epidermal
differentiation and hair follicle growth. J Invest Dermatol.
118:11–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gill RK and Christakos S: Identification
of sequence elements in mouse calbindin-D28k gene that confer
1,25-dihydroxyvitamin D3- and butyrate-inducible
responses. Proc Natl Acad Sci USA. 90:2984–2988. 1993. View Article : Google Scholar
|
|
59
|
Weber K, Erben RG, Rump A and Adamski J:
Gene structure and regulation of the murine epithelial calcium
channels ECaC1 and 2. Biochem Biophys Res Commun. 289:1287–1294.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu LJ, Sweet TB and Clapham DE:
International Union of Basic and Clinical Pharmacology. LXXVI.
Current progress in the mammalian TRP ion channel family. Pharmacol
Rev. 62:381–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Saul S, Stanisz H, Backes CS, Schwarz EC
and Hoth M: How ORAI and TRP channels interfere with each other:
interaction models and examples from the immune system and the
skin. Eur J Pharmacol. 739:49–59. 2014. View Article : Google Scholar
|
|
62
|
Leuner K, Kraus M, Woelfle U, Beschmann H,
Harteneck C, Boehncke WH, Schempp CM and Müller WE: Reduced TRPC
channel expression in psoriatic keratinocytes is associated with
impaired differentiation and enhanced proliferation. PLoS One.
6:e147162011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Christakos S, Dhawan P, Liu Y, Peng X and
Porta A: New insights into the mechanisms of vitamin D action. J
Cell Biochem. 88:695–705. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto
FL, Wong KE, Kong J and Li YC: 1,25-Dihydroxyvitamin D3
suppresses high glucose-induced angiotensinogen expression in
kidney cells by blocking the NF-{kappa}B pathway. Am J Physiol
Renal Physiol. 296:F1212–F1218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Eylenstein A, Schmidt S, Gu S, Yang W,
Schmid E, Schmidt EM, Alesutan I, Szteyn K, Regel I, Shumilina E
and Lang F: Transcription factor NF-κB regulates expression of
pore-forming Ca2+ channel unit, Orai1, and its
activator, STIM1, to control Ca2+ entry and affect
cellular functions. J Biol Chem. 287:2719–2730. 2012. View Article : Google Scholar
|
|
66
|
Borst O, Münzer P, Schmid E, Schmidt EM,
Russo A, Walker B, Yang W, Leibrock C, Szteyn K, Schmidt S, et al:
1,25(OH)2 vitamin D3-dependent inhibition of
platelet Ca2+ signaling and thrombus formation in
klotho-deficient mice. FASEB J. 28:2108–2119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ala-Houhala MJ, Karppinen T, Vähävihu K,
Kautiainen H, Dombrowski Y, Snellman E, Schauber J and Reunala T:
Narrow-band ultraviolet B treatment boosts serum 25-hydroxyvitamin
D in patients with psoriasis on oral vitamin D supplementation.
Acta Derm Venereol. 94:146–151. 2014. View Article : Google Scholar
|
|
68
|
Holick MF: Resurrection of vitamin D
deficiency and rickets. J Clin Invest. 116:2062–2072. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kerry DM, Dwivedi PP, Hahn CN, Morris HA,
Omdahl JL and May BK: Transcriptional synergism between vitamin
D-responsive elements in the rat 25-hydroxyvitamin D3
24-hydroxylase (CYP24) promoter. J Biol Chem. 271:29715–29721.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lehmann B, Tiebel O and Meurer M:
Expression of vitamin D3 25-hydroxylase (CYP27) mRNA
after induction by vitamin D3 or UVB radiation in
keratinocytes of human skin equivalents – a preliminary study. Arch
Dermatol Res. 291:507–510. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Okuda KI: Liver mitochondrial P450
involved in cholesterol catabolism and vitamin D activation. J
Lipid Res. 35:361–372. 1994.PubMed/NCBI
|
|
72
|
Varshney P, Narasimhan A, Mittal S, Malik
G, Sardana K and Saini N: Transcriptome profiling unveils the role
of cholesterol in IL-17A signaling in psoriasis. Sci Rep.
6:192952016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Matsumoto K, Hashimoto K, Nishida Y,
Hashiro M and Yoshikawa K: Growth-inhibitory effects of
1,25-dihydroxyvitamin D3 on normal human keratinocytes
cultured in serum-free medium. Biochem Biophys Res Commun.
166:916–923. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Datta Mitra A, Raychaudhuri SP, Abria CJ,
Mitra A, Wright R, Ray R and Kundu-Raychaudhuri S:
1α,25-Dihydroxyvitamin-D3-3-bromoacetate regulates
AKT/mTOR signaling cascades: a therapeutic agent for psoriasis. J
Invest Dermatol. 133:1556–1564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hegyi Z, Zwicker S, Bureik D, Peric M,
Koglin S, Batycka-Baran A, Prinz JC, Ruzicka T, Schauber J and Wolf
R: Vitamin D analog calcipotriol suppresses the Th17
cytokine-induced proinflammatory S100 'alarmins' psoriasin (S100A7)
and koebnerisin (S100A15) in psoriasis. J Invest Dermatol.
132:1416–1424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jinquan T, Vorum H, Larsen CG, Madsen P,
Rasmussen HH, Gesser B, Etzerodt M, Honoré B, Celis JE and
Thestrup-Pedersen K: Psoriasin: a novel chemotactic protein. J
Invest Dermatol. 107:5–10. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hoffmann HJ, Olsen E, Etzerodt M, Madsen
P, Thøgersen HC, Kruse T and Celis JE: Psoriasin binds calcium and
is upregulated by calcium to levels that resemble those observed in
normal skin. J Invest Dermatol. 103:370–375. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
de Koning HD, Kamsteeg M, Rodijk-Olthuis
D, van Vlijmen Willems IM, van Erp PE, Schalkwijk J and Zeeuwen PL:
Epidermal expression of host response genes upon skin barrier
disruption in normal skin and uninvolved skin of psoriasis and
atopic dermatitis patients. J Invest Dermatol. 131:263–266. 2011.
View Article : Google Scholar
|
|
79
|
Harder J, Dressel S, Wittersheim M, Cordes
J, Meyer-Hoffert U, Mrowietz U, Fölster-Holst R, Proksch E,
Schröder JM, Schwarz T, et al: Enhanced expression and secretion of
antimicrobial peptides in atopic dermatitis and after superficial
skin injury. J Invest Dermatol. 130:1355–1364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dalbeth N, Pool B, Smith T, Callon KE,
Lobo M, Taylor WJ, Jones PB, Cornish J and McQueen FM: Circulating
mediators of bone remodeling in psoriatic arthritis: implications
for disordered osteoclastogenesis and bone erosion. Arthritis Res
Ther. 12:R1642010. View
Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG
and Schwarz EM: Mechanisms of TNF-alpha- and RANKL-mediated
osteoclastogenesis and bone resorption in psoriatic arthritis. J
Clin Invest. 111:821–831. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Paruchuri V, Prasad A, McHugh K, Bhat HK,
Polyak K and Ganju RK: S100A7-downregulation inhibits epidermal
growth factor-induced signaling in breast cancer cells and blocks
osteoclast formation. PLoS One. 3:e17412008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cubillos S, Jaradat SW, Walther M,
Truta-Feles K, Koehler MJ and Norgauer J: Association of S100A7
gene polymorphisms with manifestations of common types of
psoriasis: effect on serum calcium levels. Exp Dermatol.
24:894–896. 2015. View Article : Google Scholar : PubMed/NCBI
|