Introduction
Inflammation is a defense mechanism of the innate
immune system against invading agents such as bacteria, viruses,
and fungi (1–4). Defense processes in the innate
immune system are mediated by the production of various
inflammatory mediators, such as nitric oxide (NO), and
prostaglandin E2 (PGE2), as well as the
expression of various pro-inflammatory cytokines, such as
interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α
(5–10). However, excessive amounts of these
mediators cause severe inflammatory diseases, including septic
shock, rheumatoid arthritis, systemic lupus erythematosus, cancer
and inflammatory bowel disease, although the enhanced production of
inflammatory mediators is important for host defense against
external stimuli (10–16). Macrophages are the major cells
that induce inflammatory responses by producing the various
inflammatory mediators listed above. Therefore, investigation of
the agents that inhibit the excessive production of inflammatory
mediators in activated macrophages may be a viable strategy for
developing effective anti-inflammatory agents.
Thunbergia alata (Acanthaceae), commonly
known as Black-eyed Susan vine, is a native plant of East Africa
and has been naturalized in other parts of the world, including
Brazil, Hawaii, Eastern Australia, and Southern USA. This plant was
traditionally used to treat inflammatory diseases. In Uganda, mild
infusions of the leaves and stems of T. alata are orally
administered to treat fever and malaria (17). Moreover, the infusion of leaves
was also introduced to treat diarrhea (18). Jeruto et al reported that
cough, flu, and backache were relieved by the oral administration
of the infusion of T. alata leaves in Kenya (19). Other researchers reported that in
Kenya, topical application of pounded leaves of T. alata was
successful in the treatment of boils on the skin (20). Furthermore, several studies have
elucidated the pharmacological properties of T. alata
including antimicrobial, antiviral, antifungal and sun protective
effects (21–23). According to phytochemical
research, T. alata contains phenolic compounds, such as
caffeoylmalic, feruloylmalic, and p-coumaroylmalic acids,
iridoid glucosides such as alatoside and thunaloside,
stilbericoside, 6-epi-stilbericoside and thunbergioside (24,25). Of these compounds, caffeoylmalic
acid has been reported to exert anti-inflammatory, antioxidant and
anti-spasmodic effects (26–28).
Although this plant has been used traditionally and
evaluated for its pharmacological activities, no systemic studies
on the immunomodulatory effects of the extract are available. In
the present study, we aimed to confirm the ethnopharmacological
benefits of T. alata in severe inflammatory states using
lipopolysaccharide (LPS)-activated macrophages. Furthermore, we
aimed to elucidate the precise mechanism of action responsible for
the anti-inflammatory effects of T. alata as there are no
prior reports regarding the mechanism of action of the methanol
extract of T. alata (MTA).
Materials and methods
Preparation of MTA
A methanol extract (voucher no: KRIB0043981) of
T. alata (Acanthaceae) collected from Lam Dong (Vietnam) was
purchased from the International Biological Material Research
Center [http://www.ibmrc.re.kr, Korea Research
Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea].
The concentrated methanol extract was manufactured according to a
standard protocol of KRIBB. Briefly, the leaves and stems of plants
(>1 kg by dry weight) were dried at room temperature (RT),
treated with methanol (HPLC grade), and sonicated several times at
50°C for 3 days. The extracts were filtered to remove solid
substances and concentrated with reduced pressure at 50°C. A stock
solution (200 mg/ml) of the extract was prepared in
dimethylsulfoxide (DMSO), and this was stored at −20°C prior to
use.
Cell culture
RAW 264.7 macrophages, a mouse monocyte/macrophage
cell line, were obtained from the American Type Cell Collection
(ATCC; Manassas, VA, USA) and cultured in Dulbecco's modified
Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (both
from GE Healthcare, Milwaukee, WI, USA), 50 U/ml penicillin, and 50
μg/ml streptomycin (Gibco-BRL, Grand Island, NY, USA) at
37°C in humidified air containing 5% CO2.
Antibodies
Rabbit anti-inhibitor of κB (IκB)α (sc-371),
anti-p38 (sc-535), anti-c-Jun N-terminal kinase (JNK; sc-571),
anti-signal transducer and activator of transcription 3 (STAT3;
sc-482), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
sc-25778) antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Rabbit anti-inducible NO synthase (iNOS;
2982), anti-cyclooxygenase (COX)-2 (4842), anti-phospho IκBα
(2859), anti-phospho p38 (9212), anti-extracellular
signal-regulated kinase (ERK; 9102), anti-phospho JNK
(Thr184/Tyr185; 9251), and anti-phospho STAT3 (Tyr705; 9131) and
mouse anti-phospho ERK (Thr202/Tyr204; 9106), were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell viability assay
The RAW 264.7 macrophages were seeded in 96-well
plates (4×104/well). Following adhesion overnight, cells
were treated with LPS (1 μg/ml) and various concentrations
of MTA for 24 h. EZ-Cytox solution (Daeil Lab, Seoul, Korea),
containing a water soluble tetrazolium salt, was added to each well
for 2 h at 37°C and 100 μl of supernatants were transferred
to a new 96-well plate. The absorbance was measured at 450 nm (650
nm as a reference absorbance) using a Synergy H1 microplate reader
(Bio-Tek Instruments, Winooski, VT, USA).
Nitrite assay
The RAW 264.7 macrophages seeded in 96-well plates
(4×104/well) overnight were incubated with MTA and LPS
(1 μg/ml) for an additional 24 h. Following incubation, the
levels of NO were determined by assaying the culture supernatants
for nitrite using Griess reagent (1% sulfanilamide, 0.1%
N-1-naphthylenediamine dihydrochloride and 2.5% phosphoric acid). A
standard curve for the calculation of NO production was acquired by
measuring the absorbance of fixed NaNO2 standard
solution. The absorbance was measured at 540 nm using a Synergy H1
microplate reader after incubation for 10 min.
Enzyme-linked immunosorbent assay
(ELISA)
The RAW 264.7 cells were stimulated with LPS (1
μg/ml) and MTA for 24 h. After stimulation, the supernatants
were obtained, and a sandwich ELISA was performed to determine the
quantities of TNF-α and IL-6 in culture supernatants using
Ready-SET-Go!® ELISA kits (eBioscience, San Diego, CA,
USA) with an antibody specific to each mediator. Briefly, the plate
was pre-coated with coating antibody in the supplied buffer.
Following incubation overnight at 4°C, the plate was washed with 1X
phosphate-buffered saline Tween (PBST) several times and treated
with 1X assay diluents for 1 h to block non-specific binding. After
removal of the assay diluents, each well was incubated with diluted
supernatants or standard solutions for 2 h at RT. After washing
with 1X PBST, the plate was treated with biotinylated secondary
antibody solution for 1 h. Subsequent replacement with horseradish
peroxidase (HRP)-streptavidin solution was performed after washing
several times. Following incubation at RT for 30 min, the
3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution was added
to a washed plate. The reaction was stopped by adding 1N phosphoric
acid (H3PO4) after a 10 min incubation in
dark conditions and the optical density of the individual wells was
determined at 450 nm using a Synergy H1 microplate reader.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
RAW 264.7 macrophages were treated with LPS (50
ng/ml) and various concentrations of MTA. After 3 h incubation,
total RNA was prepared from the cells using AccuZol (Bioneer,
Daejeon, Korea) and reverse transcribed into complementary DNA
(cDNA) using a TOPscript™ cDNA Synthesis kit (Enzynomics, Daejeon,
Korea). PCR amplification of the cDNA was then performed using a
PCR Premix (Bioneer). The sequences of PCR primers used were listed
in our previous study (29). The
PCR was run for 17–25 cycles of 94°C (30 sec), 60°C (30 sec), and
72°C (30 sec) using a Bioer thermal cycler (Bioer Technology Co.,
Hangzhou, China). After amplification, 10 μl of the PCR
products was separated in 1.5% (w/v) agarose gels and stained with
ethidium bromide.
Reverse transcription-quantitative PCR
(RT-qPCR)
PCR amplification of the cDNA was performed using a
qPCR Premix, iTaq™ Universal SYBR-Green Supermix (Bio-Rad,
Hercules, CA, USA). The PCR was run for 40 cycles of denaturation
at 94°C (5 sec) and annealing/extension at 60°C (30 sec) using a
CFX Connect™ Real-Time thermal cycler (Bio-Rad). The results were
normalized with multiple reference genes, β-actin and
GAPDH, and were expressed as the relative gene expressions
to the LPS-treated group (100%). The PCR primers used were listed
in our previous study (29).
Preparation of total cell lysates
RAW 264.7 cells were treated with MTA and LPS (1
μg/ml) for 15 min [for IκBs and mitogen-activated protein
kinases (MAPKs)] or 24 h (for iNOS, COX-2 and STAT3) and washed
with ice-cold PBS several times. Cells were lysed in lysis buffer
containing 0.5% IGEPAL® CA-630, 0.5% Triton X-100, 150
mM NaCl, 20 mM Tris-HCl (pH 8.0), 1 mM ethylenediaminetetraacetic
acid (EDTA), 1% glycerol, 1 mM phenylmethylsulfonyl fluoride
(PMSF), 10 mM NaF, and 1 mM Na3VO4. The
supernatants were collected in each microtube and centrifuged at
15,814 × g for 30 min at 4°C.
Western blot analysis
After boiling the mixture of lysates and sample
buffers, aliquots of the samples were separated in a 10% sodium
dodecyl sulfate (SDS)-polyacrylamide gel and transferred onto
nitrocellulose membranes with transfer buffer [192 mM glycine, 25
mM Tris-HCl (pH 8.8), and 20% MeOH (v/v)]. After blocking with 5%
non-fat dried milk in 1X Tris-buffered saline Tween (TBST)
solution, the membrane was incubated overnight at 4°C with the
primary antibodies which were diluted in 5% BSA- or 5% skim milk-1X
TBST solution. After washing with TBST, each membrane was incubated
for 1 h with secondary peroxidase-conjugated immunoglobulin G (IgG,
1:5,000). After washing several times, the protein bands were
detected using an enhanced chemiluminescence (ECL) solution. In the
present study, protein levels were quantified by scanning and
analyzing western blots with LabWorks software (UVP Inc., Upland,
CA, USA).
The blots shown in Fig. 2D, 3C and 5C were obtained from the same cell
lysates and therefore the identical GAPDH blot was used. In
addition, the blots shown in Fig. 5A
and B were also obtained from the same cell lysates.
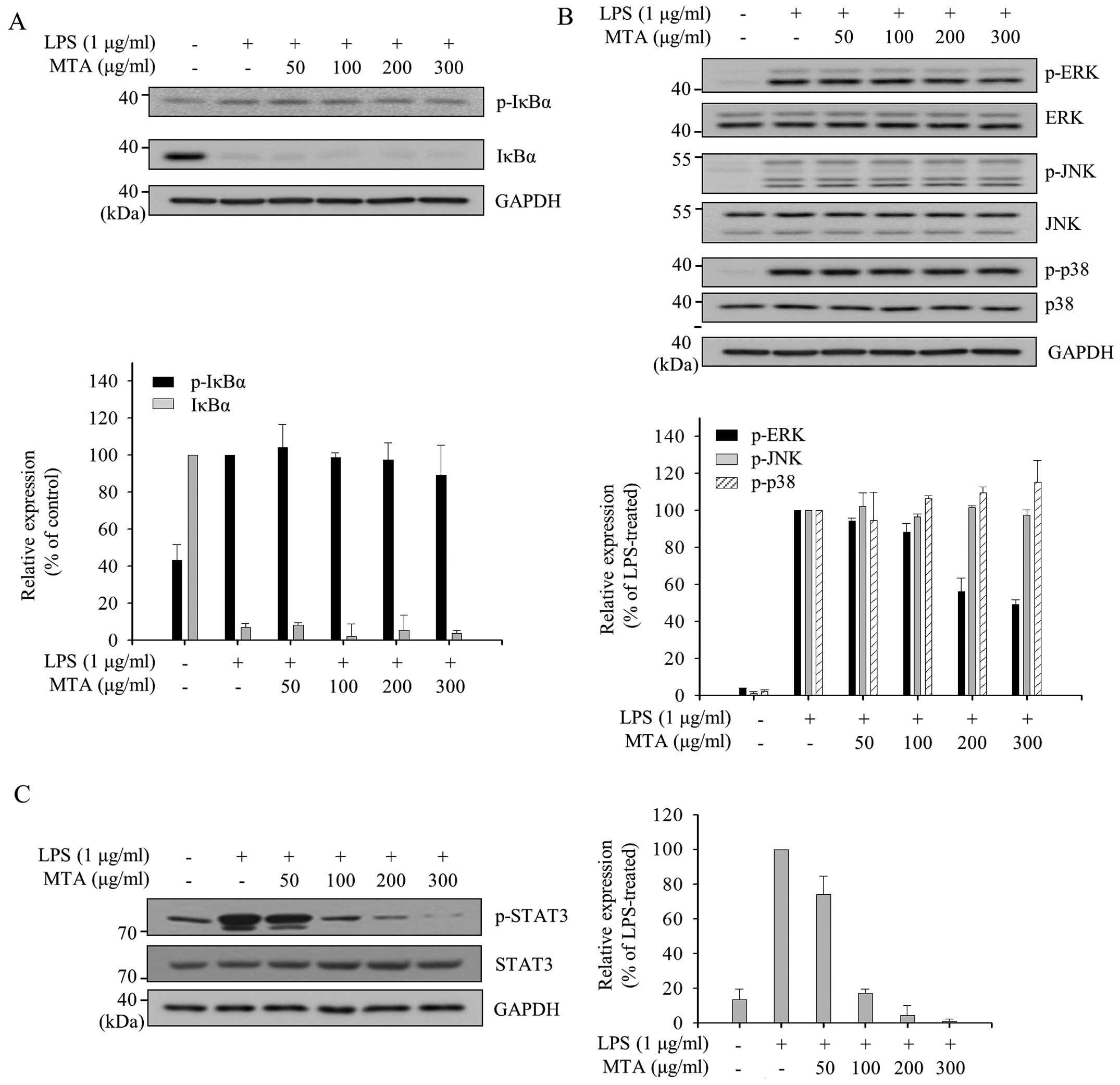 | Figure 5Differential effects of a methanol
extract of Thunbergia alata (MTA) on nuclear factor-κB
(NF-κB) and mitogen-activated protein kinase (MAPK) activation. RAW
264.7 macrophages were pre-treated with various concentrations of
MTA (50, 100, 200, and 300 μg/ml) for 1 h and then
stimulated with lipopolysaccharide (LPS) for 15 min (A and B) or 24
h (C). Total cell lysates were prepared and subjected towestern
blot analysis. The expression of (A) phosphorylated inhibitor of κB
protein (p-IκBα), IκBα, (B) p-c-Jun N-terminal kinase (JNK), JNK,
p-extracellular signal-regulated kinase (ERK), ERK, p-p38, and p38
were detected using specific antibodies. Relative expression levels
of IκBα and p-IκBα were normalized to glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) levels. Levels of p-MAPKs were normalized to
the corresponding MAPK levels. Quantitative analyses of
phosphorylation and protein levels are shown after normalization
(lower panels). (C) Total cell lysates were prepared after 24 h
treatment and subjected to western blot analysis. The
phosphorylation and total protein levels of signal transducer and
activator of transcription 3 (STAT3) was detected using specific
antibodies. Levels of p-STAT3 were normalized to the total STAT3
levels and were shown as a bar graph representing ratio to
LPS-treated group (right panel). |
Statistical analysis and experimental
replicates
The graph data are represented as the means ±
standard error of the mean (SEM). Determination of significant
differences between experimental conditions were assessed by the
Mann-Whitney U test which was performed using Prism 3.0 (GraphPad
Software, San Diego, CA, USA) and p<0.01 was considered to
indicate a statistically significant difference. The data from 9
replicates were analyzed, including three independent experiments
with three replicates in each.
Results
Determination of the non-cytotoxic
concentration of MTA in macrophages
We first examined the effect of MTA on the cell
viability of RAW 264.7 macrophages in order to determine the
maximal effective concentration that exhibits no cytotoxicity. RAW
264.7 cells were treated with various concentrations of MTA in the
presence of LPS for 24 h and cell viability was accessed by
measuring the formation of formazan. As shown in Fig. 1, cell viability did not decrease
up to 300 μg/ml of MTA. However, a notable reduction in cell
viability was observed at a concentration of 400 μg/ml of
MTA. We subsequently performed all experiments using 300
μg/ml of MTA to exclude the possibility that the
anti-inflammatory effect of MTA in activated macrophages was
induced through a cytotoxic effect.
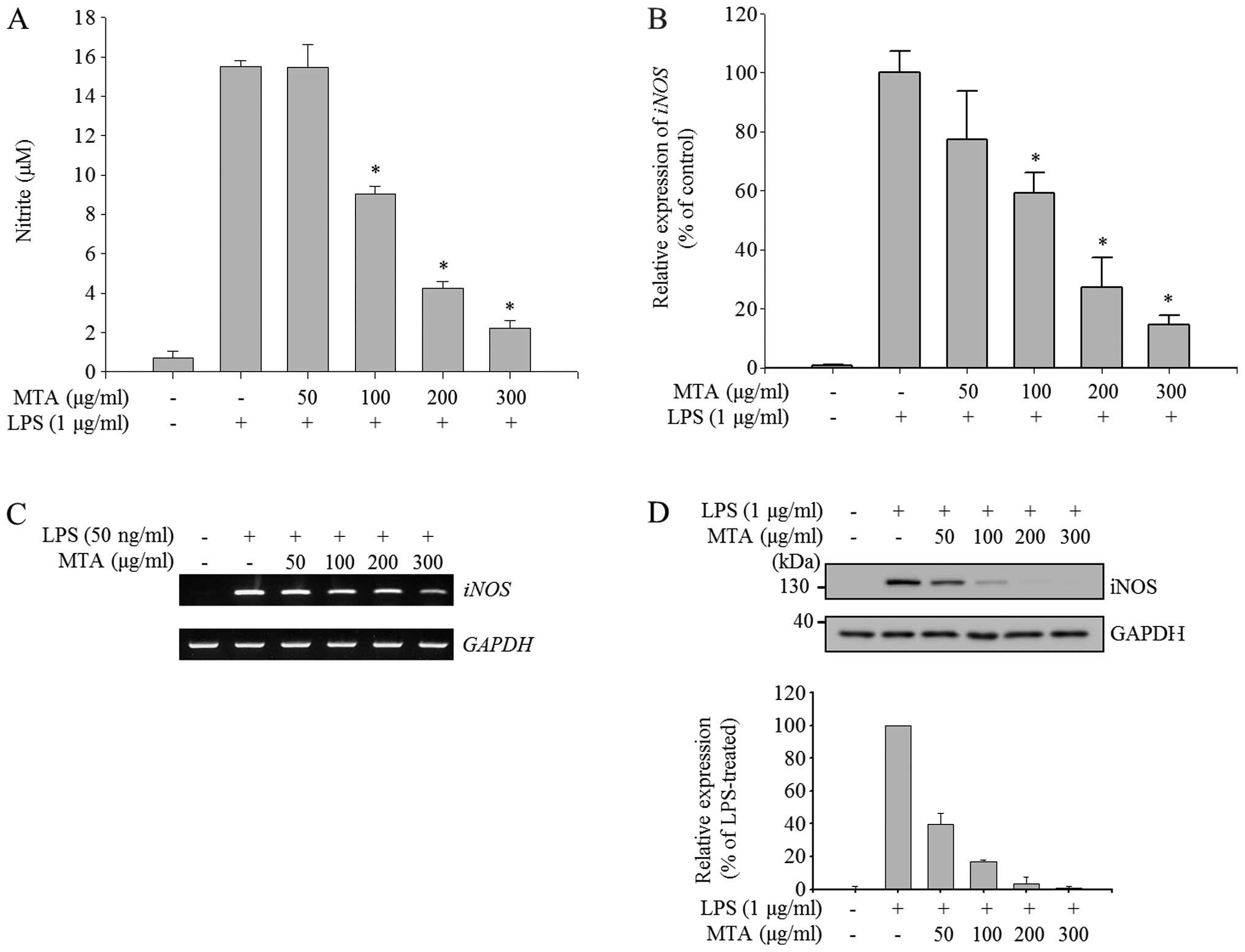
Inhibitory effect of MTA on the
production of NO in LPS-stimulated macrophages
To evaluate the anti-inflammatory effects of MTA in
macrophages, NO production and the expression of iNOS, the enzyme
responsible for NO production, were measured in LPS-stimulated RAW
264.7 macrophages. LPS stimulation highly induced the production of
NO, as shown in Fig. 2A. The
induction of NO was gradually reduced by MTA in a dose-dependent
manner; and the quantity of NO produced at a concentration of 300
μg/ml of MTA was as low as that of the unstimulated control.
We then examined whether MTA regulates the expression of iNOS at
the mRNA and protein level since iNOS is a key enzyme for the
production of NO in LPS-stimulated macrophages. RT-qPCR analysis
revealed that LPS-induced expression of iNOS was alleviated
by MTA treatment in a dose-dependent manner (Fig. 2B); and semi-quantitative PCR
result produced a similar profile to the qPCR data (Fig. 2C). Furthermore, the increased
protein expression of iNOS through LPS treatment was
dose-dependently reduced by MTA treatment (Fig. 2D). These results suggest that MTA
negatively regulates the production of NO by regulating iNOS at the
transcriptional level.
No effect of MTA on the expression of
COX-2 in LPS-stimulated macrophages
Since COX-2 is the enzyme responsible for the
production of PGE2, which induces fever during
inflammation, we explored the anti-inflammatory effect of MTA by
evaluating the mRNA and protein expression of COX-2. As shown in
Fig. 3A and B, LPS induced
COX-2 gene expression but MTA treatment did not lead to
suppression of LPS-induced COX-2 expression. Similar to the
RT-qPCR and semi-quantitative PCR data, MTA did not regulate
LPS-induced COX-2 expression at the protein level (Fig. 3C). The majority of natural
extracts that exhibit anti-inflammatory effects have been shown to
suppress PGE2 production through the inhibition of COX-2
expression (30,31). These results suggest that MTA does
not regulate the production of PGE2 since COX-2 expression in
activated macrophages is not inhibited by MTA.
Differential regulation of inflammatory
cytokine production by MTA in LPS-stimulated macrophages
To further investigate the anti-inflammatory effects
of MTA in activated macrophages, the profiles of proinflammatory
cytokines, IL-1β, IL-6, and TNF-α, were measured in the presence or
absence of MTA in LPS-stimulated RAW 264.7 macrophages. Cytokine
secretion, including IL-6 and TNF-α, was highly increased by LPS
treatment (Fig. 4A and B).
Notably, MTA treatment specifically inhibited the production of
IL-6 in a dose-dependent manner but did not regulate that of TNF-α
(Fig. 4A and B). To determine
whether the effect of MTA on the production of pro-inflammatory
cytokines is closely regulated at the transcriptional level, the
mRNA expression of pro-inflammatory cytokines was assessed in the
presence or absence of MTA in LPS-treated RAW 264.7 macrophages. As
shown in Fig. 4C, RT-qPCR
analysis revealed that MTA treatment inhibited the LPS-stimulated
expression of IL-6 and IL-1β but did not reduce that
of TNF-α. Similarly, semi-quantitative PCR analysis showed
that MTA selectively reduced the mRNA expression of IL-6 and
IL-1β (Fig. 4D). Taken
together, these results suggest that MTA negatively regulates the
LPS-induced production of pro-inflammatory cytokines by
transcriptional repression of IL-6 and IL-1β but not
TNF-α.
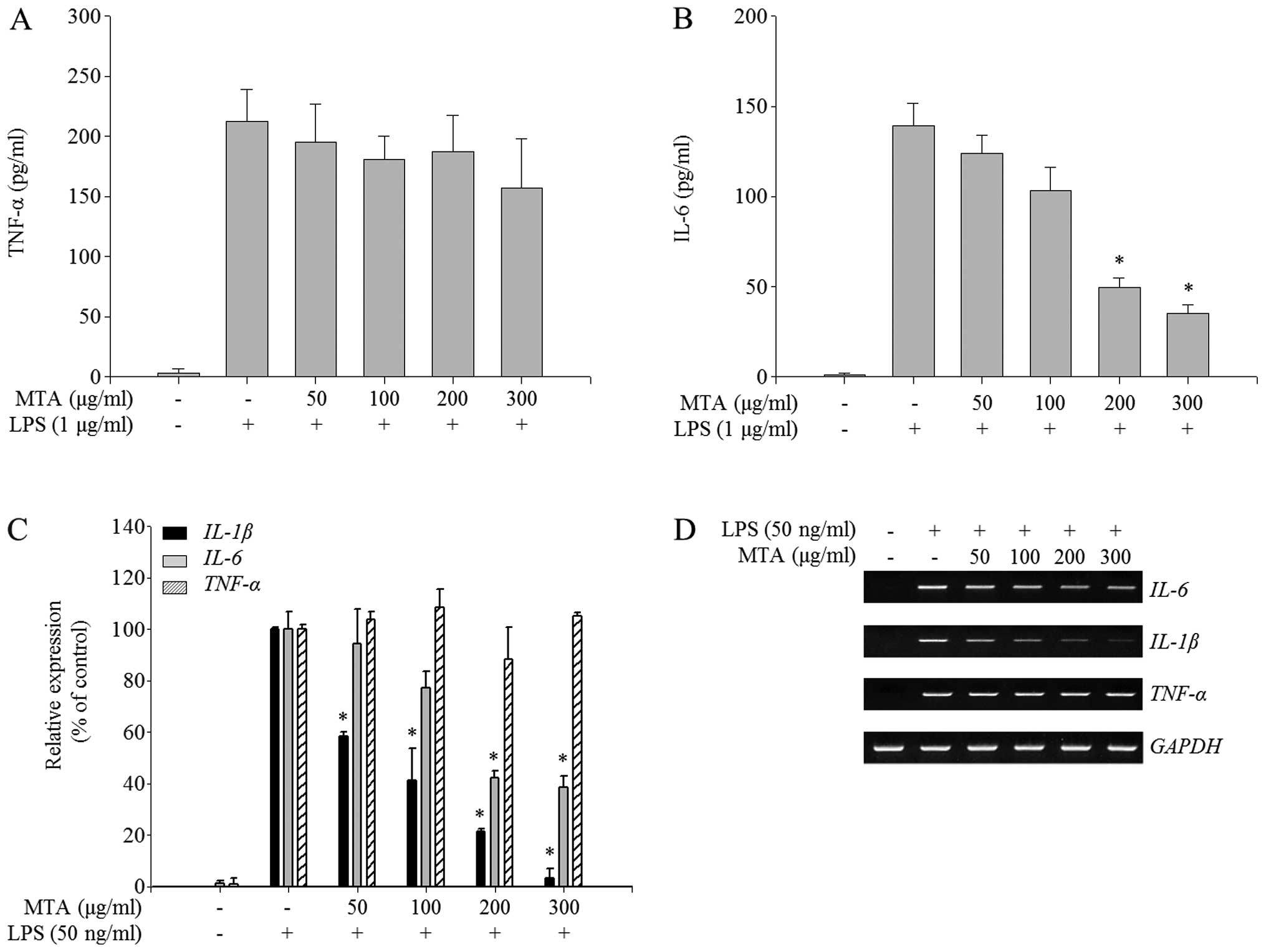 | Figure 4Inhibitory effect of a methanol
extract of Thunbergia alata (MTA) on the production of
pro-inflammatory cytokines. RAW 264.7 macrophages were treated
simultaneously with lipopolysaccharide (LPS) and MTA (50, 100, 200,
and 300 μg/ml) for the indicated times. (A and B) After 24 h
of treatment, enzyme-linked immunosorbent assays (ELISAs) were used
to measure levels of (A) tumor necrosis factor (TNF)-α and (B)
interleukin (IL)-6. The secretion of each cytokine was determined
using a standard curve. Data represent the means ± SEM.
*p<0.01 relative to the LPS-treated control. (C and
D) After 6 h of treatment, total RNA was extracted and reverse
transcribed to cDNA. (C) IL-1β, TNF-α and IL-6
were amplified by RT-qPCR, and the expression of IL-1β,
TNF-α and, IL-6 in each sample were compared to those
of the LPS-treated control. Data represent the means ± SEM.
*p<0.01 relative to the LPS-treated control. (D)
IL-1β, TNF-α and IL-6 were amplified by
semi-quantitative PCR and detected using a gel documentation
system. |
Inhibitory effect of MTA on the
activation of ERK and STAT3 in LPS-stimulated macrophages
The production of inflammatory mediators by LPS in
macrophages is mainly induced through the subsequent activation of
nuclear factor-κB (NF-κB) and MAPKs, including p38, ERK, and JNK.
The dissociation of IκBα from NF-κB by the phosphorylation of IκBα
is required for the activation of NF-κB, and subsequent
translocation of NF-κB leads to the activation of inflammatory
target genes. We therefore measured the phosphorylation levels of
IκBα in order to examine whether the inhibitory effects of MTA in
LPS-stimulated macrophages occur through the NF-κB signaling
pathway. As shown in Fig. 5A, LPS
treatment induced the phosphorylation of IκBα approximately
two-fold compared to the untrated group and reduced the expression
of IκBα due to phosphorylation-dependent degradation. However, MTA
treatment did not change the levels of p-IκBα and IκBα, suggesting
that MTA does not play a regulatory role in NF-κB signaling. The
regulation of MAPK signaling, another major LPS-induced
inflammatory signaling pathway, by MTA was also investigated. As
shown in Fig. 5B, the LPS-induced
phosphorylation of ERK was reduced by MTA without changing the
protein level of ERK, but the phosphorylations of other MAPKs, JNK
and p38, were unchanged by MTA. Collectively, these results
suggested that MTA exhibits anti-inflammatory properties by
inhibiting ERK signaling.
The differential regulation of pro-inflammatory
cytokines by MTA may be due to different transcription factor
binding regions in the gene promoter of each cytokine. Previous
studies revealed that the STAT protein-binding region is contained
in the IL-6 and IL-1β promoter regions but not in the TNF-α
promoter region (32). This
suggests that specific regulation of IL-6 and IL-1β production by
MTA in macrophages may be regulated through the inactivation of
STAT signaling. We accordingly measured the change in STAT3
phosphorylation status at Tyr705 following MTA treatment of
LPS-stimulated RAW 264.7 macrophages since STAT3 signaling is
activated through phosphorylation at Tyr705. LPS-induced
phosphorylation of STAT3 at Tyr705 was decreased by MTA in a
dose-dependent manner (Fig. 5C).
This result suggests that the differential regulation of
pro-inflammatory cytokines by MTA is due to negative modulation of
STAT3 activation.
Discussion
It is necessary to develop novel anti-inflammatory
agents since steroidal drugs such as glucocorticoids have severe
adverse effects (33–36). Non-steroidal anti-inflammatory
drugs (NSAIDs) such as aspirin and indomethacin were developed and
many studies have focused on the development of novel drugs capable
of functioning as anti-inflammatory cytokine agents (37). Due to these concerns, native
plants and their active constituents are receiving greater
attention nowadays since anecdotal evidence regarding traditional
usage as a medicine indicates the development of relatively fewer
side effects (38). Many
phytochemicals have been identified as anti-inflammatory drug
candidates and are under investigation for clinical use (39,40). In this study, we examined the
anti-inflammatory effects of MTA and the underlying molecular
mechanism responsible for these effects.
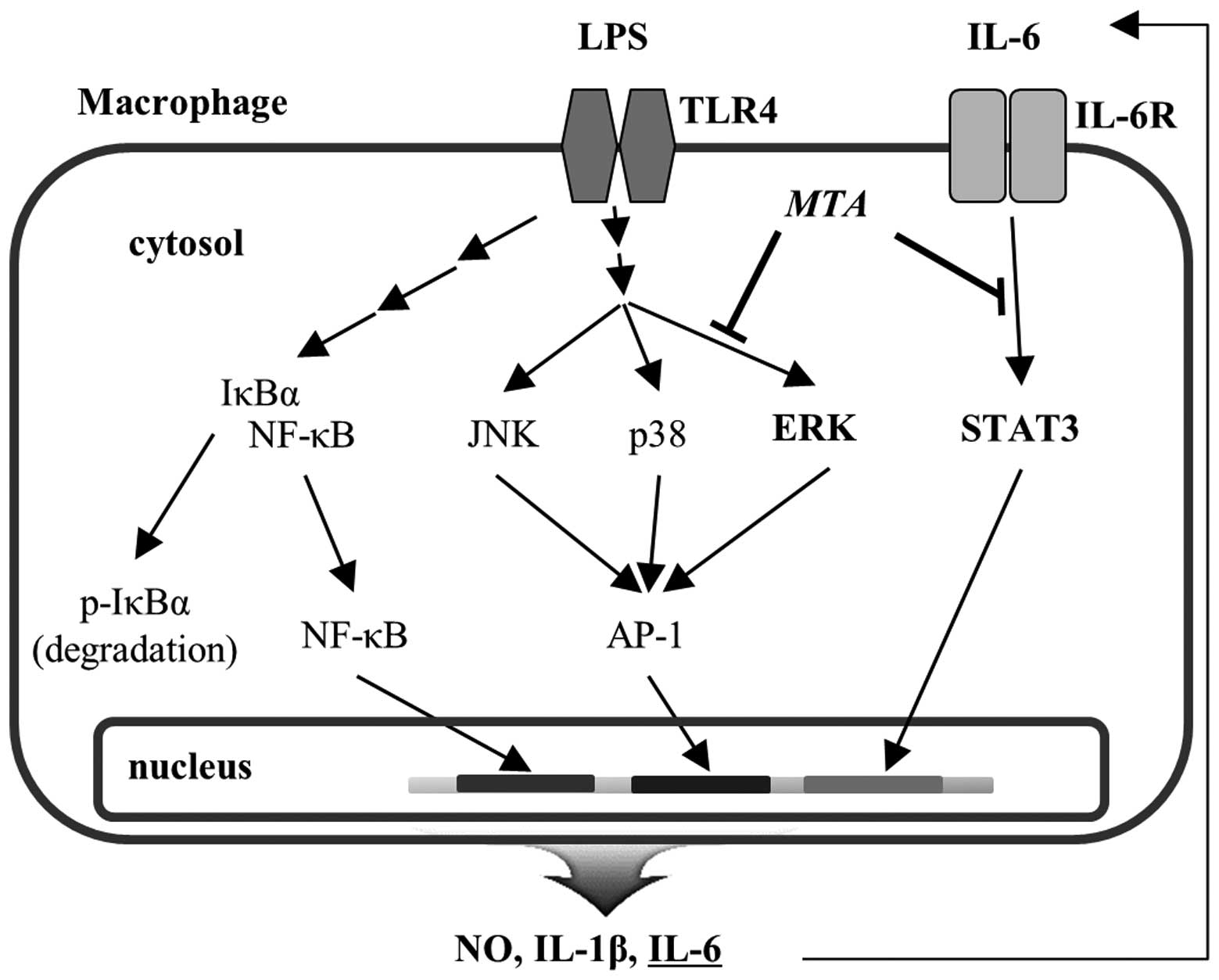
The activation of NF-κB and MAPKs is primed by LPS
ligation to Toll-like receptors. However, the activities of NF-κB
and MAPKs are regulated by different upstream kinases, such as IκB
kinases for NF-κB and MAPK kinases for MAPKs. Therefore, the data
in Fig. 5A and B suggest that MTA
regulates ERK activation specifically for its anti-inflammatory
properties without disturbing the activation of other MAPKs and
NF-κB in LPS-stimulated macrophages. Previous studies that describe
the differential regulation of MAPKs by anti-inflammatory
compounds, support our hypothesis (41–43). Several components, such as
curcumin, quercetin and resveratrol from plants, inhibit ERK
activity to exhibit various pharmacological effects in inflammatory
diseases (44–46).
TNF-α production increases through enhanced
activator protein 1 (AP-1) AP-1 transcriptional activity by MAPKs.
Therefore, as expected, LPS treatment induced TNF-α production in
RAW 264.7 cells. However, LPS-induced TNF-α production was not
inhibited by MTA treatment despite the reduced ERK activity
(Figs. 4A and 5B). It remains unclear the reason why
TNF-α induction was not suppressed through inhibition of ERK by MTA
treatment. As shown in Fig. 5B,
the phosphorylation of JNK and p38 was not inhibited by high
concentrations of MTA (300 μg/ml). These data suggest that
reduced AP-1 transcriptional activity through MTA-mediated ERK
inhibition may be recovered due to the functional redundancy of JNK
and p38 that are not inhibited by MTA.
As shown in the blots of Fig. 5B and C, the effects of MTA on the
regulation of both ERK and STAT3 were notably different. MTA
treatment at low doses almost completely inhibited the
phosphorylation of STAT3 (Fig.
5C). However, phosphorylated ERK was only 50% reduced when
LPS-stimulated cells were treated with the maximal concentration of
MTA (Fig. 5B), suggesting that
ERK is less sensitive to MTA than STAT3. Since the major regulatory
pathway for STAT3 activation is mediated by autocrine IL-6 via
LPS-activated NF-κB and MAPK signal transduction, these results
suggest that MTA selectively inhibits the IL-6-mediated STAT3
pathway at low concentrations but inhibits both ERK and STAT3
pathways at high concentrations. The differential regulation of
inflammatory mediators by MTA is likely due to the different
actions that are regulated by different concentrations of MTA. The
proposed regulatory mechanisms for the anti-inflammatory effects of
MTA are shown in Fig. 6.
Collectively, the findings of the present study
suggest that MTA selectively inhibits the production of various
inflammatory mediators by reducing the activation of ERK and STAT3
signaling pathways in macrophages. Further studies are necessary in
order to elucidate more fully the mechanisms of action of MTA
involved in the regulation of severe inflammatory states. Although
we elucidated the mechanism of action of MTA in murine macrophages
as an inhibitor of ERK and STAT3 activation, it is also necessary
to clarify the major phytochemicals that mediate the
anti-inflammatory effects of MTA.
Acknowledgments
The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Ministry of
Science, ICT and Future Planning (NRF-2015R1A2A2A11001446,
NRF-2015R1A5A1008958) and by the Ministry of Education, Science and
Technology (NRF-2013R1A1A2062389).
References
|
1
|
Boscá L, Zeini M, Través PG and Hortelano
S: Nitric oxide and cell viability in inflammatory cells: a role
for NO in macrophage function and fate. Toxicology. 208:249–258.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujihara M, Muroi M, Tanamoto K, Suzuki T,
Azuma H and Ikeda H: Molecular mechanisms of macrophage activation
and deactivation by lipopolysaccharide: roles of the receptor
complex. Pharmacol Ther. 100:171–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones BW, Heldwein KA, Means TK, Saukkonen
JJ and Fenton MJ: Differential roles of Toll-like receptors in the
elicitation of proinflammatory responses by macrophages. Ann Rheum
Dis. 60(Suppl 3): iii6–iii12. 2001.
|
|
6
|
Jones BW, Means TK, Heldwein KA, Keen MA,
Hill PJ, Belisle JT and Fenton MJ: Different Toll-like receptor
agonists induce distinct macrophage responses. J Leukoc Biol.
69:1036–1044. 2001.PubMed/NCBI
|
|
7
|
Rhee SH and Hwang D: Murine Toll-like
receptor 4 confers lipopolysaccharide responsiveness as determined
by activation of NF kappa B and expression of the inducible
cyclooxygenase. J Biol Chem. 275:34035–34040. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schroder K, Sweet MJ and Hume DA: Signal
integration between IFNgamma and TLR signalling pathways in
macrophages. Immunobiology. 211:511–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stalińska K, Guzdek A, Rokicki M and Koj
A: Transcription factors as targets of the anti-inflammatory
treatment. A cell culture study with extracts from some
Mediterranean diet plants. J Physiol Pharmacol. 56(Suppl 1):
157–169. 2005.
|
|
10
|
Szabó C and Thiemermann C: Regulation of
the expression of the inducible isoform of nitric oxide synthase.
Adv Pharmacol. 34:113–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clancy RM, Amin AR and Abramson SB: The
role of nitric oxide in inflammation and immunity. Arthritis Rheum.
41:1141–1151. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guadagni F, Ferroni P, Palmirotta R,
Portarena I, Formica V and Roselli M: Review. TNF/VEGF cross-talk
in chronic inflammation-related cancer initiation and progression:
an early target in anticancer therapeutic strategy. In Vivo.
21:147–161. 2007.PubMed/NCBI
|
|
13
|
Kröncke KD, Fehsel K and Kolb-Bachofen V:
Inducible nitric oxide synthase in human diseases. Clin Exp
Immunol. 113:147–156. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nathan C and Xie QW: Nitric oxide
synthases: roles, tolls, and controls. Cell. 78:915–918. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nathan C and Xie QW: Regulation of
biosynthesis of nitric oxide. J Biol Chem. 269:13725–13728.
1994.PubMed/NCBI
|
|
16
|
Nishimoto N and Kishimoto T: Interleukin
6: From bench to bedside. Nat Clin Pract Rheumatol. 2:619–626.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamill FA, Apio S, Mubiru NK, Mosango M,
Bukenya-Ziraba R, Maganyi OW and Soejarto DD: Traditional herbal
drugs of southern Uganda, I. J Ethnopharmacol. 70:281–300. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tabuti JR, Lye KA and Dhillion SS:
Traditional herbal drugs of Bulamogi, Uganda: plants, use and
administration. J Ethnopharmacol. 88:19–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeruto P, Lukhoba C, Ouma G, Otieno D and
Mutai C: An ethnobotanical study of medicinal plants used by the
Nandi people in Kenya. J Ethnopharmacol. 116:370–376. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okello SV, Nyunja RO, Netondo GW and
Onyango JC: Ethnobotanical study of medicinal plants used by
Sabaots of Mt. Elgon Kenya. Afr J Tradit Complement Altern Med.
7:1–10. 2009.PubMed/NCBI
|
|
21
|
Patil SB, Lende MY, Thakur VS, Naikwade NS
and Magdum CS: Sun protective activity of the hydroalchoholic
extracts of two medicinal flowers. Asian J Pharm Res. 2:37–38.
2012.
|
|
22
|
Vlietinck AJ, Van Hoof L, Totté J, Lasure
A, Vanden Berghe D, Rwangabo PC and Mvukiyumwami J: Screening of
hundred Rwandese medicinal plants for antimicrobial and antiviral
properties. J Ethnopharmacol. 46:31–47. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jenifer S, Priya S, Laveena DK, Singh JS
and Jeyasree J: Sensitivity patterns of some flowering plants
against Salmonella typhi and Pseudomonas aeruginosa. J Pharm Pharm
Sci. 3:1212–1220. 2014.
|
|
24
|
Housti F, Andary C, Gargadennec A and
Amssa M: Effects of wounding and salicylic acid on
hydroxycinnamoylmalic acids in Thunbergia alata. Plant Physiol
Biochem. 40:761–769. 2002. View Article : Google Scholar
|
|
25
|
Damfort S, Frederiksen LB and Jensen SR:
Alatoside and thunaloside, two iridoid glucosides from Thunbergia
alata. Phytochemistry. 35:1259–1261. 1994. View Article : Google Scholar
|
|
26
|
Boegge SC, Kesper S, Verspohl EJ and
Nahrstedt A: Reduction of ACh-induced contraction of rat isolated
ileum by coptisine, (+)-caffeoylmalic acid, Chelidonium majus, and
Corydalis lutea extracts. Planta Med. 62:173–174. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kimura Y, Okuda H, Okuda T, Hatano T and
Arichi S: Studies on the activities of tannins and related
compounds, X. Effects of caffeetannins and related compounds on
arachidonate metabolism in human polymorphonuclear leukocytes. J
Nat Prod. 50:392–399. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pearce G, Johnson S and Ryan CA:
Purification and characterization from tobacco (Nicotiana tabacum)
leaves of six small, wound-inducible, proteinase isoinhibitors of
the potato inhibitor II family. Plant Physiol. 102:639–644. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho YC, Ju A, Kim BR and Cho S:
Anti-inflammatory effects of Crataeva nurvala Buch. Ham. are
mediated via inactivation of ERK but not NF-κB. J Ethnopharmacol.
162:140–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee CW, Park SM, Kim YS, Jegal KH, Lee JR,
Cho IJ, Ku SK, Lee JY, Ahn YT, Son Y, et al: Biomolecular evidence
of anti-inflammatory effects by Clematis mandshurica Ruprecht root
extract in rodent cells. J Ethnopharmacol. 155:1141–1155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu T, Lee S, Yang WS, Jang HJ, Lee YJ, Kim
TW, Kim SY, Lee J and Cho JY: The ability of an ethanol extract of
Cinnamomum cassia to inhibit Src and spleen tyrosine kinase
activity contributes to its anti-inflammatory action. J
Ethnopharmacol. 139:566–573. 2012. View Article : Google Scholar
|
|
32
|
Lee C, Lim HK, Sakong J, Lee YS, Kim JR
and Baek SH: Janus kinase-signal transducer and activator of
transcription mediates phosphatidic acid-induced interleukin
(IL)-1beta and IL-6 production. Mol Pharmacol. 69:1041–1047.
2006.
|
|
33
|
Rubaltelli FF, Chiti G and Dani C: Adverse
effects of prenatal glucocorticoid treatment in the preterm infant.
Acta Biomed Ateneo Parmense. 68(Suppl 1): 35–38. 1997.PubMed/NCBI
|
|
34
|
Münstedt K, Borces D, Bohlmann MK, Zygmunt
M and von Georgi R: Glucocorticoid administration in antiemetic
therapy: is it safe? Cancer. 101:1696–1702. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Manelli F and Giustina A:
Glucocorticoid-induced osteoporosis. Trends Endocrinol Metab.
11:79–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van Raalte DH, Ouwens DM and Diamant M:
Novel insights into glucocorticoid-mediated diabetogenic effects:
towards expansion of therapeutic options? Eur J Clin Invest.
39:81–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dinarello CA: Anti-inflammatory agents:
present and future. Cell. 140:935–950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bassett IB, Pannowitz DL and Barnetson RS:
A comparative study of tea-tree oil versus benzoylperoxide in the
treatment of acne. Med J Aust. 153:455–458. 1990.PubMed/NCBI
|
|
39
|
Naksuriya O, Okonogi S, Schiffelers RM and
Hennink WE: Curcumin nanoformulations: a review of pharmaceutical
properties and preclinical studies and clinical data related to
cancer treatment. Biomaterials. 35:3365–3383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ranjan AP, Mukerjee A, Helson L, Gupta R
and Vishwanatha JK: Efficacy of liposomal curcumin in a human
pancreatic tumor xenograft model: inhibition of tumor growth and
angiogenesis. Anticancer Res. 33:3603–3609. 2013.PubMed/NCBI
|
|
41
|
Burk DR, Senechal-Willis P, Lopez LC,
Hogue BG and Daskalova SM: Suppression of
lipopolysaccharide-induced inflammatory responses in RAW 264.7
murine macrophages by aqueous extract of Clinopodium vulgare L.
(Lamiaceae). J Ethnopharmacol. 126:397–405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Bargouti M, Zughaier S, Zheng Z,
Liu Y, Sangadala S, Boden SD and Titus L: Osteoinductive LIM
mineralization protein-1 suppresses activation of NF-kappaB and
selectively regulates MAPK pathways in pre-osteoclasts. Bone.
46:1328–1335. 2010. View Article : Google Scholar
|
|
43
|
Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo
L and Yin Z: Chlorogenic acid inhibits lipopolysaccharide-induced
cyclooxygenase-2 expression in RAW264.7 cells through suppressing
NF-kappaB and JNK/AP-1 activation. Int Immunopharmacol.
9:1042–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Min Z, Kang L, Lin L, Jinghua F, Junna S
and Baolin L: Resveratrol restores lysophosphatidylcholine-induced
loss of endothelium-dependent relaxation in rat aorta tissue
coinciding with inhibition of extracellular-signal-regulated
protein kinase activation. Phytother Res. 24:1762–1768. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang J and Dong S: ICAM-1 and IL-8 are
expressed by DEHP and suppressed by curcumin through ERK and p38
MAPK in human umbilical vein endothelial cells. Inflammation.
35:859–870. 2012. View Article : Google Scholar
|