Introduction
Diabetes mellitus, which is a complex metabolic
disease, is characterized by increased blood glucose levels, which
are caused by the lack of insulin production or resistance to
insulin (1). The most common
forms are type 1 diabetes (T1D) and type 2 diabetes (T2D) (1,2).
T1D is mainly caused by autoimmune β cell destruction-induced
insulin deficiency, while T2D often results from defects in insulin
sensitivity and β cell dysfunction (1,3,4).
However, the molecular mechanisms through which β cell dysfunction
occurs have not yet been fully elucidated. Thus, the understanding
of the underlying mechanisms may aid in the development of novel
therapeutic strategies for diabetes.
MicroRNAs (miRNAs or miRs), a class of non-coding
RNAs, 18–25 nucleotides in length, are able to suppress gene
expression by targeting the complementary regions of mRNAs and
inhibiting protein translation (5). By negatively mediating their target
genes, miRs act as key regulators in a variety of physiological,
pathological and biological processes, including development,
metabolic disorders and tumorigenesis, as well in diseases, such as
diabetes (6–9). Deregulations of miRs have been
observed in patients with diabetes, and specific miRs have been
demonstrated to be involved in the regulation of pancreatic
development and function (6,10,11). For instance, Jacovetti et
al found that β cell maturation was associated with alterations
in the expression of miRs induced by the nutritional transition
that occurs at weaning, and suggested that miRs play a central role
in post-natal β cell maturation and in the determination of adult
functional β cell mass (12). The
serum levels of miR-15a have been shown to be reduced in patients
with T2D and in individuals with impaired fasting glucose
(IFG)/impaired glucose tolerance (IGT), and a lower miR-15a
expression has been shown to be significantly associated with T2D
and pre-diabetes (13). However,
the miR-mediated effectors or signaling pathways that play key
roles in diabetes have not yet been fully investigated.
miR-19a-3p, a member of the miR-17-92 miR cluster,
has been previously found to be a regulator of the expression of
5-lipoxygenase, a key enzyme in leukotriene biosynthesis (14). Moreover, it has been previoulsy
suggested to be associated with several types of human cancer, such
as breast cancer (15),
astrocytoma (16), gastric cancer
(17), skin cancer (18) and colorectal adenocarcinoma
(19). Recently, miR-19a-3p was
found to be upregulated in patients with gestational diabetes
mellitus (20). However, its
levels have been shown to be downregulated in the livers of db/db
mice, and miR-19a-3p has been suggested to promote glycogenesis in
hepatocytes through the downregulation of phosphatase and tensin
homolog (PTEN) expression (21).
Furthermore, miR-19a has been shown to promote cell proliferation
and angiogenesis by regulating the PI3K/AKT pathway associated with
diabetes-associated pancreatic cancer (22–24). However, the detailed role of
miR-19a-3p in the regulation of β cell proliferation and function,
as well as the underlying mechanisms, remain largely unknown.
In this study, we aimed to examine the role of
miR-19a-3p in diabetes. We found that miR-19a-3p was significantly
downregulated in diabetic patients, and that it enhanced cell
proliferation and insulin secretion, while it inhibited the
apoptosis of pancreatic β cells by directly targeting suppressor of
cytokine signaling 3 (SOCS3). Accordingly, we suggest that
miR-19a-3p may serve as a potential candidate for the clinical
management of diabetes.
Materials and methods
Blood sample collection
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Chongqing Medical University
(Chongqing, China), and was carried out in accordance with the
Declaration of Helsinki. Written informed consent was obtained from
all study subjects prior to enrollment. Blood samples from patients
with T2D (n=45) and normal subjects (n=20) were collected from the
First Affiliated Hospital of Chongqing Medical University from
November, 2012 to February, 2014. Patients with serious liver or
kidney diseases, malignancy and acute heart failure were
excluded.
Determination of glucose and SOCS3 levels
in blood samples
The glucose levels in the blood samples were
determined by the routine laboratory method. The blood glucose
levels were examined at the Department of Clinical Laboratory of
our hospital using the Glucose Assay kit (BioVision, San Francisco,
CA, USA), according to the manufacturer's instructions. Plasma
SOCS3 levels were analyzed by enzyme-linked immunosorbent assay
(ELISA), according to the manufacturer's instructions using the
SOCS-3 ELISA kit (Ybiotech, Shanghai, China). Briefly, an equal
amount of protein (50 µg) was diluted with sample dilution
buffer to 100 µl, and then added in each well, which was
incubated for 2.5 h at room temperature with gentle shaking. The
solution was discarded and washed for 4 times with wash solution.
After the final wash, any remaining liquid was removed by
decanting. The plate was then inverted and blotted against clean
paper towels. SOCS3 detection antibody was then added followed by
incubation for 1 h at room temperature with gentle shaking.
Subsequently, 100 µl of HRP-streptavidin solution was added
followed by incubation for 45 min at room temperature with gentle
shaking. This was followed by another wash and 100 µl of TMB
one-Step substrate reagent was added to each well followed by
incubation for 30 min at room temperature in the dark with gentle
shaking. Subsequently, 50 µl of stop solution was added and
read at 450 nm immediately. A standard curve was constructed using
various dilutions of the SOCS3 standard preparation in the kit. The
content of SOCS3 was determined by extrapolation of the absorbance
to the standard curve.
Cell culture
The pancreatic β cell lines, INS-1 and MIN6, were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 25 mM glucose and 15% fetal
bovine serum (FBS) (both from Life Technologies, Grand Island, NY,
USA) and 5.5 mM 2-mercaptoethanol (Thermo Fisher Scientific,
Waltham, MA, USA). INS-1 and MIN6 cells were collected and then
suspended using DMEM with 10% FBS. The cells were then seed into a
6-well plate (300,000 cells per well), and cultured to 70%–80%
confluence.
Cell transfection
miR-negative control (miR-NC), miR-19a-3p mimic
(both from GenePharma, Shanghai, China), siRNA targeting SOCS3
(SOCS3 siRNA), non-specific siRNA (NC siRNA), the pc-DNA3.1-SOCS3
plasmid and pc-DNA3.1 vector (NC), and Lipofectamine 2000 were
diluted with OPTI-MEM (both from Life Technologies). The diluted
Lipofectamine 2000 was added to the respective diluted plasmid,
miR, or siRNA. Following incubation at room temperature for 20 min,
the above mixture was added to the cell suspension, which was then
incubated at 37°C, 5% CO2 for 6 h. Subsequently, the
transfection mixture was replaced by DMEM with 10% FBS.
Untransfected cells were used as controls. Following transfection
for 48 h, the following assays were conducted:
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Life Technologies). RT-qPCR was used to examine the
relative miR-19a-3p expression using the mirVana™ real-time RT-PCR
microRNA detection kit (Life Technologies), in accordance with the
manufacturer's instructions. U6 was used as an internal reference.
The specific primers for miR-19a-3p and U6 were purchased from
Genecopoeia, Guangzhou, China. The relative mRNA expression of
SOCS3 was detected by quantitative PCR (qPCR) using the standard
SYBR-Green RT-PCR kit (Takara, Otsu, Japan) in accordance with the
manufacturer's instructions. Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as an internal reference gene. The
specific primers for SOCS3 were as follows: forward,
5′-ATGGTCACCCACAGCAAGTTT-3′ and reverse,
5′-TCCAGTAGAATCCGCTCTCCT-3′. The specific primers for GAPDH were as
follows: forward, 5′-TGGCCTTCCGTGTTCCTAC-3′ and reverse,
5′-GAGTTGCTGTTGAAGTCGCA-3′. The relative expression level was
quantified using the the 2−ΔΔCt method.
Western blot analysis
The cells were lysed in the protein lysis buffer [50
mM Tris/HCl, pH 8.0, 250 mM NaCl, 1% NP-40, 0.5% (w/v) sodium
deoxycholate, 0.1% sodium dodecylsulfate, 1% PMSF and 1X
phosphatase inhibitor cocktail]. The protein concentration was
determined using the BCA Protein assay kit (Pierce Chemical,
Rockford, IL, USA). Protein (60 µg) was separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), transferred onto a PVDF membrane (Life Technologies),
and then blocked in 5% non-fat dried milk (Yili, Beijing, China) in
TBST for 2 h. The PVDF membrane was then incubated with primary
antibodies against SOCS3 (ab16030) and GAPDH (ab37168; both from
Abcam, Cambridge, MA, USA) at 4°C overnight, and then washed with
TBST 4 times. The PVDF membrane was then incubated with the
corresponding secondary antibody (goat monoclonal anti-rabbit IgG;
ab190492; Abcam) for 1 h at room temperature, and then washed with
TBST 3 times. The immune complexes were then detected using the ECL
Western Blotting kit (Pierce Chemical) and X-film (Kodak, Tokyo,
Japan). ImageJ software was used to analyze the relative protein
expression, represented as the density ratio versus GAPDH.
Detection of glucose-stimulated insulin
secretion
The cells were seeded in a 96-well plate and
cultured overnight. The cells were then treated with basal glucose
(3.3 mmol/l) or stimulatory glucose (16.7 mmol/l) for 1 h.
Subsequently, the insulin level was measured by ELISA. In brief,
the cells in each well were sonicated in acid ethanol, followed by
3 freeze/thaw cycles, and then centrifuged for 5 min at 10,000 x g.
The supernatant was used to measure the insulin level by ELISA as
described above.
Determination of cell proliferation
The cells (2×103) in each group (miR-NC,
miR-19a-3p mimic, SOCS3 siRNA, NC siRNA, miR-19a-3p mimic + SOCS3,
ormiR-19a-3p mimic + NC vector) were plated into a 96-well plate
and cultured for 1 to 5 days at 37°C with 5% CO2.
Subsequently, 20 µl of
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
5 mg/ml; Sigma, St. Louis, MO, USA) were added. Followoing
incubation at 37°C for 4 h, 150 µl of dimethyl sulfoxide
(DMSO) were added. Following incubation at room temperature for 10
min, the formazan production was detected by determining the
optical density (OD) at 570 nm using the Elx800 enzyme immunoassay
analyzer (BioTek Instruments, Inc., Winooski, VT, USA).
Apoptosis assay
The cell apoptotic levels were examined using the
Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(BD Pharmingen, San Diego, CA, USA), according to the
manufacturer's instructions. The cells in each group were
re-suspended in 1X binding buffer solution with Annexin V-FITC and
PI and incubated for 15 min at room temperature in the dark. The
apoptotic rate was determined using the EPICS Altra flow cytometer
(Beckman Coulter, Brea, CA, USA).
Bioinformatics analysis
Bioinformatics analysis was conducted to predict the
putative targets of miR-19a-3p using TargetScan 4.2 online software
(www.targetscan.org).
Luciferase reporter assay
The wild-type (WT) of SOCS3 3′-UTR was constructed
by PCR and inserted into the pMIR-REPORT miRNA Expression Reporter
vector (Ambion, Carlsbad, CA, USA). The mutant type (MT) of SOCS3
3′-UTR was constructed using the Easy Mutagenesis system kit
(Promega, Madison, WI, USA), in accordance with the manufacture's
instructions, and inserted into the pMIR-REPORT miRNA Expression
Reporter vector. 293 cells (Cell Bank of the Chinese Academy of
Sciences) were plated in 96-well plates, and co-transfected with
the WT SOCS3-3′UTR or MT SOCS3-3′UTR plasmid (300 ng), and miR-NC
or miR-19a-3p mimic (100 nM), using Lipofectamine 2000, in
accordance with the manufacture's instructions. The dual-luciferase
reporter assay system (Promega) was used to determine the activity
of Renilla luciferase and Firefly luciferase following
co-transfection for 48 h. The Renilla luciferase activity
was normalized to the Firefly luciferase activity.
Statistical analysis
The data of at least 3 independent experiments are
expressed as the means ± SD. GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA) was used to perform statistical
analysis. The differences were analyzed using the Student's t-test
or one-way ANOVA. The association between the miR-19a-3p level and
the blood glucose or SOCS3 levels was analyzed using Spearman's
correlation coefficient. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-19a-3p expression is significantly
downregulated in diabetic patients
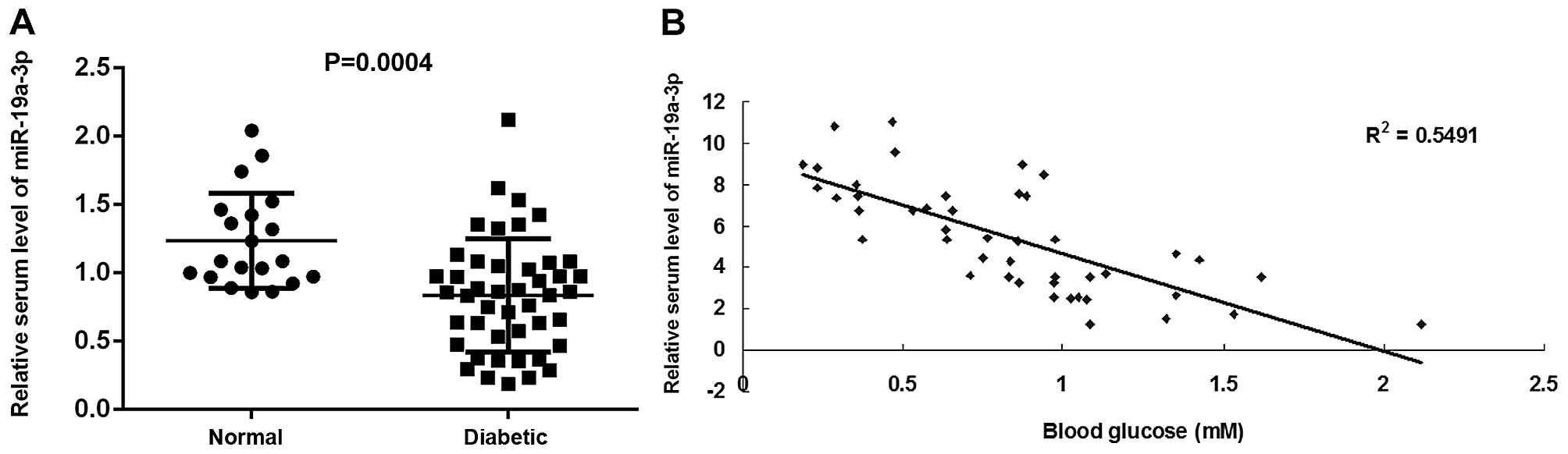
To reveal the function of miR-19a-3p in diabetes, we
first conducted RT-qPCR to examine the plasma miR-19a-3p level in
diabetic patients and normal subjects. Our data demonstrated that
the miR-19a-3p level was significantly decreased in the blood of
diabetic patients, when compared with that of normal subjects
(Fig. 1A). In addition, we
observed a significant inverse correlation between the plasma
miR-19a-3p level and the blood glucose concentration among these
diabetic patients (Fig. 1B).
These data suggest that the downregulation of miR-19a-3p expression
plays a role in the progression of diabetes.
miR-19a-3p enhances the proliferation and
insulin secretion, while it inhibits the apoptosis of pancreatic β
cells
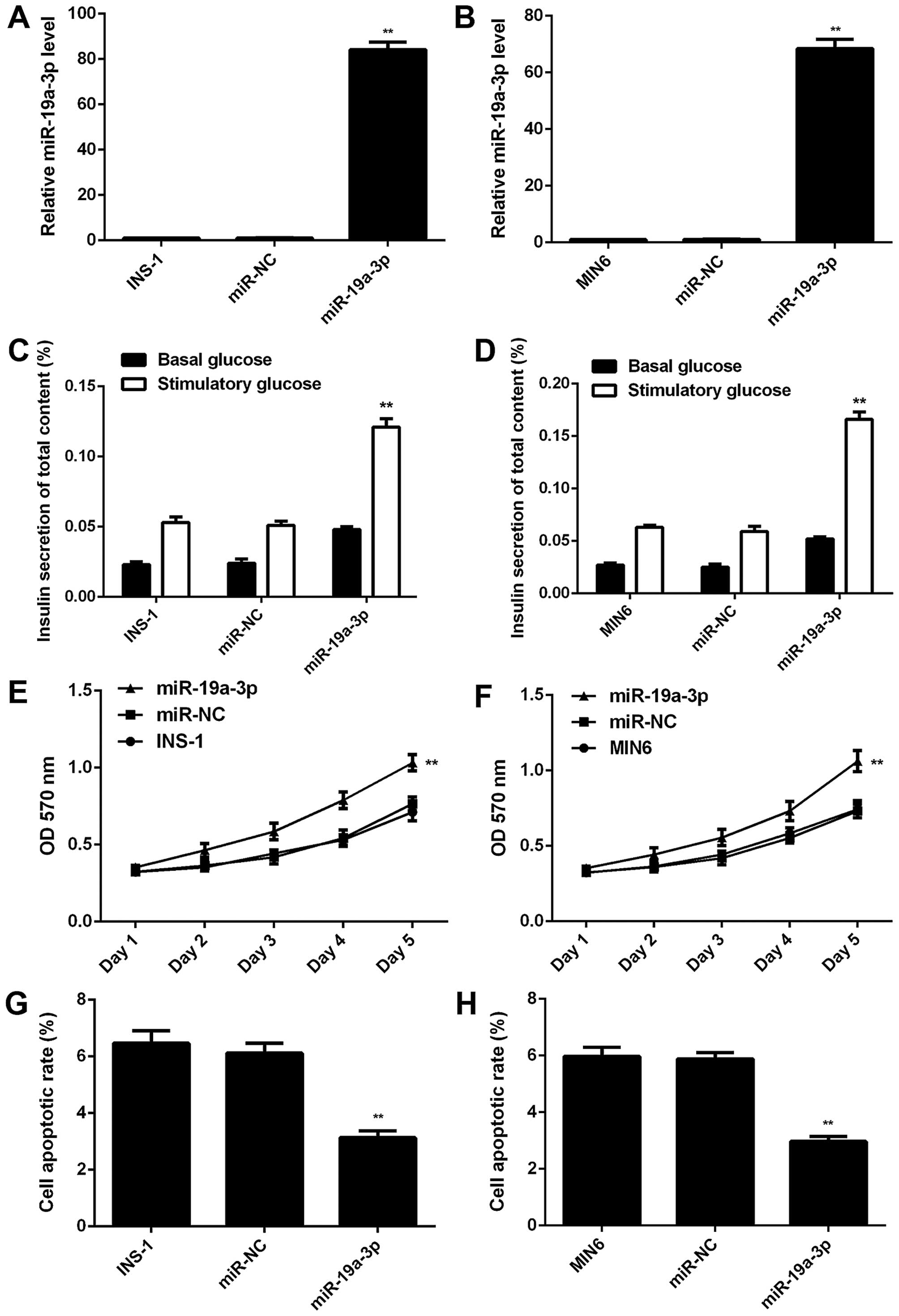
The glucose-responsive pancreatic β cell lines,
INS-1 and MIN6, were used in our in vitro experiments to
investigate the exact role of miR-19a-3p in pancreatic β cells. As
miR-19a-3p was found to be significantly downregulated in diabetic
patients, we transfected the INS-1 and MIN6 cells with miR-19a-3p
mimic to restore its expression level. Following transfection, qPCR
was conducted to examine the expression level of miR-19a-3p. Our
data indicated that the level of miR-19a-3p was significantly
higher in the cells transfected with miR-19a-3p mimic compared to
the control or negative control (NC) groups (Fig. 2A and B). However, transfection
with miR-NC did not alter the miR-19a-3p level in the INS-1 and
MIN6 cells (Fig. 2A and B). We
then found that the insulin secretion in response to glucose
stimulation was significantly increased in the
miR-19a-3p-overexpressing INS-1 and MIN6 cells, when compared with
the control and NC groups (Fig. 2C
and D), indicating that miR-19a-3p induced insulin secretion
under conditions of glucose stimulation. We further examined the
effect of miR-19a-3p on the proliferation and apoptosis of
pancreatic β cells. Our data indicated that the overexpression of
miR-19a-3p markedly enhanced cell proliferation (Fig. 2E and F), while it suppressed the
apoptosis of the INS-1 and MIN6 cells (Fig. 2G and H), when compared with the
control and NC groups. Based on these data, it is thus evident that
miR-19a-3p enhances the proliferation and insulin secretion, while
it inhibits the apoptosis of pancreatic β cells.
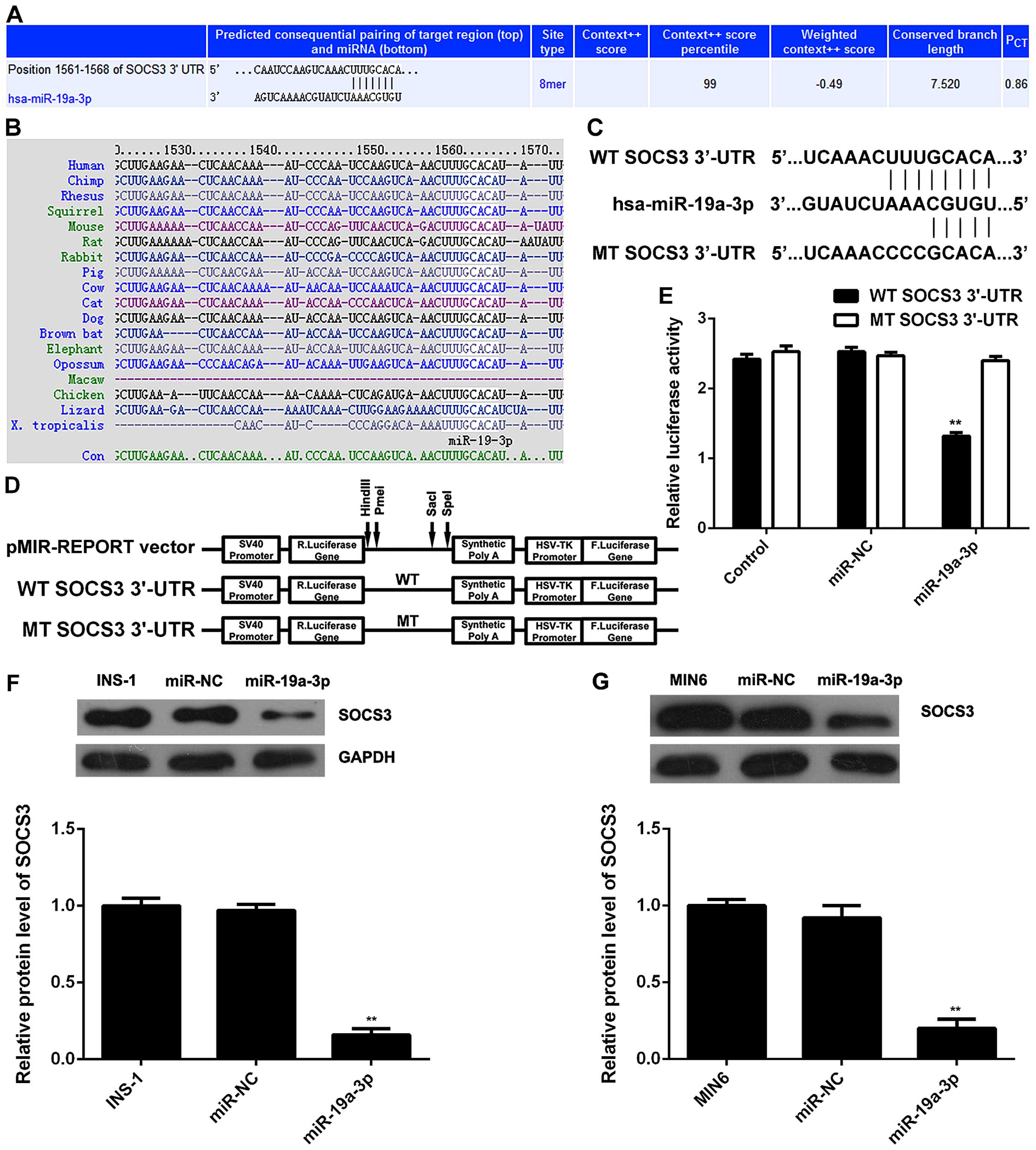
SOCS3 is a target gene of miR-19a-3p in
pancreatic β cells
As miRs function by regulating the expression of
their target genes, we then used TargetScan software to analyze the
putative target genes of miR-19a-3p. SOCS3 was predicated to be a
potential target gene of miR-19a-3p with evolutionary conservation
(Fig. 3A and B). To verify their
targeting relationship, the WT SOCS3 3′-UTR containing the
predicated miR-19a-3p binding sites or the MT SOCS3 3′-UTR without
the predicated miR-19a-3p binding sites were subcloned into the
luciferase reporter vector, respectively (Fig. 3C and D). Luciferase reporter assay
was then conducted, and our data indicated that co-transfection
with the WT SOCS3 3′-UTR vector and miR-19a-3p mimic led to a
significant decrease in luciferase activity; however, the
luciferase activity was not altered in the 293 cells co-transfected
with the MT SOCS3 3′-UTR vector and miR-19a-3p mimic (Fig. 3E). Accordingly, SOCS3 is a direct
target gene of miR-19a-3p. As miRs negatively mediate the
expression of their target genes at the post-transcriptional level,
we then examined the protein expression of SOCS3 in
miR-19a-3p-overexpressing INS-1 and MIN6 cells. We observed that
the protein level of SOCS3 was markedly decreased following the
upregulation of miR-19a-3p, when compared with the control and NC
groups (Fig. 3F and G).
Accordingly, the overexpression of miR-19a-3p inhibited the protein
expression of SOCS3 in pancreatic β cells.
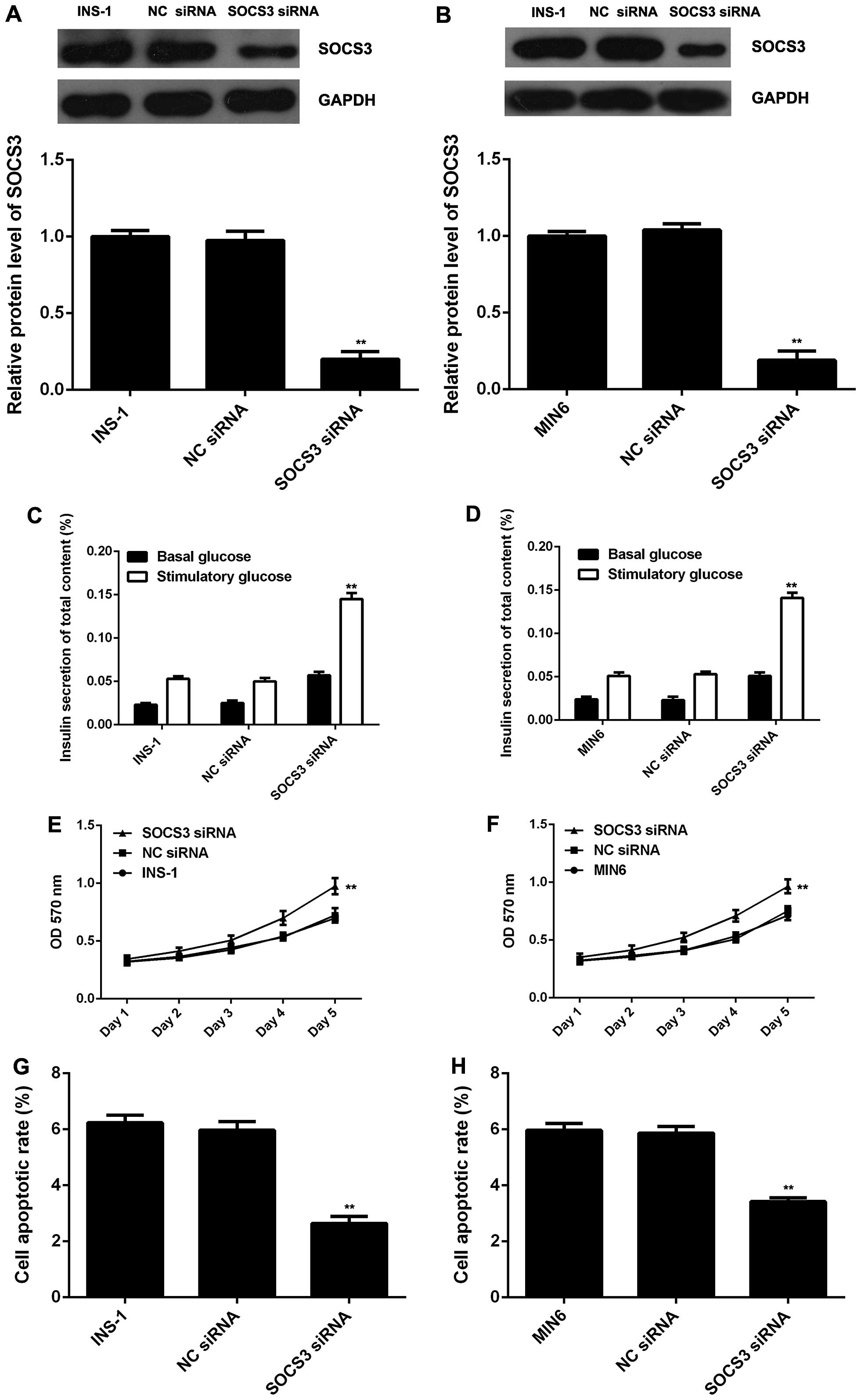
SOCS3 is involved in the
miR-19a-3p-mediated proliferation and apoptosis of, as well as in
insulin secretion by pancreatic β cells
As the overexpression of miR-19a-3p led to a
significant decrease in the protein level of SOCS3, we investigated
whether the downregulation of SOCS3 has similar effects as
miR-19a-3p overexpression in pancreatic β cells. SOCS3 siRNA was
used to transfect the INS-1 and MIN6 cells, and the protein level
of SOCS3 was found to be signifi-cantly decreased following
transfection (Fig. 4A and B). We
further found that the knockdown of SOCS3 led to a significant
increase in glucose-stimulated insulin secretion and cell
proliferation, as well as to a marked decrease in the apoptosis of
the INS-1 and MIN6 cells (Fig.
4C–H); these effects were very similar to those observed with
miR-19a-3p overexpression. According to these data, we hypothesized
that SOCS3 may act as a downstream effector of SOCS3 in pancreatic
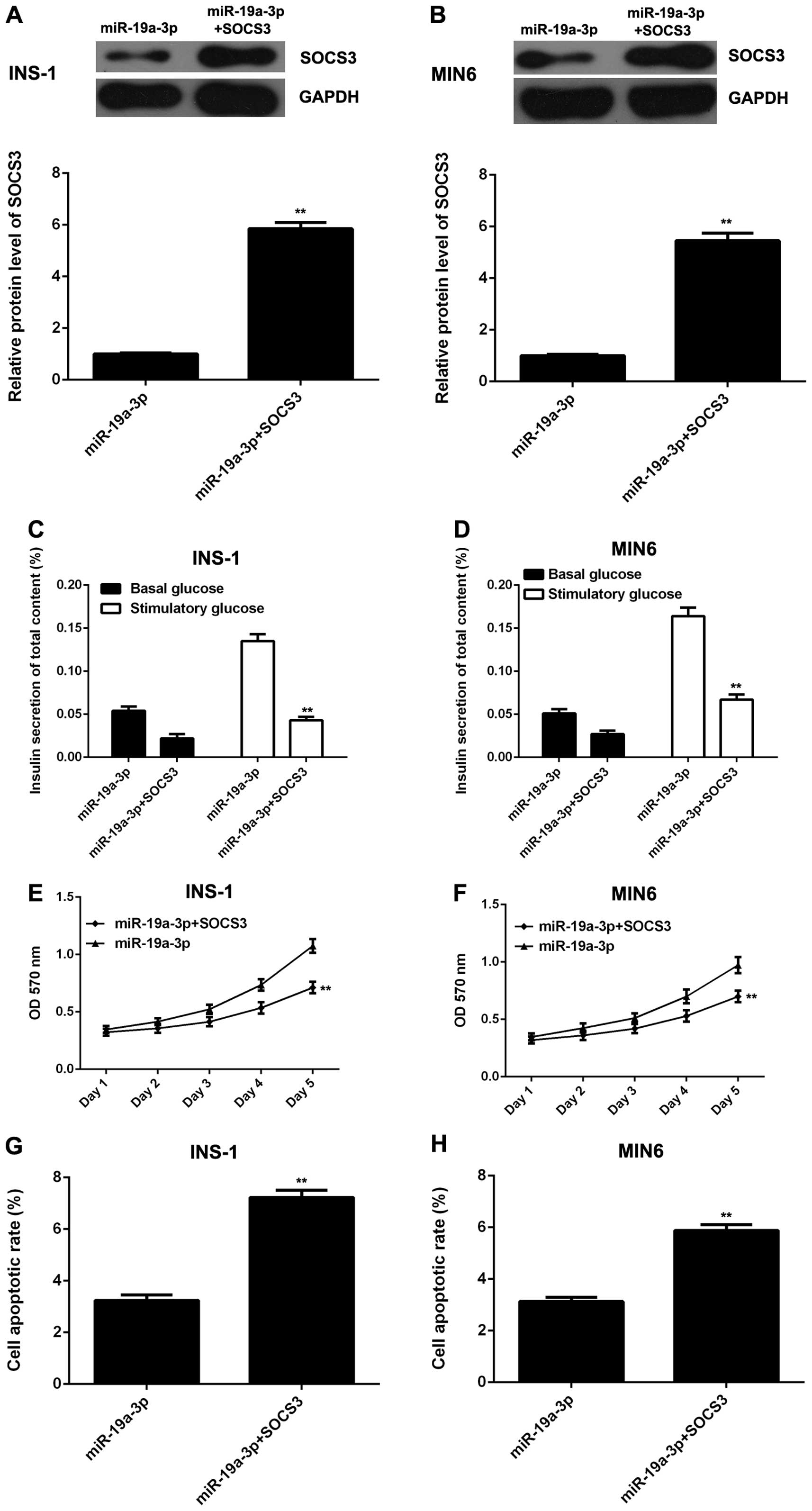
β cells. To verify this hypothesis, the pcDNA3.1-SOCS3 plasmid was
used to transfect miR-19a-3p-overexpressing INS-1 and MIN6 cells in
order to restore the expression of SOCS3. Following transfection,
western blot analysis was used to examine the protein level of
SOCS3. As shown in Fig. 5A and B,
the SOCS3 protein level was significantly higher in the INS-1 and
MIN6 cells co-transfected with the miR-19a-3p mimic and SOCS3
plasmid, when compared to the cells transfected with miR-19a-3p and
the NC vector. We then examined the glucose-stimulated insulin
secretion, cell proliferation and cell apoptosis in each group. Our
data revealed that compared with the cells transfected with
miR-19a-3p and the NC vector, the INS-1 and MIN6 cells
co-transfected with the miR-19a-3p mimic and SOCS3 plasmid
exhibited a decrease in glucose-stimulated insulin secretion and
cell proliferation, and an increase in apoptosis (Fig. 5C–H). Taken together, these data
demonstrate that miR-19a-3p enhances the proliferation and insulin
secretion, while it inhibits the apoptosis of pancreatic β cells by
directly targeting SOCS3.
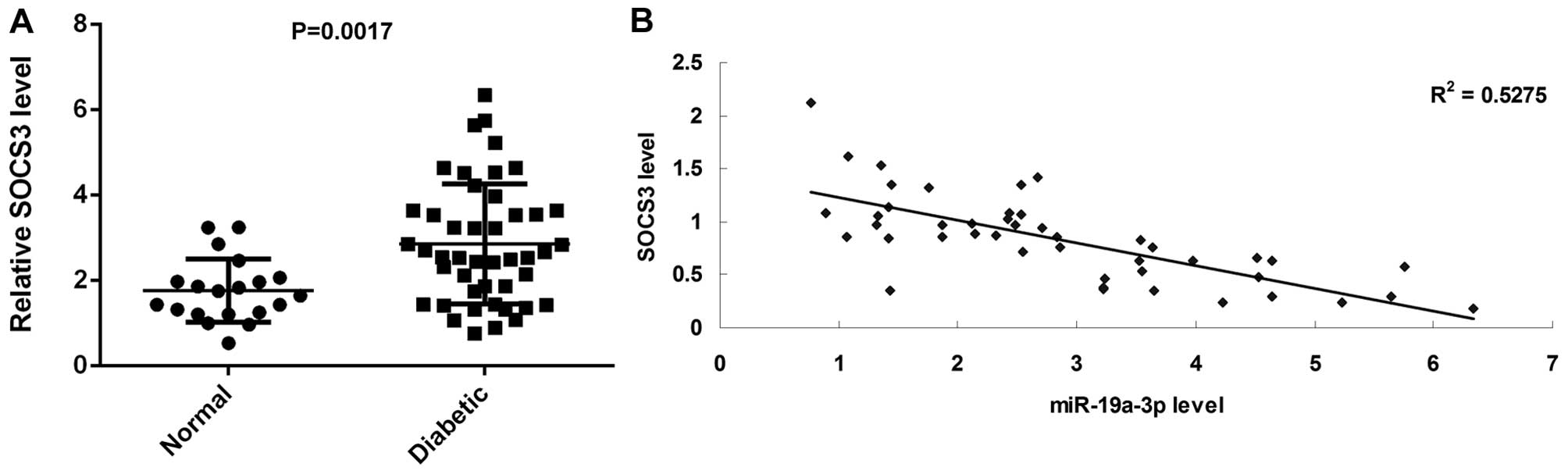
SOCS3 is upregulated in diabetic patients
and its plasma level inversely correlates with miR-19a-3p
Finally, we examined the plasma SOCS3 level using
ELISA. We found that the SOCS3 level was significantly increased in
the blood samples of diabetic patients compared with those of
normal subjects (Fig. 6A). After
that, we analyzed the correlation between the plasma level of SOCS3
and miR-19a-3p. Our data indicated a significant negative
correlation between the protein level of SOCS3 and the miR-19a-3p
level in the blood samples of diabetic patients (Fig. 6B). Therefore, we suggest that the
dysregulation of the miR-19a-3p/SOCS3 axis is involved in the
development of T2D.
Discussion
Diabetes poses one of the most severe threats to
human health worldwide, as it can induce high incidence rates of a
number of complications. In recent years, various miRs, such as
miR-375, miR-7, miR-124a2, miR-195, miR-126, miR-9, miR-96 and
miR-34a have been reported to regulate the expression of a variety
of genes that participate in the regulation of cell proliferation
and apoptosis, as well as glucose-stimulated insulin secretion, and
thus may play a role in pancreatic development and diabetes
(22). For instance, treatment
with interleukin (IL)-1β has been shown to enhance the expression
of miR-101a and miR-30b in pancreatic β cells, and the two miRs
have been shown to inhibit the insulin content, and to increase β
cell death (25). Recently,
miR-19a was found to be downregulated in the livers of diabetes
db/db mice and mice injected with IL-6, an inducer of insulin
resistance (21). However, the
exact role of miR-19a in the regulation of pancreatic β cell
proliferation, apoptosis and insulin secretion has not been studied
to date, at least to the best of our knowledge.
In the present study, the data from RT-qPCR
demonstrated that the miR-19a-3p level was significantly decreased
in the blood of diabetic patients compared with that of normal
subjects. Moreover, a significant inverse correlation was observed
between the plasma miR-19a-3p level and the blood glucose
concentration among the diabetic patients, suggesting that the
dysregulation of miR-19a-3p is associated with the progression of
diabetes. INS-1 and MIN6 are glucose-responsive murine pancreatic β
cell lines. Therefore, to further reveal the role of miR-19a-3p in
pancreatic β cell function, the INS-1 and MIN6 cells were
transfected with miR-19a-3p mimic. We found that the overexpression
of miR-19a-3p promoted the proliferation and insulin secretion,
while it suppressed the apoptosis of INS-1 and MIN6 cells, which
further supports the notion that miR-19a-3p plays a role in
diabetes, consistent with the clinical data.
Previous studies have mainly focused on the role of
miR-19a-3p in human cancers. Yang et al demonstrated that
miR-19a-3p inhibited breast cancer progression and metastasis by
inducing macrophage polarization through the targeting of the
oncogene, Fra-1 (15). These data
indicate that miR-19a-3p participates in the regulation of cell
motility. Moreover, miR-19a-3p expression has been shown to be
significantly downregulated following multifractionated radiation
in breast cancer cells, and that it may contribute to
radiosensitivity and can be used as a biomarker for radiotherapy
(26). Furthermore, miR-19a-3p
has been shown to be downregulated in non-melanoma skin cancer,
suggesting a tumor suppressor role (18). On the contrary, a high serum level
of miR-19a-3p was found to be significantly associated with a poor
survival of astrocytoma patients, suggesting that it may play an
oncogenic role in astrocytoma (16). In addition, Zheng et al
identified a four-miRNA panel, including miR-19a-3p, miR-223-3p,
miR-92a-3p and miR-422a with a high diagnostic accuracy for
early-stage colorectal adenocarcinoma (19). In the present study, we found that
miR-19a-3p promoted the proliferation and insulin secretion, while
it suppresses the apoptosis of pancreatic β cells. Jiang et
al reported that miR-19a protected endothelial cells from
lipopolysaccharide (LPS)-induced apoptosis through the apoptosis
signal-regulating kinase 1 (ASK1)/p38 pathway (27). Therefore, the ASK1/p38 pathway may
also be involved in the protective effects of miR-19a-3p against
pancreatic β cell apoptosis; this warrants further investigation in
future studies.
SOCS3 is a member of the SOCS family, which are
negative regulators of cytokine signal transduction and inhibit the
cytokine-induced activation of signal transducer and activator of
transcription (Stat) signaling (28). SOCS3 has been demonstrated to be
associated with the development of leptin resistance and the
inhibition of insulin (29,30). The muscle-specific overexpression
of SOCS3 in mice has been shown to lead to impaired systemic and
muscle-specific glucose homeostasis and insulin function, as well
as to decreased basal and leptin-stimulated activity and the
phosphorylation of α2 AMP-activated protein kinase (α2AMPK) and
acetyl-CoA carboxylase (31).
Moreover, muscle SOCS3 overexpression also suppresses
leptin-regulated genes involved in fatty acid oxidation and
mitochondrial function (31). In
the present study, we identified SOCS3 as a direct target gene of
miR-19a-3p, and the overexpression of miR-19a-3p led to a
significant decrease in the protein level of SOCS3. Moreover, we
found that the effects of miR-19a-3p on the proliferation,
apoptosis and insulin secretion of pancreatic β cells occurred
directly through the targeting of SOCS3. it has also previously
been reported that SOCS3 is involved in the regulation of
pancreatic cell proliferation and apoptosis. The restoration of
SOCS3 expression using a demethylating agent
(5-aza-2′-deoxycytidine), was shown to markedly suppress the
proliferation and to induce the apoptosis of methylated pancreatic
cells (32). In addition, we
observed a negative correlation between miR-19a-3p expression and
the SOCS3 level in the plasma samples of patients with diabetes,
which further supports the notion that the protective role
miR-19a-3p in diabetes at least partly involves the inhibition of
SOCS3 expression.
In conclusion, the findings of the present study
demonstrate that miR-19a-3p is significantly downregulated in
diabetic patients, and that its expression is inversely correlated
with the blood glucose concentration. Moreover, our data indicate
that miR-19a-3p significantly enhances the proliferation and
insulin secretion, while it inhibits the apoptosis of pancreatic β
cells (INS-1 and MIN6) by directly targeting SOCS3. Accordingly,
miR-19a-3p may become a potential candidate for the treatment of
diabetes. However, further studies are warranted to verify our
findings.
References
|
1
|
Kaul K, Apostolopoulou M and Roden M:
Insulin resistance in type 1 diabetes mellitus. Metabolism.
64:1629–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chatterjee S and Davies MJ: Current
management of diabetes mellitus and future directions in care.
Postgrad Med J. 91:612–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keane KN, Cruzat VF, Carlessi R, de
Bittencourt PI Jr and Newsholme P: Molecular events linking
oxidative stress and inflammation to insulin resistance and β-cell
dysfunction. Oxid Med Cell Longev. 2015:1816432015. View Article : Google Scholar
|
|
4
|
Berge LI and Riise T: Comorbidity between
type 2 diabetes and depression in the adult population: Directions
of the association and its possible pathophysiological mechanisms.
Int J Endocrinol. 2015:1647602015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhatia P, Raina S, Chugh J and Sharma S:
miRNAs: early prognostic biomarkers for type 2 diabetes mellitus?
Biomark Med. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Libânio D, Dinis-Ribeiro M and
Pimentel-Nunes P: Helicobacter pylori and microRNAs: Relation with
innate immunity and progression of preneoplastic conditions. World
J Clin Oncol. 6:111–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinez-Sanchez A, Nguyen-Tu MS and
Rutter GA: DICER inactivation identifies oancreatic β-cell
'Disallowed' genes targeted by MicroRNAs. Mol Endocrinol.
29:1067–1079. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Latreille M, Herrmanns K, Renwick N,
Tuschl T, Malecki MT, McCarthy MI, Owen KR, Rülicke T and Stoffel
M: miR-375 gene dosage in pancreatic β cells: Implications for
regulation of β cell mass and biomarker development. J Mol Med
Berl. 93:1159–1169. 2015. View Article : Google Scholar
|
|
12
|
Jacovetti C, Matkovich SJ, Rodriguez-Trejo
A, Guay C and Regazzi R: Postnatal β cell maturation is associated
with islet-specific microRNA changes induced by nutrient shifts at
weaning. Nat Commun. 6:80842015. View Article : Google Scholar
|
|
13
|
Al-Kafaji G, Al-Mahroos G, Alsayed NA,
Hasan ZA, Nawaz S and Bakhiet M: Peripheral blood microRNA-15a is a
potential biomarker for type 2 diabetes mellitus and pre-diabetes.
Mol Med Rep. 12:7485–7490. 2015.PubMed/NCBI
|
|
14
|
Busch S, Auth E, Scholl F, Huenecke S,
Koehl U, Suess B and Steinhilber D: 5-Lipoxygenase is a direct
target of miR-19a-3p and miR-125b-5p. J Immunol. 194:1646–1653.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Zhang Z, Chen C, Liu Y, Si Q,
Chuang TH, Li N, Gomez-Cabrero A, Reisfeld RA, Xiang R and Luo Y:
MicroRNA-19a-3p inhibits breast cancer progression and metastasis
by inducing macrophage polarization through downregulated
expression of Fra-1 proto-oncogene. Oncogene. 33:3014–3023. 2014.
View Article : Google Scholar
|
|
16
|
Zhi F, Shao N, Wang R, Deng D, Xue L, Wang
Q, Zhang Y, Shi Y, Xia X, Wang S, et al: Identification of 9 serum
microRNAs as potential noninvasive biomarkers of human astrocytoma.
Neuro Oncol. 17:383–391. 2015.
|
|
17
|
Ibarrola-Villava M, Llorca-Cardeñosa MJ,
Tarazona N, Mongort C, Fleitas T, Perez-Fidalgo JA, Roselló S,
Navarro S, Ribas G and Cervantes A: Deregulation of ARID1A, CDH1,
cMET and PIK3CA and target-related microRNA expression in gastric
cancer. Oncotarget. 6:26935–26945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balci S, Ayaz L, Gorur A, Yildirim Yaroglu
H, Akbayir S, Dogruer Unal N, Bulut B, Tursen U and Tamer L:
microRNA profiling for early detection of nonmelanoma skin cancer.
Clin Exp Dermatol. 41:346–351. 2016. View Article : Google Scholar
|
|
19
|
Zheng G, Du L, Yang X, Zhang X, Wang L,
Yang Y, Li J and Wang C: Serum microRNA panel as biomarkers for
early diagnosis of colorectal adenocarcinoma. Br J Cancer.
111:1985–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Y, Tian F, Li H, Zhou Y, Lu J and Ge
Q: Profiling maternal plasma microRNA expression in early pregnancy
to predict gestational diabetes mellitus. Int J Gynaecol Obstet.
130:49–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dou L, Meng X, Sui X, Wang S, Shen T,
Huang X, Guo J, Fang W, Man Y, Xi J and Li J: miR-19a regulates
PTEN expression to mediate glycogen synthesis in hepatocytes. Sci
Rep. 5:116022015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chakraborty C, George Priya Doss C and
Bandyopadhyay S: miRNAs in insulin resistance and
diabetes-associated pancreatic cancer: The 'minute and miracle'
molecule moving as a monitor in the 'genomic galaxy'. Curr Drug
Targets. 14:1110–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Wang L, Mo Q, Jia A, Dong Y and
Wang G: A positive feedback loop of p53/miR-19/TP53INP1 modulates
pancreatic cancer cell proliferation and apoptosis. Oncol Rep.
35:518–523. 2016.
|
|
24
|
Tan Y, Yin H, Zhang H, Fang J, Zheng W, Li
D, Li Y, Cao W, Sun C, Liang Y, et al: Sp1-driven up-regulation of
miR-19a decreases RHOB and promotes pancreatic cancer. Oncotarget.
6:17391–17403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng Y, Wang Z, Tu Y, Shen H, Dai Z, Lin
J and Zhou Z: miR-101a and miR-30b contribute to inflammatory
cytokine- mediated β cell dysfunction. Lab Invest. 95:1387–1397.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leung CM, Chen TW, Li SC, Ho MR, Hu LY,
Liu WS, Wu TT, Hsu PC, Chang HT and Tsai KW: MicroRNA expression
profiles in human breast cancer cells after multifraction and
single-dose radiation treatment. Oncol Rep. 31:2147–2156.
2014.PubMed/NCBI
|
|
27
|
Jiang WL, Zhang YF, Xia QQ, Zhu J, Yu X,
Fan T and Wang F: MicroRNA-19a regulates lipopolysaccharide-induced
endothelial cell apoptosis through modulation of apoptosis
signal-regulating kinase 1 expression. BMC Mol Biol. 16:112015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim MH, Kim MS, Kim W, Kang MA, Cacalano
NA, Kang SB, Shin YJ and Jeong JH: Suppressor of cytokine signaling
(SOCS) genes are silenced by DNA hypermethylation and histone
deacetylation and regulate response to radiotherapy in cervical
cancer cells. PLoS One. 10:e01231332015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wunderlich CM, Hövelmeyer N and Wunderlich
FT: Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity.
JAKSTAT. 2:e238782013.PubMed/NCBI
|
|
30
|
Krebs DL and Hilton DJ: A new role for
SOCS in insulin action. Suppressor of cytokine signaling. Sci STKE.
2003:PE62003.PubMed/NCBI
|
|
31
|
Yang Z, Hulver M, McMillan RP, Cai L,
Kershaw EE, Yu L, Xue B and Shi H: Regulation of insulin and leptin
signaling by muscle suppressor of cytokine signaling 3 (SOCS3).
PLoS One. 7:e474932012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Zhou H, Han Y, Liu X, Wang M, Wang
X, Yin G, Li X and Xiang M: SOCS3 methylation in synergy with Reg3A
over-expression promotes cell growth in pancreatic cancer. J Mol
Med Berl. 92:1257–1269. 2014. View Article : Google Scholar
|