Introduction
Primary liver cancer is fifth most common type of
cancer and has the third highest rates of mortality worldwide.
Hepatocellular carcinoma (HCC) represents approximately 85% of all
primary liver cancer cases. Although the incidence of some types of
cancer is decreasing, the incidence of HCC is increasing worldwide
(1,2). A number of risk factors have been
identified to increase the risk of HCC. Non-alcoholic fatty liver
disease (NAFLD) is one of these risk factors. The histological
changes occurring in NAFLD range over a wide spectrum, extending
from simple steatosis to non-alcoholic steatohepatitis (NASH),
liver cirrhosis and liver failure, and sometimes even HCC (3). NAFLD occurs in patients with
components of metabolic syndrome, such as type 2 diabetes mellitus
(T2DM), obesity, hypertension and hyperlipidemia (3). Therefore, hyperlipidemia also
represents a patient population at risk for HCC that can readily be
identified.
Statins are effective drug for patients with
hyperlipidemia. The drugs inhibit 3-hydroxy-3-methyl-glutaryl
coenzyme A (HMG-CoA) reductase, a key enzyme that catalyzes the
rate-limiting step within the cholesterol biosynthetic pathway.
Previous studies have shown that statins can exert effects separate
their lipid-lowering properties. These non-lipid effects include
antioxidant effects (4),
anti-inflammatory effects (5) and
the upregulation of endothelial nitric oxide synthase (6). Rosuvastatin (Ros), a HMG-CoA
reductase inhibitor, has exhibited a more potent affinity for the
active site of HMG-CoA reductase than other statins. In addition,
the hepatic uptake of Ros in rats has been found to be more
selective and efficient than that with other drugs (7). Furthermore, the cytoprotective
effects of Ros against ischemic injury have been clearly reported
(8–11). Thus, in this study, we aimed to
determine the role of Ros as a preventive drug in HCC associated
with NAFLD.
Materials and methods
Chemicals and diets
Rosuvastatin Calcium was purchased from AvaChem
Scientific (San Antonio, TX, USA). The experimental diets
[high-fat-diet (HFD)] were purchased from Oriental Yeast Co., Ltd.
(Tokyo, Japan). The energy content of the diet was 5.1 kcal/g, with
56.7% of calories from fat, 20.1% of calories from protein, and
23.2% of calories from carbohydrate, plus vitamins and minerals as
recommended. The diets were freshly prepared each day. Ros was
administered orally by premixing with the HFD to a concentration of
0.00125% to a concentration of 0.00125%, as these concentrations of
the drug have been administered to patients with
hyperlipidemia.
Animals
STAM mice, a NASH-cirrhosis-hepatocarcino genic
model, were purchased from STELIC Co., Ltd. (Tokyo, Japan). The
mouse model was established according to a previously described
protocol (12). Briefly, pregnant
C57BL/6 mice were purchased from CLEA-Japan (Tokyo, Japan) and
2-day-old male pups were injected with streptozotocin (200
µg/mouse) and fed a HFD (HFD-32; CLEA-Japan) from the age of
4 weeks. This mouse model progresses from NAFLD to NASH at 8 weeks
of age and develops HCC at 16 weeks of age (12).
Experimental design
After weaning, 16 mice were divided into 2
experimental groups. The experimental designs were as follows:
5-week-old male STAM mice, which developed T2DM and NAFLD by being
fed a HFD, were divided into a group in which a HFD was given to
the mice for 15 weeks (n=8) as controls (HFD group); in the other
group, the mice were fed a HFD supplemented with 0.00125% Ros for
15 weeks (n=8) (Ros group). The mice were allowed free access to
food, with a 12-h light/12-h dark cycle under conditions of
controlled temperature (22±1°C) and humidity (50±10%). Food intake
was measured daily, while individual body weight was recorded once
a week. The 8 mice from each group were fasted overnight prior to
euthanasia (by cervical dislocation). All mice were sacrificed
after completing their respective dietary regimens, and the livers
of the individual animals were weighed. The livers were removed,
the samples were placed in formalin and the remainder were
snap-frozen and stored at −80°C. All surgical and experimental
procedures were performed according to the guidelines for the care
and use of animals and approved by the Osaka Medical College Ethics
Committee.
Assay for plasma hepatic and metabolic
parameters
Blood samples were obtained by cardiac puncture and
separated by centrifugation (12,000 rpm, 15 min) as plasma. The
levels of blood biochemical parameters, including aspartate
aminotransferase (AST), alanine aminotransferase (ALT), free fatty
acid (FFA), triglyceride (TG) and total cholesterol (T-CHO) were
measured by a local laboratory specified in clinical analyses
(Oriental Yeast Co., Ltd.).
Assay for hepatic lipid content
The hepatic tissues were homogenized using a Janke
& Kunkel Polytron homogenizer (Ultra-Turrax TP18/1051; IKA
Labortechnik, Staufen, Germany) in buffer (pH 7.4) containing 20 mM
Tris-HCl, 1 mM EGTA, 2 mM EDTA, and treated with protease inhibitor
(2 µg/ml, leupeptin cocktail). Hepatic TG levels were
measured by a local laboratory that specifies in clinical analyses
(SRL Co. Ltd., Tokyo, Japan).
Histological analysis of hepatic
tissue
The liver sections were examined blindly from
different lobes of each mouse. Liver tissues were fixed in 10%
buffered formaldehyde, and then embedded in paraffin. A 4-mm-thick
section cut from a paraffin-embedded block was stained with
hematoxylin and eosin (H&E; Applied Medical Research, Osaka,
Japan).
Reverse transcription-quantitative PCR
(RT-qPCR)
Tissue specimens were preserved in RNAlater reagent
(Qiagen, Valencia, CA, USA) until the isolation of the total RNA.
Total RNA was isolated from the liver tissues using a QIAshredder
and an RNeasy kit (Qiagen). cDNA was prepared using the TaqMan
reverse transcriptase kitQiagen (Qiagen). Quantitative (real-time)
PCR (qPCR) was performed using the StrataScript First Strand cDNA
synthesis kit and FullVelocity SYBR-Green qPCR Master Mix
(Stratagene, La Jolla, CA, USA) according to the manufacturer's
instructions. The primers used for qPCR were designed using Beagon
Designer software version 2.12, according to the parameters
outlined in the Bio-Rad iCycler Manual, using reference mRNA
sequences accessed through GenBank and as shown in Table I. All probes used in the TaqMan
Gene Expression assays were purchased from Applied Biosystems
(Foster City, CA, USA). PCR reactions were carried out in the
iCycler Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA).
PCR products were detected using the iCycler IQ Real-Time PCR
detection system (Bio-Rad). The relative amount of mRNA was
calculated by comparative cycle time determination with the
ribosomal protein, RPL32 as the invariant control. Gene expression
values were calculated based on the ΔΔCt method. The results were
expressed as a fold increase in expression relative to the control
group.
 | Table ISequences of primers used for the
RT-qPCR. |
Table I
Sequences of primers used for the
RT-qPCR.
| Gene | Primer sequences
(sense) | Primer sequences
(antisense) |
|---|
| TNF-α |
5′-ACCTTGTTGCCTCCTCTT-3′ |
5′-GTTCAGTGATGTAGCGACAG-3′ |
| IL-1β |
5′-TCCAGGATGAGGACATGAGCAC-3′ |
5′-GAACGTCACACACCAGCAGGTTA-3′ |
| IL-6 |
5′-TTCCTCACTGTGGTCAGA-3′ |
5′-CATTCATATTGTCAGTTCTTCGTA-3′ |
| IFN-γ |
5′-CGGCACAGTCATTGAAAGCCTA-3′ |
5′-GTTGCTGATGGCCTGATTGTC-3′ |
| SREBP-1c |
5′-GGTACCTGCGGGACAGCTTA-3′ |
5′-CCGTGAGCTACCTGGACTGAA-3′ |
| FAS |
5′-TACAGATGGCAGCAAGGA-3′ |
5′-TGATACAGAGAGCAGATGAGT-3′ |
| PPAR-α |
5′-ATGGCAGCAATATCAGAG-3′ |
5′-AGCAGTAAAGTATCATATCAAAG-3′ |
| PPAR-γ |
5′-GAAGACAGAGACAGACAT-3′ |
5′-GCAATCAATAGAAGGAACA-3′ |
| SCD-1 |
5′-CTGGCTGGAGAGTCATCA-3′ |
5′-TAACGAGGACGACAATACAATC-3′ |
| AMPKα1 |
5′-CCTTCGGGAAAGTGAAGGT-3′ |
5′-GAATCTTCTGCCGGTTGAGT-3′ |
| Gclc |
5′-ATGATAGAACACGGGAGGAGAG-3′ |
5′-TGATCCTAAAGCGATTGTTCTTC-3′ |
| GST |
5′-CCTCCCCACAGTGAAGAAGT-3′ |
5′-CAACACATTTTGCGTCATCA-3′ |
| TGF-β1 |
5′-GCGATACCTCAGCAACCG-3′ |
5′-CTAAGGCGAAAGCCCTCAAT-3′ |
| TIMP1 |
5′-GCATCTCTGGCATCTGGCATC-3′ |
5′-GCGGTTCTGGGACTTGTGGGC-3′ |
| MMP-13 |
5′-CCTTCTGGTCTTCTGGCACAC-3′ |
5′-GGCTGGGTCACACTTCTCTGG-3′ |
| Type 1 collagen
α1 |
5′-ACCTGTGTGTTCCCTACTCA-3′ |
5′-GACTGTTGCCTTCGCCTCTG-3′ |
| EGFR |
5′-TCTTCAAGGATGTGAAGTGTG-3′ |
5′-TGTACGCTTTCGAACAATGT-3′ |
| VEGFR |
5′-TACTGCTGTACCTCCACCTCCACCATG-3′ |
5′-TCACTTCATGGGACTTCTGCTCT-3′ |
| PDGFR |
5′-TCCTTCTACCACCTCAGCGAG-3′ |
5′-CCGGATGGTCACTCTTTAGGAAG-3′ |
Statistical analysis
Data are presented as the means ± standard error of
the mean. Statistical analyses were performed using Student's
t-test. Values of p<0.05 were considered to indicate
statistically significant differences.
Results
Effect of diets on the liver/body weight
ratio of the mice in each experimental group
As shown Table
II, the ratio of liver weight to body weight did not differ
significantly between the 2 groups of mice (7.2±0.56 and 7.5±0.04%
in the HFD and Ros group, respectively; p<0.05).
 | Table IIPlasma and hepatic biochemical
parameters and liver weight/body weight ratios of the HFD group and
Ros group. |
Table II
Plasma and hepatic biochemical
parameters and liver weight/body weight ratios of the HFD group and
Ros group.
| Parameter | HFD group
(n=8) | Ros group
(n=8) |
|---|
| Liver/body weight
ratio (%) | 7.2±0.56 | 7.5±0.04 |
| AST (IU/l) | 189.75±441.19 |
138.13±42.84a |
| ALT (IU/l) | 59.88±20.32 | 39.75±6.92a |
| T-CHO (mg/dl) | 161.88±52.14 |
109.13±14.64a |
| FFA
(µEq/l) | 514.5±144.15 |
350.75±119.7a |
| TG (mg/dl) | 150.13±68.16 | 39.0±8.29a |
| Hepatic TG
(mg/dl) | 38.11±2.44 | 29.99±1.22a |
Plasma and hepatic biochemical
parameters
To examine whether Ros, as a preventive drug for the
development of HCC associated with NAFLD, affected liver damage and
steatosis in our mouse experimental groups, we quantified the
plasma levels of AST, ALT, T-CHO, TG and FFA. The plasma AST (HFD,
189.75±441.19 vs. Ros, 138.13±42.84 IU/l; p<0.05) and ALT levels
(HFD, 59.88±20.32 vs. Ros, 39.75±6.92 IU/l; p<0.05) differed
significantly between the Ros group and HFD group (Table II). Mice fed the diet containing
Ros had lower plasma levels of T-CHO (HFD, 161.88±52.14 vs. Ros,
109.13±14.64 mg/dl; p<0.05), FFA (HFD, 514.5±144.15 vs. Ros,
350.75±119.7 µEq/l; p<0.05) and TG (HFD, 150.13±68.16 vs.
Ros, 39.0±8.29 mg/dl; p<0.05) (Table II). Mice fed the diet containing
Ros also had a lower hepatic TG content (HFD, 38.11±2.44 vs. Ros,
29.99±1.22 mg/dl; p<0.05) (Table
II).
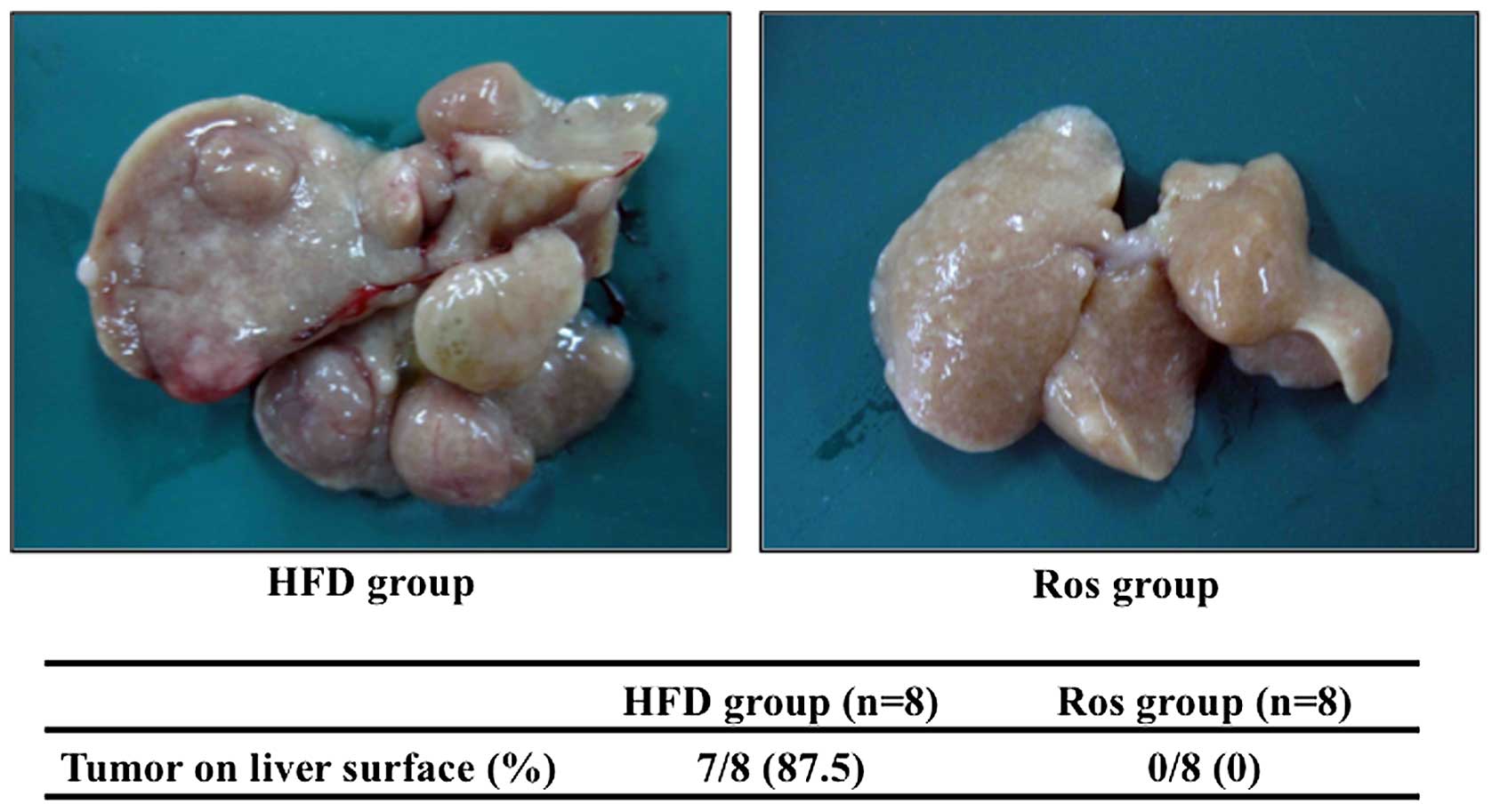
Macroscopic examinations
Numerous tumors on the liver surface were observed
in 4 out of 5 mice in the HFD group (Fig. 1). On the other hand, no tumors on
the liver surface were observed in the mice in the Ros group
(Fig. 1).
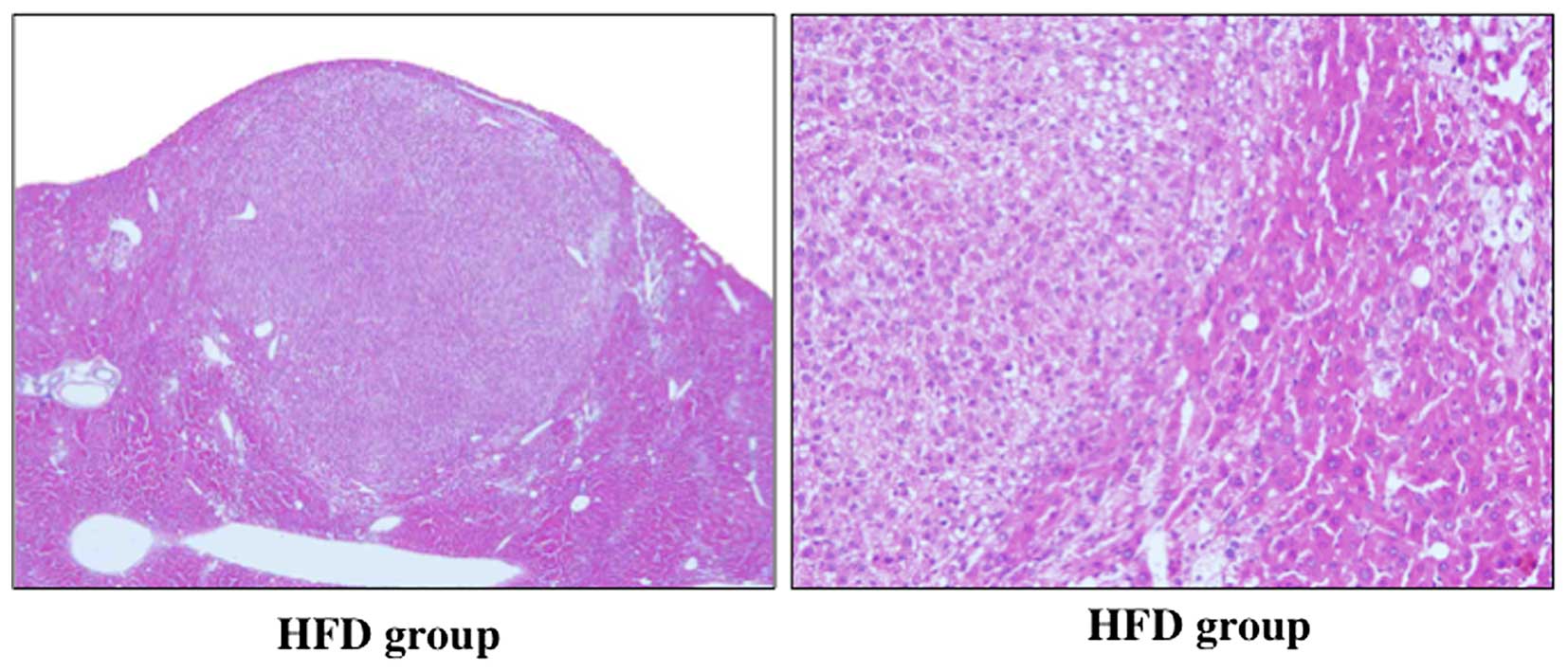
Histological analysis
Mild hepatic steatosis was observed in the 2 groups
(Fig. 2). Hepatic steatosis was
however, decreased in the Ros group compared to the HFD group.
Although large fatty droplets were observed in the HFD group, no
large fatty droplets were observed in the Ros group. Histological
findings in the liver of two groups did not show clear inflammatory
cell infiltration. The cells in the tumor in HFD group had high
nuclear/cytoplasmic ratio, and this finding did not contradict the
findings of HCC. These cells were well differentiated. Therefore,
histological findings revealed that the tumors were HCC (Fig. 3). On the other hand, histological
examinations did not reveal any HCCs in the Ros group (Fig. 2).
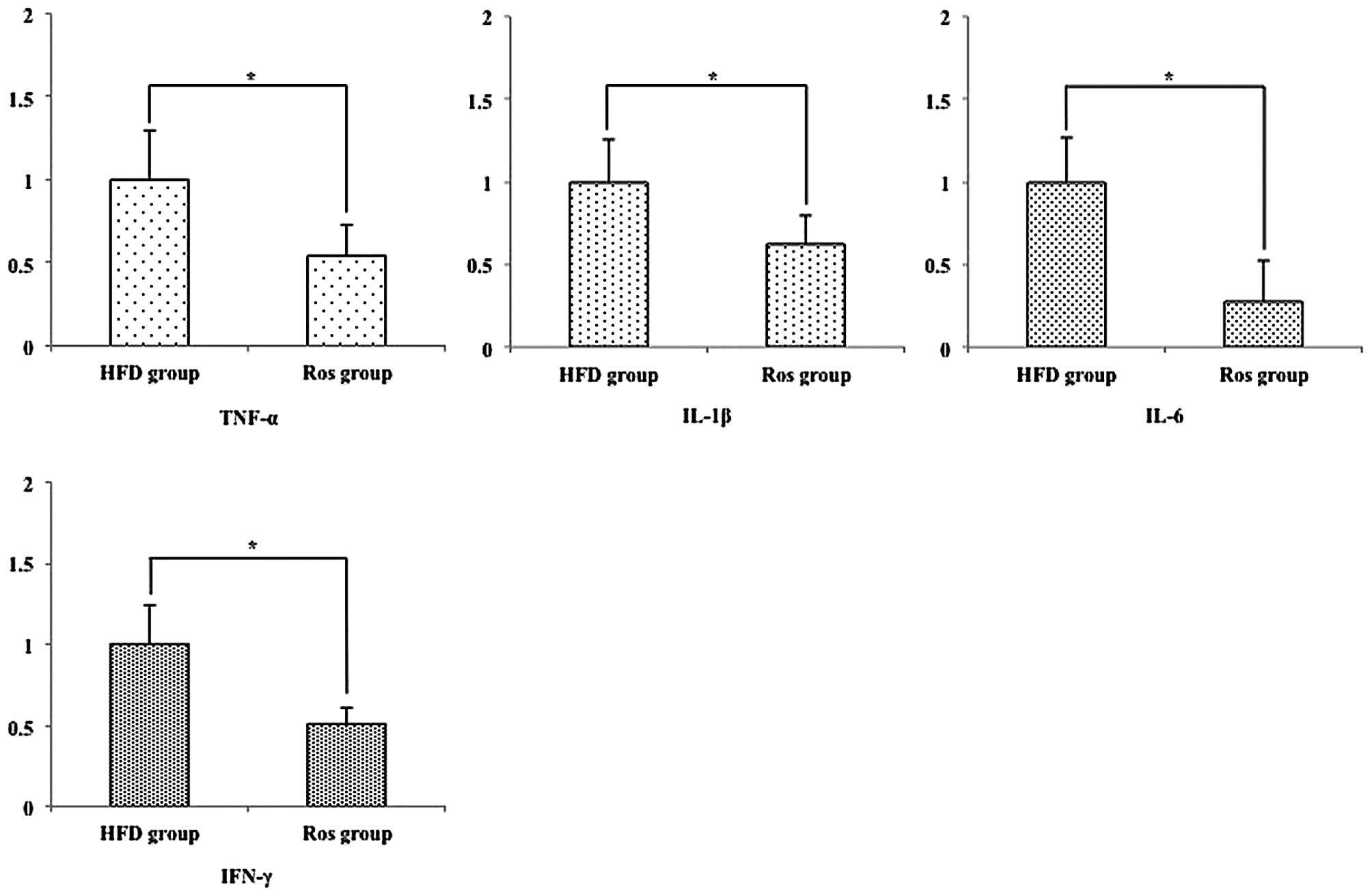
Hepatic pro-inflammatory mRNA
expression
A previous study demonstrated that several
pro-inflammatory cytokines are associated with the development of
NASH (13). Therefore, we
examined the expression levels of hepatic pro-inflammatory
cytokines. The relative hepatic mRNA expression levels of tumor
necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and interferon
(IFN)-γ were significantly decreased in the Ros group compared with
the HFD group (all p<0.05) (Fig.
4).
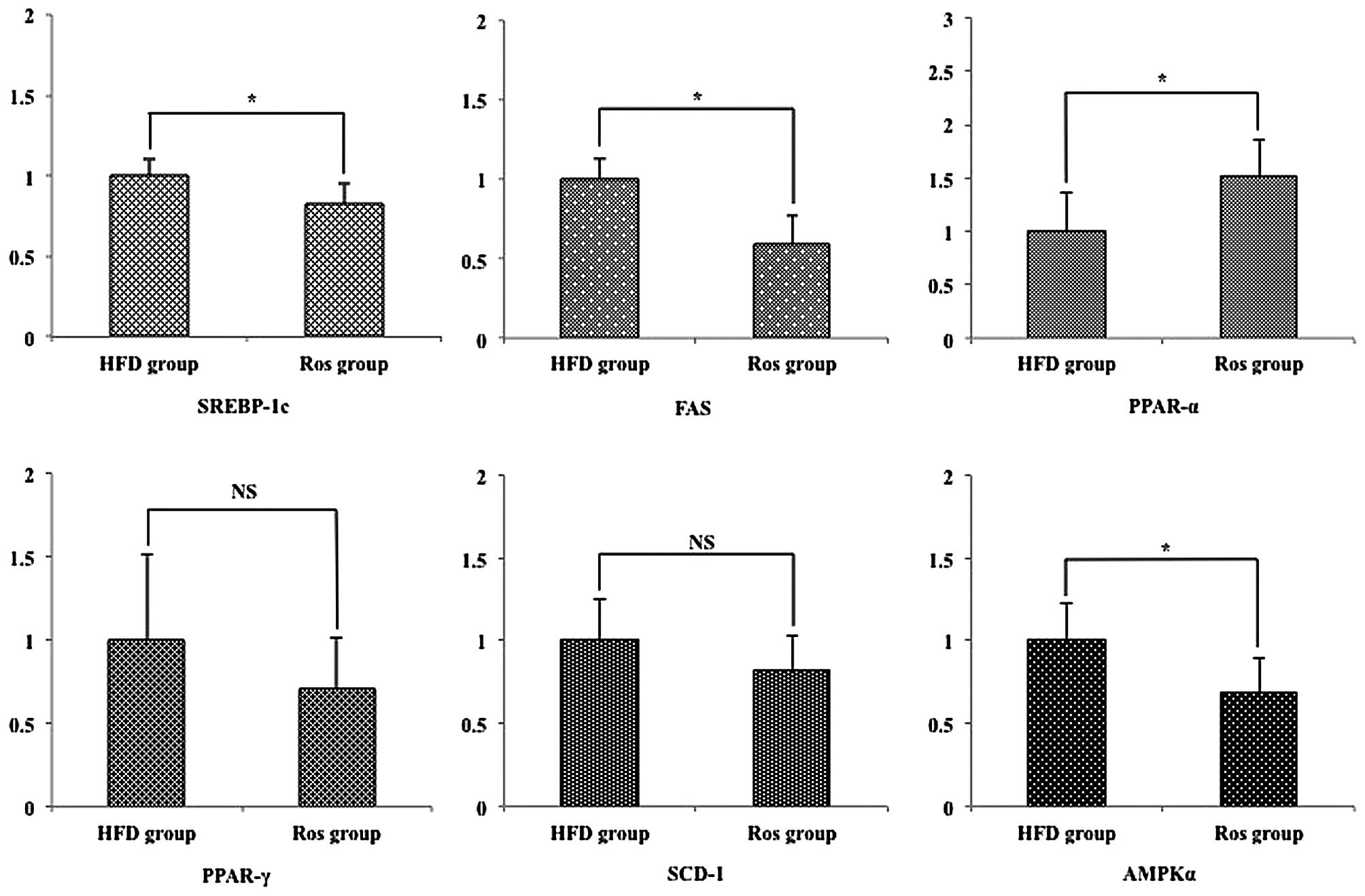
Hepatic lipogenic-related mRNA
expression
The differences in the levels of plasma TG and FFA
between the 2 groups and the effect of Ros on the development of
NAFLD suggest the expression of cytokines involved in the
development of NAFLD. Sterol regulatory element binding protein-1c
(SREBP-1c) is well known to be involved in these states (14). The relative hepatic mRNA
expression levels of SREBP-1c were significantly decreased in the
Ros group compared with the HFD group (p<0.05; Fig. 5). Furthermore, it has been
reported that SREBP-1 is regulated by a pathway of AMP-activated
protein kinase (AMPK) (15).
Since fatty acid synthase (FAS) and stearoyl-CoA desaturase-1
(SCD-1) may be critical to the role of triglyceride accumulation in
hepatocytes (16), we examined
the expression levels of these 3 genes. The relative hepatic mRNA
expression levels of FAS were significantly decreased in the Ros
group compared with the HFD group. However, there was no
significant difference in the hepatic mRNA expression levels of
SCD-1 between the 2 groups (Fig.
5). Peroxisome proliferator-activated receptors (PPARs) are
nuclear transcription factors that include 3 subtypes: α, β and γ.
PPAR-α is a member of the PPAR subfamily of nuclear receptors that
transcriptionally promotes peroxisomal, microsomal and
mitochondrial oxidation (17).
PPAR-γ, another member of the PPAR subfamily of nuclear receptors,
transcriptionally activates adipocyte differentiation (18). Thus, we examined the hepatic mRNA
expression levels of these genes. The relative hepatic mRNA
expression levels of PPAR-α were significantly increased in the Ros
group compared with the HFD group (Fig. 5). On the other hand, there was no
significant difference in the relative hepatic mRNA expression
levels of PPAR-γ between the 2 groups (Fig. 5).
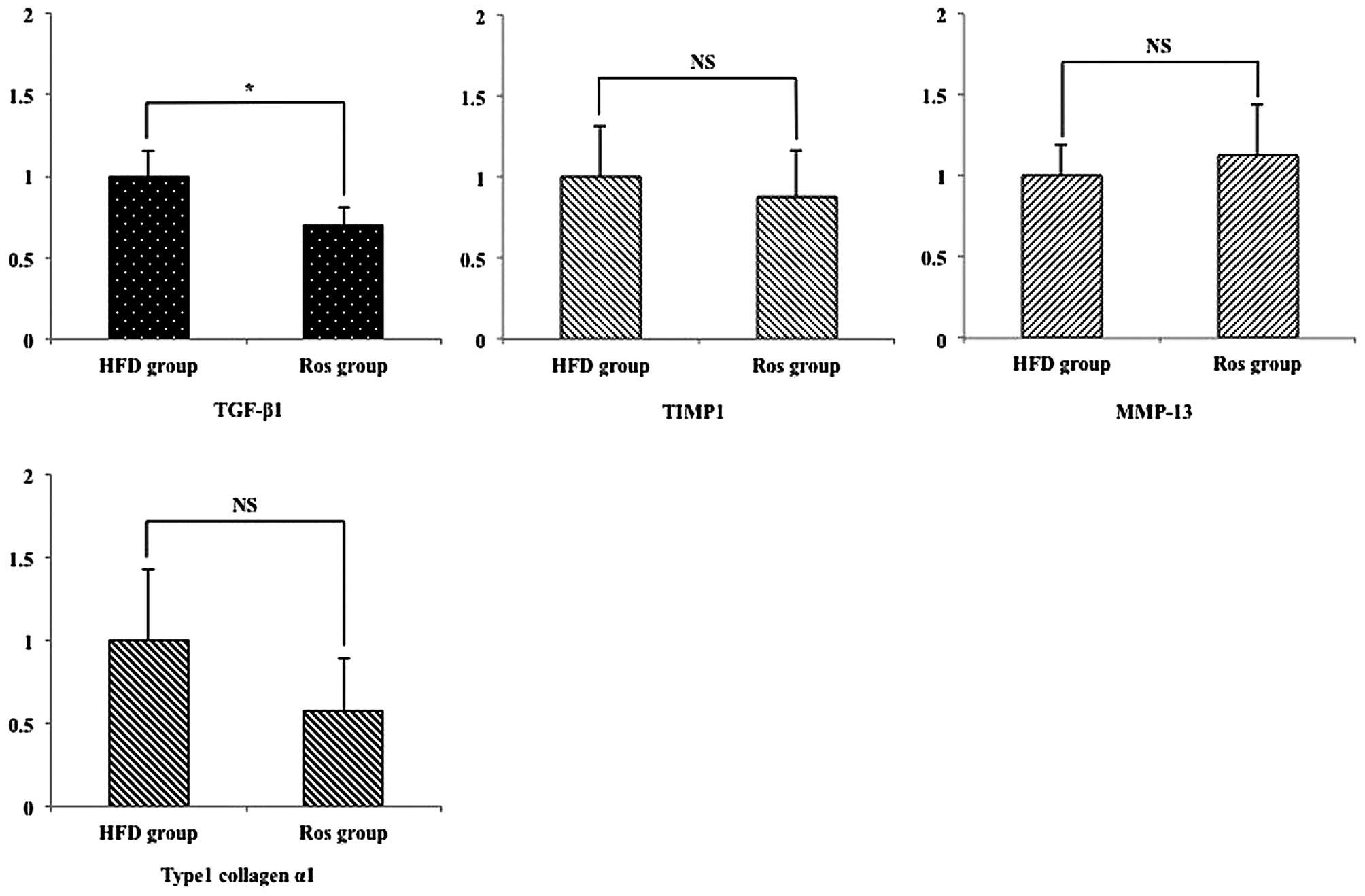
Hepatic pro-fibrogenic mRNA
expression
Transforming growth factor (TGF)-β1 is produced by
Kupffer cells and activates hepatic stellate cells (HSCs) that play
a role in fibrogenesis in the liver (19). Thus, we examined the expression
levels of TGF-β1 and fibrogenesis-related genes produced from HSCs,
such tissue inhibitors of matrix metalloproteinase (TIMP)-1, matrix
metalloproteinase (MMP)-13 and type 1 collagen α1 (19). The relative hepatic mRNA
expression levels of TGF-β1 were significantly decreased in the Ros
group compared with the HFD group (Fig. 6). However, there was no
significant difference in the relative mRNA expression levels of
the other genes between the 2 groups (Fig. 6).
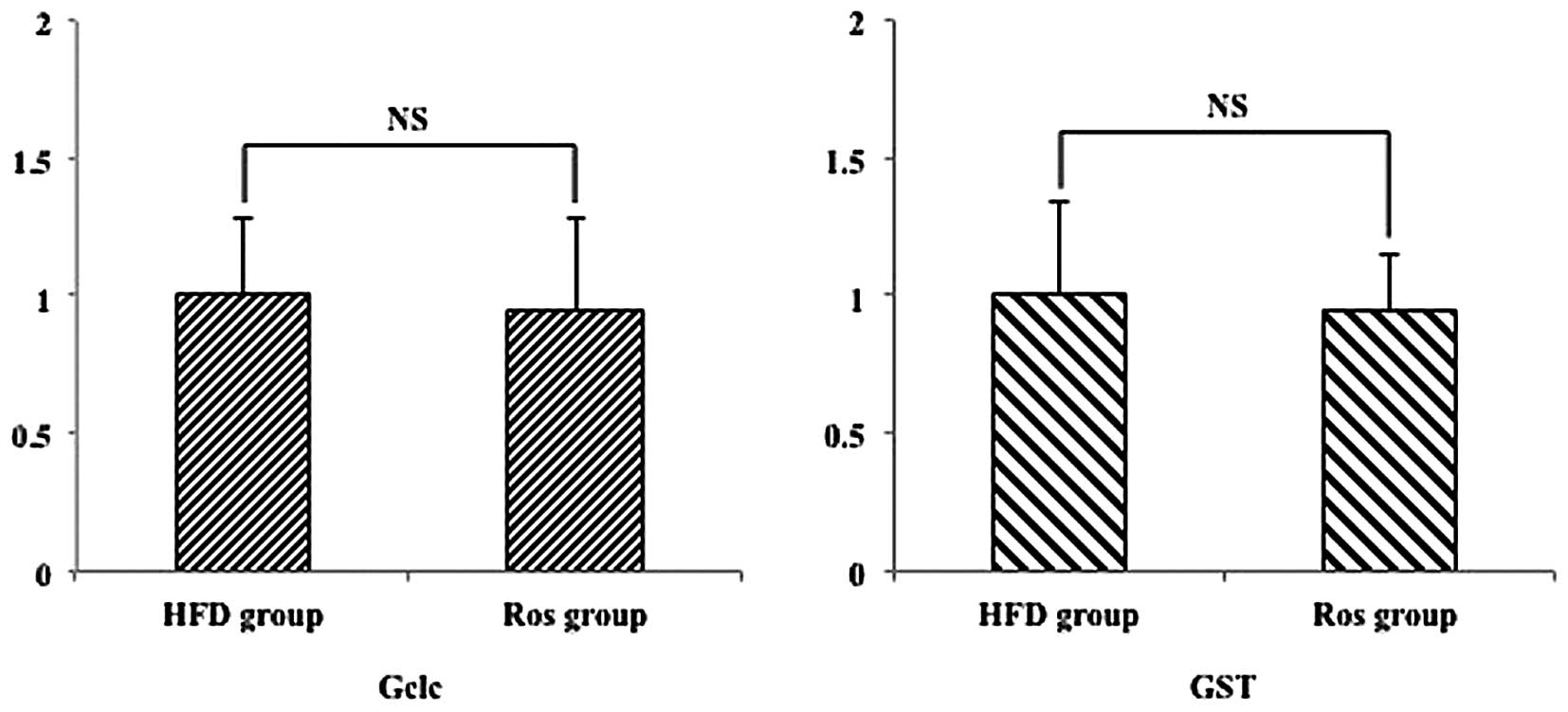
Hepatic antioxidant-related mRNA
expression
To determine whether Ros detoxifies reactive oxygen
species in the Ros group, we then performed RT-qPCR to quantify the
expression levels of antoxidant genes, such as GCL catalytic
subunit (Gclc) and glutathione S-transferase (GST) (20). The relative hepatic expression of
these genes did not differ significantly between the 2 groups
(Fig. 7).
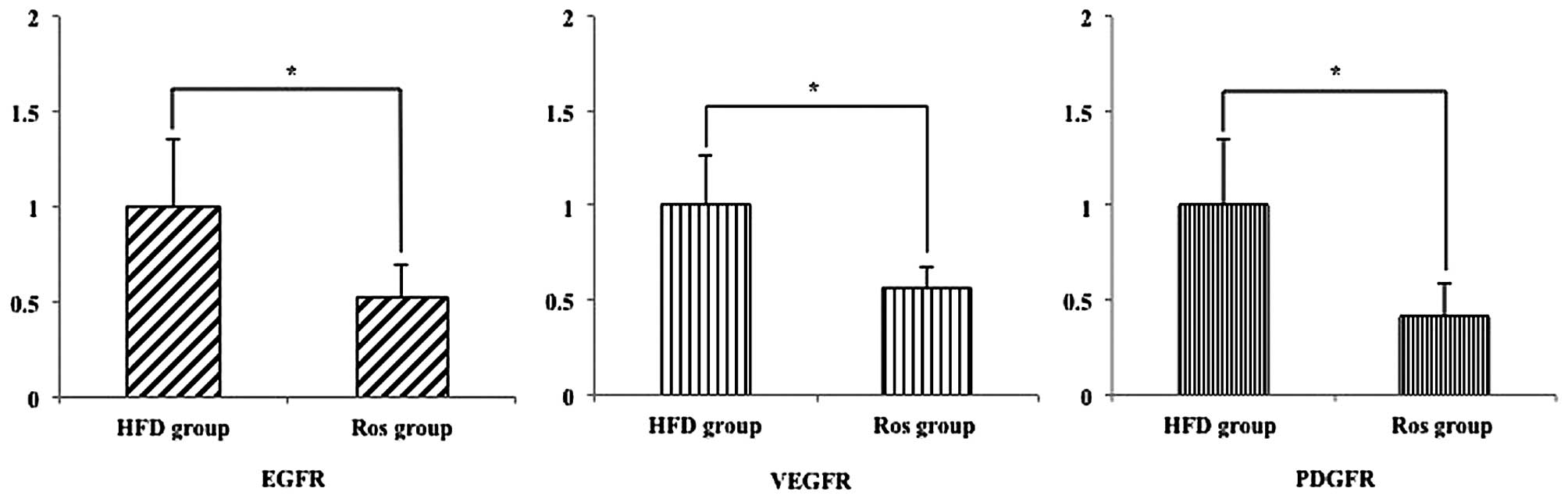
Hepatic developing HCC-related mRNA
expression
Sorafenib is a useful drug for the treatment of HCC
as it inhibits the epidermal growth factor (EGF), vascular
endothelial growth factor (VEGF) and platelet-derived growth factor
(PDGF) (21,22). Thus, we examined whether Ros
inhibits the development of these genes. The relative hepatic mRNA
expression levels of EGFR, VEGER and PDGFR were significantly
decreased in the Ros group compared with the HFD group (p<0.05;
Fig. 8).
Discussion
HCC typically has a poor prognosis, and the majority
of patients are diagnosed with progressive liver cancer with a
5-year survival rate of approximately 2%. Therefore, the prevention
of HCC is important for reducing its mortality and morbidity. It is
considered necessary to identify patients at risk of the occurrence
of HCC and to develop a safe chemopreventive agent. Hyperlipidemia
is one of the risk factors responsible for the occurrence of NASH,
which ultimately causes HCC. An increase in the extent of
hyperlipidemia translates to an increase in the risk of the
occurrence of HCC. Ros is a hypolipidemic agent. In addition to its
anti-hyperlipidemic effect, it has been shown to exert
anti-inflammatory and anti-arteriosclerotic effectsin
cardiovascular diseases both in vivo and in vitro
(4–6). Although Ros is commonly used in
hyperlipidemic patients, no study has been reported to date on the
effect of the drug on the occurrence of HCC in the presence of an
underlying NASH, at least to the best of our knowledge. The present
study showed the potential protective effect of Ros against HCC or
tumor formation. Our data also showed that this protective effect
is not only partially mediated by its anti-inflammatory effect and
the downregulation of genes involved in new lipogenesis, but may
also be mediated by the downregulation of vascular proliferative
factors.
It has beent demonstrated that Ros exerted
anti-inflammatory effects not only in cardiovascular diseases, but
also on factors involved in the occurrence and progression of NASH
(4–6). These findings are consistent with
the biochemistry measurements of AST and ALT in our study. In
fibrogenesis, however, Ros exerted its effect on TGF-β1, but not on
stellate cell-derived factors.
Our results also suggested that Ros exerted its
effect on the expression of lipid-related genes. Biochemical
analyses revealed that the serum and liver TG levels decreased in
the Ros group. To elucidate the mechanism involved, we measured the
hepatic expression of some lipogenic and lipid oxidation-related
genes in both groups. The results of RT-qPCR assay confirmed that
the administration of Ros significantly affected the expression of
a number of lipid-related genes. In particular, SREBP-1c (23), a key transcription activator in
hepatic lipid synthesis, was significantly decreased in the Ros
group, compared with the HFD group. The multi-subunit enzyme, AMPK,
is recognized as a major regulator in the hepatic lipid synthesis
pathway and fatty acid oxidation (24,25). We found that the expression of the
AMPKα1 subunit was higher in the Ros group than in the HFD group.
The molecule PPAR-α is a member of the PPAR subfamily of nuclear
receptors that transcriptionally promotes peroxisomal, microsomal
and mitochondrial oxidation (17). PPAR-α expression in the liver was
significantly higher in the Ros group than in the HFD group. By
contrast, no significant difference was observed between the two
groups as regards the expression of PPAR-γ (18), another member of the PPAR
subfamily, which transcriptionally initiates adipocyte
differentiation.
It has previously been shown that the administration
of eicosapentaenoic acid (EPA), a hypolipidemic agent, to Pten
knockout mice induces the expression of antioxidant genes and
inhibits the formation of reactive oxygen species. Thus, we
considered whether Ros exerts an antioxidant effect such as EPA. In
our study, no significant expression of antioxidant genes, such as
Gclc and GST, was induced in the livers of mice treated with Ros
(20). These findings suggest
that Ros has no antioxidant effect on STAM mouse livers.
Importantly, Ros suppressed the occurrence of
hepatic adenoma and HCC in the STAM mice used in this study. While
this effect has been reported for EPA (26), it has not even investigated for
Ros. EPA has been reported to have an antitumor effect originating
from cell death induction and cell growth inhibition. In addition
to focusing our attention to the already demonstrated effect of Ros
on cardiovascular diseases, we investigated the expression of
vascular proliferative factors, based on the fact that sorafenib,
the only oral treatment for progressive HCC, has a suppressive
effect on tumor vessel proliferation. Our results indicated that
the expression of vascular proliferative factors, including EGF,
VEGF and PDGF, was inhibited by almost 50% in the Ros group,
compared with the HFD group. This finding suggests that the
hypolipidemic agent, Ros, may have an antitumor effect, in addition
to its antihyperlipidemic effect, against HCC associated with
NASH.
Ros is a drug used in the treatment of many
hyperlipidemic patients. In the present study, we demonstrated that
the administration of Ros is a potential treatment for NASH and its
final stage, HCC. We suggest that the effects of Ros be evaluated
in future prospective and retrospective randomized
placebo-controlled clinical studies.
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
NAFLD
|
nonalcoholic fatty liver disease
|
|
HMG-CoA
|
3-hydroxy-3-methylglutaryl coenzyme
A
|
|
TNF
|
tumor necrosis factor
|
|
IL
|
interleukin
|
|
TGF
|
transforming growth factor
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
|
EGFR
|
epidermal growth factor receptor
|
|
PDGF
|
platelet-derived growth factor
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
Ros
|
Rosuvastatin
|
|
HFD
|
high-fat-diet
|
|
AST
|
spartate aminotransferase
|
|
ALT
|
alanine aminotransferase
|
|
FFA
|
free fatty acid
|
|
TG
|
triglyceride
|
|
T-CHO
|
total cholesterol
|
|
TG
|
tissue triglyceride
|
|
H&E
|
hematoxylin and eosin
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
IFN
|
interferon
|
|
SREBP-1c
|
sterol regulatory element binding
protein-1c
|
|
AMPK
|
AMP-activated protein kinase
|
|
FAS
|
fatty acid synthase
|
|
SCD-1
|
stearoyl-CoA desaturase-1
|
|
PPAR
|
peroxisome proliferator-activated
receptor
|
|
HSCs
|
hepatic stellate cells
|
|
TIMP
|
tissue inhibitors of matrix
metalloproteinases
|
|
MMP
|
matrix metalloproteinases
|
|
Gclc
|
GCL catalytic subunit
|
|
GST
|
glutathione S-transferase
|
|
EPA
|
eicosapentaenoic acid
|
Acknowledgments
The authors would like to thank Yukio Nakahira and
Eiko Koubayashi, at the Osaka Medical College, for providing them
with technical support.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ludwig J, Viggiano TR, McGill DB and Oh
BJ: Nonalcoholic steatohepatitis: Mayo Clinic experiences with a
hitherto unnamed disease. Mayo Clin Proc. 55:434–438.
1980.PubMed/NCBI
|
|
4
|
Aviram M, Rosenblat M, Bisgaier CL and
Newton RS: Atrovastatin and gemfibrozil metabolities, but not the
parent drugs are potent antioxidants against lipoprotein oxidation.
Antherosclerosis. 138:272–280. 1998.
|
|
5
|
Ridker PM, Cannon CP, Morrow D, Rifai N,
Rose LM, McCabe CH, Pfeffer MA and Braunwald E; Pravastatin or
Atorvastatin Evaluation and Infection Therapy-Thrombolysis in
Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators:
C-reactive protein levels and outcomes after statin therapy. N Engl
J Med. 352:20–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laufs U, La Fata V, Plutzky J and Liao JK:
Upregulation of endothelial nitric oxide synthase by HMG CoA
reductase inhibitors. Circulation. 97:1129–1135. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nezasa K, Higaki K, Matsumura T, Inazawa
K, Hasegawa H, Nakano M and Koike M: Liver-specific distribution of
rosuvastatin in rats: Comparison with pravastatin and simvastatin.
Drug Metab Dispos. 30:1158–1163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ikeda Y, Young LH and Lefer AM:
Rosuvastatin, a new HMG-CoA reductase inhibitor, protects ischemic
reperfused myocardium in normocholesterolemic rats. J Cardiovasc
Pharmacol. 41:649–656. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bulhak A, Sjoquist PO and Pernow J:
Rosuvastatin protects the myocardium against ischaemia-reperfusion
injury via inhibition of GGPP synthesis. Cardiovasc J S Afr.
15:S112004.
|
|
10
|
Weinberg EO, Scherrer-Crosbie M, Picard
MH, Nasseri BA, MacGillivray C, Gannon J, Lian Q, Bloch KD and Lee
RT: Rosuvastatin reduces experimental left ventricular infarct size
after ischemia-reperfusion injury but not total coronary occlusion.
Am J Physiol Heart Circ Physiol. 288:H1802–H1809. 2005. View Article : Google Scholar
|
|
11
|
Bulhak AA, Gourine AV, Gonon AT, Sjöquist
PO, Valen G and Pernow J: Oral pre-treatment with rosuvastatin
protects porcine myocardium from ischaemia/reperfusion injury via a
mechanism related to nitric oxide but not to serum cholesterol
level. Acta Physiol Scand. 183:151–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takakura K, Koido S, Fujii M, Hashiguchi
T, Shibazaki Y, Yoneyama H, Katagi H, Kajihara M, Misawa T, Homma
S, et al: Characterization of non-alcoholic steatohepatitis-derived
hepatocellular carcinoma as a human stratification model in mice.
Anticancer Res. 34:4849–4855. 2014.PubMed/NCBI
|
|
13
|
Gao B: Innate immunity and
steatohepatitis: A critical role of another toll (TLR-9).
Gastroenterology. 139:27–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horton JD, Shah NA, Warrington JA,
Anderson NN, Park SW, Brown MS and Goldstein JL: Combined analysis
of oligonucleotide microarray data from transgenic and knockout
mice identifies direct SREBP target genes. Proc Natl Acad Sci USA.
100:12027–12032. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foretz M, Ancellin N, Andreelli F,
Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S and Viollet
B: Short-term overexpression of a constitutively active form of
AMP-activated protein kinase in the liver leads to mild
hypoglycemia and fatty liver. Diabetes. 54:1331–1339. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohen P and Friedman JM: Leptin and the
control of metabolism: Role for stearoyl-CoA desaturase-1 (SCD-1).
J Nutr. 134:2455S–2463S. 2004.PubMed/NCBI
|
|
17
|
Yu S, Rao S and Reddy JK: Peroxisome
proliferator-activated receptors, fatty acid oxidation,
steatohepatitis and hepatocarcinogenesis. Curr Mol Med. 3:561–572.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tontonoz P, Hu E, Graves RA, Budavari AI
and Spiegelman BM: mPPAR gamma 2: Tissue-specific regulator of an
adipocyte enhancer. Genes Dev. 8:1224–1234. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Friedman SL: The cellular basis of hepatic
fibrosis. Mechanism and treatment strategies. N Engl J Med.
328:1828–1835. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaparro M, González Moreno L,
Trapero-Marugán M, Medina J and Moreno-Otero R: Review article:
Pharmacological therapy for hepatocellular carcinoma with sorafenib
and other oral agents. Aliment Pharmacol Ther. 28:1269–1277. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siegelin MD, Raskett CM, Gilbert CA, Ross
AH and Altieri DC: Sorafenib exerts anti-glioma activity in vitro
and in vivo. Neurosci Lett. 478:165–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tontonoz P, Kim JB, Graves RA and
Spiegelman BM: ADD1: A novel helix-loop-helix transcription factor
associated with adipocyte determination and differentiation. Mol
Cell Biol. 13:4753–4759. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hardie DG and Carling D: The AMP-activated
protein kinase–fuel gauge of the mammalian cell? Eur J Biochem.
246:259–273. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Winder WW and Hardie DG: AMP-activated
protein kinase, a metabolic master switch: Possible roles in type 2
diabetes. Am J Physiol. 277:E1–E10. 1999.PubMed/NCBI
|
|
26
|
Ishii H, Horie Y, Ohshima S, Anezaki Y,
Kinoshita N, Dohmen T, Kataoka E, Sato W, Goto T, Sasaki J, et al:
Eicosapentaenoic acid ameliorates steatohepatitis and
hepatocellular carcinoma in hepatocyte-specific Pten-deficient
mice. J Hepatol. 50:562–571. 2009. View Article : Google Scholar : PubMed/NCBI
|