Introduction
Retinal pigment epithelial cells (RPE cells), which
are located between the choroids and the neurosensory retina, form
the outer blood-retinal barrier and play a crucial role in the
pathological processes that leads to the loss of vision. RPE cells
are activated by the breakdown of the outer blood-retinal barrier,
and can undergo proliferation and migration and secrete
extracellular matrix (ECM) molecules in vitreoretinal disorders,
such as proliferative diabetic retinopathy (DR), proliferative
vitreoretinopathy (PVR) and age-related macular degeneration (AMD)
(1–3). RPE cells are known to contribute to
inflammation and fibrosis in vitreoretinal disorders (4) and in the formation of fibrotic
membranes (5).
It has been documented that epithelial-mesenchymal
transition (EMT) plays a role in the fibrosis of various organs,
such as the kidneys, lungs and liver (6–9).
There is evidence to suggest that kidney proximal tubule epithelial
cells undergo EMT to induce interstitial fibrosis in diabetic
nephropathy (6,10). As shown in a previous study, in
diabetic nephropathy, the expression of mesenchymal proteins was
detected in the kidney sections of diabetic patients, and the
alterations in mesenchymal proteins in tubular epithelial cells
were well correlated with the declining renal function (11). Since cells undergoing EMT will
lose their normal functions and mediate fibrosis in diabetic
nephropathy, we speculated that mesenchymal transition may be
involved in the development of RPE cell-related diseases.
EMT is a multi-step morphogenetic process during
which epithelial cells lose their epithelial properties and acquire
mesenchymal characteristics. Static epithelial cells lose cell to
cell junctions, and consequently they lose apico-basal polarity to
become migratory mesenchymal-like cells (12,13). EMT occurs in three different
biological settings with very different functional consequences
(14). Type 1 EMT is invloved in
original embryonic development and postnatal growth (12,15). Type 2 EMT participates in wound
healing, tissue regeneration and organ fibrosis. Oncogenic (type 3)
EMT enables epithelial cells to acquire invasive mesenchymal
phenotype characteristics that are essential in the metastatic
spread (16). The most
characterized transcription factors in the regulation of EMT are
Snail, Slug, Twist, zinc finger E-box-binding homeobox (ZEB)1 and
ZEB2 (12,14).
EMT is triggered by inflammatory cytokines,
cytotoxic stress and DNA damage in tissue repair and tissue
fibrosis (17,18). Abundant evidence indicates that
hyperglycemia is etiologically related to human aging and diseases,
including DR and AMD (19), and
high glucose is a predictor of progression to late AMD (20). Therefore, the aim of this study
was to examine the effects of high glucose on EMT in RPE cells and
to determine its pathogenic role.
Materials and methods
Materials and antibodies
L-glucose and D-glucose were purchased from Sigma
(St. Louis, MO, USA). AKT inhibitor IV and the extracellular
signal-regulated kinase (ERK) inhibitor, U0126, were obtained from
Millipore (Billerica, MA, USA) and Selleckchem (Houston, TX, USA)
respectively. Antibodies to α-smooth muscle actin (α-SMA; A2547)
and β-actin (A5441) were purchased from Sigma-Aldrich. The antibody
against phosphorylated (p-)ERK (sc-7383) was from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies against
E-cadherin (610181), vimentin (550513), N-cadherin (610920) and
fibronectin (610077) were obtained from BD Biosciences (Franklin
Lakes, NJ, USA). Antibodies against Snail (3879S), β-catenin
(9582S) and p-AKT (4060S) were from Cell Signaling Technology
(Danvers, MA, USA). The antibody against connective tissue growth
factor (CTGF; ab6992) was purchased from Abcam (Cambridge, MA,
USA). ZO1 antibody (40-2200) was obtained from Invitrogen Life
Technologies, Carlsbad, CA, USA. Goat anti-mouse (PI-2000) or
anti-rabbit (PI-1000) horseradish peroxidase (HRP)-labeled
secondary antibodies were from Vector Laboratories (Burlingame, CA,
USA). Alexa Fluor 488 goat anti-rabbit/anti-mouse (A21206/A21202),
Alexa Fluor 594 goat anti-rabbit/anti-mouse (A21207/A21203)
antibodies and 4′,6-diamidino-2-phenylindole (DAPI; D1306) were
from Life Technologies (St. Louis, MO, USA).
Cell culture
ARPE19, a cell line derived from human retinal
pigment epithelium (RPE) was obtained from ATCC (Manassas, VA, USA)
and cultured in Dulbecco's modified Eagle's medium (DMEM)
containing 10% heat inactivated fetal bovine serum (FBS) and 100
U/ml penicillin/streptomycin (Invitrogen Life Technologies). The
cells were maintained at 37°C in a humidified atmosphere with 5%
CO2.
Immunofluorescence staining
Immunofluorescence staining was performed as
previously described (21). For
immunocytochemistry, the cells grown in 4-well glass slide chambers
to 60% confluence were exposed to 25 mM high glucose for 48 h. The
cells were then incubated with the primary antibodies specific for
vimentin, N-cadherin and α-SMA at a dilution of 1:200 overnight at
4°C. The secondary antibodies (Alexa Flour 488/Alexa Flour 594 goat
anti-rabbit/anti-mouse) were then added at a dilution of 1:200 for
1 h. Slides were prepared with a mounting medium containing DAPI to
counterstain the nucleus.
Western blot analysis
The cells were lysed for total protein extraction
using RIPA buffer. The protein concentration was determined using a
Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA,
USA) according to the manufacturer's instructions. The aliquots of
equal amounts of protein were resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto a PVDF membrane (Bio-Rad Laboratories). After
blocking with 5% non-fat dry milk in Tris-buffered saline Tween-20
(TBST) for 1 h, the membrane was incubated overnight at 4°C with
various primary antibodies. After washing with TBST, the membrane
was incubated with the appropriate secondary antibody for 2 h. The
membrane was again washed with TBST, and immunoblots were developed
with the enhanced chemiluminescent reagents from Pierce/Thermo
Fisher Scientific (Waltham, MA, USA) according to the
manufacturer's instructions. Images were acquired using ImageQuant
Las 4000 mini (GE Healthcare Bio-sciences, Pittsburgh, PA, USA) and
densitometry was performed using ImageJ software and normalized to
the β-actin levels.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cultured cells
using TRIzol reagent according to the manufacturer's instructions
(Invitrogen Life Technologies). Total RNA (500 ng) was used for
reverse transcription using the PrimeScript® RT reagent
kit (perfect real-time) (Takara Bio Inc., Otsu, Japan). The cDNA
was used for quantitative PCR (qPCR) using SYBR® Premix
Ex Taq™ (rerfect real-time) (Takara Bio Inc.) and a Roche
capillary-based LightCycler® 2.0 system (Roche
Diagnostics, Indianapolis, IN, USA). The specificity of the
amplification reactions was confirmed by melting curve analysis.
All expression data were normalized to those for β-actin. The data
were quantified by the comparative threshold cycle (Ct) method for
relative gene expression. The PCR cycling conditions were as
follows: 95°C for 30 sec, 95°C for 5 sec and 60°C for 45 sec for 40
cycles. Primer sequences are as follows: human snail forward,
TGCGCTACTGCTGCGCGAAT and reverse, GGGCTGCTGGAAGGTAAACTCTGGA;
β-actin forward, GCACTCTTCCAGCCTTCCTT and reverse, GTTGG
CGTACAGGTCTTTGC.
Wound healing assay
The cells were seeded in each well of a 6-well
culture plate and then cultured for 24 h until they reached
approximately 80% confluence. The cells were starved in DMEM for 24
h and then exposed to L-glucose as a control and D-glucose (25 mM)
for 48 h. Images of the wells under a microscope (Zeiss Axio
Observer Z1, Carl Zeiss Meditec AG, Jena, Germany) were acquired
the indicated time points after the wound scratch was made. The
migration rate of the cells was calculated as the distance traveled
by the cells from the wound edge to the cell-free space.
RNA interference
Oligonucleotides matching the selected regions of
human Snail and scrambled siRNAs that were used as a negative
control were purchased from RiboBio (Guangzhou, China). The cells
were transfected with siRNA oligonucleotides at a final
concentration of 100 nM with HiPerFect (Qiagen, Carson City, CA,
USA) according to the manufacturer's instructions. The cells were
transfected with siRNA oligonucleotides for 24 h then followed by
incubation in the presence of high glucose for an additional 48
h.
Cell transfection with overexpression
vector
Full-length Snail cDNA was a gift from Professor Jun
Li (Sun Yat-sen University, Guangzhou, China). The pCR3.1-vector
and pCR3.1-Snail plasmid were transfected into the cells using
Lipofectamine 2000 according to the manufacturer's instructions
(Invitrogen Life Technologies). After 48 h, the cells were
harvested, and the expression of proteins was determined using
western blot analysis.
Statistical analysis
Data are presented as the means ± SD. Comparisons
were performed by a two-tailed paired Student's t-test. A value of
p<0.05 was considered to indicate a statistically significant
difference.
Results
EMT is induced by high glucose in RPE
cells
RPE cells are the key component of the outer
blood-retina barrier and the main contributor to the development of
fibrotic tissue in the retina (22). Therefore, we evaluated the direct
effect of high glucose on mesenchymal transition in RPE cells. As
shown in Fig. 1, compared to
exposure to L-glucose as an osmotic control, exposure to 25 mM high
glucose for 48 h elevated the levels of N-cadherin, β-catenin,
fibronectin and decreased the levels of E-cadherin and ZO-1 in the
RPE cells (Fig. 1A). Moreover,
immunofluorescence staining revealed that the cells exposed to high
glucose had more intensive vimentin, N-cadherin and α-SMA signals
compared with the control cells (Fig.
1B).
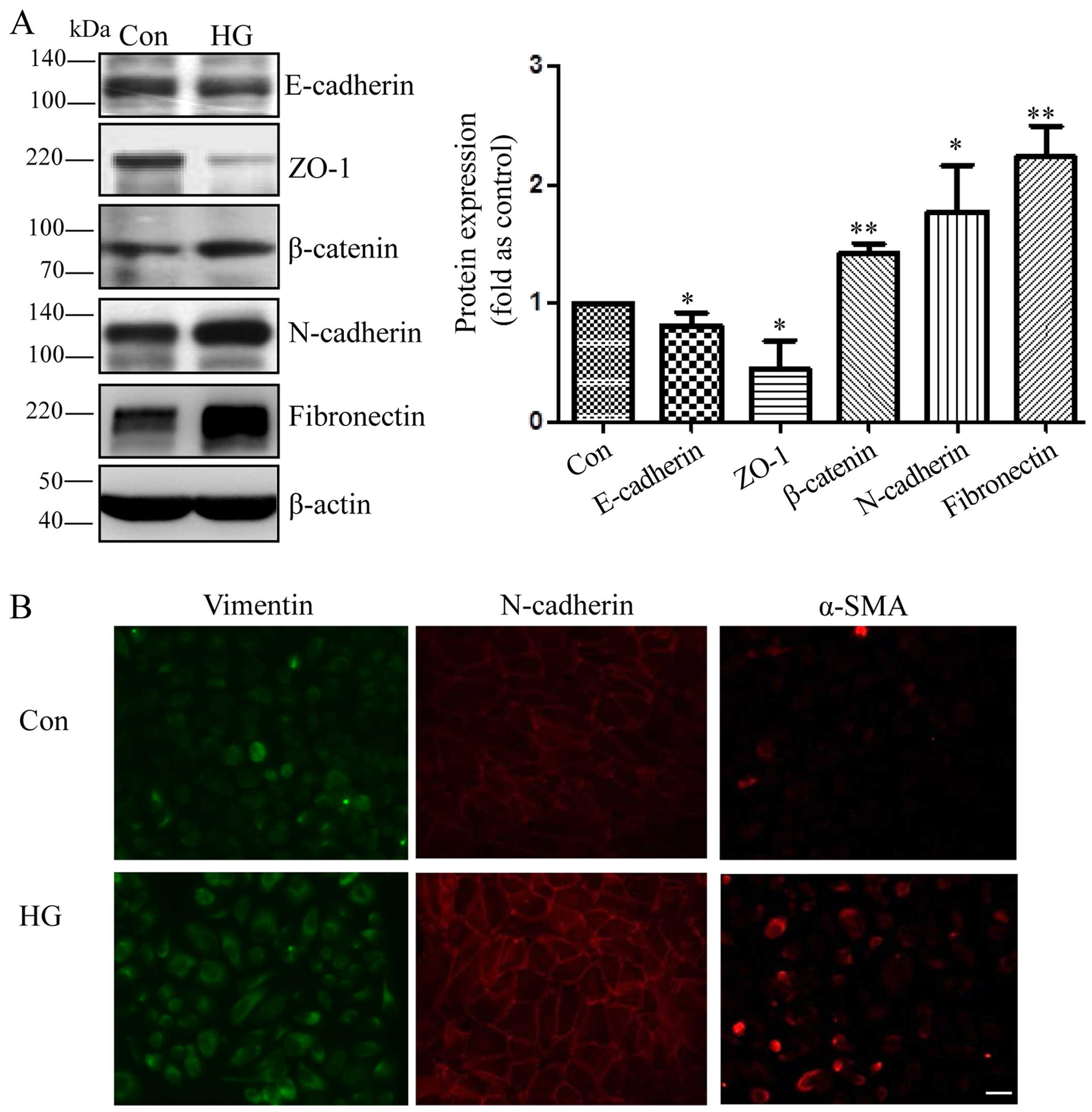 | Figure 1Mesenchymal transition of retinal
pigment epithelial cells (RPE cells) induced by exposure to high
glucose. (A) Representative western blots of E-cadherin, Zonula
occludens-1 (ZO-1), β-catenin, N-cadherin, fibronectin in the cells
exposed to 25 mM D-glucose (HG) and L-glucose as an osmotic control
for the indicated periods of time (48 h). The bands were quantified
relative to β-actin (means ± SD, **p<0.01,
*p<0.05, n=3). (B) RPE cells were grown on glass
coverslips for 24 h, starved for 24 h, and then incubated with 25
mM D-glucose (HG) and L-glucose as a control for 48 h. Compared
with the control cells, the HG-exposed cells displayed an increased
expression of vimentin, N-cadherin and α-SMA, as shown by
immunofluorescence staining. Original magnification, ×400. |
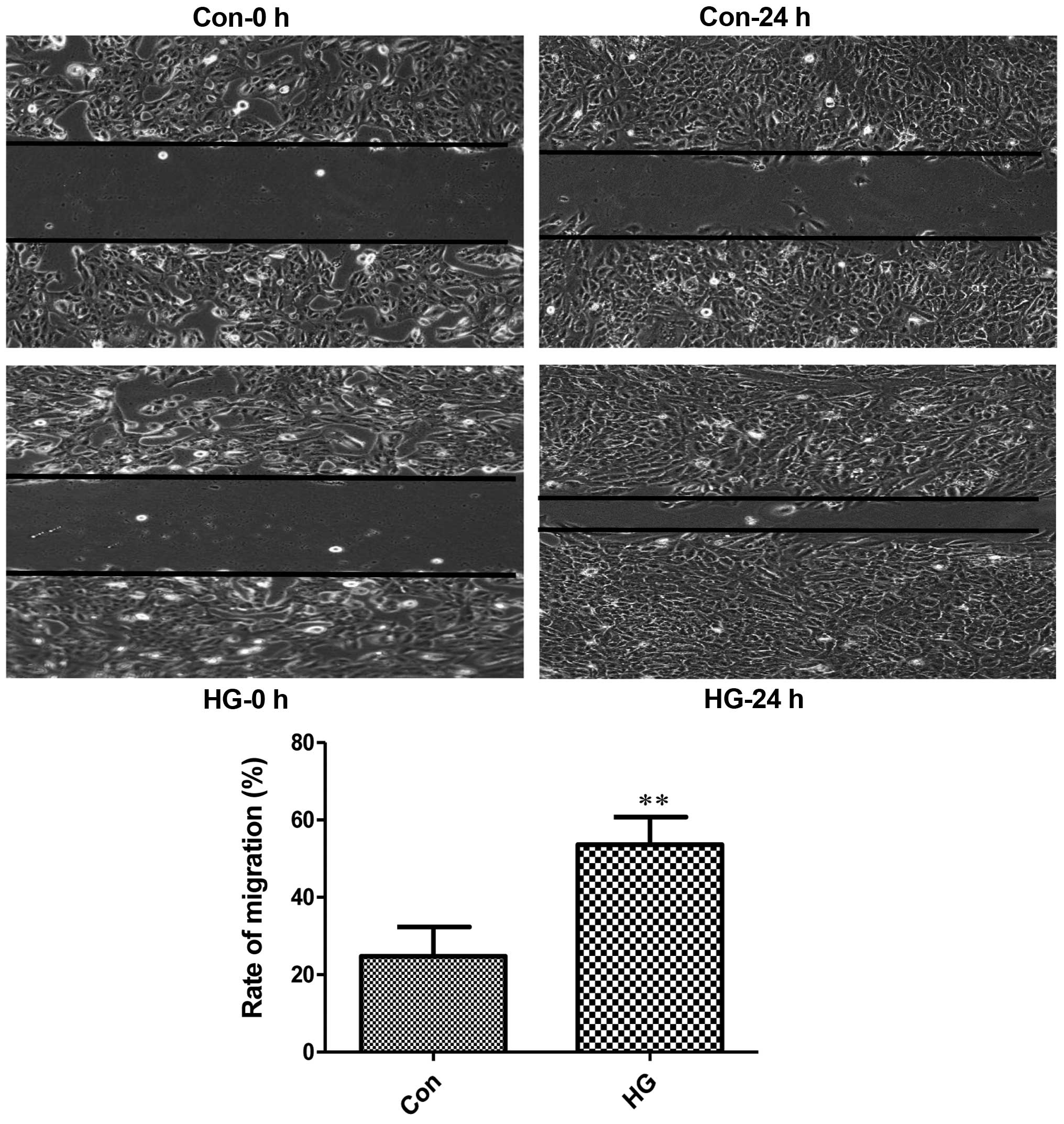
It is well known that EMT can increase cell motility
(23–25). Normal RPE cells are quiescent
without migration (26,27). In this study, the number of
migrated cells in the high glucose-exposed RPE cells was
considerably higher than the mean number of migrated control cells
(Fig. 2). These observations
indicated that the RPE cells exposed to high glucose underwent
mesenchymal transition and migration was initiated.
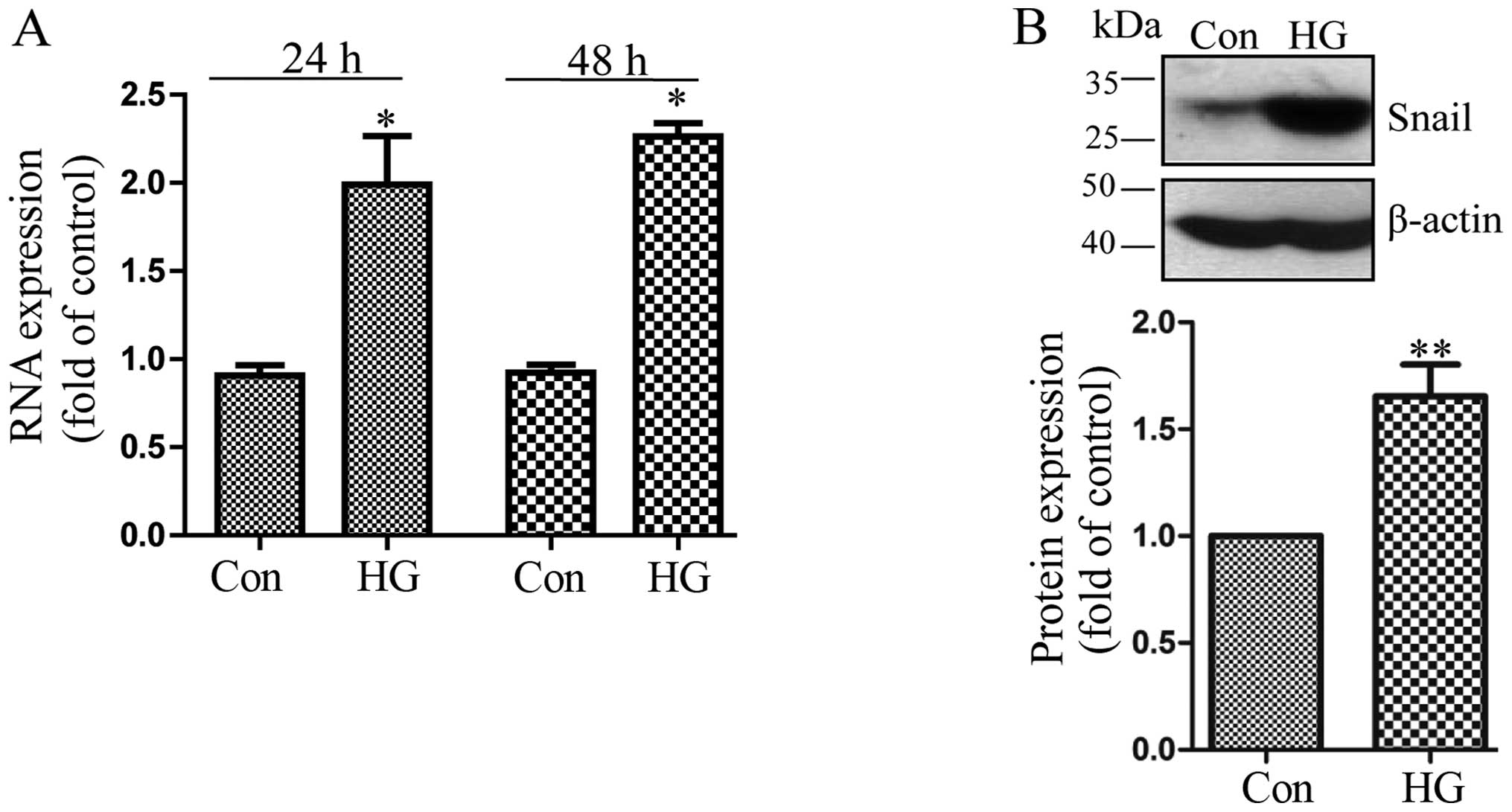
Exposure to high glucose induces the
upregulation of Snail
Snail is a classical transcription factor involved
in EMT in tumors (14).
Therefore, in this study, we investigated whether the expression of
Snail was upregulated by exposure of the cells to high glucose. We
found that compared to the control cells, high glucose increased
the mRNA expression of Snail 24 h following exposure and reached
the highest level at 48 h (Fig.
3A). Likewise, the promoting effect of high glucose on the
Snail protein level at 48 h was also confirmed by western blot
analysis (Fig. 3B).
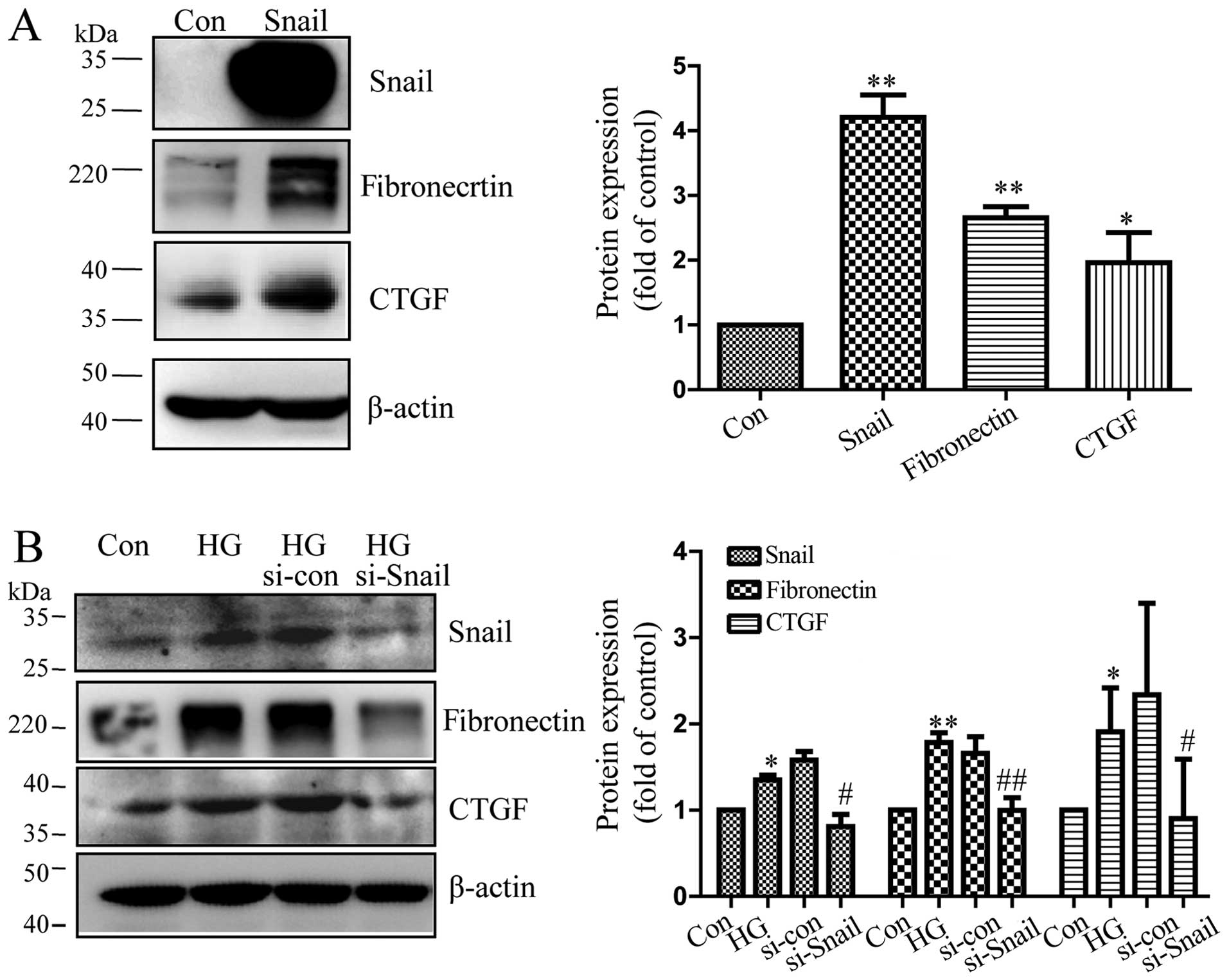
Snail promotes the expression of
cytokines in RPE cells
CTGF and fibronectin are important profibrotic
growth factors that induce the production of ECM components and
angiogenesis (28,29). CTGF and fibronectin have been
implicated in the pathological progress in patients with
vitreoretinal disorders (30–34) and is induced by high glucose
(31,35). It has been reported that EMT is
associated with fibrogenesis in diabetic nephropathy and other
organs (6–9). In order to fully understand the
pathogenic role of mesenchymal transition in RPE cells, we
transfected the cells with Snail expression vector. Our results
revealed that the protein level of Snail was upregulated in the
cells transfected with the Snail overexpression vector compared to
the cells transfected with the empty vector (Fig. 4A). In addition, with the
overexpression of Snail, the expression of fibronectin and CTGF
also increased in RPE cells, as shown by western blot analysis
(Fig. 4A). Furthermore, compared
to controls transfected with scrambled siRNA, the silencing of
Snail decreased expression of CTGF and fibronectin in RPE cells,
which had been increased by high glucose (Fig. 4B). These data suggested that
mesenchymal transition in RPE cells may contribute to fibrosis by
promoting the secretion of important cytokines.
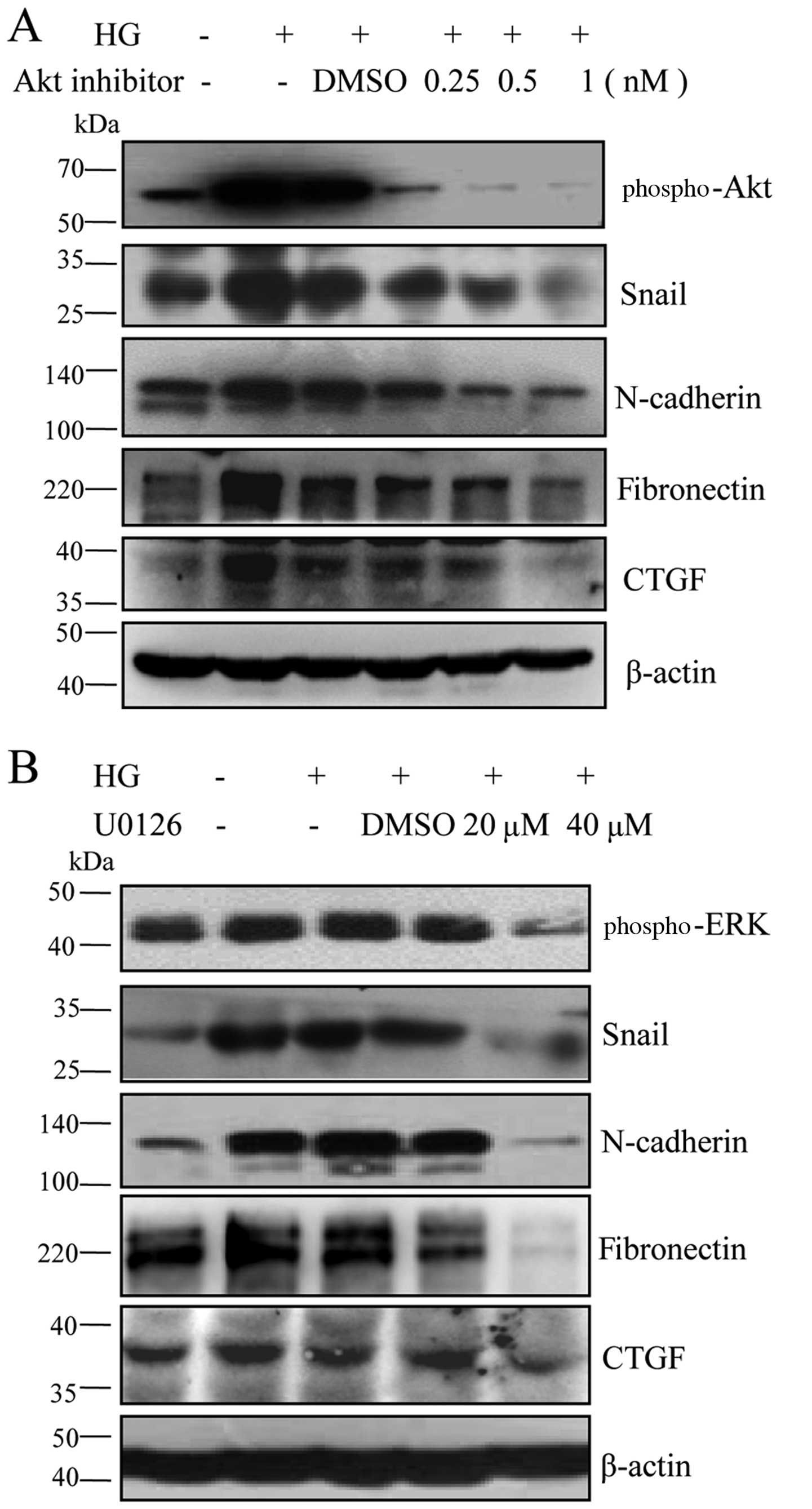
The AKT and ERK signaling pathways
mediate the expression of mesenchymal markers induced by high
glucose in RPE cells
The activation AKT and ERK plays a critical role in
the process of epithelial-mesenchymal transition (36–38). The AKT and ERK pathways have been
recently recognized as new players in retinal disorders (39–41). These findings led us to
hypothesize that the AKT and ERK signaling pathways regulate the
expression of mesenchymal markers in RPE cells. To confirm the
hypothesis, we used the AKT, AKT inhibitor IV and the ERK
inhibitor, U0126, to block these signaling pathways. Our results
revealed that AKT inhibitor IV and U0126 downregulated the levels
of Snail and N-cadherin in a dose-dependent manner, followed by a
decrease in the levels of CTGF and fibronectin (Fig. 5). These data indicated that the
AKT and ERK signaling pathways were involved in the high
glucose-induced mesenchymal transition and fibrosis in RPE
cells.
Discussion
The present study reports that hyperglycemia induces
mesenchymal transition in cultured RPE cells. The overexpression of
Snail increased the protein levels of fibronectin and CTGF.
Likewise, the silencing of Snail using siRNA decreased the
expression of fibronectin and CTGF which was induced by high
glucose in RPE cells. Mechanism experiments indicated that blockade
of the AKT and ERK signaling pathways using chemical inhibitors
decreased the expression of Snail, as well as that of fibronectin
and CTGF, which had been induced by high glucose in RPE cells.
EMT is observed in the process of renal interstitial
fibrosis, of pulmonary fibrosis, of liver fibrosis, or in specific
ocular tissue (6–11). Most vision loss occurs following
the transition from a disease of inflammation to a disease of
neovascular fibrosis (42). RPE
cells form the outer blood retinal barrier from the choroidal
capillary bed by separating the outer retina. The dysfunction of
the RPE can result in retinal edema, detachment or degeneration
(43). Normal RPE cells are
quiescent without proliferation or migration abilities (26,27). In this study, we found that high
glucose induced the expression of mesenchymal makers in RPE cells
(Fig. 1). Subsequently, the
activated RPE cells induced by high glucose underwent migration
(Fig. 2). Moreover, it has been
proposed that hyperglycemia increases superoxide production, which
in turn initiates accelerated advanced glycation end-product (AGE)
formation and exacerbates interrelated pathogenic responses. AGEs
are one of the important factors involved in the pathogenesis of
diseases of the eye, and it has been demonstrated that AGE mimetic
administration induces the breakdown of RPE function in RPE cells
(44). We speculated that the
pathogenic role of AGE in RPE was partially ascribed to the
induction of mesenchymal transition. In this study, we verified
that AGE-stimulated cells displayed an altered mesenchymal
morphology with a decreased expression of E-cadherin and an
increased expression of vimentin by immunofluorescence staining.
AGE significantly elevated the Snail mRNA level (data not
shown).
Cao et al newly proved the existence
endothelial to mesenchymal transition (EndMT) in diabetic retinas
(45). Our data, together with
their study extend our understaning of mesenchymal transition
specific to diabetic retinas. These observations indicate that
retinal cells in the setting of hyperglycemia undergo mesenchymal
transition, and this may be the initial and key event that is
responsible for cellular dysfunction and the development of
vitreoretinal diseases.
In this study, we demonstrated that hyperglycemia
induced the cell transition from a normal phenotype to a
mesenchymal phenotype and promoted Snail expression (Fig. 3). It would be of interest to
determine the consequence of this transition concerning the
pathogenic progress. In this study, to the best of our knowledge,
we demonstrate for the first time that Snail regulated the
expression of CTGF and fibronectin, which are important fibrogenic
factors produced by RPE cells (Fig.
4). Due to the location of these cells, we hypothesized that
the occurrence of mesenchymal transition in RPE cells would lead to
the production of cytokines that results in indirect effects on the
retina. Moreover, we hypothesized that mesenchymal transition leads
to the cellular dysfunction partly through abnormal cytokine
secretion and may participate in the functions of retinal cells,
namely their fuctions other than fibrosis, such as intraretinal
micovasular abnormalities.
This point warrants further investigation.
Furthermore, recent studies have reported that CTGF itself induces
EMT in renal cells (46,47). If this is also the case in retinal
cells, we can assume that CTGF and Snail form a positive loop,
resulting in a vicious circle of the development of vitreoretinal
disorders.
Transforming growth factor (TGF)-β has been shown to
play a central role in initiating EMT, and has been extensively
studied. Therefore, we expected to elucidate a novel mechanism
other than TGF-β, which could modulate mesenchymal transition in
RPE cells. Recent data indicate that the normal epithelial
phenotype and cell proliferation and migration appear to be
associated with the activation of AKT and ERK via their
phosphorylation (36,38). The connection between AKT and
Snail and cell-cell adhesion plays a role in various tumors, as
well as in the repair of normal tissue after wounding (48). Moreover, the Ras-ERK pathway is
required for EMT, and it cooperates with other pathways to
upregulate the expression of EMT-related genes, including
mesenchymal genes and transcriptional repressors (e.g. Snail, Slug,
Twist and ZEB) (37). However,
these studies were confined to EMT in tumors. It is unknown as to
which signaling pathways are involved in mesenchymal transition in
RPE cells. In this study, we found that high glucose induced AKT
and ERK phosphorylation followed by the induction of Snail and
N-cadherin expression, as well as that of fibrogenic factors, while
the blockade of the signaling pathways decreased the expression of
Snail, N-cadherin, fibronectin and CTGF (Fig. 5). These findings indicated a novel
mechanism through which the AKT and ERK signaling pathways modulate
RPE dysfunction, relying on the regulation of mesenchymal
transition, and that the signaling pathways may cooperate with each
other.
In conclusion, the findings of our study, to the
best of our knowledge, demonstrate for the first time that high
glucose induces mesenchymal transition in RPE cells and suggest
that the AKT and ERK signaling pathways regulate the expression of
mesenchymal markers in RPE cells.
Acknowledgments
This study was supported by the National Nature
Science Foundation of China, grant nos. 81200706, 81172163,
81272338, 81272515, 81400639, 81370945, 81471033, 81572342,
81570871 and 81570764; the National Key Sci-Tech Special Project of
China, grant no. no. 2013ZX09102-053; the Program for Doctoral
Station in University, grant nos. 20120171110053 and
20130171110053; the Fundamental Research Funds for the Central
Universities' Youth Cultivation Project of China, grant no.
50000-3161046; the Guangdong Natural Science Fund, grant nos.
S2012040006986, S2012010009250 and 2015A030313103; and the Key
Sci-Tech Research Project of Guangzhou Municipality, China, grant
nos. 2011Y1-00017-8, 12A52061519 and 201508020033.
References
|
1
|
Campochiaro PA: Pathogenic mechanisms in
proliferative vitreoretinopathy. Arch Ophthalmol. 115:237–241.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Esser P, Heimann K, Bartz-schmidt KU,
Fontana A, Schraermeyer U, Thumann G and Weller M: Apoptosis in
proliferative vitreoretinal disorders: Possible involvement of
TGF-beta-induced RPE cell apoptosis. Exp Eye Res. 65:365–378. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller H, Miller B and Ryan SJ: The role
of retinal pigment epithelium in the involution of subretinal
neovascularization. Invest Ophthalmol Vis Sci. 27:1644–1652.
1986.PubMed/NCBI
|
|
4
|
Bastiaans J, van Meurs JC, van
Holten-Neelen C, Nijenhuis MS, Kolijn-Couwenberg MJ, van Hagen PM,
Kuijpers RWAM, Hooijkaas H and Dik WA: Factor Xa and thrombin
stimulate proinflammatory and profibrotic mediator production by
retinal pigment epithelial cells: A role in vitreoretinal
disorders? Graefes Arch Clin Exp Ophthalmol. 251:1723–1733. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qin D, Zhang GM, Xu X and Wang LY: The
PI3K/Akt signaling pathway mediates the high glucose-induced
expression of extracellular matrix molecules in human retinal
pigment epithelial cells. J Diabetes Res. 2015:9202802015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carew RM, Wang B and Kantharidis P: The
role of EMT in renal fibrosis. Cell Tissue Res. 347:103–116. 2012.
View Article : Google Scholar
|
|
7
|
Nowrin K, Sohal SS, Peterson G, Patel R
and Walters EH: Epithelial-mesenchymal transition as a fundamental
underlying pathogenic process in COPD airways: Fibrosis, remodeling
and cancer. Expert Rev Respir Med. 8:547–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chapman HA: Epithelial-mesenchymal
interactions in pulmonary fibrosis. Annu Rev Physiol. 73:413–435.
2011. View Article : Google Scholar
|
|
9
|
Lee SJ, Kim KH and Park KK: Mechanisms of
fibrogenesis in liver cirrhosis: The molecular aspects of
epithelial-mesenchymal transition. World J Hepatol. 6:207–216.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srivastava SP, Koya D and Kanasaki K:
MicroRNAs in kidney fibrosis and diabetic nephropathy: Roles on EMT
and EndMT. BioMed Res Int. 2013:1254692013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y: New insights into
epithelial-mesenchymal transition in kidney fibrosis. J Am Soc
Nephrol. 21:212–222. 2010. View Article : Google Scholar
|
|
12
|
Samatov TR, Tonevitsky AG and Schumacher
U: Epithelial-mesenchymal transition: Focus on metastatic cascade,
alternative splicing, non-coding RNAs and modulating compounds. Mol
Cancer. 12:1072013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalluri R and Weinberg RA: The basics of
epithelial- mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaffer CL, Thompson EW and Williams ED:
Mesenchymal to epithelial transition in development and disease.
Cells Tissues Organs. 185:7–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirasawa M, Noda K, Noda S, Suzuki M,
Ozawa Y, Shinoda K, Inoue M, Ogawa Y, Tsubota K and Ishida S:
Transcriptional factors associated with epithelial-mesenchymal
transition in choroidal neovascularization. Mol Vis. 17:1222–1230.
2011.PubMed/NCBI
|
|
18
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiu CJ and Taylor A: Dietary
hyperglycemia, glycemic index and metabolic retinal diseases. Prog
Retin Eye Res. 30:18–53. 2011. View Article : Google Scholar
|
|
20
|
Ghaem Maralani H, Tai BC, Wong TY, Tai ES,
Li J, Wang JJ and Mitchell P: Metabolic syndrome and risk of
age-related macular degeneration. Retina. 35:459–466. 2015.
View Article : Google Scholar
|
|
21
|
Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B,
Gao G and Ma JX: The pathogenic role of the canonical Wnt pathway
in age-related macular degeneration. Invest Ophthalmol Vis Sci.
51:4371–4379. 2010. View Article : Google Scholar :
|
|
22
|
Snead DR, James S and Snead MP:
Pathological changes in the vitreoretinal junction 1: Epiretinal
membrane formation. Eye (Lond). 22:1310–1317. 2008. View Article : Google Scholar
|
|
23
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Williams E, Williams G, Gour BJ, Blaschuk
OW and Doherty P: A novel family of cyclic peptide antagonists
suggests that N-cadherin specificity is determined by amino acids
that flank the HAV motif. J Biol Chem. 275:4007–4012. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Wever O, Westbroek W, Verloes A,
Bloemen N, Bracke M, Gespach C, Bruyneel E and Mareel M: Critical
role of N-cadherin in myofibroblast invasion and migration in vitro
stimulated by colon-cancer-cell-derived TGF-beta or wounding. J
Cell Sci. 117:4691–4703. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bharti K, Nguyen MT, Skuntz S, Bertuzzi S
and Arnheiter H: The other pigment cell: specification and
development of the pigmented epithelium of the vertebrate eye.
Pigment Cell Res. 19:380–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:845–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Winkler JL, Kedees MH, Guz Y and Teitelman
G: Inhibition of connective tissue growth factor by small
interfering ribonucleic acid prevents increase in extracellular
matrix molecules in a rodent model of diabetic retinopathy. Mol
Vis. 18:874–886. 2012.PubMed/NCBI
|
|
29
|
Austin BA, Liu B, Li Z and Nussenblatt RB:
Biologically active fibronectin fragments stimulate release of
MCP-1 and catabolic cytokines from murine retinal pigment
epithelium. Invest Ophthalmol Vis Sci. 50:2896–2902. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kothary PC, Badhwar J, Weng C and Del
Monte MA: Impaired intracellular signaling may allow upregulation
of CTGF-synthesis and secondary peri-retinal fibrosis in human
retinal pigment epithelial cells from patients with age-related
macular degeneration. Adv Exp Med Biol. 664:419–428. 2010.
View Article : Google Scholar
|
|
31
|
Tikellis C, Cooper ME, Twigg SM, Burns WC
and Tolcos M: Connective tissue growth factor is upregulated in the
diabetic retina: Amelioration by angiotensin-converting enzyme
inhibition. Endocrinology. 145:860–866. 2004. View Article : Google Scholar
|
|
32
|
Kuiper EJ, Witmer AN, Klaassen I, Oliver
N, Goldschmeding R and Schlingemann RO: Differential expression of
connective tissue growth factor in microglia and pericytes in the
human diabetic retina. Br J Ophthalmol. 88:1082–1087. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cherian S and Roy S, Pinheiro A and Roy S:
Tight glycemic control regulates fibronectin expression and
basement membrane thickening in retinal and glomerular capillaries
of diabetic rats. Invest Ophthalmol Vis Sci. 50:943–949. 2009.
View Article : Google Scholar
|
|
34
|
Roy S, Cagliero E and Lorenzi M:
Fibronectin overexpression in retinal microvessels of patients with
diabetes. Invest Ophthalmol Vis Sci. 37:258–266. 1996.PubMed/NCBI
|
|
35
|
Hughes JM, Kuiper EJ, Klaassen I, Canning
P, Stitt AW, Van Bezu J, Schalkwijk CG, Van Noorden CJ and
Schlingemann RO: Advanced glycation end products cause increased
CCN family and extracellular matrix gene expression in the diabetic
rodent retina. Diabetologia. 50:1089–1098. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martinez G and de Iongh RU: The lens
epithelium in ocular health and disease. Int J Biochem Cell Biol.
42:1945–1963. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Neuzillet C, Tijeras-Raballand A, de
Mestier L, Cros J, Faivre S and Raymond E: MEK in cancer and cancer
therapy. Pharmacol Ther. 141:160–171. 2014. View Article : Google Scholar
|
|
38
|
Yuan L, Hu J, Luo Y, Liu Q, Li T, Parish
CR, Freeman C, Zhu X, Ma W, Hu X, et al: Upregulation of heparanase
in high-glucose-treated endothelial cells promotes endothelial cell
migration and proliferation and correlates with Akt and
extracellular-signal-regulated kinase phosphorylation. Mol Vis.
18:1684–1695. 2012.PubMed/NCBI
|
|
39
|
Qin D, Zheng XX and Jiang YR: Apelin-13
induces proliferation, migration, and collagen I mRNA expression in
human RPE cells via PI3K/Akt and MEK/Erk signaling pathways. Mol
Vis. 19:2227–2236. 2013.PubMed/NCBI
|
|
40
|
Sasore T, Reynolds AL and Kennedy BN:
Targeting the PI3K-Akt-mTOR pathway in ocular neovascularization.
Adv Exp Med Biol. 801:805–811. 2014. View Article : Google Scholar
|
|
41
|
Yuan Z, Feng W, Hong J, Zheng Q, Shuai J
and Ge Y: p38MAPK and ERK promote nitric oxide production in
cultured human retinal pigmented epithelial cells induced by high
concentration glucose. Nitric Oxide. 20:9–15. 2009. View Article : Google Scholar
|
|
42
|
Radeke MJ, Radeke CM, Shih YH, Hu J, Bok
D, Johnson LV and Coffey PJ: Restoration of mesenchymal retinal
pigmented epithelial cells by TGFβ pathway inhibitors: Implications
for age-related macular degeneration. Genome Med. 7:582015.
View Article : Google Scholar
|
|
43
|
Simó R, Villarroel M, Corraliza L,
Hernández C and Garcia-Ramírez M: The retinal pigment epithelium:
Something more than a constituent of the blood-retinal barrier -
implications for the pathogenesis of diabetic retinopathy. J Biomed
Biotechnol. 2010:1907242010. View Article : Google Scholar
|
|
44
|
Dahrouj M, Desjardins DM, Liu Y, Crosson
CE and Ablonczy Z: Receptor mediated disruption of retinal pigment
epithelium function in acute glycated-albumin exposure. Exp Eye
Res. 137:50–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cao Y, Feng B, Chen S, Chu Y and
Chakrabarti S: Mechanisms of endothelial to mesenchymal transition
in the retina in diabetes. Invest Ophthalmol Vis Sci. 55:7321–7331.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sonnylal S, Xu S, Jones H, Tam A, Sreeram
VR, Ponticos M, Norman J, Agrawal P, Abraham D and de Crombrugghe
B: Connective tissue growth factor causes EMT-like cell fate
changes in vivo and in vitro. J Cell Sci. 126:2164–2175. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang Z, Sun L, Nie H, Liu H, Liu G and
Guan G: Connective tissue growth factor induces tubular epithelial
to mesenchymal transition through the activation of canonical Wnt
signaling in vitro. Ren Fail. 37:129–135. 2015. View Article : Google Scholar
|
|
48
|
Qiao M, Sheng S and Pardee AB: Metastasis
and AKT activation. Cell Cycle. 7:2991–2996. 2008. View Article : Google Scholar : PubMed/NCBI
|