Introduction
Neonatal hypoxia/ischemia and subsequent brain
damage continue to be an alarming socio-sanitary problem, being
considered the single-most important cause of acute mortality and
chronic disability in newborns worldwide (1–3).
Although improved obstetric and neonatal intensive care practices
have led to increased survival, infants born very preterm are prone
to disorders of cerebral development, including impaired cognition
and behavior, epilepsy and cerebral palsy (4–6).
Similarly, infants who suffer brain injury from hypoxia/ischemia
during critical developmental periods of cerebral circuit formation
are also at an increased risk of developing seizures,
neuropsychiatric conditions and cognitive disorders (7). The severity of neonatal
encephalopathy depends on the intensity, duration and location of
the insult (8,9). Approximately 15–20% of affected
newborns will die in the post-natal period and an additional 25%
will develop severe and permanent neuropsychological sequelae
(10). Only are a small
percentage of infants with severe injury survive without any
handicaps (10,11).
Ciliary neurotrophic factor (CNTF) is a member of
NTF family originally isolated from chick embryo ciliary neurons,
which: i) promote survival and/or differentiation in many cell
types; and ii) have been demonstrated to have therapeutic potential
in neurodegenerative diseases and the injured central nervous
system (CNS) (12). CNTF exerts
its biological functions by binding to high or low affinity
receptor complexes consisting of CNTFR·gp130·LIFR or
IL-6R·gp130·LIFR, respectively (13). Recent data indicate that distinct
intracellular signaling pathways mediate diverse neuroprotective
processes in response to CNTF. There is evidence to indicate that
Janus kinase 2 (JAK2)/signal transducer and activator of
transcription 3 (STAT3), mitogen-activated protein kinase
(MAPK)-extracellular signal-regulated protein kinase (ERK)1/2, as
well as phosphatidylinositol-3-kinase (PI3K)/Akt, play important
roles in promoting neuronal survival and process the outgrowth
response to CNTF (14–17). STAT3 is known to modulate injury
following an imbalance between pro- and anti-inflammatory cytokines
in peripheral and CNS injury, rendering it a potential molecule for
study. It has been demonstrated that CNTF plays a role in neural
stem/progenitor cell (NSP) cell responses to hypoxia/ischemia
(18). As a major transducer of
CNTF-mediated neuroprotective activity, the activation of the
JAK2/STAT3 axis by CNTF has been demonstrated to be responsible for
the neuroprotective effects against the pathogenesis of Alzheimer's
disease (AD) (14). It also
points towards a significant role of STAT3 signaling following
micro- and astrogliosis in the pathophysiology of neonatal
hypoxia-related brain injury (19). However, whether the JAK/STAT3 axis
mediated by CNTF is responsible for the neuroprotective effects in
hypoxic injury remains unknown.
The aim of this study was to investigate whether
CNTF plays its neuroprotective role following hypoxic injury
through the activation of STAT3 signaling. Firstly, to determine
whether CNTF exerts its effects via STAT3 following hypoxia,
cultured neurons from the cerebral cortex of mice were prepared and
a neuronal model of hypoxia was established. The neurons exposed to
hypoxia were pre-treated with CNTF and transfected with small
interference RNA targeting STAT3 (STAT3 siRNA) using polybrene, or
with STAT3Tyr705 mutant or STAT3Ser727 mutant
using an electroporation system. The survival, proliferation, and
neurite outgrowth of neurons subjected to different treatments were
also determined. Reverse transcription-quantitive PCR (RT-qPCR) and
western blot analysis were employed to examine the expression
levels of STAT3, p-STAT3Tyr705 and
p-STAT3Ser727 following treatment with CNTF and other
treatments.
Materials and methods
Ethics statement
Animal use and care were carried out in accordance
with the animal care guidelines, which conformed to the Guide for
the Care and Use of Laboratory Animals published by the US National
Institutes of Health (NIH Publication no. 85-23, revised 1996). The
Ethics Committee of Harbin Medical University specifically approved
this study (Permit number: 2014ME1028). All efforts were made to
minimize animal suffering.
Neuronal culture
Pregnant mice were housed with free access to food
and water and exposed to a 12-h light/dark cycle at the Animal
Central of Harbin Medical University, Harbin, China. A total of 20
neonatal C57BL/6J mice (during postnatal 24 h), which were
purchased from Harbin Medical University, were then employed in the
present study for cell culture. The mice were sacrificed by
decapitatation and disinfected in 75% ethanol. The hippocampus was
then completely removed following craniotomy. The hippocampus was
dissected into slices at a volume of 1 mm3 using an
anatomic microscope in PBS and then digested for 30 min at 37°C
incubator with 2 mg/ml papain (Roche Diagnostics GmbH, Mannheim,
Germany) containing 2 µl/ml DNAase. Termination of the
digestion was performed by the addition of an equal amount of DMEM
including 10% FBS and 1% penicillin-streptomycin solution. The cell
suspension was centrifuged at 1,000 rpm for 10 min at 4°C. After
discarding the supernatant, the cells were re-suspended in the same
medium by gently pipetting up and down and seeded on a 24-well
plate at a density of 2×105 cells/ml. The medium was
replaced with neurobasal medium (Gibco-BRL, Grand Island, NY, USA)
supplemented with 2% B27 and 1% penicillin-streptomycin
solution.
Neuronal model of hypoxia
Hypoxia was achieved by placing the neurons in a
modular incubator chamber (Billups-Rothenberg, Del Mar, CA, USA)
that was then flushed for 5 min (20 l/min) with a gas mixture of
90% N2, 5% CO2 and 5% O2. These
conditions are reported by the manufacturer to render the hypoxia
chamber completely purged.
Experimental grouping
The cultured neurons were divided into different
groups as follows: i) the normal untreated neurons; ii) the hypoxia
group (the neurons were exposed to hypoxia as described above);
iii) the hypoxia + CNTF-treated group [CNTF (recombinant human
CNTF; Novoprotein Scientific, Inc., Summit, NJ, USA) was added to
the culture exposed to hypoxia at a concentration of 100 ng/ml
(20)]; the iv) si-STAT3 group
[neurons were exposed to hypoxia, treated with CNTF and transfected
with STAT3 siRNA (hypoxia + CNTF + STAT3 siRNA)]; v) the si-STAT3
control group [neurons were exposed to hypoxia, treated with CNTF
and transfected with control siRNA (hypoxia + CNTF + control STAT3
siRNA; STAT3-si-Non)]; vi) the STAT3Tyr705 mutant group
[neurons were exposed to hypoxia, treated with CNTF and transfected
with STAT3 Y705F mutant (STAT3Tyr705 mutant) (hypoxia +
CNTF + STAT3Tyr705 mutant)]; vii) the
STAT3Ser727 mutant group [neurons were exposed to
hypoxia, treated with CNTF and transfected with STAT3 S727A mutant
(STAT3Ser727 mutant) (hypoxia + CNTF +
STAT3Ser727 mutant)]; and viii) STAT3 mutant control
group [neurons were exposed to hypoxia, treated with CNTF and
transfecfed with the blank pcDNA3 vector (hypoxia + CNTF + blank
vector pcDNA3)]. The STAT3 Y705F and S727A mutants, and pcDNA3
vector were purchased from Shenggong Biotechnology, Co. (Shanghai,
China).
STAT3 gene knockdown
According to the CDS of STAT3 recorded in
Nucleotide, we predesigned siRNA targeting the mouse STAT3 gene
(GenBank accession no. U06922.1) using the online system
RNAiDesigner (http://RNAiDesigner.invitrogen.com). The siRNA
sequences targeting STAT3 were as follows: si-1,
5′-CCACGTTGGTGTTTCATAA-3′; si-2, 5′-GGGTGAAATTGACCAGCAA-3′; si-3,
5′-GCAGATG TTGGAGCAGCAT-3′; and si-4, 5′-CCAGATGCGGAGAAGATT-3′. A
scrambled non-target siRNA was also used as a control
(STAT3-si-Non). The lentivirus was packaged in PC12 cells
(purchased from the Cell Bank of the Chinese Academy of Sciences,
Shanghai, China) using Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) and viral titers were determined.
The interference efficiency of si-1–4 targeting STAT3 in the PC12
cells was determined by reverse transcription-quantitative PCR
(RT-qPCR) and western blot analysis. The target siRNA-2 was
selected for further investigation as it had the highest
interference efficiency. The neuronal cells exposed to hypoxia were
then infected with 1×106 recombinant
lentivirus-transducing units containing the target siRNA or
non-target siRNA in the presence of 6 µg/ml polybrene
(Sigma-Aldrich, St. Louis, MO, USA), respectively.
RT-qPCR
The expression levels of STAT3 in the neuronal cells
in each group were detected by RT-qPCR. RNA was extracted from the
cells using TRIzol reagent (Invitrogen Life Technologies). Total
RNA (2 µg) was used for cDNA synthesis using moloney murine
leukemia virus reverse transcriptase (MMLV-RT; Takara Bio, Inc.,
Otsu, Japan), and the reverse transcript was used as the template
for RT-qPCR using a Tower qRT-PCR system (Analytik Jena, Jena,
Germany). qPCR was conducted using 2X Mix SYBR-Green I (Biosea
Biotechnology, Co., Ltd., Beijing, China) (10 µl), primer
(0.25 µl, 10 pmol/l), template DNA (1 µl) and sterile
water (8.5 µl). All PCR reactions included initial
denaturation and multiple cycles at (95°C for 3 min); 37 cycles at
95°C for 10 sec, 54°C for 10 sec, and 72°C for 30 sec; followed by
95°C for 10 sec, 65°C for 5 sec and a final 95°C for 15 sec. The
primer for each gene was synthesized by Invitrogen Life
Technologies. The qPCR primers used to quantify GAPDH expression
were s follows: forward, 5′-CGAGATCCCTCCAAAATCAA-3′ and reverse,
5′-TTCACACCCATGACGAACAT-3′; and for STAT3 forward,
5′-TCAGTGGAACCAGCTGCA-3′ and reverse, 5′-AGAATCAAGCAGTTTCTG-3′. The
expression of STAT3 was normalized to endogenous GAPDH expression.
The Ct value was defined as the number of cycles required for the
fluorescent signal to cross the threshold (i.e., exceed the
background level). The correlation between the Ct value and the DNA
copy number was calculated as follows: Ct = −3.347424 × log copy
number + 35.885406, as previously described (21).
Transfection of neurons with
STAT3Tyr705 mutant and STAT3Ser727
mutant
The Neon™ electroporation transfection system
(Invitrogen, Eugene, OR, USA) was used to transfect the STAT3
mutant-pcDNA vector into the cultured neurons. Approximately 10
million cells were harvested, pelleted at 800 rpm and washed with
1X Dulbecco's phosphate-buffered saline (DPBS containing NaCl,
Na2PO4 and KCl, but not Ca2+ and
Mg2+) prior to re-suspending in resuspension buffer R,
provided by the manufacturer. STAT3 mutants (100 nM) were then
mixed with the suspended neurons and loaded into a 100 µl
Neon tip. The neurons were then transfected with the STAT3
mutant-pcDNA vector via the Neon electroporation system at 1150
V/30 msec for 2 pulses. The cells were then cultured at 37°C/5%
CO2/95% humidity for 48 h prior to harvesting and
further analysis.
Western blot analysis
The STAT3 and p-STAT3 levels in the different groups
were determined by western blot analysis. Briefly, the cells were
lysed for 30 min in Cytobuster protein extraction buffer (Novagen,
Madison, WI, USA) and centrifuged at 12,000 rpm. The supernatant
was collected, total protein was measured, and 50 µg were
used for 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). The protein was then transferred to a
nitrocellulose (NC) membrane and sealed with Tris-buffered saline
and Tween-20 (TBST) containing 5% non-fat milk powder. The membrane
was subsequently incubated with goat anti-rat STAT3 (1:1,000) and
rabbit anti-p-STAT3 (Tyr705, rabbit mAb no. 9145 and Ser727, mouse
mAb no. 9136) antibodies (both from Cell Signaling Technology,
Inc., Danvers, MA, USA), and mouse anti-rat GAPDH (1:500, sc-81545;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) antibody at
4°C overnight. After washing in TBST, the membrane was incubated
with horseradish peroxidase (HRP)-conjugated secondary antibody
(1:2,000; A0208; Beyotime Institute of Biotechnology, Shanghai,
China) at 25°C, and the protein quantity was determined using
electrochemiluminescence (ECL) technique (Bestbio Biotechnology,
Co., Ltd., Shanghai, China). The results were photographed using
the JS gel imaging system and the grey density was calculated using
SensiAnsys software (both from Shanghai Peiqing Science and
Technology, Co., Ltd., Shanghai, China).
Determination of neuronal survival
Cell survival was evaluated by means of the trypan
blue staining. In brief, cell numbers were determined by dispersing
the neurons in trypsin and by counting using a coulter counter
(model Z; Beckman Coulter, Palo Alto, CA, USA). These experiments
were performed in triplicate in 24-well plates.
Assay for neurite outgrowth
For the evaluation of neurite outgrowth, thye cells
treated as indicated and observed under a phase-contrast microscope
(Leica DMi8; Leica Microsystems, Wetzlar, Germany) and the cell
bodies and neurites were counted. The ratio between neurites and
cell bodies was calculated yielding the average of neurites per
neuron.
Statistical analysis
Data are presented as the means ± SD. The
comparisons of the mRNA levels and protein concentrations in the
different groups were analyzed by one-way analysis of variance
(ANOVA). Five independent experiments were performed. Statistical
analyses were performed using GraphPad Prism, version 5.0 software
(GraphPad Software, Inc., San Diego, CA, USA). A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
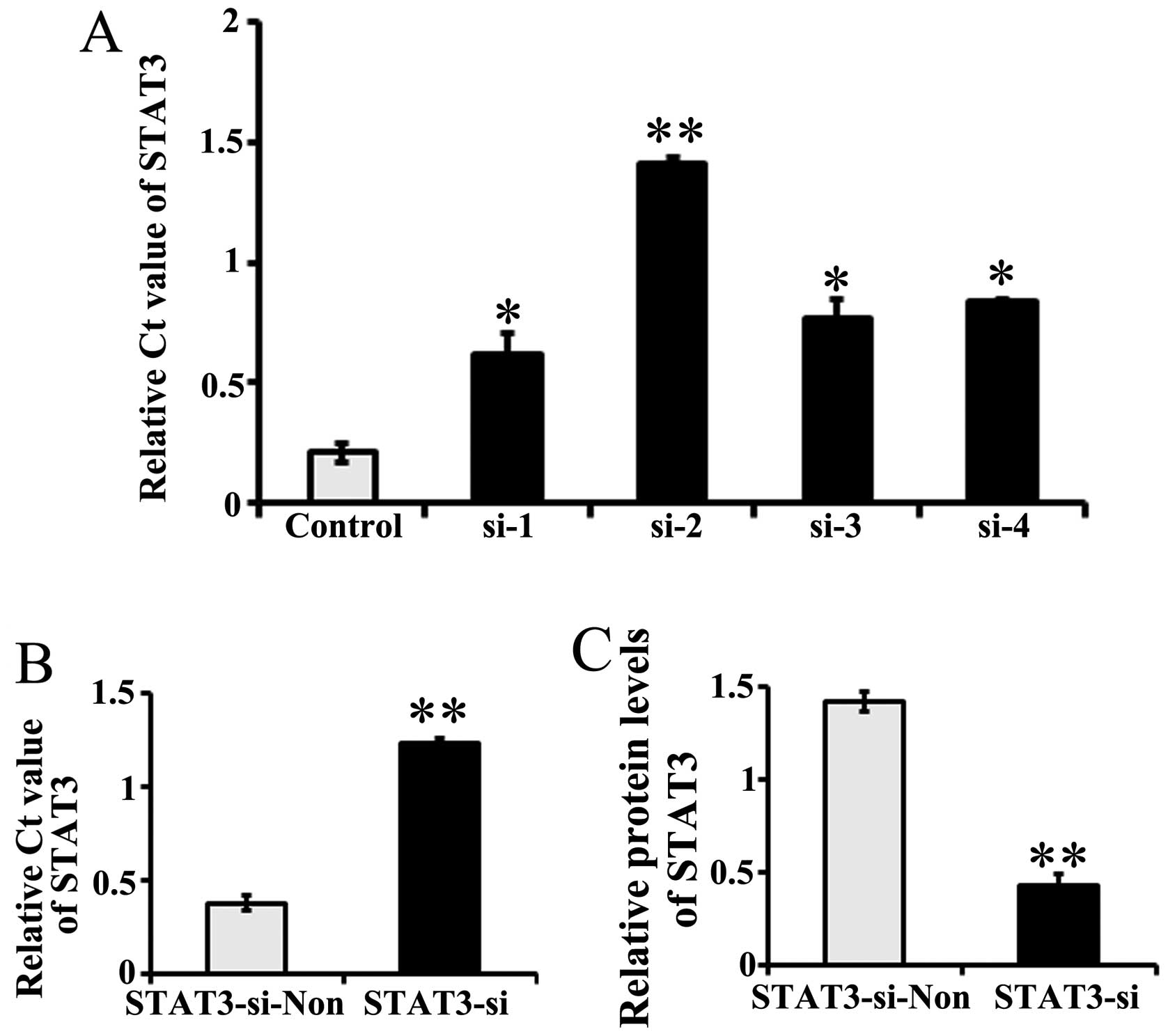
Transfection with specific siRNA
targeting STAT3 suppresses STAT3 expression
The stable transfection of 4 siRNAs targeting STAT3
(si-1, si-2, si-3 and si-4) in PC12 cells resulted in the
inhibition of STAT3 expression by >80% (Fig. 1A). Considering the highest
expression inhibition rates observed for STAT3, si-2 was selected
as the target siRNA for use in the following experiments.
The cultured neurons were then stably transfected
with STAT3 si-2 (named STAT3-si). Negative control neurons were
transfected with non-target siRNA (recorded as STAT3-si-Non). The
STAT3 mRNA levels, as detected by RT-qPCR, were significantly lower
(as indicated by the higher Ct value) in the STAT3
siRNA-transfected neurons than in the control siRNA-transfected
ones (P=0.00013, P<0.01; Fig.
1B). Western blotting found that the level of immunoreactive
protein was significantly downregulated in STAT3-si-2 transfected
neurons relative to the controls (P=0.00002, P<0.01; Fig. 1C).
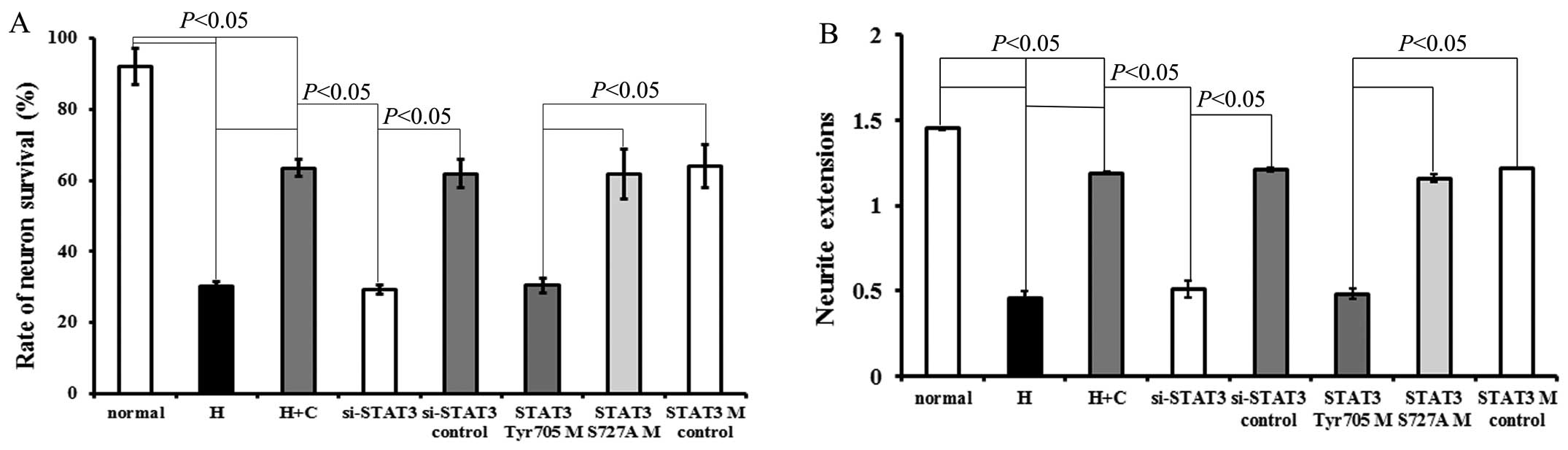
Effects of CNTF on survival and neurite
growth of neurons exposed to hypoxia
Exposure to hypoxia decreased the survival rate (vs.
normal, P=0.00012, P<0.05; Fig.
2A) and neurite length of neurons (vs. normal, P=0.00012,
P<0.05; Fig. 2B). The results
revealed that CNTF had a significant protective effect on neuronal
survival under hypoxic conditions (Fig. 2A). In addition, the effects of
CNTF on neurite growth were investigated. Following culture with
CNTF for 48 h, the hypoxia-exposed neurons (hypoxia + CNTF)
displayed outgrowth in the form of neurite extensions, when
compared with that of the hypoxia-exposed neurons not treated with
CNTF (P=0.0003, P<0.05; Figs.
2B and 3).
Transfection of the hypoxia-exposed neurons with
STAT3 siRNA or STAT3Tyr705 mutant neutralized the
protective effects induced by treatment with CNTF (compared with
the si-STAT3 control group, P=0.0015, P<0.05; or compared with
the STAT3 mutant control group, P=0.00025, P<0.05) (Figs. 2 and 3).
There was no significant difference between the
STAT3 mutant control group and the si-STAT3 control group
(P>0.05). Neither the STAT3 mutant control group nor the
si-STAT3 control group exhibited a significant difference compared
with the hypoxia + CNTF group (P>0.05). Transfection with
STAT3Ser727 mutant did not exert any exert any
significant effecft on survival or neurite outgrowth compared to
the hypoxia-exposed neurons or to the neurons transfected with the
control siRNA or mutant (Fig. 2
and 3). Thus, our results suggest
that CNTF protects neurons from hypoxic injury through
STAT3Tyr705, but not through STAT3Ser727.
CNTF exerts protective effects against
hypoxic injury to neurons through STAT3
As previously demonstrated, STAT3pTyr705
is an indicator of its transcriptional activation (22–24). In this study, to gain further
insight into the association between STAT3 activation and CNTF
treatment, recombinant human CNTF was used to treat neurons and the
activation state of STAT3 (STAT3pTyr705) was monitored
by western blot analysis. The level of STAT3pSer727 was
also detected.
Treatment with CNTF induced a significant increase
in the levels of STAT3 and STAT3pTyr705, and in the
STAT3pTyr705/STAT3 ratio, but not in the levels of
STAT3pSer727 in the cerebral cortex neurons under
hypoxic conditions (hypoxia + CNTF group vs. hypoxia group,
P=0.00017, P<0.05) (Figs. 4
and 5).
 | Figure 4Ciliary neurotrophic factor (CNTF)
exerts its effects via signal transducer and activator of
transcription factor 3 (STAT3). (A) Expression of STAT3,
STAT3pTyr705 and STAT3pSer727 in the
different groups detected by western blot analysis. Lane 1, normal
group; lane 2, hypoxia group; lane 3, hypoxia + CNTF group; lane 4,
si-STAT3 group; lane 5, si-STAT3 control group; lane 6,
STAT3Tyr705 mutant group; lane 7, STAT3Ser727
mutant group; and lane 8, STAT3 mutant control group. (B-D)
Quantitative analysis of the relative protein levels of STAT3,
STAT3pTyr705 and STAT3pSer727 in the
different groups determined by western blot analysis. GAPDH was
used as an internal control. Five independent experiments were
performed. The values plotted are the means ± SD. |
Moreover, the blocking of STAT3 signaling by STAT3
siRNA in the neurons exposed to hypoxia and treated with CNTF
(si-STAT3 group) prevented the CNTF-induced increase in the levels
of STAT3pTyr705 (Fig.
4). Conversely, transfection with STAT3Tyr705 mutant
suppressed STAT3 signaling which was activated in the neurons
treated with CNTF (STAT3Tyr705 mutant group vs. STAT3
mutant control group, P=0.0006, P<0.05). However, the
above-mentioned suppressive effects were not observed in the
neurons transfected with STAT3pSer727 mutant (Fig. 4).
There was no significant difference between the
STAT3 mutant control group and the si-STAT3 control group
(P>0.05). Neither the STAT3 mutant control group nor the
si-STAT3 control group exhibited a significant difference compared
with the hypoxia + CNTF group (P>0.05).
Discussion
The present data revealed that treatment with CNTF:
i) protected neurons from hypoxic injury by promoting survival and
neurite growth; ii) induced a significant increase in the levels of
STAT3 and STAT3pTyr705, and in the
STAT3pTyr705/STAT3 ratio, but not in the levels of
STAT3pSer727 in hypoxic cerebral cortex neurons. The
blocking of STAT3 signaling using STAT3 siRNA prevented the
CNTF-induced increase in the levels of STAT3pTyr705.
Transfection of the hypoxic neurons treated with CNTF with STAT3
siRNA or STAT3Tyr705 mutant neutralized the protective
effects exerted by CNTF. These results demonstrated that CNTF
exerted neuroprotective effects against hypoxic injury through the
activation of STAT3pTyr705.
Our data demonstrated that treatment with CNTF
protected the neurons from hypoxic injury by promoting survival
rate and neurite growth. Hypoxia-associated brain damage results in
immediate neuronal injury and in the exhaustion of cellular energy
stores, which lead to a multi-faceted cascade of biochemical
events, biological injury and neuronal death (25,26). NTFs are essential proteins for the
maintenance and survival of neurons in both developing and mature
nervous systems (27,28). Currently, CNTF is the only known
factor which shows direct trophic effects on muscle and nerve
system, and may have therapeutic effects on motor neuron diseases,
nerve damage and muscular atrophy (29). Our data confirmed the fact that
CNTF is an important neurocytokine for the survival and neurite
growth of neurons following hypoxic injury.
Further experiments revealed that CNTF induced the
phosphorylation of STAT3 in neurons under hypoxic conditions;
however, the promoting effects of CNTF on survival and neurite
growth of neuron was attenuated by transfection with STAT3 siRNA or
STAT3Tyr705 mutant, but not by transfection with
STAT3Ser727 mutant. These data demonstrated that CNTF
exerted neuroprotective effects under hypoxic conditions through
the activation of STAT3/STAT3pTyr705. It has been
demonstrated that the cellular response to CNTF is mediated by a
receptor complex consisting of the signal transducers glycoprotein
130 (gp130) and LIF receptor β (β-receptor components) and CNTFRα
(30–32). The dimerization of the β-receptor
components results in the phosphorylation of JAK (33) followed by signal transduction,
including the STAT proteins (32,33). The JAK/STAT pathway is considered
to be the primary cytokine signaling pathway among other pathways,
such as the Ras-mitogen-activated protein (Ras-MAP) kinase pathway,
including ERK1 and ERK2 (MAPK/ERK kinase system) and the cell
line-dependent PI3K pathway (PI3K/Akt system) (32,34). It has been established that the
JAK2/STAT3 pathway is mainly involved inthe survival of neurons in
response to CNTF (35,36). It has also been demonstrated that
the STAT3 and PI3K/Akt pathways, but not the MEK/MAPK signaling
play a major role in mediating the survival response of neurons by
cytokines (37) with STAT3,
specifically activated by CNTF, leading to increased neuronal
survival (38). Phosphorylated
STAT3 dimerizes and translocates to the nucleus to regulate target
gene transcription (39). In
addition, CNTF can also trigger and activate the PI3K/Akt or
MEK/ERK pathways, either concomitantly or independently of the
JAK2/STAT3 signaling pathway (40,41). Moreover, STAT3 is phosphorylated
at Tyr705 upon the activation of cytokine and growth factor
receptors, resulting in its homodimerization and nuclear
translocation to activate the transcription of downstream
responsive genes (22,24). Once activated, STAT3 mediates
multiple biological functions, including the promotion of cell
proliferation, angiogenesis and metastasis, the inhibition of
differentiation and antitumor immune responses (42–44). Given these, the present study
indicated that the protective roles of CNTF were dependent on
STAT3/STAT3pTyr705-mediated neuronal survival and
proliferation under hypoxic conditions.
In conclusion, the findings of our study
demonstrated that the treatment of neurons exposed to hypoxia with
CNTF: i) protected cultured neurons from hypoxic injury by
promotion survival and neurite growth; ii) induced the
phosphorylation of STAT3. However, the promoting effects of CNTF on
survival and neurite growth of neurons were suppressed by
transfection with STAT3 siRNA or STAT3Tyr705 mutant, but
not by transfection with STAT3Ser727 mutant. Taken
together, the findings of the present study demonstrate that
CNTF-mediated neuron survival and proliferation under hypoxic
conditions is mediated by the activation of
STAT3/STAT3pTyr705.
Acknowledgments
This study was supported by the Health and Family
Planning Commission of Heilongjiang Province (no. 2013097). We
would like to thank the Labreal Bioscience and Technology, Ltd.,
Co., Kunming, China for their valuable contribution to parts of the
experimental design.
References
|
1
|
du Plessis AJ and Volpe JJ: Perinatal
brain injury in the preterm and term newborn. Curr Opin Neurol.
15:151–157. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Azra Haider B and Bhutta ZA: Birth
asphyxia in developing countries: current status and public health
implications. Curr Probl Pediatr Adolesc Health Care. 36:178–188.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang H, Lei JJ and Zhang YH: Protective
effect of topiramate on hypoxic-ischemic brain injury in neonatal
rat. Asian Pac J Trop Med. 7:496–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marín-Padilla M: Perinatal brain damage,
cortical reorganization (acquired cortical dysplasias), and
epilepsy. Adv Neurol. 84:153–172. 2000.PubMed/NCBI
|
|
5
|
Robinson S: Systemic prenatal insults
disrupt telencephalon development: implications for potential
interventions. Epilepsy Behav. 7:345–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Volpe JJ: Brain injury in premature
infants: a complex amalgam of destructive and developmental
disturbances. Lancet Neurol. 8:110–124. 2009. View Article : Google Scholar :
|
|
7
|
Martinez-Biarge M, Diez-Sebastian J,
Rutherford MA and Cowan FM: Outcomes after central grey matter
injury in term perinatal hypoxic-ischaemic encephalopathy. Early
Hum Dev. 86:675–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferriero DM: Neonatal brain injury. N Engl
J Med. 351:1985–1995. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Juul SE and Ferriero DM: Pharmacologic
neuroprotective strategies in neonatal brain injury. Clin
Perinatol. 41:119–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levene ML, Kornberg J and Williams TH: The
incidence and severity of post-asphyxial encephalopathy in
full-term infants. Early Hum Dev. 11:21–26. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cerio FG, Lara-Celador I, Álvarez A and
Hilario E: Neuroprotective therapies after perinatal
hypoxic-ischemic brain injury. Brain Sci. 3:191–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skaper SD, Selak I, Manthorpe M and Varon
S: Chemically defined requirements for the survival of cultured
8-day chick embryo ciliary ganglion neurons. Brain Res.
302:281–290. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wagener EM, Aurich M, Aparicio-Siegmund S,
Floss DM, Garbers C, Breusing K, Rabe B, Schwanbeck R, Grötzinger
J, Rose-John S and Scheller J: The amino acid exchange R28E in
ciliary neurotrophic factor (CNTF) abrogates interleukin-6
receptor-dependent but retains CNTF receptor-dependent signaling
via glycoprotein 130 (gp130)/leukemia inhibitory factor receptor
(LIFR). J Biol Chem. 289:18442–18450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang K, Zhou F, Zhu X, Zhang K, Huang B
and Zhu L and Zhu L: Neuroprotective properties of ciliary
neurotrophic factor on retinoic acid (RA)-predifferentiated SH-SY5Y
neuroblastoma cells. Folia Neuropathol. 52:121–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Askvig JM and Watt JA: The MAPK and PI3K
pathways mediate CNTF-induced neuronal survival and process
outgrowth in hypothalamic organotypic cultures. J Cell Commun
Signal. 9:217–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Q, Chen P, Di G, Zhang Y, Wang Y, Qi
X, Duan H and Xie L: Ciliary neurotrophic factor promotes the
activation of corneal epithelial stem/progenitor cells and
accelerates corneal epithelial wound healing. Stem Cells.
33:1566–1576. 2015. View Article : Google Scholar
|
|
17
|
Severi I, Senzacqua M, Mondini E, Fazioli
F, Cinti S and Giordano A: Activation of transcription factors
STAT1 and STAT5 in the mouse median eminence after systemic ciliary
neurotrophic factor administration. Brain Res. 1622:217–229. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Covey MV and Levison SW: Leukemia
inhibitory factor participates in the expansion of neural
stem/progenitors after perinatal hypoxia/ischemia. Neuroscience.
148:501–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shrivastava K, Llovera G, Recasens M,
Chertoff M, Giménez-Llort L, Gonzalez B and Acarin L: Temporal
expression of cytokines and signal transducer and activator of
transcription factor 3 activation after neonatal hypoxia/ischemia
in mice. Dev Neurosci. 35:212–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwieger J, Warnecke A, Lenarz T, Esser
KH and Scheper V: Neuronal survival, morphology and outgrowth of
spiral ganglion neurons using a defined growth factor combination.
PLoS One. 10:e01336802015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin JC, Xing YL, Xu WM, Li M, Bo P, Niu YY
and Zhang CR: Evaluation of galactomannan enzyme immunoassay and
quantitative real-time PCR for the diagnosis of invasive pulmonary
aspergillosis in a rat model. J Microbiol Biotechnol. 24:1044–1050.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quesnelle KM, Boehm AL and Grandis JR:
STAT-mediated EGFR signaling in cancer. J Cell Biochem.
102:311–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frank DA: STAT3 as a central mediator of
neoplastic cellular transformation. Cancer Lett. 251:199–210. 2007.
View Article : Google Scholar
|
|
24
|
Germain D and Frank DA: Targeting the
cytoplasmic and nuclear functions of signal transducers and
activators of transcription 3 for cancer therapy. Clin Cancer Res.
13:5665–5669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Northington FJ, Chavez-Valdez R and Martin
LJ: Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol.
69:743–758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Zhang J, Chai S and Wang X:
Progesterone alleviates hypoxic-ischemic brain injury via the
Akt/GSK-3β signaling pathway. Exp Ther Med. 8:1241–1246.
2014.PubMed/NCBI
|
|
27
|
Linker R, Gold R and Luhder F: Function of
neurotrophic factors beyond the nervous system: inflammation and
autoimmune demyelination. Crit Rev Immunol. 29:43–68. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maisonpierre PC, Belluscio L, Squinto S,
Ip NY, Furth ME, Lindsay RM and Yancopoulos GD: Neurotrophin-3: a
neurotrophic factor related to NGF and BDNF. Science.
247:1446–1451. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davis S, Aldrich TH, Valenzuela DM, Wong
VV, Furth ME, Squinto SP and Yancopoulos GD: The receptor for
ciliary neurotrophic factor. Science. 253:59–63. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ip NY, Nye SH, Boulton TG, Davis S, Taga
T, Li Y, Birren SJ, Yasukawa K, Kishimoto T, Anderson DJ, et al:
CNTF and LIF act on neuronal cells via shared signaling pathways
that involve the IL-6 signal transducing receptor component gp130.
Cell. 69:1121–1132. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sleeman MW, Anderson KD, Lambert PD,
Yancopoulos GD and Wiegand SJ: The ciliary neurotrophic factor and
its receptor, CNTFR alpha. Pharm Acta Helv. 74:265–272. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turnley AM and Bartlett PF: Cytokines that
signal through the leukemia inhibitory factor receptor-beta complex
in the nervous system. J Neurochem. 74:889–899. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stahl N, Boulton TG, Farruggella T, Ip NY,
Davis S, Witthuhn BA, Quelle FW, Silvennoinen O, Barbieri G,
Pellegrini S, et al: Association and activation of Jak-Tyk kinases
by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 263:92–95.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boulton TG, Stahl N and Yancopoulos GD:
Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin
6/oncostatin M family of cytokines induces tyrosine phosphorylation
of a common set of proteins overlapping those induced by other
cytokines and growth factors. J Biol Chem. 269:11648–11655.
1994.PubMed/NCBI
|
|
35
|
Kaur N, Kim IJ, Higgins D and Halvorsen
SW: Induction of an interferon-γ Stat3 response in nerve cells by
pre-treatment with gp130 cytokines. J Neurochem. 87:437–447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaur N, Wohlhueter AL and Halvorsen SW:
Activation and inactivation of signal transducers and activators of
transcription by ciliary neurotrophic factor in neuroblastoma
cells. Cell Signal. 14:419–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alonzi T, Middleton G, Wyatt S, Buchman V,
Betz UA, Müller W, Musiani P, Poli V and Davies AM: Role of STAT3
and PI 3-kinase/Akt in mediating the survival actions of cytokines
on sensory neurons. Mol Cell Neurosci. 18:270–282. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schweizer U, Gunnersen J, Karch C, Wiese
S, Holtmann B, Takeda K, Akira S and Sendtner M: Conditional gene
ablation of Stat3 reveals differential signaling requirements for
survival of motoneurons during development and after nerve injury
in the adult. J Cell Biol. 156:287–297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: a
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sango K, Yanagisawa H, Komuta Y, Si Y and
Kawano H: Neuroprotective properties of ciliary neurotrophic factor
for cultured adult rat dorsal root ganglion neurons. Histochem Cell
Biol. 130:669–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rhee KD, Goureau O, Chen S and Yang XJ:
Cytokine-induced activation of signal transducer and activator of
transcription in photoreceptor precursors regulates rod
differentiation in the developing mouse retina. J Neurosci.
24:9779–9788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leeman RJ, Lui VW and Grandis JR: STAT3 as
a therapeutic target in head and neck cancer. Expert Opin Biol
Ther. 6:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Johnston PA and Grandis JR: STAT3
signaling: anticancer strategies and challenges. Mol Interv.
11:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Regis G, Pensa S, Boselli D, Novelli F and
Poli V: Ups and downs: the STAT1:STAT3 seesaw of interferon and
gp130 receptor signalling. Semin Cell Dev Biol. 19:351–359. 2008.
View Article : Google Scholar : PubMed/NCBI
|