Introduction
Endoprosthetic surgery in orthopedic and trauma
patients is known to be one of the most successful reconstructive
approaches, yet wear particle-induced osteolysis (PIO)-mediated by
ultrahigh molecular weight polyethylene (UHMWPE) particles remains
the major cause of implant loosening (1–3).
Depending on the composition of the bearing couple, macrophages
phagocytose released wear particles, which leads to an aseptic
inflammatory response and subsequently induces osteolysis in the
bone-implant interface (4–6).
In order to face the problem of PIO, different approaches have been
introduced.
In the field of biotribology, major improvements of
endoprosthetic components have been presented in the past as an
attempt to reduce wear and osteolysis. Thus, the development of new
implant designs, materials, sterilisation techniques, oxidation
resistance and articulating surface treatments have contributed to
a reduction of wear, delamination and structural fatigue (7). As shown in hip and knee simulator
studies, the development of improved inserts from conventional
UHMWPE towards highly cross-linked UHMWPE is associated with a
significant reduction of wear, while clinical investigations have
observed reduced osteolysis (8,9).
The incorporation of vitamin E (α-tocopherol) into
UHMWPE (VE-UHMWPE) either by diffusion following irradiation or
blending followed by irradiation is a novel modification made in an
aim to increase oxidation resistance and improve the mechanical
properties of UHMWPE (10,11).
As described previously, vitamin E has a positive impact in the
process of ageing of UHMWPE which is related to a chemical reaction
cascade between the macromolecules and oxygen. Irradiation
processes from sterilisation or crosslinking generate free bonds
(radicals) on the molecules which react with oxygen. One possible
outcome of these reaction cascades is chain scission of the
macromolecules, leading to mechanical property degradation. Vitamin
E can donate hydrogen to react with the free bonds and interrupt
this reaction cascade (12).
In a recent study, vitamin E supplementation was
shown to have neuroprotective characteristics mediated via
calcitonin gene-related peptide (CGRP) nerve fibers which can be of
interest (13). Neuropeptides,
such as α-CGRP have also been detected in the synovial fluid and
periarticular tissue of loosened implants which strengthens the
impact of the local neurogenic environment in the process of
osteolysis and may open new regenerative therapy approaches
(14,15). α-CGRP belongs to the calcitonin
(CT) peptide family which is a generated analog to calcitonin by
alternative splicing of the CALCA gene (16). Previous experiments using knockout
mouse models with isolated or combined deficiency of CT/α-CGRP have
provided further insight on the impact of the CT family peptides,
whereas α-CGRP turned out to play an important role in the process
of UHMWPE particle induced osteolysis (17–19).
Therefore, the aim of the present study was to
further clarify the impact of VE-UHMWPE wear particles on the
osseous microenvironment of an established murine calvaria model
and to identify the potential modulatory pathways.
Materials and methods
Animals
All experiments were performed and registered in
accordance to the local authorities (Reference nos.
55.2-1-54-2532-232-2013) similar to the NIH guidelines for the care
and use of laboratory animals (NIH Publication #85-23 Rev. 1985).
As described previously, a murine calvaria model of UHMWPE
particle-induced osteolysis was established using 54 male C57BL/6
mice provided by Charles River Laboratories, Inc. (Sulzfeld,
Germany) (17,18). The animals were delivered at the
age of 10 weeks and surgery was performed at the age of 12 weeks.
The mice were divided into 3 groups as follows: one group was
subjected to sham operation (SHAM group), the animals in the second
group were treated with UHMWPE particles and the animals in the
third group were treated with vitamin E-blended UHMWPE (VE-UHMWPE)
particles. Furthermore, the animals were divided into subgroups
according to the different durations of the experimental duration
(7, 14 and 28 days). Thus, each group consisted of 6 mice. During
the experimental period, food (V 1534-300; Ssniff Spezialdiäten
GmbH, Soest, Germany) and water was supplied ad libitum. The
animals were kept under specific pathogen-free (SPF) conditions.
Post-operative analgesia was achieved using metamizole dissolved in
the animals' drinking water (1.2 mg/ml). The animals were
sacrificed at the end of the experimental duration, depending on
the group.
Particles
The treated mice in the second group received
conventional UHWMPE particles, whereas the animals in the third
group received crosslinked VE-UHMWPE particles obtained from
Aesculap (Tuttlingen, Germany) and generated as previously
described (20). The equivalent
circle diameter (ECD) of the UHMWPE particles [detected according
to ASTM F 1877 (21)] was 1
μm, whereas 90% of the particles had a size <2 μm.
Testing for cytotoxicity and endotoxin purity was performed
according to the United States Pharmacopeial Convention (USP) given
an endotoxin level <20.0 USP endotoxins per device and no
cytotoxic reactivity.
Surgical procedure
All mice were anaesthetised by an intraperitoneal
injection of fentanyl [0.05 mg/kg body weight (BW)], midazolam (5
mg/kg BW) and medetomidine (0.5 mg/kg BW). After shaving the head
and sterile draping, a 1 cm skin incision over the calvarian
sagittal midline suture was made and a 1×1 cm area of the
periosteum was exposed and left intact as described previously
(19). According to the
experimental design, the animals in group A were subjected to sham
operation only (SHAM group), the animals in group B received ~30
μl (2×108 particles/1,000 μl) of dried
UHMWPE particles implanted periostally, and the animals in group C
received an equivalent amount of VE-UHMWPE wear particles. The
incision was closed using a 4-0 Ethilon skin suture (Ethicon,
Sommerville, NJ, USA). The animals were sacrificed in a
CO2 chamber following an experimental period of 7, 14 or
28 days according to their group.
Micro-computed tomography (μ-CT)
Microstructural differences of the different
calvaria were assessed using high-resolution μ-CT (SkyScan
1072; SkyScan, Aartselaar, Belgium). Following sacrifice, the mouse
skulls were separated using a sharp cutter at the neck for
decapitation and fixed in 4% paraformaldehyde for 24 h and stored
in 70% of ethanol thereafter. During scanning, the skulls were
placed in a tightly fitting rigid plastic tube inside the scanner's
chamber to avoid artifacts. With a resolution set at 19 μm
at a source voltage of 80 kV and 100 μA, scanning was
performed with rotation of the specimens in equiangular steps of
0.9°. Three-dimensional images were computed using the program
Cone-beam Reconstruction (SkyScan). Quantitative analysis was
performed using CT Analyser (CTAn; SkyScan) with a fixed volume of
interest (VOI) having set a rectangular region of interest (ROI) of
4×4 mm centering the midline suture in the 2D-reconstructed
cross-sectional slices. Within the VOI, the parameters of the
osseous micro-architecture (BV/TV) were obtained as previously
described (22).
Three-dimensional images of the calvaria were generated using CT
Vol (SkyScan).
Histological analysis
After μ-CT analysis, the decapitated skulls
were prepared for histological processing; therefore, the calvaria
were removed as an elliptical plate of bone defined by the foramen
magnum, auditory canals and orbits. The scalp was kept in order to
protect the calvaria in the operated area. The samples were then
dehydrated in a graded alcohol series and embedded in a plastic
embedding system based on methyl meth-acrylate (MMA)
'Technovit-9100' (Heraeus-Kulzer, Wehrheim, Germany). Following
complete polymerization, the samples were cut using a 'charly'
diamond saw (Walter Messner GmbH, Norderstedt, Germany) in the
coronar plane of the calvaria. Of these samples, 5-μm-thick
slices were cut using a Reichert-Jung rotary microtome
(Reichert-Jung, Nussloch, Germany).
Masson-Goldner trichrome and toluidine blue staining
were performed (Carl Roth GmbH, Karlsruhe, Germany) according to
standard protocols. Tartrate resistant acid phosphatase (TRAP)
staining was completed with a Napthol AS-BI phosphoric acid
solution (N-2250; Sigma-Aldrich Chemie GmbH, Munich, Germany) on
randomly selected slices from each group. Osteoclasts were detected
in a set field of view following digital photography at a
magnification of ×10 with the midline suture in its center (Axio
Observer Z.1 AX10, connected to ZEN Imaging Software; Carl Zeiss
Microscopy GmbH, Jena, Germany). Immunohistochemistry was performed
using canine anti-mouse monoclonal tumor necrosis factor
(TNF)-α-antibodies (Cat. no. 17590-1-AP; Acris Antibodies GmbH,
Herford, Germany) in a first step conjugated with anti-canine
peroxidase polymer in a second step for 30 min (Medac GmbH, Wedel,
Germany). Color development was performed using the chromogen
Bright DAB substrate kit (Immunologic, Duiven, The Netherlands) for
10 min. Counterstaining was performed with Mayer's hemalum solution
for 5 min (Leica, Wetzlar, Germany). For α-CGRP antibody staining,
canine anti-mouse polyclonal antibodies were used for 30 min (Cat.
no. A78-128; Antibodies-online GmbH, Aachen, Germany) conjugated
with anti-canine peroxidase polymer in a second step as stated
above (Cat. no. 414141F; Medac GmbH). Similarly, counter-staining
was performed with Mayer's hemalum solution (Leica). Dehydration
was performed using a graded alcohol series. To rule out a
potential cross-reactivity of the secondary antibody (α-CGRP/TNF-α)
negative controls were generated with a dilution medium. Similarly,
a positive control was prepared for each antibody using spleen
samples of mouse cadavers.
Serum analyses
To rule out bone metabolic disorders, retro-orbital
blood collection was performed at the beginning of the experimental
period and calcium, phosphate and alkaline phosphatase levels were
quantified. Prior to sacrifice, additional blood samples were taken
via puncture of the vena cava. The serum samples were centrifuged
at 8,000 × g for 10 min, aliquoted and frozen at −70°C.
Biomarker analysis was performed according to the
manufacturer's instructions. Thus, serum α-CGRP levels were
detected using a specific enzyme-linked immunosorbent assay (ELISA)
kit provided by MyBioSource Inc. (MBS 721907; San Diego, CA, USA).
As a marker of bone resorption, Dickkopf-1 (DKK-1) levels were
detected using a DKK-1 Quantikine ELISA kit (R&D Systems,
Minneapolis, MN, USA). The serum levels of osteoprotegerin (OPG),
which is a marker of bone formation, were detected using a
TNFRSF11B Quantikine ELISA kit (R&D Systems, Minneapolis, MN,
USA).
Statistical analysis
Data are reported as the means ± standard deviation
(SD). Normally distributed results were analysed by one-way
analysis of variance (ANOVA) to identify global significant
differences between groups, followed by post-hoc analysis via
Tukey's test or Games-Howell test. In the case of significant
variance of the normal distribution, non-parametric tests were
applied using the method of Kruskal-Wallis. For statistical
analysis, SPSS 22 (IBM Germany GmbH, Ehningen, Germany) was used
and the level of significance was set at p<0.05. Graphics were
created with GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla,
CA, USA).
Results
Apart from 2 mice that suffered cardiovascular
arrest under anesthesia, all animals tolerated the surgery and the
experimental duration well.
μ-CT
Comparison of the calvarias' histomorphometry within
the mice in the SHAM group revealed no relevant differences in bone
volume density (BV/TV) between the different experimental duration
times of 7, 14 or 28 days (Fig.
1). The intragroup comparison in the UHMWPE-treated mice
revealed the smallest BV/TV in the mice within the 7-day
experimental duration group, whereas there was a trend towards an
increase in BV/TV when comparing the UHMWPE-treated mice at 7 and
14 days (p=0.101) and a significant increase in BV/TV in mice
treated with the UHMWPE particles for 28 days compared to the
animals within the 7-day experimental duration group (p<0.001).
Similarly, the animals treated with the VE-UHMWPE particles
(VE-UHMWPE) showed the lowest BV/TV in mice observed at 7 days,
whereas there was a trend indicating an increase in BV/TV when
comparing the mice treated for 7 days with those treated for 14
days (p=0.617), and a comparison of the animals treated with the
VE-UHMWPE particles for 7 days with those treated for 28 days
showed a significant increase in BV/TV (p<0.001).
Intergroup comparisons of BV/TV (Fig. 1) revealed a significantly reduced
BV/TV in the mice treated with either the UHMWPE or VE-UHMWPE
particles for 7 days compared to the mice in the SHAM group
(p<0.001). No significant difference in BV/TV was observed when
comparing the UHMWPE particle- with the VE-UHMWPE particle- treated
mice at 7 days (p=0.960). At 14 days, there was still a reduction
in BV/TV in the UHMWPE particle-treated mice (p=0.227) and
VE-UHMWPE particle-treated mice (p=0.008) compared to the mice in
the SHAM group. Whereas after a period of 28 days, the UHMWPE or
VE-UHMWPE particle-treated mice showed a significant increase in
BV/TV compared to the mice in the SHAM group (vs. UHMWPE, p=0.007;
vs. VE-UHMWPE, p=0.018).
Inflammation and osteoclast activity
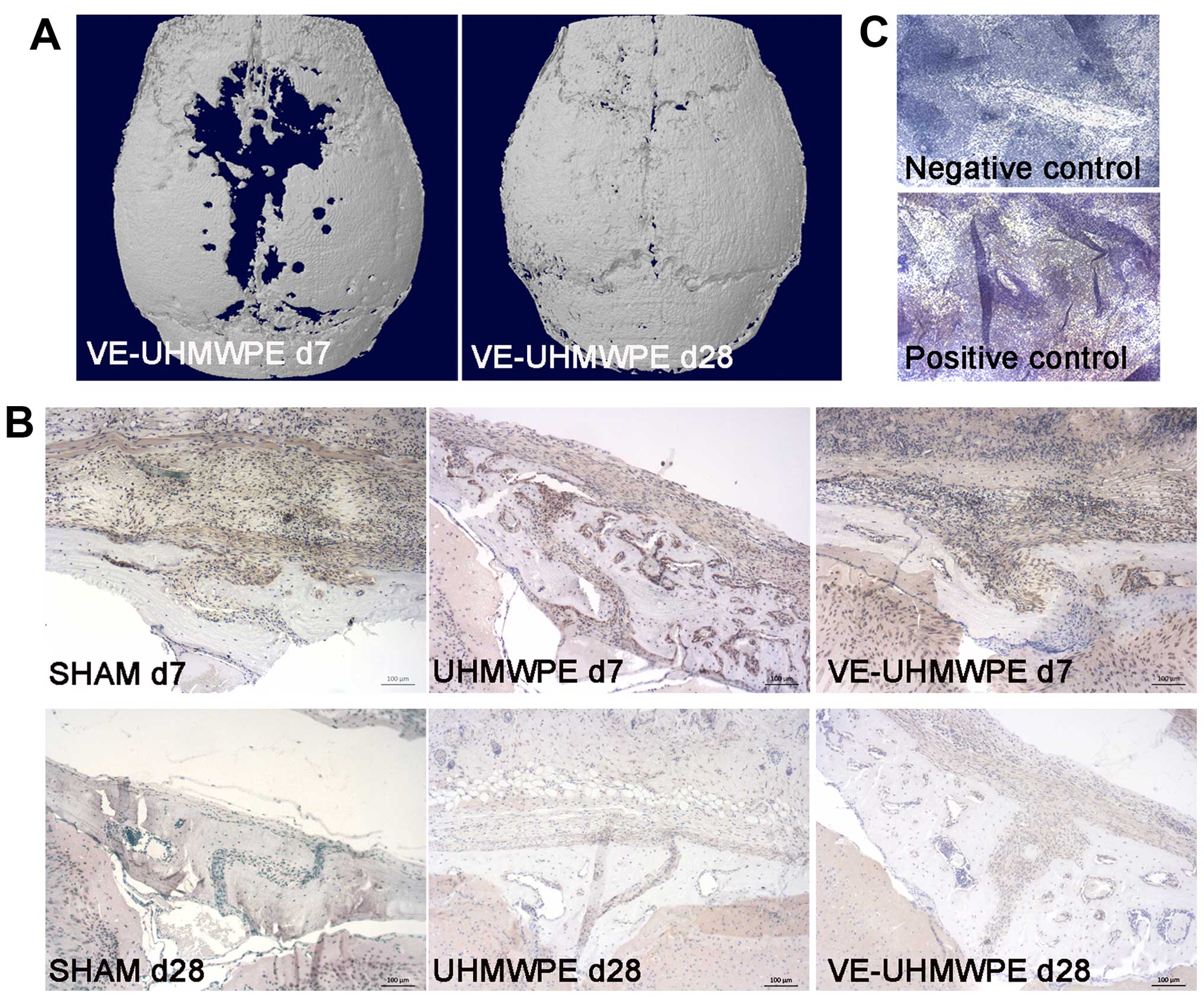
Immunohistochemistry revealed an increased TNF-α
concentration in all mice in the 7-day experimental duration group
compared those in the 28-day experimental duration group. TNF-α
antibody staining was most intense at the surface of the calvaria
in the area of the midline-suture, and there were no distinct
inter- or intra-differences observed in a comparison of either the
animals in the SHAM group or the particle treated animals (Fig. 2).
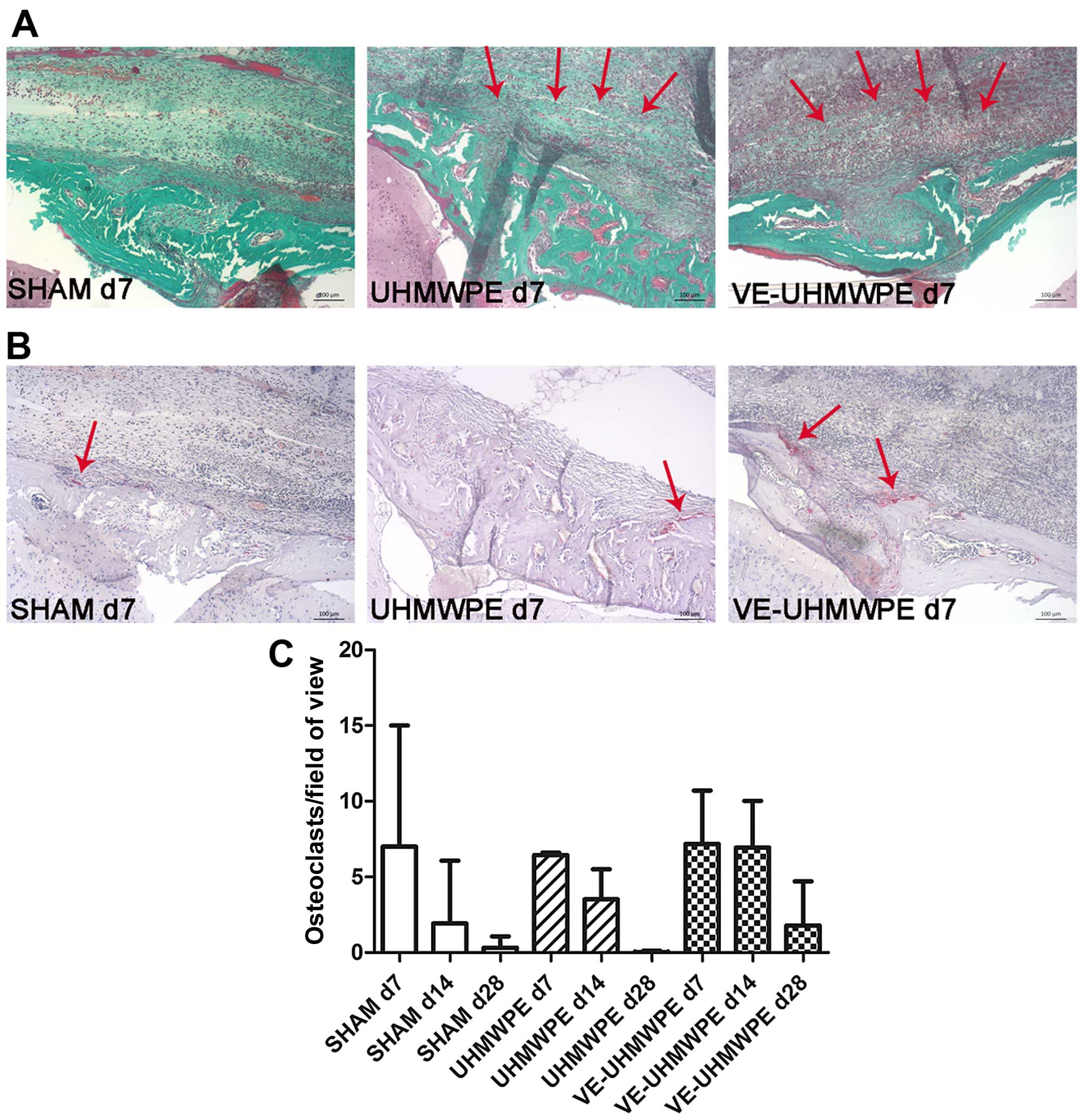
Similar to the TNF-α immunoreactivity, there were
considerably more osteoclasts detected by TRAP staining (Fig. 3B) in all animals either in the
SHAM group, or those treated with the UHMWPE or VE-UHMWPE particles
at 7 days compared to the animals treated at 28 days (Fig. 3C). Intragroup comparison revealed
a clear reduction in TRAP-positive cells after 14 days in the mice
in the SHAM group and the UHMWPE particle-treated mice compared to
their corresponding groups at 7 days, whereas a clear reduction in
TRAP-positive cells in the VE-UHMWPE particle-treated mice was
first observed after an experimental time of 28 days, although the
SD was relatively large.
Histological analysis of the soft tissues
surrounding the midline suture was associated with an increased
inflammatory response which was most obvious in the mice in the
7-day experimental duration group, as indicated by the
granulomatous tissue on Masson-Goldner-stained sections (Fig. 3A).
α-CGRP-mediated local neurogenic
microenvironment
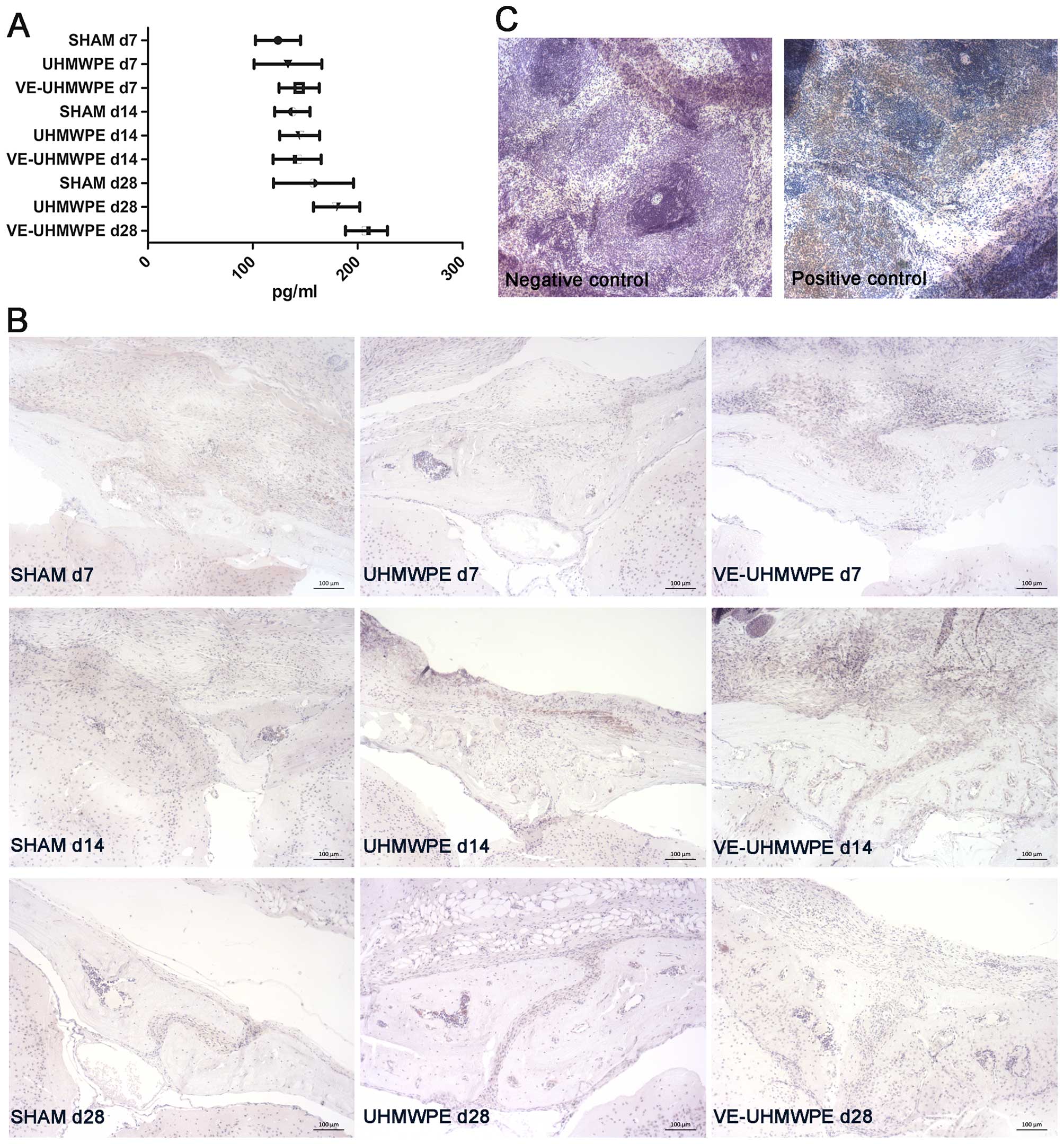
Biomarker analysis of the serum revealed an increase
in the α-CGRP levels in all groups given a longer experimental time
(Fig. 4A). In the UHMWPE and
VE-UHMWPE particle-treated mice, the detected α-CGRP levels were
significantly higher at 28 days compared to those at 14 days
(p=0.036 and p<0.001). Intergroup comparisons only revealed
significantly higher α-CGRP levels in the mice treated with the
VE-UHMWPE particles for 28 days compared to the corresponding
controls (SHAM group; p=0.009).
According to immunohistochemistry, however, α-CGRP
antibody staining revealed a more distinct α-CGRP concentration in
the UHMWPE and VE-UHMWPE particle-treated groups after a period of
14 days compared to their counterparts with an experimental time of
7 days (Fig. 4B).
Biochemical markers of bone turnover
Bone metabolic disorders were excluded at the
beginning of the experimental duration time by the detection of
calcium, phosphate and alkaline phosphatase levels. Following
dilution with saline in a ratio of 1:2, the mean calcium level
detected in serum at the beginning of the experimental time was
0.74+0.08 mmol/l, the mean phosphate level was 2.1+0.3 mg/dl and
the mean alkaline phosphatase level was 30.08+4.7 U/l. There were
no relevant inter- or intragroup differences observed in any of the
animals at the beginning of the experimental duration time (data
not shown).
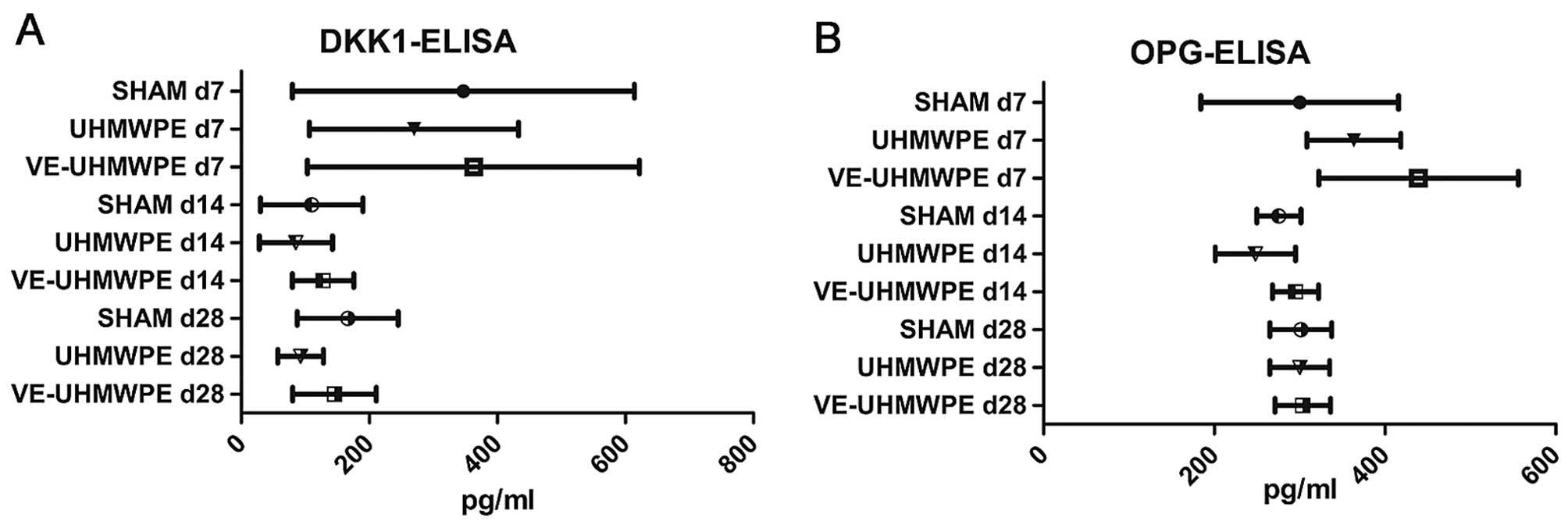
At the end of the experimental time, marker analysis
for bone resorption revealed significantly elevated DKK-1 levels in
the mice in the SHAM group and the UHMWPE particle-treated animals
at the experimental time of 7 days compared to their corresponding
groups at 14 days (p<0.05), though no significant differences
were observed in a similar comparison of the VE-UHMWPE
particle-treated mice (p=0,999; Fig.
5A). Generally, the DKK-1 levels were lowest in all groups of
mice at the experimental time of 14 days followed by a minor
increase in DKK-1 levels in the animals treated at 28 days.
Intergroup analysis, however, revealed no significant differences
between any of the groups.
Similar to the DKK-1 levels, the levels of the
marker of bone formation, OPG, were higher in all animals treated
at 7 days compared to the experimental time of 14 days (Fig. 5B). Although this difference was
only significant in the UHMWPE and VE-UHMWPE-particle treated mice
(p=0.005), there were no significant differences between the
different groups. In the treated animals at 28 days, there was a
trend towards increased OPG levels in all groups, yet there was not
statistically significant difference.
Discussion
The biotribological performance of implants in
endoprosthetic surgery is of superior importance as it contributes
to the stability and longevity of implant anchorage. Therefore, the
present study investigated the impact of VE-UHMWPE wear particles
on the osseous microenvironment compared to UHMWPE wear
particles.
In both the UHMWPE and VE-UHMWPE particle-treated
groups, significant signs of particle-induced osteolysis were
observed in the animals at 7 and 14 days compared to their
corresponding controls (SHAM group). Of note, in the treated mice
at 28 days, an increase in BV/TV was observed in both the UHMWPE
and VE-UHMWPE particle-treated mice compared to the mice in the
SHAM group, which could be attributed by a reactive osteoinduction
following an increased inflammation in the early post-operative
phase. In the present study, no significant differences in
osteolysis were observed between the UHWMPE and VE-UHMWPE
particle-treated mice, which is contradictory to the results
reported by Bichara et al, who recently found a reduced
osteolytic potential in VE-UHMWPE particle-treated mice compared to
the controls (23). Another study
on that topic published by Huang et al within this year also
found similar signs of osteolysis between mice treated with vitamin
E-containing particles and conventional UHMWPE wear particles
(24). However, both studies
investigated a murine calvaria model at a distinct time-point
(10/14 days). Furthermore, Bichara et al investigated very
young animals at the age of 8 weeks, whereas Huang et al
used wear particles generated from wearing simulators; thus, the
observed effect is likely to differ from PIO as it is typically
seen. The use of an established murine calvaria model with
different experimental duration times and more detailed
immunohistochemical investigations may provide further insight.
Diverging results may be attributed to the size of particles
implanted, as the particle size is known to have a significant
impact on the extent of the biological reaction to wear debris
(25). Wear particles in the
phagocytosable range from 0.3 to 10 μm have been associated
with the highest biological activity in in vitro studies
(26). Studies on wear particles
from retrieved human periprosthetic tissues and worn polyethylene
surfaces are consistent with an average particle size in the range
of 0.5 μm in diameter (27). However, the ECD of the wear
particles used in the present study was similar to the size of
particles used in the experiments conducted by Bichara et al
and Huang et al. Apart from the particle size, differences
in reactivity may also be associated with the composition of wear
particles. Thus, Bichara et al and Huang et al used
cross-linked UHMWPE particles in their control group, whereas
conventional UHMWPE particles were used in the present study, while
differences in reactivity of conventional vs. cross-linked UHMWPE
particles remain a matter of discussion (28). In addition, the amount of wear
particles may have attributed to the extent of osteolysis observed
in the UHMWPE and VE-UHMWPE particle-treated mice in the present
study compared to the mice in the SHAM group. Thus, as an attempt
to provoke a severe inflammatory response and to determine the
osteolytic potential of UHMWPE and VE-UHMWPE particles, an
extremely high concentration of particles was chosen in the present
study, which was adapted to the amount of wear used in previous
experiments by our group (18,19). Huang et al treated the
calvaria with 1 mg of wear particles, Bichara et al chose a
concentration of 3 mg, whereas a 30- to 10-fold higher particle
concentration was chosen in the present study. In hip arthroplasty,
a 28-mm head with linear wear of 0.05 mm/y corresponds to a
volumetric wear rate of 30 mm3/y (27).
Therefore, a closer investigation of the osseous
microenvironment and bone turnover may provide further insight into
the biological activity of VE-UHMWPE wear particles in comparison
to UHMWPE particles. As expected, the inflammatory response was
pronounced in the early post-operative stage given the higher
concentrations of TNF-α observed in all mice at day 7, accompanied
by an increased number of osteoclasts. In the particle-treated
mice, these appearances were associated with significant
osteolysis, as indicated by a reduction in BV/TV. These data are
supported by prior investigations on the inflammatory response of
polyethylene particles. For example, Takahashi et al, using
a luminescence murine calvaria model in NF-κB/luciferase transgenic
mice, found that the level of luminescence was maximal at day 7
associated with a significant correlation with pro-inflammatory
mediator mRNAs and bone resorption parameters (29). As TNF-α has been shown to be one
of the key factors in the process of osteoclastogenesis, the use of
anti-TNF agents may be a useful regenerative approach in the
treatment of joint inflammation related to osteolysis (30). Whereas investigations on the
biological reaction to wear particles have found a minor
inflammatory response of ceramic wear particles compared to
polyethylene wear particles (31), the present study revealed no
potential differences in the inflammatory response in between
UHMWPE and VE-UHMWPE wear particles.
Given the findings of α-CGRP in the synovial fluid
and periarticular tissue of loosened implants and the modulatory
effect of vitamin E on CGRP-based neuropeptides known from
different experimental setups, further investigations in the
present study aimed to identify the impact of VE-UHMWPE wear
particles on the local neurogenic environment. Thus, biomarker
analysis of serum α-CGRP showed a continuous increase in α-CGRP
levels throughout the experimental duration time in all groups
(SHAM, UHMWPE and VE-UHMWPE) and significantly higher α-CGRP levels
in the VE-UHMWPE particle-treated mice at the experimental time of
28 days, whereas minor α-CGRP antibody concentrations were
detectable for the first time via immunohistochemistry at day 14 in
the UHMWPE and VE-UHMWPE particle-treated mice. Recent
investigations by our group on haploinsufficient calcitonin
receptor knockout mice identified that regenerative strategies in
the aseptic loosening of endoprosthetic implants should focus on
the impact of α-CGRP-mediated signalling, although previous results
have to be considered (18).
Thus, experiments on α-CGRP-deficient mice have demonstrated
reduced PIO associated with decreased RANKL mRNA levels compared to
wild-type controls, presuming a catabolic effect of α-CGRP in
particle induced osteolysis (19). On the contrary, in vitro
analysis observed a time-dependent inhibitory effect of α-CGRP on
the secretion of osteolysis-associated pro-inflammatory cytokines
such as TNF-α (32). Therefore,
the observed increase in α-CGRP levels found in the present study
in the VE-UHMWPE particle-treated mice after a period of 28 days
compared to the sham-operated controls has to be interpreted with
caution. Although there is evidence, that α-CGRP has a modulatory
effect on the production of pro-inflammatory cytokines, its impact
is not yet fully understood and there are limited data on the
interaction between vitamin E and CGRP. As stated above, Tashima
et al found that vitamin E supplementation provided a
neuroprotective effect associated with a reduction in number of
CGRP nerve fiber varicosities in the jejunum in experimental
diabetes (13), although
transmission of this interaction to the process of PIO involved
uncertainties. Furthermore, the authors did not specify whether
they detected α-CGRP or β-CGRP nerve fibres, which differ from each
other.
The investigation of bone turnover may therefore be
an opportunity to further identify the impact of VE-UHMWPE
particles in the process of PIO. It is well known that osteoclast
and osteoblast activities in PIO are mediated by various
inflammatory cytokines released from macrophages following the
phagocytosis of wear particles (33). Therefore, differences in markers
of bone formation and bone resorption would be expected if there
were differences in the inflammatory response between UHMWPE and
VE-UHMWPE wear particles. In the present study, significantly
higher levels of DKK-1, a marker of bone resorption, were found in
all mice after a period of 7 days compared to serum levels detected
after a period of 14 days. Serum analysis similarly revealed
significantly higher levels of OPG, a marker of bone formation, in
particle-treated mice after a period of 7 days compared to the
controls at 14 days. Taken together, no significant differences
with regards to the inflammatory potential were observed between
the UHMWPE und VE-UHMWPE particle-treated mice. These data are in
accordance with previous in vitro testing comparing the
biocompatibility of UHMWPE and vitamin E (α-tocopherol)-stabilised
UHMWPE tablets, in which similar proliferation rates were found in
both polyethylene samples with no evidence of cytotoxicity
(34).
In conclusion, the investigation of a very high
concentration of VE-UHMWPE wear particles in an established murine
calvaria model revealed a comparable extent of PIO as observed in
UHMWPE wear particles. Only minor differences between the VE-UHMWPE
particle- compared to the UHMWPE particle-treated mice were found
with regards to an activation of inflammatory cytokines and
osteoclasts, which was most distinct in the early stages after
surgery. The impact of increased α-CGRP levels observed in the mice
treated with the VE-UHMWPE particles at 28 days and the influence
of vitamin E on CGRP requires further investigation. However, as
there were no differences in bone turnover between UHMWPE and
VE-UHMWPE particle-treated mice, vitamin E-blended UHMWPE particles
appear to have a reasonable biocompatibility. Taking the improved
data on the tribologic and mechanical properties associated with a
reduction of released wear particles into account, and given the
increased ageing resistance of vitamin E-blended UHMWPE particles,
the stabilization of polyethylene-bearing couples with vitamin E
appears to be a promising approach (12,35). Further investigations on different
concentrations of VE-UHMWPE wear particles and the identification
of potential regenerative strategies remain of interest to reduce
the extent of wear particle induced osteolysis in future.
Study limitations
There are some constraints of the experimental setup
of this study, thus a very high concentration of wear particles was
chosen as an attempt to provoke an inflammatory response which does
not resemble the amount of wear occurring in humans. Furthermore,
animals chosen for the present study were at the age of 12 weeks,
as these wild-type mice are used very frequently this provides
better comparison with other data, yet it has been shown that there
are potential differences in bone metabolism of younger and aged
mice (36). Also there may have
been compensatory effects influencing the inflammatory response
observed in the present model of particle induced osteolysis which
could have been induced by other cytokines or peptide hormones that
have not been detected.
Acknowledgments
The authors wish to thank Dr. A. Riedasch for
support during the experimental time in the laboratory of animal
science. Also many thanks to Mr. W. Wilfert at the Institute of
Laboratory Medicine for the implementation of biomarker analysis.
This study was supported by a research grant provided by the
Friedrich-Baur-Foundation (FBS). The authors J. Schwiesau and T.M.
Grupp are employes of the Aesculap AG.
References
|
1
|
Garellick G, Kärrholm J, Rogmark C,
Rolfson O and Herberts P: Swedish Hip Arthroplasty Register, Annual
Report 2011. Gothenburg, Sweden: 2012, ISBN: 978-91-980507-1-4
|
|
2
|
Jiang J, Yang CH, Lin Q, Yun XD and Xia
YY: Does arthroplasty provide better outcomes than internal
fixation a mid- and long-term followup? A meta-analysis. Clin
Orthop Relat Res. 473:2672–2679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Learmonth ID, Young C and Rorabeck C: The
operation of the century: Total hip replacement. Lancet.
370:1508–1519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drees P, Eckardt A, Gay RE, Gay S and
Huber LC: Molecular pathways in aseptic loosening of orthopaedic
endoprosthesis. Biomed Tech (Berl). 53:93–103. 2008.In German.
View Article : Google Scholar
|
|
5
|
Rubash HE, Sinha RK, Shanbhag AS and Kim
SY: Pathogenesis of bone loss after total hip arthroplasty. Orthop
Clin North Am. 29:173–186. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmalzried TP, Jasty M and Harris WH:
Periprosthetic bone loss in total hip arthroplasty. Polyethylene
wear debris and the concept of the effective joint space. J Bone
Joint Surg Am. 74:849–863. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grupp TM, Utzschneider S and Wimmer MA:
Biotribology in knee arthroplasty. BioMed Res Int. 2015:6189742015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muratoglu OK, Bragdon CR, O'Connor DO,
Jasty M and Harris WH: A novel method of cross-linking
ultra-high-molecular-weight polyethylene to improve wear, reduce
oxidation, and retain mechanical properties. Recipient of the 1999
HAP Paul Award. J Arthroplasty. 16:149–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomas GE, Simpson DJ, Mehmood S, Taylor
A, McLardy-Smith P, Gill HS, Murray DW and Glyn-Jones S: The
seven-year wear of highly cross-linked polyethylene in total hip
arthroplasty: A double-blind, randomized controlled trial using
radiostereometric analysis. J Bone Joint Surg Am. 93:716–722. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oral E, Godleski Beckos C, Malhi AS and
Muratoglu OK: The effects of high dose irradiation on the
cross-linking of vitamin E-blended ultrahigh molecular weight
polyethylene. Biomaterials. 29:3557–3560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oral E, Wannomae KK, Rowell SL and
Muratoglu OK: Diffusion of vitamin E in ultra-high molecular weight
polyethylene. Biomaterials. 28:5225–5237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwiesau J, Fritz B, Kutzner I, Bergmann
G and Grupp TM: CR TKA UHMWPE wear tested after artificial aging of
the vitamin E treated gliding component by simulating daily patient
activities. BioMed Res Int. 2014:5673742014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tashima CM, Hermes-Uliana C, Perles JV, de
Miranda Neto MH and Zanoni JN: Vitamins C and E
(ascorbate/α-tocopherol) provide synergistic neuroprotection in the
jejunum in experimental diabetes. Pathophysiology. 22:241–248.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian Y, Zeng BF, Zhang XL and Jiang Y:
High levels of substance P and CGRP in pseudosynovial fluid from
patients with aseptic loosening of their hip prosthesis. Acta
Orthop. 79:342–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saxler G, Löer F, Skumavc M, Pförtner J
and Hanesch U: Localization of SP- and CGRP-immunopositive nerve
fibers in the hip joint of patients with painful osteoarthritis and
of patients with painless failed total hip arthroplasties. Eur J
Pain. 11:67–74. 2007. View Article : Google Scholar
|
|
16
|
Schinke T, Liese S, Priemel M, Haberland
M, Schilling AF, Catala-Lehnen P, Blicharski D, Rueger JM, Gagel
RF, Emeson RB and Amling M: Decreased bone formation and osteopenia
in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner
Res. 19:2049–2056. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kauther MD, Neuerburg C, Wefelnberg F,
Bachmann HS, Schlepper R, Hilken G, Broecker-Preuss M, Grabellus F,
Schilling AF, Jäger M and Wedemeyer C: RANKL-associated suppression
of particle-induced osteolysis in an aged model of calcitonin and
α-CGRP deficiency. Biomaterials. 34:2911–2919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neuerburg C, Wedemeyer C, Goedel J,
Schlepper R, Hilken G, Schwindenhammer B, Schilling AF, Jäger M and
Kauther MD: The role of calcitonin receptor signalling in
polyethylene particle-induced osteolysis. Acta Biomater.
14:125–132. 2015. View Article : Google Scholar
|
|
19
|
Wedemeyer C, Neuerburg C, Pfeiffer A,
Heckelei A, Bylski D, von Knoch F, Schinke T, Hilken G, Gosheger G,
von Knoch M, et al: Polyethylene particle-induced bone resorption
in alpha-calcitonin gene-related peptide-deficient mice. J Bone
Miner Res. 22:1011–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Utzschneider S, Becker F, Grupp TM,
Sievers B, Paulus A, Gottschalk O and Jansson V: Inflammatory
response against different carbon fiber-reinforced PEEK wear
particles compared with UHMWPE in vivo. Acta Biomater. 6:4296–4304.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Standard Practice for Characterization of
Particles. American Society for Testing and Materials; West
Conshohocken: 2010
|
|
22
|
Wedemeyer C, Xu J, Neuerburg C,
Landgraeber S, Malyar NM, von Knoch F, Gosheger G, von Knoch M,
Löer F and Saxler G: Particle-induced osteolysis in
three-dimensional micro-computed tomography. Calcif Tissue Int.
81:394–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bichara DA, Malchau E, Sillesen NH, Cakmak
S, Nielsen GP and Muratoglu OK: Vitamin E-diffused highly
cross-linked UHMWPE particles induce less osteolysis compared to
highly cross-linked virgin UHMWPE particles in vivo. J
Arthroplasty. 29(Suppl): 232–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang CH, Lu YC, Chang TK, Hsiao IL, Su
YC, Yeh ST, Fang HW and Huang CH: In vivo biological response to
highly cross-linked and vitamin e-doped polyethylene - a
particle-Induced osteolysis animal study. J Biomed Mater Res B Appl
Biomater. 104:561–567. 2016. View Article : Google Scholar
|
|
25
|
Gallo J, Slouf M and Goodman SB: The
relationship of polyethylene wear to particle size, distribution,
and number: A possible factor explaining the risk of osteolysis
after hip arthroplasty. J Biomed Mater Res B Appl Biomater.
94:171–177. 2010.PubMed/NCBI
|
|
26
|
Green TR, Fisher J, Stone M, Wroblewski BM
and Ingham E: Polyethylene particles of a 'critical size' are
necessary for the induction of cytokines by macrophages in vitro.
Biomaterials. 19:2297–2302. 1998. View Article : Google Scholar
|
|
27
|
Bezwada HP, Nazarian D and Booth R:
Acetabular wear in total hip arthroplasty. E-Medicine.com; pp.
1–15. 2004
|
|
28
|
Catelas I, Wimmer MA and Utzschneider S:
Polyethylene and metal wear particles: Characteristics and
biological effects. Semin Immunopathol. 33:257–271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi K, Onodera S, Tohyama H, Kwon
HJ, Honma K and Yasuda K: In vivo imaging of particle-induced
inflammation and osteolysis in the calvariae of NFκB/luciferase
transgenic mice. J Biomed Biotechnol. 2011:7270632011. View Article : Google Scholar
|
|
30
|
Dong L, Wang R, Zhu YA, Wang C, Diao H,
Zhang C, Zhao J and Zhang J: Antisense oligonucleotide targeting
TNF-α can suppress Co-Cr-Mo particle-induced osteolysis. J Orthop
Res. 26:1114–1120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Warashina H, Sakano S, Kitamura S,
Yamauchi KI, Yamaguchi J, Ishiguro N and Hasegawa Y: Biological
reaction to alumina, zirconia, titanium and polyethylene particles
implanted onto murine calvaria. Biomaterials. 24:3655–3661. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jablonski H, Kauther MD, Bachmann HS,
Jager M and Wedemeyer C: Calcitonin gene-related peptide modulates
the production of pro-inflammatory cytokines associated with
periprosthetic osteolysis by THP-1 macrophage-like cells.
Neuroimmunomodulation. 22:152–165. 2015. View Article : Google Scholar
|
|
33
|
Landgraeber S, Jäger M, Jacobs JJ and
Hallab NJ: The pathology of orthopedic implant failure is mediated
by innate immune system cytokines. Mediators Inflamm.
2014:1851502014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wolf C, Lederer K, Pfragner R,
Schauenstein K, Ingolic E and Siegl V: Biocompatibility of
ultra-high molecular weight polyethylene (UHMW-PE) stabilized with
alpha-tocopherol used for joint endoprostheses assessed in vitro. J
Mater Sci Mater Med. 18:1247–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bracco P and Oral E: Vitamin E-stabilized
UHMWPE for total joint implants: A review. Clin Orthop Relat Res.
469:2286–2293. 2011. View Article : Google Scholar :
|
|
36
|
Langlois J, Zaoui A, Bichara DA, Nich C,
Bensidhoum M, Petite H, Muratoglu OK and Hamadouche M: Biological
reaction to polyethylene particles in a murine calvarial model is
highly influenced by age. J Orthop Res. 34:574–580. 2016.
View Article : Google Scholar
|