Introduction
Osteoarthritis is a degenerative disease with an
irreversible course and serious consequences that affects millions
of individuals worldwide (1).
Increasing age is one of the most common risk factors for
osteoarthritis (2). With an
increase in life expectancy, more elderly patients are likely to
develop osteoarthritis (3), and
osteoarthritis is estimated to become the fourth most disabling
disease by the year 2020 (1).
However, the pathogenesis of the disease remains poorly
understood.
Although chondrocytes, which are mainly responsible
for the anabolic-catabolic balance in cartilage, account for only
1–2% of the total cartilage volume, they play an important role in
regulating the function of articular cartilage by synthesizing the
structural components of the extracellular matrix (ECM) and
matrix-degrading proteases (3).
Chondrocytes have been found to play a pivotal role in the
pathology of osteoarthritis through chondrocyte apoptosis and
cartilage matrix degradation (4–6).
However, the molecular mechanisms underlying the development of
osteoarthritis due to chondrocyte apoptosis have not yet been
clearly elucidated.
Aquaporins (AQPs) are specific transmembrane
proteins responsible for water transport and are expressed in
articular chondrocytes (7). It
has been reported that the expression levels of AQPs are associated
with apoptosis in many types of cells. However, the role of AQPs in
the pathogenesis of osteoarthritis remains unclear (7,8).
It remains to be determined whether AQP-1 expression is altered in
chondrocytes in osteoarthritis and whether the expression levels of
AQP-1 are associated with chondrocyte apoptosis.
We have previously reported that AQP-1 mRNA
expression is increased in a rat model of osteoarthritis and
positively correlates with the mRNA expression and activity of the
the apoptotic marker, caspase-3 (9). In the present study, we further
performed RNA interference (RNAi) experiments to knock down AQP-1
and investigated the association between the expression of AQP-1
and the expression and activity of caspase-3. The aim of this study
was to further determine the role of AQP-1 expression in
chondrocyte apoptosis and to further explore the role of AQP-1 in
the pathogenesis of osteoarthritis.
Materials and methods
Animals
All experimental protocols were approved by the
Institutional Animal Care and Use Committee of Nanjing Medical
University, Nanjing, China. All procedures were carried out in
accordance with the Institutional Animal Care and Use Committee
Guide at Merck Research Laboratories (Germany). A total of 72 male
Sprague-Dawley (SD) rats (8 weeks old, weighing 286–320 g) were
obtained from the Animal Center of Nanjing Medical University
(Nanjing, China). The animals were housed at room temperature
(25°C) with 60% humidity and a 12-h light/dark cycle. The animals
were fed standard rat chow and were provided with water ad
libitum. The rats were randomly assigned to 3 groups as
follows: the control group not treated surgically (n=24), the
sham-operated group (n=24), and the osteoarthritis group
(n=24).
Establishment of rat model of
osteoarthritis
The rats were anesthetized by an intraperitoneal
injection of 10% chloral hydrate (1–2 ml/kg). The anterior cruciate
ligament and medial collateral ligament were cut, and the anterior
horn of the medial meniscus was partially removed, via the medial
parapatellar approach, as previously described (10). The anterior drawer test and the
lateral stress test were used to confirm the dissection of the
anterior cruciate ligament and medial collateral ligament. The
articular cavity was flushed with iodine and saline. The wounds
were sutured, and penicillin (80,000 U; Shanghai Nuotai Chemical
Co., Ltd., China) was administered for 3 days. For the rats in the
the sham-operated group, the articular cavity was exposed, but the
ligaments and anterior horn of the medical meniscus were not
removed. For the rats in the the control group, no
treatment/surgery was administered. The rats in the each group were
forced to move for 2 h each day by the squirrel wheel method. The
general condition of the articular cartilage, based on the color,
cracking, softening and osteophyte formation, was observed. The
rats were sacrificed by CO2 inhalation, and the knee
joints were harvested at 1, 2, 4 and 8 weeks after the
osteoarthritis model was established. The samples were stored in 4%
formaldehyde solution at −80°C until use.
Isolation and culture of chondrocytes
from rats with osteoarthritis
The cartilage was removed from the rats in the
osteoarthritis group at 8 weeks post-surgery and used for
chondrocyte isolation and culture. Chondrocytes were isolated from
the cartilage matrix by serial digestion with trypsin (Amresco,
Solon, OH, USA) and collagenase II (Sigma, St. Louis, MO, USA) and
cultured as previously described (11). The survival conditions of the
cultured cells were examined under an optical microscope (A11.1535;
Opto-Edu, Beijing, China).
Cultured chondrocytes in the logarithmic growth
phase were seeded in a 6-well plate, supplemented with 2 ml
Dulbecco's modified Eagle's medium/F-12 medium containing 15% fetal
bovine serum with 200,000 units penicillin. The cells were cultured
for 3, 5 days and 1 week in an incubator at 37°C with 5%
CO2. The medium was changed every other day.
Transfection was performed when the cells reached 80%
confluence.
Hematoxylin and eosin (H&E) and
Alcian blue staining
H&E and Alcian blue staining was used to assess
chondrocyte morphology. For H&E staining (AR1180-100; Boster,
Wuhan, China), the cells were stained with H&E for 5 min. For
Alcian blue staining, the cells were stained with Alcian blue,
using the Alcian Blue pH 2.5 Stain kit (American MasterTech, Lodi,
CA, USA).
Immunofluorescence staining
For immunofluorescence staining, the cells were
grown on glass coverslips, rinsed with phosphate-buffered saline
(PBS), and fixed in 4% paraformaldehyde for 30 min at room
temperature. The cells were then permeabilized with 1% Triton X-100
for 10 min. Following 3 washes with PBS, the cells were incubated
with primary antibody against type II collagen (rabbit anti-rat
type II collagen, 1:100 dilution; Cat no. 70R-CR008; Fitzgerald
Industries International, Acton, MA, USA) at 4°C overnight. PBS
without primary antibody was used as a negative control. After the
primary antibody was removed by washing in PBS, immunoreactivity
was detected by incubation with
fluorescein-isothiocyanate-conjugated secondary antibody (goat
anti-rabbit IgG, 1:100 dilution; 111-005-144; Jackson Immuno
Research, West Grove, PA, USA) at room temperature for 45 min.
After the coverslips were washed with PBS, the cells were
counterstained with DAPI (Sigma) and examined and photographed
under a fluorescence microscope (Olympus Corp., Tokyo, Japan).
RNAi and cell transfection
The rat cDNA sequence (GenBank NM-012778; https://www.ncbi.nlm.nih.gov/nuccore/NM_012778) was
analyzed for potential small interfering RNA (siRNA) target
sequences for AQP-1. The oligonucleotide was designed to have a
hairpin loop and cloned into the pGenesil-1 plasmid containing the
U6 promoter and green fluorescent protein (GFP) (Wuhan Cell Marker
Biotechnology Co., Ltd., Wuhan, China). As previously described
(12), the AQP-1-shRNA pGenesil-1
plasmid named AQP-l-pGenesil was used for RNAi to knock down AQP-1.
The following oligonucleotide was used for AQPl-2 (19 nt):
5′-TTCTCAAA CCACTGGATT-3′. The oligonucleotide used for scrambled
shRNA was 5′-GACTTCATAAGGCGCATGC-3′. Chondrocytes at 80% confluence
were transfected with AQP-l-pGenesil using the transfection
reagent, Lipofectamine® 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. Untransfected cells were used as empty controls, and
cells transfected with Lipofectamine 2000 were used as the empty
Lipofectamie 2000 group. At 48 h post-transfection, the transfected
cells showing GFP expression were sorted using flow cytometry
(Guava® easyCyte 8, Merck Millipore, Billerica, MA, USA)
and used in the subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the articular cartilage
using the TRIzol RNA extraction kit (Invitrogen Life Technologies).
RNA was reverse transcribed into complementary DNA (cDNA) using the
reverse transcription system (Toyobo Co., Ltd., Osaka, Japan).
Quantitative (qPCR) was performed with a 20-μl mixture
containing 2.5 μl cDNA, 0.4 μl of each primer, and 10
μl SYBR-Green (Toyobo Co., Ltd.). The following primers were
used: 5′-CATTGGCTTGTCTGTGGC-3′ (forward) and
5′-TTTGAGAAGTTGCGGGTG-3′ (reverse) for AQP-1,
5′-CTGGACTGCGGTATTGAG-3′ (forward) and 5′-GGGTGCGGTAGAGTAAGC-3′
(reverse) for caspase-3, and 5′-CAAGTTCAACGGCACGTCAA-3′ (forward)
and 5′-TGGTGAAGACGCCAGAGACTC-3′ (reverse) for
glyceraldehyde-3-phosphate dehydrogenase (GAPDH). GAPDH was used as
an internal control. The reaction conditions were as follows: 95°C
for 20 sec; 50.4°C for 20 min; 95°C for 60 sec; 95°C for 15 sec
with 40 cycles of 55°C for 15 sec and 74°C for 45 sec. The relative
expression levels of AQP-1 and caspase-3 were calculated using the
2−ΔΔCt method, as previously described (13).
Determination of caspase-3 activity
Total protein was extracted from the articular
cartilage of the rats in each group or the cultured chondrocytes.
Protein concentrations were determined using the BCA Protein Assay
kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Caspase-3
activity was measured using the Caspase-3 Colorimetric Assay kit
(Nanjing KeyGen Biotech Co., Ltd.). The plates were read at 405 nm
using a microplate spectrophotometer (Model 680; Bio-Rad, Hercules,
CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Quantitative data are
presented as the means ± standard deviation. One-way analysis of
variance (ANOVA) was used to compare the differences among groups,
followed by the post hoc Student-Newman-Keuls tests. Pearson's
correlation analysis was used to evaluate the association between
the expression of AQP-1 and caspase-3 expression or activity. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Cartilage damage in our rat model of
osteoarthritis
In the control and sham-operated groups, the surface
of the cartilage was resilient and smooth, showing no cracks,
softening, or osteophyte formation at 8 weeks group (Fig. 1A and B). At 8 weeks post-surgery,
synovial hyperplasia was observed in the osteoarthritis group. The
cartilage of the rats in the osteoarthritis group had lost the
original luster and showed obvious roughness, osteophyte formation
and cracking. The articular surface also appeared opaque (Fig. 1C).
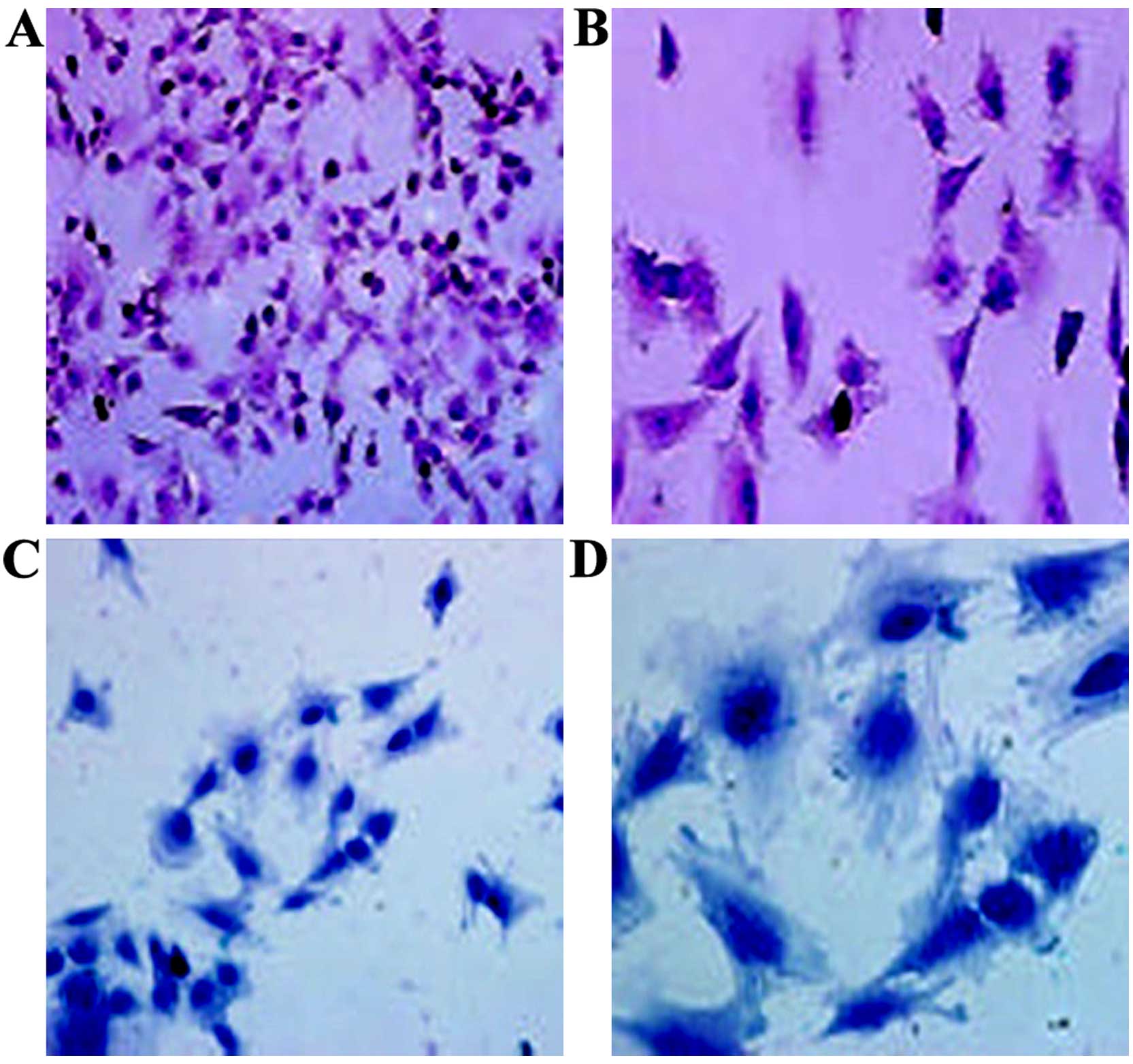
Morphology of cultured chondrocytes from
rats in our model of osteoarthritis
The chondrocytes isolated from the rats in our model
of osteoarthritis were spherical in shape with strong refractivity
and became triangular or polygonal in shape following adherence to
the culture surfaces. The cell nuclei were round or oval with 1–3
nucleoli and located in the center of the cells. Following culture
for 3 days, the cells were clustered and grew in a round or oval
shape. Following culture for 5 days, the cells overlapped and
proliferated significantly to form connections between cells.
Matrix materials were deposited around the cells. The cells grew in
a monolayer and covered the bottom of the culture bottle following
culture for 1 week (Fig. 2).
H&E staining indicated that the cartilage cells
at the 4th passage were triangular or polygonal. The nuclei had
double or multiple nucleoli. The ECM was stained red (Fig. 3A and B). Upon Alcian blue
staining, the cytoplasm and cell membrane were stained dark blue,
suggesting that the cultured chondrocytes synthesized and secreted
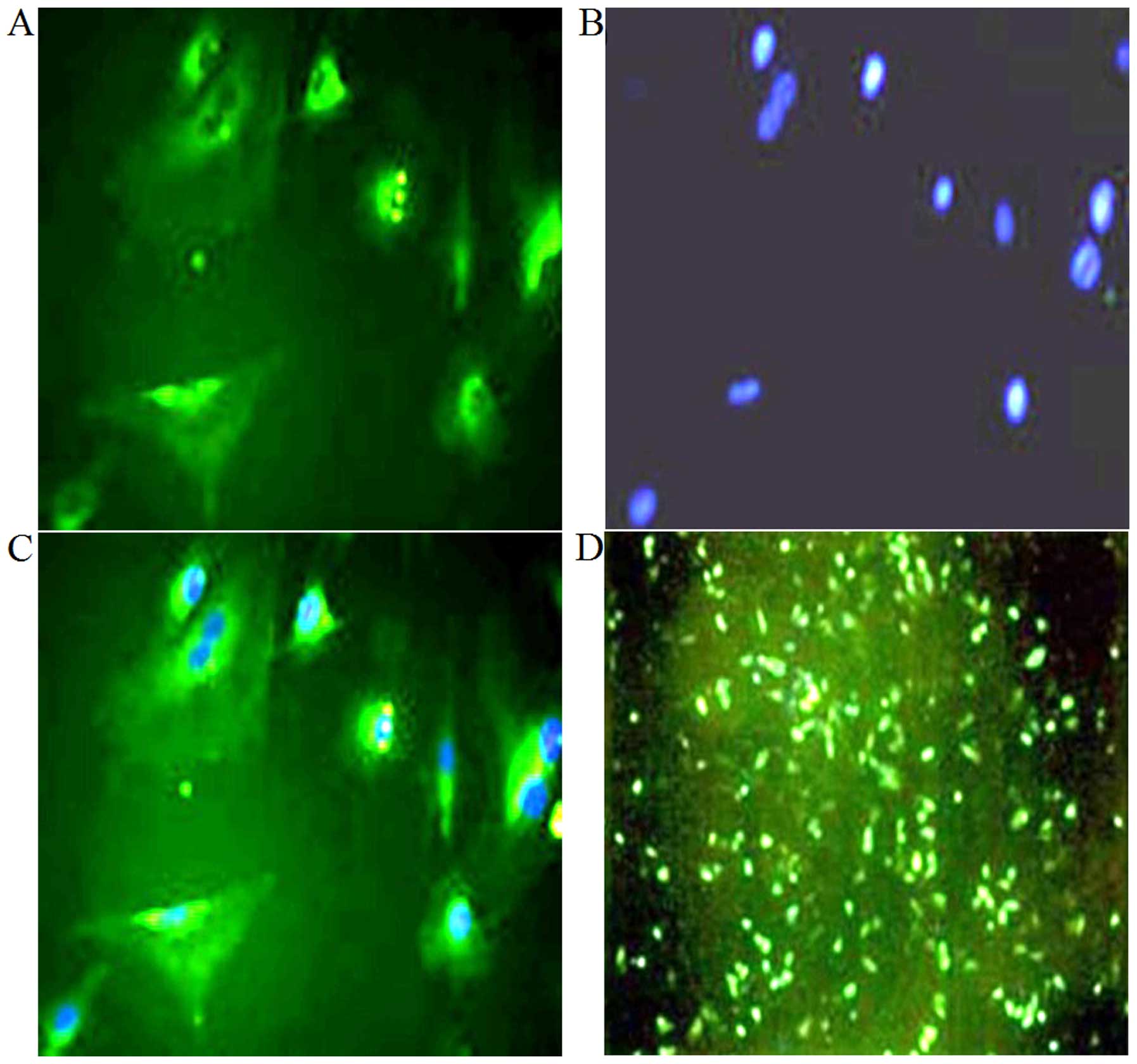
proteoglycans (Fig. 3C and D). In
addition, oositive immunofluorescence staining for type II collagen
indicated that chondrocyte-specific type II collagen was mainly
distributed in the cytoplasm and cell membrane (Fig. 4A–C).
Knockdown of AQP-1 decreases the
expression of caspase-3 in cultured chondrocytes
At 48 h post-transfection, green fluorescence was
clearly observed in the transfected cells (Fig. 4D), indicating the success of the
transfection. Flow cytometry revealed that fluorescent cells
represented 41.9% of all cells (Fig.
5B). The expression of AQP-1 was significantly decreased in the
cells transfected with AQPl-1-pGenesil-1 compared with that in
untransfected control cells, cells treated with
Lipofectamine® 2000 alone, and cells transfected with
scrambled shRNA (P<0.01, Fig.
5C). In addition, the expression and activity of caspase-3 were
significantly decreased in the cells transfected with
AQPl-1-pGenesil-1 (P<0.05, Fig. 6A
and B).
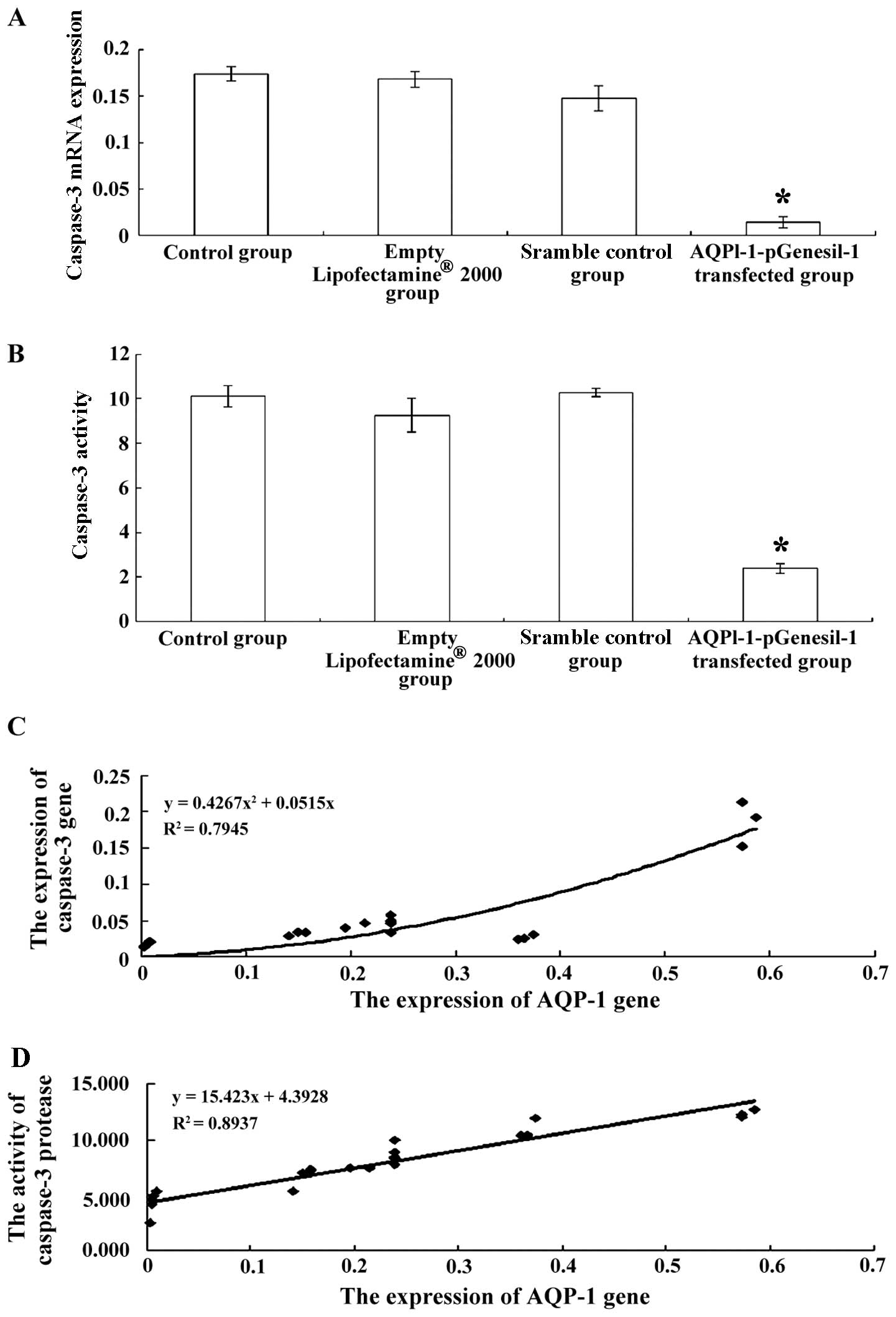 | Figure 6(A) Caspase-3 mRNA expression in
control cells, cells treated with Lipofectamine® 2000
alone, cells transfected with scrambled shRNA, and cells
transfected with AQPl-1-pGenesil-1. *P<0.01 vs.
normal control group, Lipofectamine® 2000 group, and
scrambled shRNA group. (B) Caspase-3 activity in control cells,
cells treated with Lipofectamine® 2000 alone, cells
transfected with scrambled shRNA, and cells transfected with
AQPl-1-pGenesil-1. *P<0.01 vs. normal control group,
Lipofectamine® 2000 group, and scrambled shRNA group.
(C) Correlation between the expression of AQP-1 and the expression
of caspase-3 and, (D) correlation between the expression of AQP-1
and caspase-3 activity in cultured chondrocytes. (C) Curve fitting
was carried out regression analysis using the following equation:
y=0.4267x2+0.0515x (R2=0.7945, P<0.001).
(D) Curve fitting was carried out regression analysis using the
following equation: y=15.423x+4.3928 (R2=0.8937,
P<0.001). |
Correlation between the expression of
AQP-1 and caspase-3 expression and activity
Pearson's correlation analysis was used to evaluate
the association between the expression of AQP-1 and caspase-3
expression or activity. The expression of AQP-1 positively
correlated with the expression of caspase-3 (Fig. 6C, r=0.817; P<0.001) and with
caspase-3 activity (Fig. 6C,
r=0.945; P<0.001).
Discussion
Osteoarthritis is regarded as a pathological
condition resulting from the degeneration or destruction of the
articular cartilage that covers and protects the moving joints.
However, the role of chondrocytes in osteoarthritis remains
unclear. We have previously demonstrated that the expression of
AQP-1 is upregulated in a rat model of osteoarthritis, accompanied
by an increase in caspase-3 expression and activity (9). In the present study, we found that
the knockdown of AQP-1 in chondrocytes from rats with
osteoarthritis decreased caspase-3 expression and activity, and
that the expression of AQP-1 positively correlated with caspase-3
expression and activity, suggesting that AQP-1 contributes to
chondrocyte apoptosis and to the development of osteoarthritis.
Chondrocytes are the major regulators of the process
of matrix anabolism and catabolism and, thus, are essential to
maintain the homeostasis of the cartilage matrix. Chondrocytes not
only synthesize the ECM, but also play a direct role in the
degradation process termed as 'chondrocytic chondrolysis'. Injured
chondrocytes fail to degrade the damaged matrix in osteoarthritic
cartilage and, thus, contribute to the irreversible pathological
process of osteoarthritis (3).
Chondrocyte apoptosis has been found to be a major contributor to
the progression of osteoarthritis (14–17). Caspase-3 protease is considered to
be a killer protease and a key apoptosis mediator that mediates the
terminal phase of apoptosis induced by death receptors or through
the mitochondrial pathway (18).
Several lines of evidence have indicated that activated caspase-3
protease will lead to irreversible apoptosis, and thus, the
activation of caspase-3 is regarded as a molecular marker of
apoptosis (19–22). In the present study, the
significantly upregulated expression of caspase-3 was found in
chondrocytes from rats with osteoarthritis, suggesting that
chondrocyte apoptosis plays an important role in
osteoarthritis.
It has been reported that the apoptotic volume
decrease (AVD) is a common apoptotic pathway in various cells of
many species and is a common reaction of cells to apoptosis
inducers (23). AVD is mainly
caused by the flow of monovalent cations though cation channels and
alterations in water permeability via AQPs (24–28). It has been demonstrated that water
and ion channels play a certain role in cell apoptosis in the
central nervous system, and the expression levels of AQPs,
potassium channels and chloride channels contribute to the
initiation and progression of apoptosis (27). The outflow of water molecules
mediated by AQPs has been reported to be one of the preconditions
of AVD (25). In addition, the
altered expression of AQPs in the mitochondria has been found
during the apoptotic process (28). Furthermore, the activation of AQPs
followed by mitochondrial swelling has been reported to induce the
release of cytochrome c and the activation of caspase
enzymes (28). It has also been
reported that the overexpression of AQP-1 activates intracellular
caspase-3 and induces apoptosis in vitro (24,25). In addition, a decrease in the
expression of AQP-1 in hepatocellular carcinoma cells has been
shown to be associated with resistance to apoptosis (29). Li et al reported that
α-melanocyte-stimulating hormone reduced renal tubular epithelial
cell apoptosis and prevented the downregulation of AQPs and
Na+-K+ ATP enzymes in rats with bilateral
ureteral obstruction (30). The
inhibition of AQP-1 by mercuric chloride (HgCl2) has
been reported to induce a decrease in AVD and caspase-3 activity
(27). Consistent with the
literature, in this study, we found that AQP-1 was upregulated in
rats with osteoarthritis, and that the expression of AQP-1
positively correlated with caspase-3 expression and activation in
chondrocytes, suggesting that AQP-1 promotes chondrocyte apoptosis
via the activation of caspase-3.
The findings that the knockdown of AQP-1
significantly decreased the expression of caspase-3 and activity
further confirmed that the upregulation of AQP-1 expression
activated caspase-3, and thus contributed to chondrocyte apoptosis
and to the development of osteoarthritis.
In conclusion, we previously found that the
expression of AQP-1 was upregulated in the osteoarthritic cartilage
and that it strongly correlated with caspase-3 expression and
activity (9). In the present
study, we further found that the inhibition of AQP-1 expression
using shRNA decreased the expression of caspase-3 in chondrocytes
from rats with osteoarthritis, suggesting that AQP-1 participates
in the process of chondrocyte apoptosis, and thereby contributes to
the development of osteoarthritis.
Acknowledgments
The authors appreciate the excellent technical
assistance of Dr Haibo Sun (Nanjing Medical University, Nanjing,
China). This study was supported by the special foundation for
major scientists of Nanjing City (project no. 3030930).
References
|
1
|
Tesche F and Miosge N: New aspects of the
pathogenesis of osteoarthritis: The role of fibroblast-like
chondrocytes in late stages of the disease. Histol Histopathol.
20:329–337. 2005.
|
|
2
|
Burnett BP, Levy R and Cole BJ: Metabolic
mechanisms in the pathogenesis of osteoarthritis. A review. J Knee
Surg. 19:191–197. 2006.PubMed/NCBI
|
|
3
|
Aigner T, Söder S, Gebhard PM, McAlinden A
and Haag J: Mechanisms of disease: Role of chondrocytes in the
pathogenesis of osteoarthritis - structure, chaos and senescence.
Nat Clin Pract Rheumatol. 3:391–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas CM, Fuller CJ, Whittles CE and
Sharif M: Chondrocyte death by apoptosis is associated with
cartilage matrix degradation. Osteoarthritis Cartilage. 15:27–34.
2007. View Article : Google Scholar
|
|
5
|
Lee HG and Yang JH: PKC-δ mediates
TCDD-induced apoptosis of chondrocyte in ROS-dependent manner.
Chemosphere. 81:1039–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abouheif MM, Nakasa T, Shibuya H, Niimoto
T, Kongcharoensombat W and Ochi M: Silencing microRNA-34a inhibits
chondrocyte apoptosis in a rat osteoarthritis model in vitro.
Rheumatology (Oxford). 49:2054–2060. 2010. View Article : Google Scholar
|
|
7
|
Trujillo E, González T, Marín R,
Martín-Vasallo P, Marples D and Mobasheri A: Human articular
chondrocytes, synoviocytes and synovial microvessels express
aquaporin water channels; upregulation of AQP1 in rheumatoid
arthritis. Histol Histopathol. 19:435–444. 2004.PubMed/NCBI
|
|
8
|
Geyer M, Grässel S, Straub RH, Schett G,
Dinser R, Grifka J, Gay S, Neumann E and Müller-Ladner U:
Differential transcriptome analysis of intraarticular lesional vs
intact cartilage reveals new candidate genes in osteoarthritis
pathophysiology. Osteoarthritis Cartilage. 17:328–335. 2009.
View Article : Google Scholar
|
|
9
|
Gao H, Ren G, Xu Y, Jin C, Jiang Y, Lin L,
Wang L, Shen H and Gui L: Correlation between expression of
aquaporins 1 and chondrocyte apoptosis in articular chondrocyte of
osteoarthritis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
25:279–284. 2011.In Chinese. PubMed/NCBI
|
|
10
|
Hayami T, Pickarski M, Zhuo Y, Wesolowski
GA, Rodan GA and Duong LT: Characterization of articular cartilage
and subchondral bone changes in the rat anterior cruciate ligament
transection and meniscectomized models of osteoarthritis. Bone.
38:234–243. 2006. View Article : Google Scholar
|
|
11
|
Sandell LJ, Xing X, Franz C, Davies S,
Chang LW and Patra D: Exuberant expression of chemokine genes by
adult human articular chondrocytes in response to IL-1beta.
Osteoarthritis Cartilage. 16:1560–1571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Xie CM, Li ZP, Zhu ZW, Yan YS and
Du HC: Inhibition of the aquaporin-1 gene expression by RNA
interference: experiment with cultured rat pleural mesothelial
cells. Zhonghua Yi Xue Za Zhi. 87:1773–1777. 2007.In Chinese.
PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Watrin-Pinzano A, Etienne S, Grossin L,
Gaborit N, Cournil-Henrionnet C, Mainard D, Netter P, Gillet P and
Galois L: Increased apoptosis in rat osteoarthritic cartilage
corresponds to degenerative chondral lesions and concomitant
expression of caspase-3. Biorheology. 43:403–412. 2006.PubMed/NCBI
|
|
15
|
Attur MG, Dave M, Akamatsu M, Katoh M and
Amin AR: Osteoarthritis or osteoarthrosis: The definition of
inflammation becomes a semantic issue in the genomic era of
molecular medicine. Osteoarthritis Cartilage. 10:1–4. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goggs R, Carter SD, Schulze-Tanzil G,
Shakibaei M and Mobasheri A: Apoptosis and the loss of chondrocyte
survival signals contribute to articular cartilage degradation in
osteoarthritis. Vet J. 166:140–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HA, Lee YJ, Seong SC, Choe KW and Song
YW: Apoptotic chondrocyte death in human osteoarthritis. J
Rheumatol. 27:455–462. 2000.PubMed/NCBI
|
|
18
|
Nicholson DW and Thornberry NA: Caspases:
Killer proteases. Trends Biochem Sci. 22:299–306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kliem H, Berisha B, Meyer HH and Schams D:
Regulatory changes of apoptotic factors in the bovine corpus luteum
after induced luteolysis. Mol Reprod Dev. 76:220–230. 2009.
View Article : Google Scholar
|
|
20
|
Irony-Tur-Sinai M, Lichtenstein M, Brenner
T and Lorberboum-Galski H: IL2-caspase3 chimeric protein controls
lymphocyte reactivity by targeted apoptosis, leading to
amelioration of experimental autoimmune encephalomyelitis. Int
Immunopharmacol. 9:1236–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwarz K, Simonis G, Yu X, Wiedemann S
and Strasser RH: Apoptosis at a distance: Remote activation of
caspase-3 occurs early after myocardial infarction. Mol Cell
Biochem. 281:45–54. 2006. View Article : Google Scholar
|
|
22
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar
|
|
23
|
Maeno E, Ishizaki Y, Kanaseki T, Hazama A
and Okada Y: Normotonic cell shrinkage because of disordered volume
regulation is an early prerequisite to apoptosis. Proc Natl Acad
Sci USA. 97:9487–9492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jablonski EM and Hughes FM Jr: The
potential role of caveolin-1 in inhibition of aquaporins during the
AVD. Biol Cell. 98:33–42. 2006. View Article : Google Scholar
|
|
25
|
Flamenco P, Galizia L, Rivarola V,
Fernandez J, Ford P and Capurro C: Role of AQP2 during apoptosis in
cortical collecting duct cells. Biol Cell. 101:237–250. 2009.
View Article : Google Scholar
|
|
26
|
Jablonski EM, Webb AN, McConnell NA, Riley
MC and Hughes FM Jr: Plasma membrane aquaporin activity can affect
the rate of apoptosis but is inhibited after apoptotic volume
decrease. Am J Physiol Cell Physiol. 286:C975–C985. 2004.
View Article : Google Scholar
|
|
27
|
Jessica Chen M, Sepramaniam S, Armugam A,
Shyan Choy M, Manikandan J, Melendez AJ, Jeyaseelan K and Sang
Cheung N: Water and ion channels: Crucial in the initiation and
progression of apoptosis in central nervous system? Curr
Neuropharmacol. 6:102–116. 2008. View Article : Google Scholar
|
|
28
|
Lee WK, Bork U, Gholamrezaei F and
Thévenod F: Cd(2+)-induced cytochrome c release in apoptotic
proximal tubule cells: Role of mitochondrial permeability
transition pore and Ca(2+) uniporter. Am J Physiol Renal Physiol.
288:F27–F39. 2005. View Article : Google Scholar
|
|
29
|
Jablonski EM, Mattocks MA, Sokolov E,
Koniaris LG, Hughes FM Jr, Fausto N, Pierce RH and McKillop IH:
Decreased aquaporin expression leads to increased resistance to
apoptosis in hepatocellular carcinoma. Cancer Lett. 250:36–46.
2007. View Article : Google Scholar
|
|
30
|
Li C, Shi Y, Wang W, Sardeli C, Kwon TH,
Thomsen K, Jonassen T, Djurhuus JC, Knepper MA, Nielsen S and
Frøkiaer J: alpha-MSH prevents impairment in renal function and
dysregulation of AQPs and Na-K-ATPase in rats with bilateral
ureteral obstruction. Am J Physiol Renal Physiol. 290:F384–F396.
2006. View Article : Google Scholar
|