Introduction
The β-adrenergic receptor (β-AR) belongs to the
family of G protein-coupled receptors and consists of 3 subtypes:
β1-, β2- and β3-AR. The different subtypes are associated with
different changes during the course of heart failure. the
stimulation of β1- and β2-AR produces positive chronotropic and
inotropic effects, while the activation of β3-AR decreases the
contractility of the myocardium. In the failing ventricular
myocardium, β1-AR is downregulated, whereas β2-AR exhibits little
or no change. However, β3-AR is upregulated (1–4).
Pulmonary edema is a main complication of heart failure. β-ARs also
exist in the lungs, where the dominant subtype is β2-AR (5). There are currently few available
articles on the changes in β-AR levels in the lungs during the
course of heart failure. A recent study demonstrated that the β2-AR
mRNA level was significantly decreased in a murine model of heart
failure in pulmonary tissues (6).
Our previous study demonstrated a decrease in β3-AR mRNA and
protein in the lungs of an aged rat model during heart failure
(3). However, to the best of our
knowledge, we have not found any article discussing the changes in
the levels of pulmonary β1- and β2-ARs during the course of heart
failure in aged rats. It is commonly observed that heart failure
occurs mainly in aged subjects, and aged individuals have some
physiological and pathological characters which differ from those
of young individuals (7). Aging
should be considered as an important factor when elucidating
cardiac disease mechanisms (7).
The pathogenetic role of anti-β1-AR autoantibody has
long been researched in experimental models (8,9),
and anti-β1-AR autoantibody is closely associated with cardiac
sympathetic nervous activity and reduced cardiac function in
patients with heart failure (10). Anti-β1-AR autoanti body induces
the apoptosis of adult rat cardiomyocytes (11). We hypothesized that since β2-AR
also belongs to β-ARs (the G protein-coupled receptors
characterized by 7 transmembrane domains of 22–28 amino acids and
having 3 intracellular and 3 extracellular loops), its
corresponding autoantibody would undergo similar changes during the
course of heart failure.
Fas, a type I membrane protein, is a member of the
tumor necrosis factor (TNF) gene superfamily, which is distributed
on the cytoplasmic membrane. Fas ligand (FasL), a cell surface
molecule also belonging to the tumor necrosis (TNF) family, binds
to its receptor Fas, thus activating the caspase system and
inducing apoptosis (12). The
Fas/FasL system suggests a pathophysiological role of cardiomyocyte
apoptosis in patients with worsening heart failure. Circulating
apoptotic mediators (sFas and sFasL) have been shown to be
significantly higher in patients with chronic heart failure as
compared to normal control subjects (13,14). Thus, in this study, we also
investigated the association between anti-β-AR autoantibody and the
apoptotic mediators, sFas and sFasL.
Based on these considerations, in this study, we
used aged Wistar rats to examine the expression levels of β1- and
β2-ARs in the lungs and the changes in anti-β2-AR autoantibody in a
rat model of heart failure. We also investigated apoptotic factors
(sFas and sFasL) in a rat model of heart failure. The findings of
this study suggested that the densities of pulmonary β1- and β2-ARs
decreased, while the levels of anti-β2-AR autoantibody exhibited
changes similar to those of anti-β1-AR autoantibody.
Materials and methods
Animal model
All the animal experiments were approved by the
Institutional Animal Care and Use Committee of Capital Medical
University (Beijing, China; 10-A-35). Male Wistar rats (20 months
old; body weight, 400–450 g) were housed (3 animals/cage) in a room
with a controlled temperature (22°C) and a 12-h light/12-h dark
cycle. The rats were randomly divided into 2 groups as follows: the
heart failure group (n=50) and the sham-operated group (n=30).
Endotracheal intubation was performed as previously described by
Brown et al (15). The rat
model of heart failure was established as follows: through a small
incision at the second intercostal space, the transverse aorta was
isolated. A stenosis of the ascending aorta was induced by a
ligation of the aorta (steel wire area/ascending aorta area, 75%),
as previously described (16).
The sham-operated group underwent the same surgical procedure, but
without the occlusion of the aorta. At 24 h (0 week) or 9 weeks
post-surgery, the animals (0 week, sham-operated group, n=11; heart
failure group, n=12; 9 weeks, sham-operated group, n=16; heart
failure group, n=18) were anesthetized by intraperitoneal injection
of urethane (20%, 1 g/kg) and a cannula connected to a pressure
transducer was inserted along the carotid artery into the left
ventricle to measure the primary and derived variables, including
heart rate (HR), left ventricular end-systolic pressure (LVESP),
left ventricular end-diastolic pressure (LVEDP), maximum rate of
rise of left ventricular pressure (+dp/dt max) and maximum rate of
fall of left ventricular pressure (−dp/dt max). A pressure
transducer connected to a polygraph recorder was used to measure
the pressure. The left ventricles and lungs were rapidly excised,
rinsed in ice-cold isotonic saline, and weighed and frozen in
liquid nitrogen. During the course of the experiment, 2 ml of blood
sample was obtained from the carotid artery from both groups. The
sera were separated. The sera and the tissues were stored at −80°C
until further analysis.
Determination of anti-β-ARs autoantibody
concentrations by enzyme-linked immunosorbent assay (ELISA)
Two peptides with cysteine at the terminal position,
corresponding to the sequence of the second extracellular loop of
the rat β1-AR (residues 197–222,
H-W-W-R-A-E-S-D-E-A-R-R-C-Y-N-D-P-K-C-C-D-F-V-T-N-R-C) and β2-AR
(residues 173–198,
W-Y-R-A-T-H-K-Q-A-I-D-C-Y-A-K-E-T-C-C-D-F-F-T-N-Q-A-C), were
synthesized by the Institute of Laboratory Animal Science, Chinese
Academy of Medical Sciences (CAMS) and Peking Union Medical College
(PUMC) (Beijing, China), using the solid phase method of
Merrifield. The presence of the autoantibodies was detected by
ELISA as previously described (8).
Determination of β-AR protein expression
by western blot analysis
Cardiac and pulmonary tissues (n=8 for each group)
were homogenized in ice-cold homogenization buffer separately and
then centrifuged at 12,000 × g 4°C. Total protein was isolated from
the tissues and the concentration was determined using the Bradford
method. The protein sample (80 µg) was separated by 10% sodium
dodecyl sulphate-polyacrylamide gel electrophoresis and then
transferred onto nitrocellulose membranes. The membranes were
blocked for 1 h at room temperature in TBS-T, containing 5% non-fat
milk and probed with 1:1,000 diluted primary antibodies against
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; TA-08; made in
USA, subpackaged by ZSGB-BIO, Beijing, China) and β1- and β2-ARs
[β1-AR (V-19): sc-568, β2-AR (M-20): sc-570; Santa Cruz
Biotechnology, Santa Cruz, CA, USA]. Goat anti-mouse (ZB-2305; made
in USA, subpackaged by ZSGB-BIO) or goat anti-rabbit (ZB-2301,made
in USA, subpackaged by ZSGB-BIO) antibodies (1:3,000 dilution) were
used as secondary antibodies to incubate the blots for 1 h. Bands
on the blots were visualized using western blot kit (Promega,
Madison, WI, USA) and semi-quantified using a computer image
analysis system (Quantity One software v4.62; Bio-Rad, Hercules,
CA, USA).
Measurement of serum sFas/sFasL
levels
The serum concentrations of sFas and sFasL were
measured using commercially available ELISA kits, according to the
manufacturer's instructions. The kits were obtained from Shanghai
Senxiong Biotech Industry Co., Ltd. (Shanghai, China). Each group
consisted of 8 rats.
Data analysis
Data are expressed as the means ± SD. The mean of
antibody titers is represented by geometric means. A statistical
comparison of group means was performed using the Student's t-test.
A statistical comparison of the positive ratios of autoantibodies
was performed using the Chi-square test. Data analysis was
performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
on a personal computer. A value of P<0.05 was considered to
indicate a statistically significant difference, and a value of
P<0.01 was considered to indicate a highly statistically
significant difference.
Results
Mortality rate of aged rats
The mortality rate in the heart failure group was
significantly higher than that in the sham-operated group
(P<0.01, 40 vs. 10%) during the post-operative period (0–9
weeks). The major causes of death were over-anesthesia in the
sham-operated group and heart failure in the heart failure group
(Table I).
 | Table IMortality of aged rats. |
Table I
Mortality of aged rats.
| Time | Sham-operated
group
(n=30) | Heart failure
group
(n=50) |
|---|
| 0 week (within 24
h) | 3 (10%) | 4 (8%) |
| 1 week (24 h to 7
days) | 0 | 3 (6%) |
| 2 weeks | 0 | 2 (4%) |
| 3 weeks | 0 | 1 (2%) |
| 4 weeks | 0 | 1 (2%) |
| 5 weeks | 0 | 0 |
| 6 weeks | 0 | 1 (2%) |
| 7 weeks | 0 | 0 |
| 8 weeks | 0 | 3 (6%) |
| 9 weeks | 0 | 5 (10%) |
| Total | 3 (10%) | 20 (40%) |
Changes in cardiac function
The changes in cardiac function are presented in
Table II. At 9 weeks
post-surgery, the cardiac function parameters, LVESP and the
maximum rate of rise or fall of left ventricular pressure absolute
values, were significantly decreased in the rats in the heart
failure group as compared to the control animals (P<0.01). LVEDP
was significantly increased (P<0.01) in the heart failure group,
which indicated the occurrence of heart failure.
 | Table IIChanges in cardiac function. |
Table II
Changes in cardiac function.
| Group | Time
(weeks) | n | HR
(bpm) | LVESP
(mmHg) | LVEDP
(mmHg) |
+dp/dtmax
(mmHg/msec) |
−dp/dtmax
(mmHg/msec) |
|---|
| Sham | 0 | 11 | 327±16 | 127.7±5.9 | 1.07±0.10 | 9.84±0.72 | −6.41±0.72 |
| 9 | 16 | 325±13 | 129.7±5.8 | 1.15±0.14 | 9.46±0.54 | −6.10±0.41 |
| HF | 0 | 12 | 332±18 | 131.2±4.8 | 1.13±0.13 | 9.56±0.49 | −6.00±0.65 |
| 9 | 18 | 291±16a | 91.7±7.2a | 10.53±0.60a | 4.23±0.07a | −2.61±0.25a |
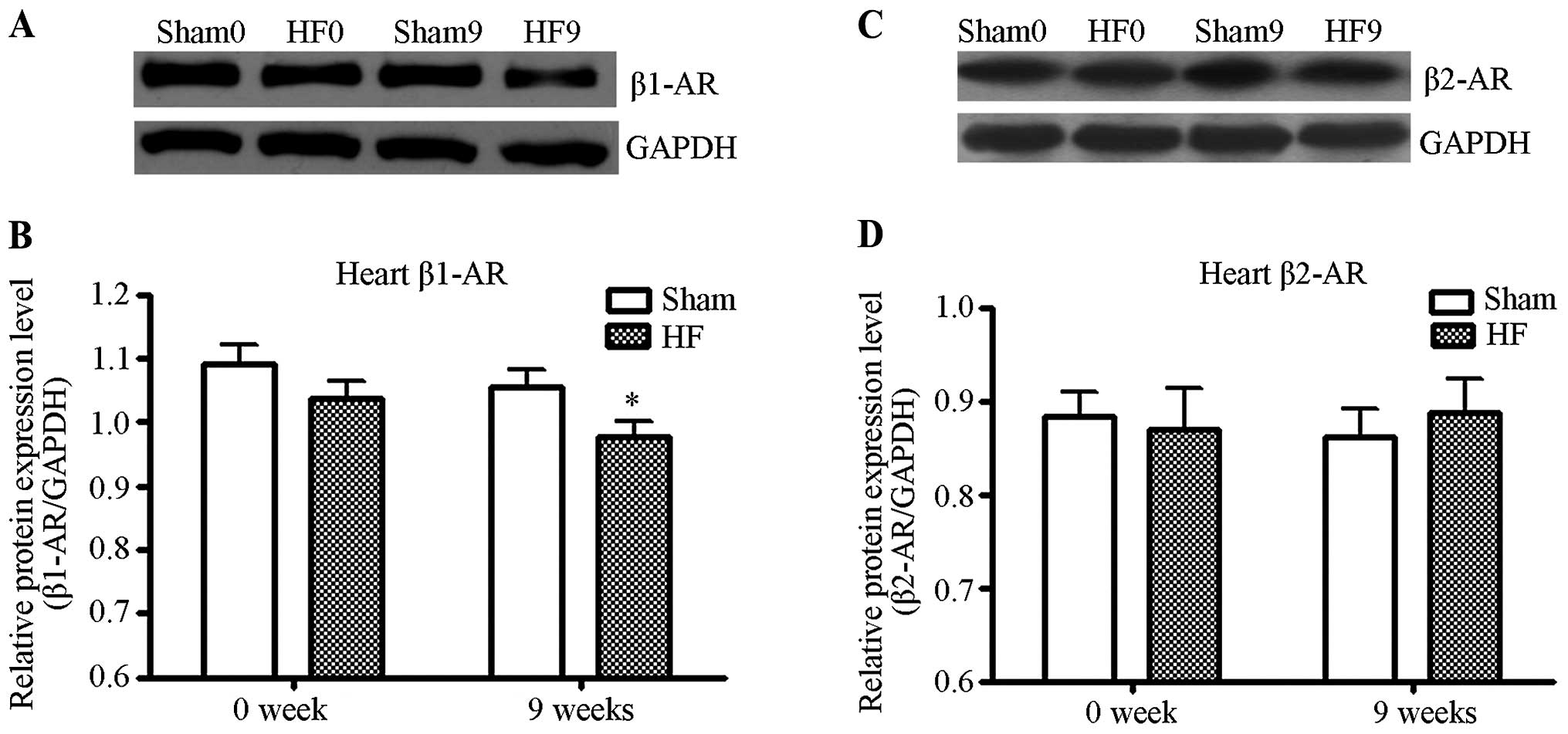
The protein levels of β1- and β2-ARs. The
protein levels of β1- and β2-ARs in the left ventricle of the rats
from the control and heart failure groups were quantified by
western blot analysis. As shown in Fig. 1, the density of β1-AR protein in
the heart failure group decreased significantly as compared to the
control (P<0.01). The density of β2-AR protein however, did not
exhibit any significant change in the heart failure group, as
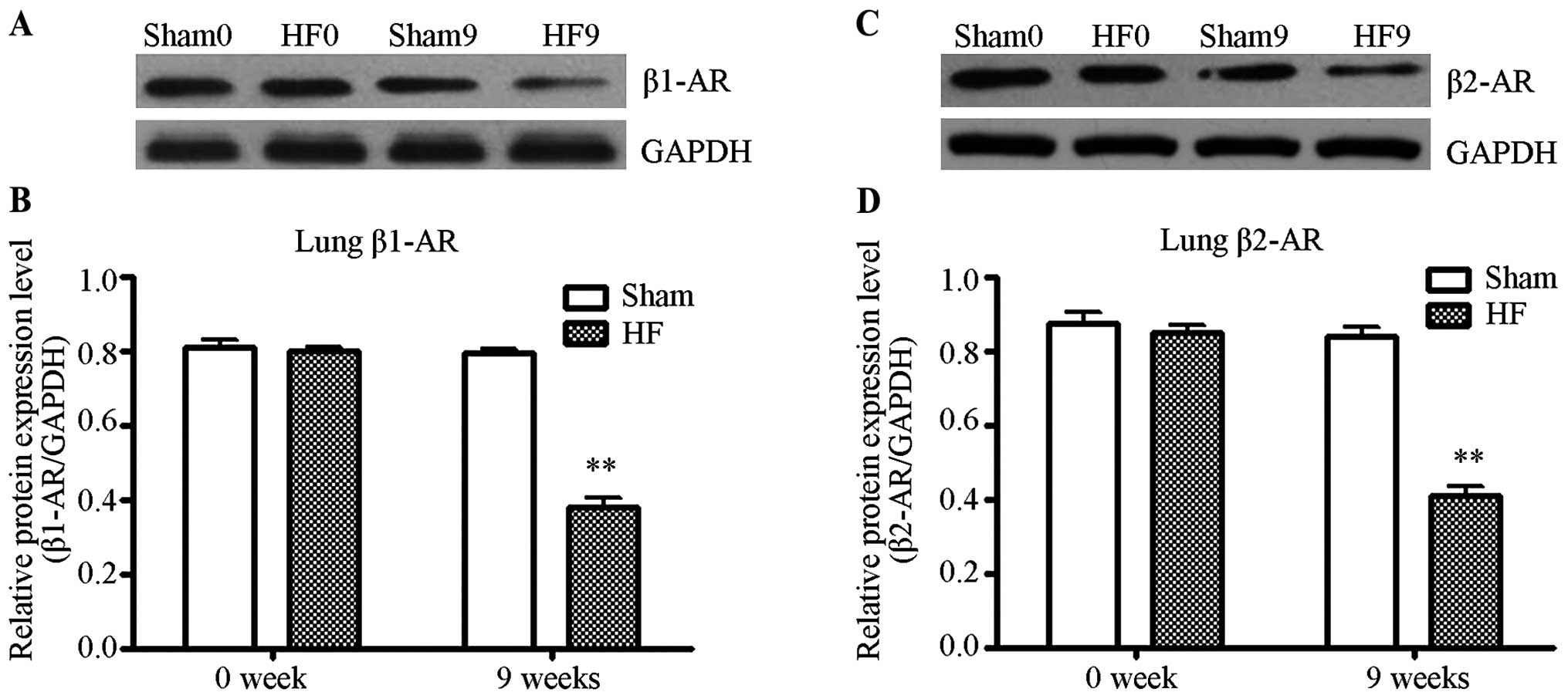
compared to the control (P>0.05). The protein levels of β1- and
β2-ARs in the lungs of rats from the control and heart failure
groups were quantified by western blot analysis. As shown in
Fig. 2, at 9 weeks post-surgery,
the density of β1- and β2-AR protein in the heart failure group
decreased significantly as compared to the control (P<0.01).
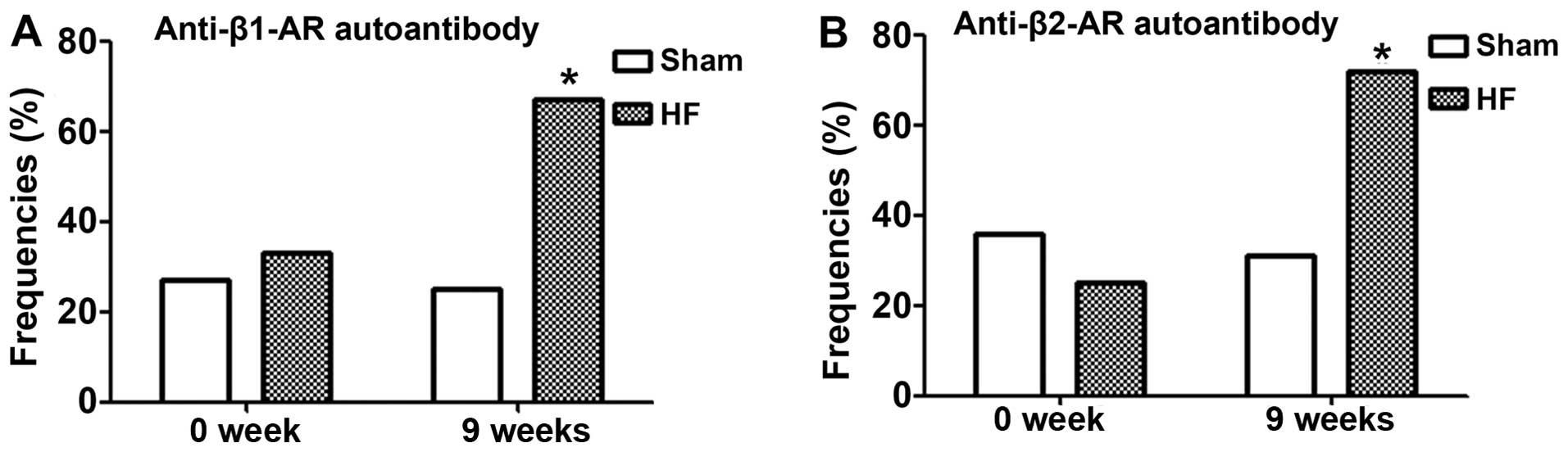
Changes in the frequencies of the
occurrence and titres of autoantibodies
At 9 weeks post-surgery, when cardiac function
exhibited significant changes, the positive ratios of the anti-β1-
and -β2-AR autoantibodies were increased from 25 and 31.3 to 66.7
and 72.2%, respectively (P<0.05; Fig. 3). The anti-β1- and -β2-AR antibody
titers were increased from 1:(39.8±1.6) and 1:(45.7±1.8) to
1:(117.5±1.8) (P<0.05) and 1:(112.2±2.0) (P<0.05),
respectively (Fig. 4).
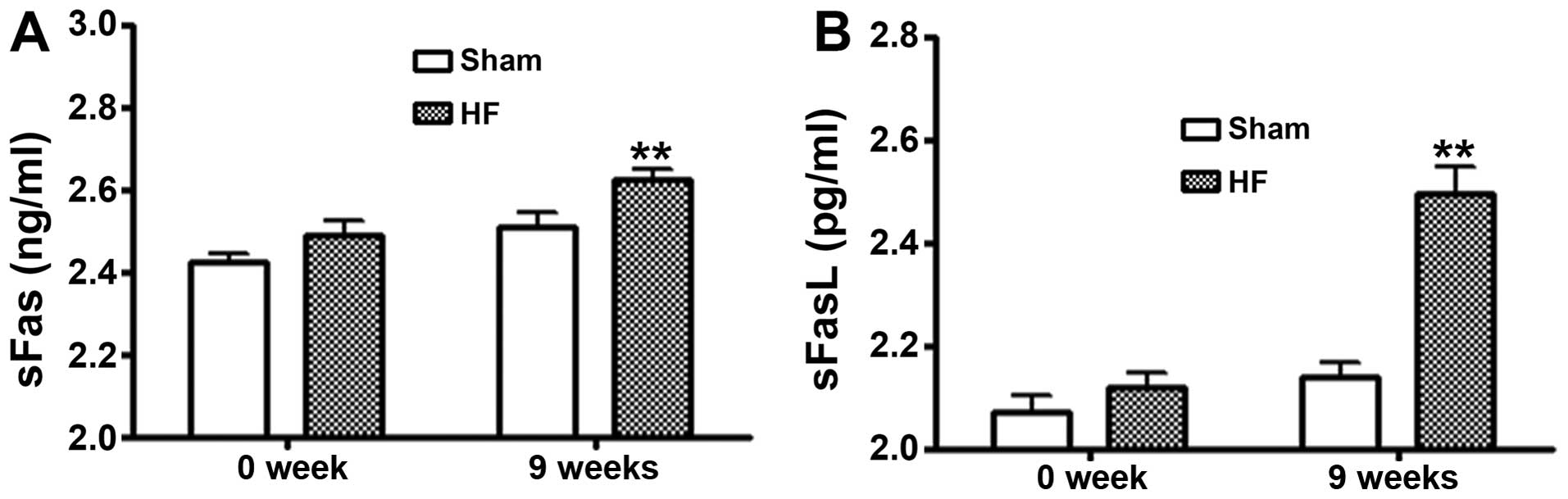
Serum levels of sFas/sFasL
The levels of sFas/sFasL in serum from the rats in
the control and heart failure groups were measured by ELISA. As
shown in Fig. 5, at 9 weeks
post-surgery the levels of sFas/sFasL in the heart failure group
were significantly increased as compared to the control
(P<0.01).
Levels of sFas/sFasL at 9 weeks
post-surgery in the heart failure group
There were 18 animals at 9 weeks post-surgery in the
heart failure group. We defined the 12 animals with positive
anti-β1-AR autoantibody as the group with positive anti-β1-AR
autoantibody, and the 6 animals with negative anti-β1-AR
auto-antibody as the group with negative anti-β1-AR autoantibody.
We defined the 13 animals with positive anti-β2-AR autoantibody as
the group with positive anti-β2-AR autoantibody, and the 5 animals
with negative anti-β2-AR autoantibody as the group with negative
anti-β2-AR autoantibody. There were no differences observed in the
levels of sFas or sFasL between the groups with positive and
negative anti-β1-AR autoantibody. In addition, there were no
differences observed in the levels of sFas or sFasL between the
groups with positive and negative anti-β2-AR autoantibody (Fig. 6).
Discussion
The data from the present study provided three
important observations: i) a decrease in the pulmonary β1- and
β2-AR protein levels during the development of heart failure; ii)
at 9 weeks post-surgery, both the frequency of the occurrence and
the titres of anti-β2-AR autoantibody were significantly increased
as compared to those of the control; and iii) at 9 weeks
post-surgery, the levels of sFas/sFasL in the heart failure group
decreased significantly as compared to those of the control. From
these results, we can draw some important conclusions.
Changes in the densities of pulmonary β1-
and β2-ARs the rat model of heart failure
Studies carried out on adult rats have demonstrated
that in the failing ventricular myocardium, β1-AR is downregulated,
whereas β2-AR exhibits little or no change (1,2).
Similar results were observed in aged rats. Pulmonary edema is the
main threatening complication of heart failure. Since β-ARs also
exist in the lungs, we wished to determine whether pulmonary β1-
and β2-ARs experience any changes in a rat model of heart failure.
Fewer studies have related the changes in the levels of pulmonary
β-ARs during the course of heart failure. In 1999, Borst et
al studied the changes in the total AR density in a rat model
of heart failure and concluded that the density was significantly
decreased (17). However, due to
the presence of 3 subtypes of β-ARs, studies on the changes of
different subtypes are mandatory. Our study demonstrated that in an
aged rat model of heart failure, the levels of both pulmonary β1-
and β2-ARs were significantly decreased, and the changes in these
levels differed from those in the heart. The respiratory system is
characterized by the prevalence of β2-AR. β2-AR plays a key role in
both the regulation of airway smooth muscle tone and lung fluid
clearance, which influences gas diffusion (18,19). β1-AR is also present in the lungs,
and accounts for 10 and 30% of the ARs on submucosal glands and
alveolar walls, respectively (20). Some studies have indciated that
both β1- and β2-AR agonists increase alveolar fluid clearance in
experimental models (21,22). The decrease in the levels of
pulmonary β1- and β2-ARs during the course of heart failure may
increase the airway smooth muscle tone and reduce lung fluid
clearance. This change may aggravate pulmonary edema to a certain
extent.
Changes in the levels of anti-β2-AR
autoantibody during the course of heart failure
We chose to use aged 20-month-old Wistar rats as
experiment animals and established a model of heart failure by
aortic binding. We found that both the frequency of the occurrence
and the titre of anti-β1-AR autoantibody were significantly
increased as compared to the control. More importantly, it was
observed that anti-β2-AR autoantibody exhibited a similar change to
that of anti-β1-AR autoantibody. Thus, it was opined that
anti-β1-AR autoantibody was closely associated with cardiac
sympathetic nervous activity and cardiac event in patients with
chronic heart failure (10,23,24). Heart failure can trigger
anti-β1-AR autoantibody and conversely, anti-β1-AR autoantibody can
aggravate heart failure. Carvedilol (β-AR blocker) has been shown
to be effective in improving cardiac dysfunction and reversing
remodeling in patients positive for anti-β-AR autoantibody
(25). Stavrakis et al
(26) demonstrated that the
co-existence of anti-β2-AR autoantibody partially suppressed the
effect of the anti-β1-AR autoantibody, although the study was
carried out on patients with cardiomyopathy and not on patients
with heart failure. Our study demonstrated that heart failure can
also trigger anti-β2-AR autoantibody. Since anti-β1-AR autoantibody
can aggravate heart failure and the co-existence of anti-β2-AR
autoantibody can partially suppress the effect of the anti-β1-AR
autoantibody, we opined that anti-β2-AR autoantibody can alleviate
heart failure to a certain extent during the course of heart
failure. The rate of autoantibodies against two types of β-ARs was
24.6%, which indicated a multiplicity of autoimmune response in
heart failure.
Changes exhibited by β-ARs and their
corresponding autoantibodies during the development of heart
failure
Few studies have examined the changes in β-ARs and
the changes in autoantibodies simultaneously (3,27).
In this study, we investigated β-ARs and autoantibodies
simultaneously and found that, although the two types of β-ARs
exhibited different changes during the development of heart
failure, their corresponding autoantibodies exhibited similar
changes. Genetic and pharmacological approaches have demonstrated
that the β1-AR constitutes approximately 70–80% of the cardiac β-AR
complement and plays a predominant role in mediating cardiac
inotropic and chronotropic responses to catecholamines (28). In the failing ventricular
myocardium and lungs, β1-AR is down-regulated. Therefore, the
change in the levels of anti-β1-AR autoantibody was found to be
related with the change in the levels of β1-AR in the heart and
lungs. β2-AR is the main β-AR subtype in the lungs (6). In the failing ventricular
myocardium, the β2-AR protein level was unaltered. Pulmonary
β-receptor density was significantly decreased in a rat model of
heart failure (29). Thus, the
change in anti-β2-AR autoantibody was mainly associated with the
changes in β2-AR in the lungs. Since it was established that the
co-existence of anti-β2-AR autoantibody partially suppressed the
effect of anti-β1-AR autoantibody and affected the constriction of
the heart, we speculated that anti-β2-AR autoantibody may act on
β2-AR in the lungs and affect the regulation of airway smooth
muscle tone and lung fluid clearance. Thus, anti-β2-AR autoantibody
may affect the course of heart failure, not only through the heart,
but also through the lungs. We only proved the existence of
anti-β2-AR autoantibody in a model of heart failure; further
studies are required to investigate anti-β2-AR autoantibody in more
detail.
Association between anti-β-AR
autoantibody and the levels of sFas/sFasL during the course of
heart failure
With the increase in the frequencies and titres of
autoantibodies, the sFas and sFasL levels were also elevated. These
exert differential effects on the course of apoptosis. Human sFasL
induces apoptosis in vitro and in vivo (30), but sFas inhibits the apoptosis of
muscle cells in cell culture (31), and improves the survival of
animals with heart failure (32).
Norepinephrine stimulates apoptosis via β1-AR and inhibits
apoptosis via β2-AR. β1-AR plays a predominant role in the adult
rat cardiac myocyte system, and the net effect of norepinephrine
results in an increase in the frequency of apoptosis (33). Anti-β1-AR autoantibody induces the
apoptosis of adult rat cardiomyocytes via the protein kinase A
cascade (11) and mediates
dilated cardiomyopathy agonistically by inducing cardiomyocyte
apoptosis (34). Although
anti-β1-AR autoantibody induces apoptosis, we did not determine any
difference in sFas or sFasL between the groups with positive and
negative anti-β1-AR autoantibody. We were not able to find any
difference in sFas or sFasL between the groups with positive and
negative anti-β2-AR autoantibodies.
In conclusion, the findings of our study suggested
that during the development of heart failure, the densities of
pulmonary β1- and β2-ARs decreased and the changes were different
from those occurring in the heart. The levels of anti-β2-AR
autoantibody exhibited similar changes to those of anti-β1-AR
autoantibody during the course of heart failure. When the
frequencies and titres of autoantibodies increased, the levels of
sFas and sFasL were also elevated; however, there was no definite
association between anti-β-AR autoantibody and the levels of
sFas/sFasL.
Acknowledgments
The authors of this study acknowledge Ms. Xiu-Lan
Liu, Ms. Jing Chang and Ms. Ling-Qiao Lu for their contributions to
the data collection. There has been no financial assistance with
this project.
References
|
1
|
Bristow MR, Ginsburg R, Umans V, Fowler M,
Minobe W, Rasmussen R, Zera P, Menlove R, Shah P and Jamieson S:
Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing
and failing human ventricular myocardium: coupling of both receptor
subtypes to muscle contraction and selective beta 1-receptor
down-regulation in heart failure. Circ Res. 59:297–309. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engelhardt S, Böhm M, Erdmann E and Lohse
MJ: Analysis of beta-adrenergic receptor mRNA levels in human
ventricular biopsy specimens by quantitative polymerase chain
reactions: progressive reduction of beta 1-adrenergic receptor mRNA
in heart failure. J Am Coll Cardiol. 27:146–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miao G, Chen Z, Fang X, Liu M, Hao G, An
H, Zhang Z, Lu L, Zhang J and Zhang L: Relationship between the
autoantibody and expression of β3-adrenoceptor in lung and heart.
PLoS One. 8:e687472013. View Article : Google Scholar
|
|
4
|
Grossini E, Surico D, Mary DA, Molinari C,
Surico N and Vacca G: In anesthetized pigs human chorionic
gonadotropin increases myocardial perfusion and function through a
β-adrenergic-related pathway and nitric oxide. J Appl Physiol
(1985). 115(4): 422–35. 2013. View Article : Google Scholar
|
|
5
|
Mak JC, Nishikawa M, Haddad EB, Kwon OJ,
Hirst SJ, Twort CH and Barnes PJ: Localisation and expression of
beta-adrenoceptor subtype mRNAs in human lung. Eur J Pharmacol.
302:215–221. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rinaldi B, Capuano A, Gritti G, Donniacuo
M, Scotto Di Vettimo A, Sodano L, Rafaniello C, Rossi F and Matera
MG: Effects of chronic administration of β-blockers on airway
responsiveness in a murine model of heart failure. Pulm Pharmacol
Ther. 28:109–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horn MA, Graham HK, Richards MA, Clarke
JD, Greensmith DJ, Briston SJ, Hall MC, Dibb KM and Trafford AW:
Age-related divergent remodeling of the cardiac extracellular
matrix in heart failure: collagen accumulation in the young and
loss in the aged. J Mol Cell Cardiol. 53:82–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwata M, Yoshikawa T, Baba A, Anzai T,
Nakamura I, Wainai Y, Takahashi T and Ogawa S: Autoimmunity against
the second extracellular loop of beta(1)-adrenergic receptors
induces beta-adrenergic receptor desensitization and myocardial
hypertrophy in vivo. Circ Res. 88:578–586. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jahns R, Boivin V, Hein L, Triebel S,
Angermann CE, Ertl G and Lohse MJ: Direct evidence for a beta
1-adrenergic receptor-directed autoimmune attack as a cause of
idiopathic dilated cardiomyopathy. J Clin Invest. 113:1419–1429.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aso S, Yazaki Y, Kasai H, Takahashi M,
Yoshio T, Yamamoto K and Ikeda U: Anti-beta 1-adrenoreceptor
autoantibodies and myocardial sympathetic nerve activity in chronic
heart failure. Int J Cardiol. 131:240–245. 2009. View Article : Google Scholar
|
|
11
|
Staudt Y, Mobini R, Fu M, Felix SB, Kühn
JP and Staudt A: Beta1-adrenoceptor antibodies induce apoptosis in
adult isolated cardiomyocytes. Eur J Pharmacol. 466:1–6. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng QZ, Zhao YS and Abdelwahid E: The
role of Fas in the progression of ischemic heart failure:
prohypertrophy or proapoptosis. Coron Artery Dis. 19:527–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adamopoulos S, Parissis J, Karatzas D,
Kroupis C, Georgiadis M, Karavolias G, Paraskevaidis J, Koniavitou
K, Coats AJ and Kremastinos DT: Physical training modulates
proinflammatory cytokines and the soluble Fas/soluble Fas ligand
system in patients with chronic heart failure. J Am Coll Cardiol.
39:653–663. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinugawa T, Kato M, Yamamoto K, Hisatome I
and Nohara R: Proinflammatory cytokine activation is linked to
apoptotic mediator, soluble Fas level in patients with chronic
heart failure. Int Heart J. 53:182–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown RH, Walters DM, Greenberg RS and
Mitzner W: A method of endotracheal intubation and pulmonary
functional assessment for repeated studies in mice. J Appl Physiol
(1985). 87:2362–2365. 1999.
|
|
16
|
Chen Z, Miao G, Liu M, Hao G, Liu Y, Fang
X, Zhang Z, Lu L, Zhang J and Zhang L: Age-related up-regulation of
beta3-adrenergic receptor in heart-failure rats. J Recept Signal
Transduct Res. 30:227–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Borst MM, Beuthien W, Schwencke C, LaRosée
P, Marquetant R, Haass M, Kübler W and Strasser RH: Desensitization
of the pulmonary adenylyl cyclase system: a cause of airway
hyperresponsiveness in congestive heart failure? J Am Coll Cardiol.
34:848–856. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carstairs JR, Nimmo AJ and Barnes PJ:
Autoradiographic visualization of beta-adrenoceptor subtypes in
human lung. Am Rev Respir Dis. 132:541–547. 1985.PubMed/NCBI
|
|
19
|
Agostoni P, Contini M, Cattadori G,
Apostolo A, Sciomer S, Bussotti M, Palermo P and Fiorentini C: Lung
function with carvedilol and bisoprolol in chronic heart failure:
is beta selectivity relevant? Eur J Heart Fail. 9:827–833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruffin RE, McIntyre EL, Latimer KM, Ward
HE, Crockett AJ and Alpers JH: Assessment of beta-adrenoceptor
antagonists in asthmatic patients. Br J Clin Pharmacol. 13(Suppl
2): 325S–335S. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakuma T, Tuchihara C, Ishigaki M, Osanai
K, Nambu Y, Toga H, Takahashi K, Ohya N, Kurihara T and Matthay MA:
Denopamine, a beta(1)-adrenergic agonist, increases alveolar fluid
clearance in ex vivo rat and guinea pig lungs. J Appl Physiol
(1985). 90:10–6. 2001.
|
|
22
|
Lasnier JM, Wangensteen OD, Schmitz LS,
Gross CR and Ingbar DH: Terbutaline stimulates alveolar fluid
resorption in hyperoxic lung injury. J Appl Physiol (1985). 81(4):
1723–9. 1996.
|
|
23
|
Liu J, Wang Y, Chen M, Zhao W, Wang X,
Wang H, Zhang Z, Zhang J, Xu L, Chen J, et al: The correlation
between peripartum cardiomyopathy and autoantibodies against
cardiovascular receptors. PLoS One. 9:e867702014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshikawa T, Baba A and Nagatomo Y:
Autoimmune mechanisms underlying dilated cardiomyopathy. Circ J.
73:602–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagatomo Y, Yoshikawa T, Okamoto H,
Kitabatake A and Hori M; Japanese Chronic Heart Failure Study
Investigators: Presence of autoantibody directed against
β1-adrenergic receptors is associated with amelioration of cardiac
function in response to carvedilol: Japanese chronic heart failure
(J-CHF) study. J Card Fail. 21:198–207. 2015. View Article : Google Scholar
|
|
26
|
Stavrakis S, Kem DC, Patterson E, Lozano
P, Huang S, Szabo B, Cunningham MW, Lazzara R and Yu X: Opposing
cardiac effects of autoantibody activation of β-adrenergic and M2
muscarinic receptors in cardiac-related diseases. Int J Cardiol.
148:331–336. 2011. View Article : Google Scholar
|
|
27
|
Sterin-Borda L, Gorelik G, Postan M,
Gonzalez Cappa S and Borda E: Alterations in cardiac
beta-adrenergic receptors in chagasic mice and their association
with circulating beta-adrenoceptor-related autoantibodies.
Cardiovasc Res. 41:116–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lohse MJ, Engelhardt S and Eschenhagen T:
What is the role of beta-adrenergic signaling in heart failure?
Circ Res. 93:896–906. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu HR, Zhao RR, Jiao XY, Wang YY and Fu
M: Relationship of myocardial remodeling to the genesis of serum
autoantibodies to cardiac beta(1)-adrenoceptors and muscarinic type
2 acetyl-choline receptors in rats. J Am Coll Cardiol.
39:1866–1873. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanaka M, Suda T, Yatomi T, Nakamura N and
Nagata S: Lethal effect of recombinant human Fas ligand in mice
pretreated with Propionibacterium acnes. J Immunol. 158:2303–2309.
1997.PubMed/NCBI
|
|
31
|
Vasudevan SS, Lopes NH, Seshiah PN, Wang
T, Marsh CB, Kereiakes DJ, Dong C and Goldschmidt-Clermont PJ:
Mac-1 and Fas activities are concurrently required for execution of
smooth muscle cell death by M-CSF-stimulated macrophages.
Cardiovasc Res. 59:723–733. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Takemura G, Kosai K, Takahashi T,
Okada H, Miyata S, Yuge K, Nagano S, Esaki M, Khai NC, et al:
Critical roles for the Fas/Fas ligand system in postinfarction
ventricular remodeling and heart failure. Circ Res. 95:627–636.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Communal C, Colucci WS and Singh K: p38
mitogen-activated protein kinase pathway protects adult rat
ventricular myocytes against beta-adrenergic receptor-stimulated
apoptosis. Evidence for Gi-dependent activation. J Biol Chem.
275:19395–19400. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jane-wit D, Altuntas CZ, Johnson JM, Yong
S, Wickley PJ, Clark P, Wang Q, Popović ZB, Penn MS, Damron DS, et
al: Beta 1-adrenergic receptor autoantibodies mediate dilated
cardiomyopathy by agonistically inducing cardiomyocyte apoptosis.
Circulation. 116:399–410. 2007. View Article : Google Scholar : PubMed/NCBI
|