Introduction
The large intestine contains five types of
enteroendocrine cells: namely serotonin-, polypeptide YY (PYY)-,
oxyntomodulin (enteroglucagon)-, pancreatic polypeptide (PP)- and
somatostatin-producing cells (1).
In addition, chromogranin A (CgA) is expressed by all
enteroendocrine cells and is used as a common marker for them
(2). The interaction between
enteroendocrine and immune cells during inflammation was recently
discussed, and this interaction is thought to play a pivotal role
in the inflammatory process (3).
Patients with inflammatory bowel disease (IBD), as
well as animal models of human IBD have been shown to have abnormal
enteroendocrine cells (4–24). The nature of the changes in
enteroendocrine cells differs between ulcerative colitis (UC),
Crohn's disease (CD) and microscopic colitis (4–23).
The mechanisms underlying such abnormalities are not yet known.
However, a recent study using an animal model of human UC, namely
dextran sulfate sodium-induced colitis, found that the
abnormalities in enteroendocrine cells strongly correlated with the
abnormal differentiation progeny of stem cells (25). It has been suggested that the
abnormalities in the enteroendocrine cells in this animal model are
caused by an abnormal stem cell differentiation progeny toward
enteroendocrine cells (25).
Trinitrobenzene sulfonic acid (TNBS)-induced colitis
in experimental animals is commonly used as an animal model of
human CD (26). The
enteroendocrine cells in this animal model have been reported to be
abnormal (27). The treatment
colitis with activator protein 1 (AP-1) and nuclear factor-κB
inhibitors, which are potent anti-inflammatory agents, has been
shown to restore enteroendocrine cells to normal levels (27).
The aim of the present study was to determine
whether the changes in the densities of enteroendocrine cells in
TNBS-induced colitis involve stem cell differentiation and/or the
cellular expression of enteroendocrine cell hormones.
Materials and methods
Rats
A total of 40 male Wistar rats (Hannover GALAS;
Taconic Farms Inc., Lille Skensved, Denmark) with a mean body
weight of 200 g (range, 160–250 g) were housed in Macrolon III
cages with water and food available ad libitum. They were
fed a standard diet (B&K Universal, Nittedal, Norway) and were
kept at a temperature of 18–22°C, a relative humidity of 50–60%,
and under a 12/12-h light/dark cycle. The rats were allowed to
acclimatize to the conditions in the animal house for at least 7
days prior to being used in the experiments. The rats were divided
into the following 4 groups containing 10 animals in each: i) the
control group; ii) the group with TNBS-induced colitis with no
treatment (TNBS group); iii) the group with TNBS-induced colitis
treated with 3-[(dodecylthiocarbonyl)-methyl]-glutarimide (DTCM-G;
an activator protein-1 inhibitor) (DTCM-G group); and iv) the group
with TNBS-induced colitis treated with
dehydroxymethylepoxyquinomicin (DHMEQ; a nuclear factor-κB
inhibitor) (DHMEQ group).
This study was performed in accordance with the
Directive for the Protection of Vertebrate Animals used for
Experimental and other Scientific Purposes (86/609/EEC), in
compliance with the Helsinki Declaration. The Local Ethics
Committee for Experimental Animals at the University of Bergen
(Bergen, Norway) approved the study.
Use of TNBS to induce colitis
Colitis was induced in the rats in the TNBS, DTCM-G
and DHMEQ groups as previously described (28) using a single dose of TNBS
(Sigma-Aldrich Logistik, Steinheim, Germany). The animals were
anesthetized with isoflurane (Schering-Plough Pharmaceuticals,
North Wales, PA, USA), and TNBS was administered into the colon at
8 cm from the anal margin (25 mg/animal in 50% ethanol solution;
0.5 ml/rat), followed by 2 ml of air. TNBS was administered via an
8.5-cm-long, 2.5-mm-diameter round-tipped Teflon feeding tube
(AngTheo, Lidingö, Sweden). The animals were kept in the prone
position with their hind legs raised for approximately 3 min
following the administration of TNBS. They were supervised until
recovery and then monitored daily. The rats in the control group
were subjected to the same procedure as the rats in the TNBS group,
except that 0.9% saline was introduced into the colon instead of
TNBS.
Treatment with DTCM-G and DHMEQ
Three days following the administration of TNBS, the
rats were treated as follows: those in the control and TNBS groups
received 0.5 ml of the vehicle [0.5% carboxymethyl cellulose
(CMC)], those in the DTCM-G group received DTCM-G at 20 mg/kg body
weight in 0.5% CMC, and those in the DHMEQ group received DHMEQ at
15 mg/kg body weight in 0.5% CMC. All injections were administered
intraperitoneally twice daily for 5 days. The synthesis of DTCM-G
and DHMEQ is described in detail elsewhere (29–34). Animals exhibiting signs of pain
were administered a subcutaneous injection of 1 ml of a 0.3-g/ml
Temgesic solution (Merck Pharmaceuticals, Kenilworth, NJ, USA). At
the end of the experiments, the animals were sacrificed by the
inhalation of CO2 and tissue samples were obtained from
the colon.
Histopathological and immunohistochemical
examinations
The colonic tissues were fixed in 4% buffered
paraformaldehyde overnight, embedded in paraffin, and cut into
5-μm-thick sections, which were stained with hematoxylin and
eosin (ThermoFischer Scientific, Waltham. MA, USA). Inflammation
was evaluated using the scoring system of Hunter et al
(35). The sections were also
immunostained using the ultraView Universal DAB Detection kit
(version 1.02.0018) and the BenchMark Ultra IHC/ISH staining module
(both from Venata Medical Systems, Basel, Switzerland). The
sections were incubated with the primary antibodies for 32 min at
37°C. Details of the primary antibodies used are presented in
Table I.
 | Table IPrimary antibodies used in
immunohistochemical staining. |
Table I
Primary antibodies used in
immunohistochemical staining.
| Antibodies raised
against | Source | Code no. | Working
dilution | Type of
antibody | Detects |
|---|
| N-terminal of
purified CgA | Dako (Glostrup,
Denmark) | M869 | 1:1,000 | Monoclonal, raised
in mouse | CgA |
| Serotonin | Dako | 5HT-209 | 1:1,500 | Monoclonal, raised
in mouse | Serotonin |
| PYY | Alpha-Diagnostica
(San Antonio, TX, USA) | PYY 11A | 1:1,000 | Polyclonal, raised
in rabbit | PYY |
| Porcine
glicentin/glucagon | Acris antibodies
(Herford, Germany) | BP508 | 1:800 | Polyclonal, raised
in rabbit | Oxyntomodulin
(enteroglucagon) |
| Synthetic human
PP | Diagnostic
Biosystems (Pleasanton, CA, USA) | #114 | 1:400 | Polyclonal, raised
in rabbit | PP |
| Synthetic human
somatostatin | Dako | A566 | 1:200 | Polyclonal, raised
in rabbit | Somatostatin |
| Residues 5-21 [APQP
GLASPDSPHDPCK] of the human, mouse and rat Msi1 | Novus Biologicals
Europe (Abingdon, UK) | NB100-1759 | 1:100 | Polyclonal, raised
in rabbit | Msi1 |
| Sy\nthetic peptide
surrounding amino acid 190 of human Math1 | BioVision
(Milpitas, CA, USA) | 3658-100 | 1:50 | Polyclonal, raised
in rabbit | Math1 |
| KLH-conjugated
synthetic peptide between 40–69 amino acids from the N-terminal
region of human Neurog3 | ThermoFisher
Scientific (Oslo, Norway) | BT-B56180 | 1:50 | Polyclonal, raised
in rabbit | Neurog3 |
| Recombinant
full-length human NeuroD1 | Nordic BioSite
(Täby, Sweden) | PA5-11893 | 1:100 | Polyclonal, raised
in rabbit | NeuroD1 |
Morphometry
The endocrine cells were quantified using image
analysis software (version 1.7, cellSens; Olympus, Tokyo, Japan).
The numbers of endocrine, and Musashi (Msi1)-, Math1-, Neurogenin3
(Neurog3)- and NeuroD1-positive cells were counted manually. The
area of epithelial cells was determined manually by drawing an
enclosed region using the computer mouse.
The densities of endocrine cells were expressed as
the number of immunoreactive endocrine cells per square millimeter
of epithelium, the density of Msi1 cells was expressed as the
number of immunoreactive cells per crypt, and the densities of
Math1, Neurog3 and NeuroD1 cells were expressed as the number of
immunoreactive cells per field. Quantification was performed in 10
randomly chosen microscopic fields using a ×40 objective. The
measurements were made by the same individual (M.E.-S.) who was
blind to the identities of the slides.
Statistical analysis
Differences between the control, TNBS, DTCM-G and
DHMEQ groups were analyzed using the Kruskal-Wallis non-parametric
test, with Dunn's test as a post-test. The correlations between
abnormalities in the densities of PYY/oxyntomodulin-,
CgA/serotonin- and PP/somatostatin-positive cells were determined
using the non-parametric Spearman correlation test. The data are
presented as the mean ± SEM values. Probability values of P<0.05
were considered to indicate statistically significant
differences.
Results
Two animals died spontaneously in the TNBS group.
There were no deaths in the other 3 groups.
Histopathological and immunohistochemical
examinations
The histopathological inflammation scores were
6.4±1.1, 1.8±1.2 and 2.3±0.9 in the TNBS, DTCM-G and DHMEQ groups,
respectively (Kruskal-Wallis test, P=0.002). Dunn's test showed
that the scores differed between the TNBS group, and the DTCM-G and
DHMEQ groups (P=0.04 and 0.02, respectively) (data not shown).
CgA-, serotonin-, PYY-, oxyntomodulin-, PP-,
somatostatin-, Msi1-, Math1-, Neurog3- and NeuroD1-positive cells
were found in all the colonic tissues from the rats in all groups.
The CgA-, serotonin-, PYY-, oxyntomodulin-, PP- and
somatostatin-positive cells were located mostly in the crypts of
Lieberkühn. Msi1-positive cells were found exclusively in the
crypts of Lieberkühn. Msi1-positive cells in rats with colitis
tended to accumulate at the margins of deep ulcers. Math1-,
Neurog3- and NeuroD1-positive cells were observed in the crypts and
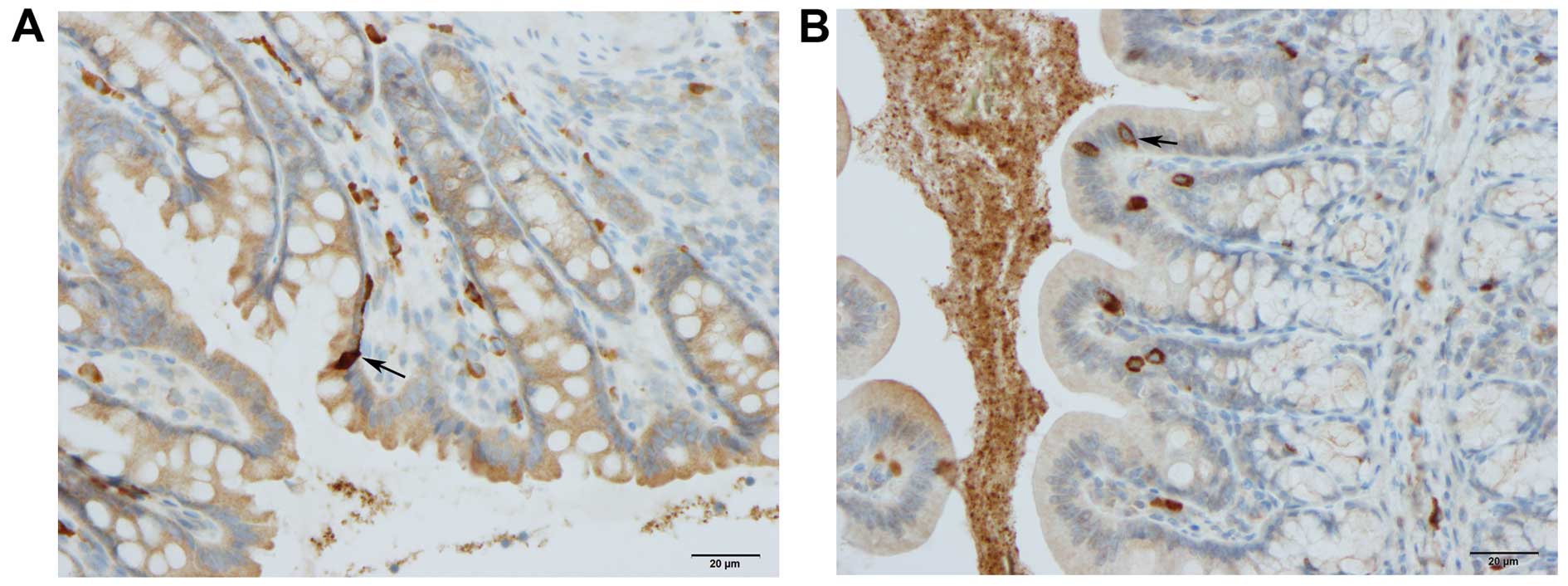
alongside the gland of Lieberkühn (Figs. 2 and 6).
Morphometry
The results of the quantification of the different
types of endocrine cells, stem cells and differentiation
progenitors in all 4 experimental groups are summarized in Tables II and III.
 | Table IIDensities of colonic enteroendocrine
cells in the 4 experimental groups. |
Table II
Densities of colonic enteroendocrine
cells in the 4 experimental groups.
| Endocrine cell
type | Controls | TNBS | DTCM-G | DHMEQ |
|---|
| CgA-positive | 111.8±17.9 | 51.0±21.1a | 104.0±14.6 | 107.6±17.1 |
|
Serotonin-positive | 40.6±6.6 | 62.6±7.5b | 39.6±6.2 | 37.0±5.4 |
| PYY-positive | 87.3±2.7 | 14.1±3.0c | 83.8±4.0 | 83.9±2.6 |
|
Oxyntomodulin-positive | 44.8±3.7 | 78.3±6.8c | 48.3±4.4 | 49.3±3.8 |
| PP-positive | 58.0±3.5 | 31.8±7.5c | 69.3±6.2 | 60.4±4.4 |
|
Somatostatin-positive | 43.6±3.2 | 69.9±7.8b | 40.5±3.2 | 43.7±5.1 |
 | Table IIIDensities of colonic stem cells and
differentiation progenitors in the 4 experimental groups. |
Table III
Densities of colonic stem cells and
differentiation progenitors in the 4 experimental groups.
| Cell type | Controls | TNBS | DTCM-G | DHMEQ |
|---|
| Msi1-positive | 4.8±0.5 | 1.9±0.3b | 4.9±0.5 | 4.5±0.5 |
| Math1-positive | 72.2±8.5 | 98.8±12.7 | 97.2±9.3 | 98.3±12.7 |
|
Neurog3-positive | 70.9±11.2 | 43.6±3.8a | 106.0±12.6 | 79.5±12.0 |
|
NeuroD1-positive | 68.8±10.4 | 44.5±7.2a | 107.5±11.7 | 81.0±12.4 |
The Kruskal-Wallis test showed that there were
significant differences between the experimental groups regarding
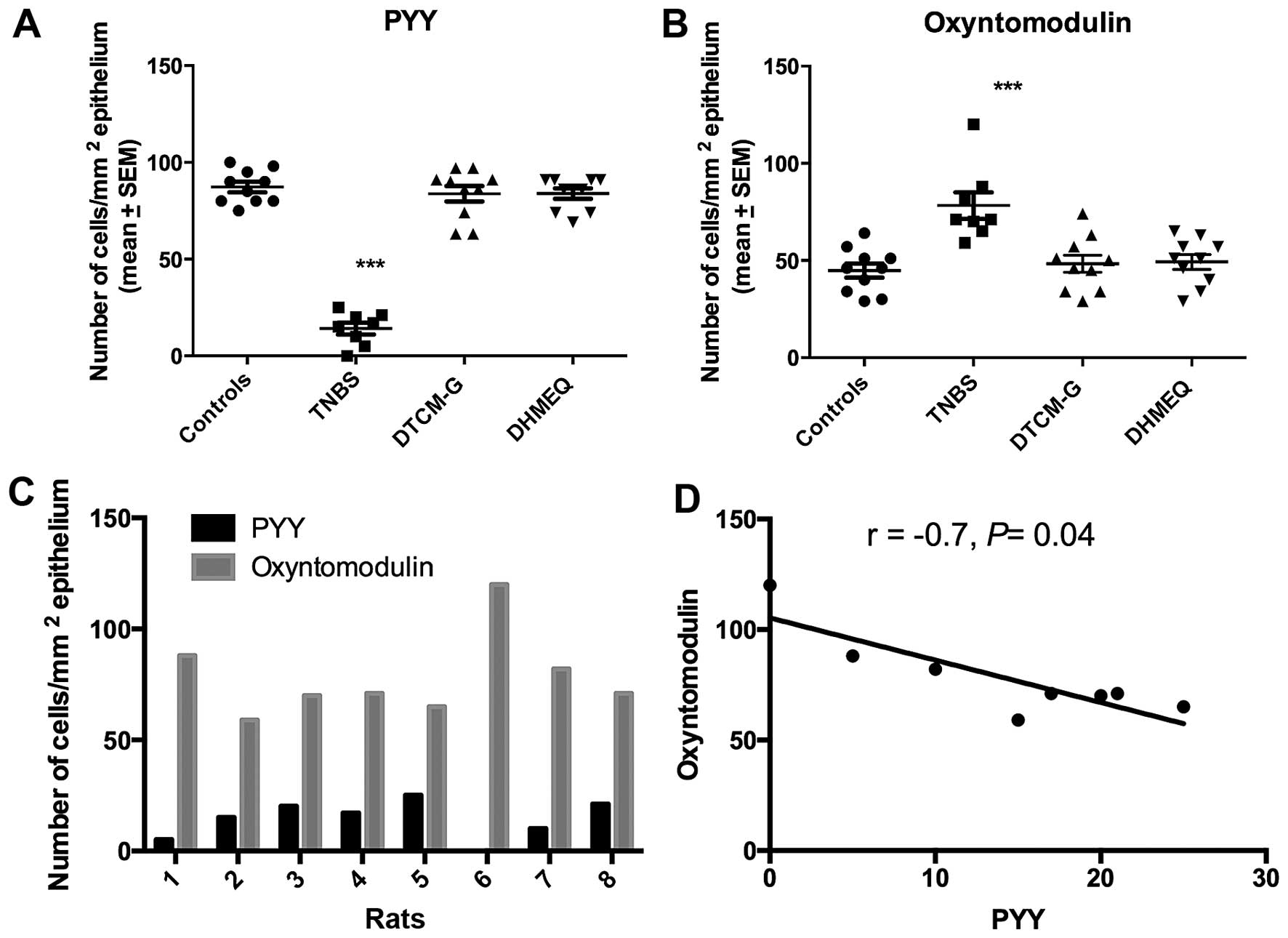
both PYY- and oxyntomodulin-positive cells (P=0.0003 and 0.001,
respectively). Whereas the density of PYY-positive cells was
significantly reduced in the TNBS group relative to the controls,
the density of oxyntomodulin-positive cells was significantly
increased (P<0.0001 for in both) (Figs. 1 and 2). The density of PYY-positive cells
inversely correlated with the density of oxyntomodulin-positive
cells (r=−0.7, P=0.04).
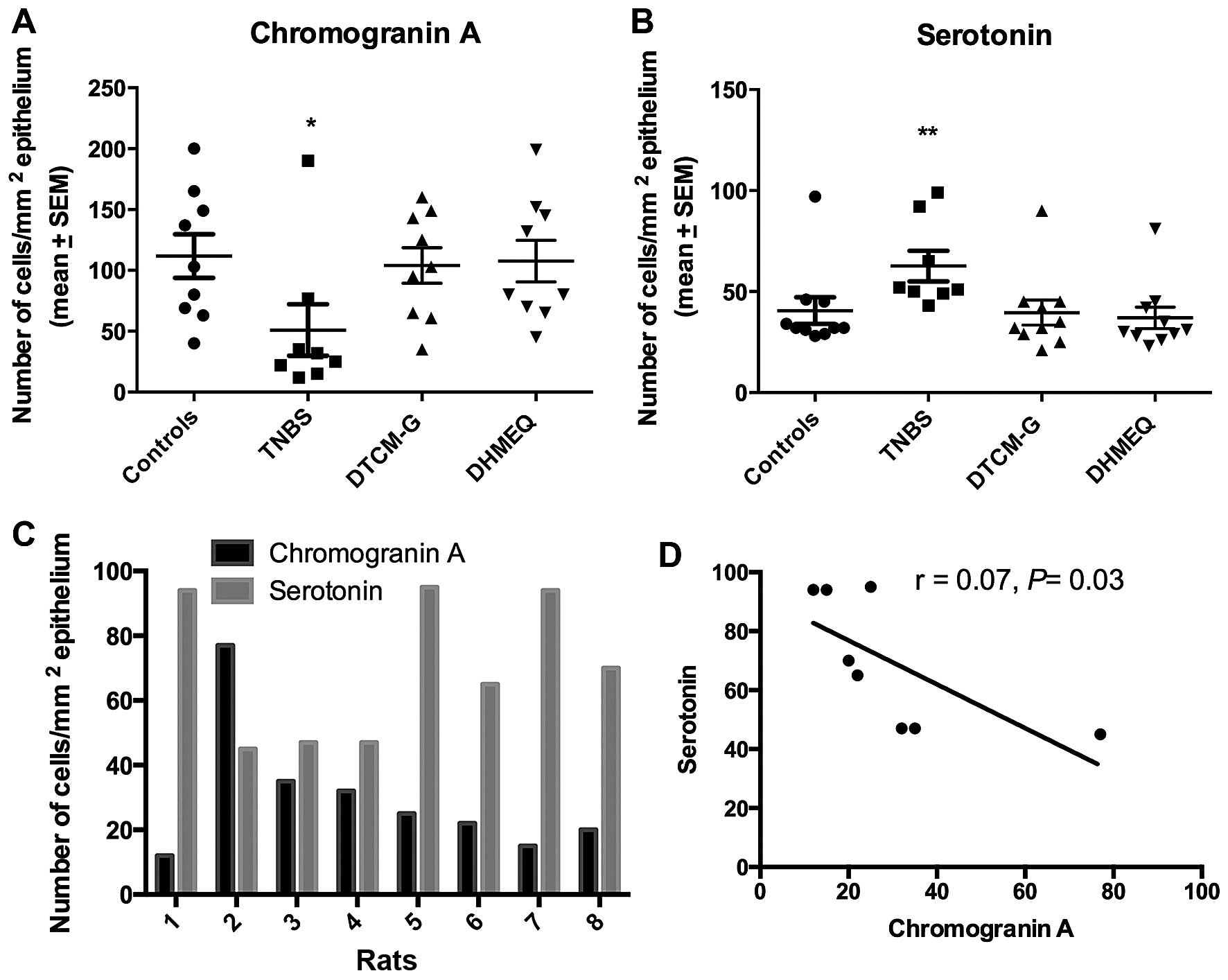
The densities of CgA- and serotonin-positive cells
differed significantly between the control, TNBS, DTCM-G and DHMEQ
groups (P=0.04 and 0.006, respectively). In the TNBS group, the
density of CgA-positive cells was significantly reduced (P=0.02)
and that of serotonin-positive cells was increased (P=0.004)
(Fig. 3). The density of
CgA-positive cells inversely correlated with the density of
serotonin-positive cells (r=−0.7, P=0.03).
The Kruskal-Wallis test showed that there were
significant differences in both the PP-positive and
somatostatin-positive cell densities between the control and
experimental groups (P= 0.002 and 0.01, respectively). While the
density of PP-positive cells was reduced in the TNBS group relative
to controls (P=0.001) (Fig. 4),
that of somatostatin-positive cells was increased (P=0.006). The
density of PP-positive cells inversely correlated with the density
of serotonin-positive cells (r=−0.8, P=0.004).
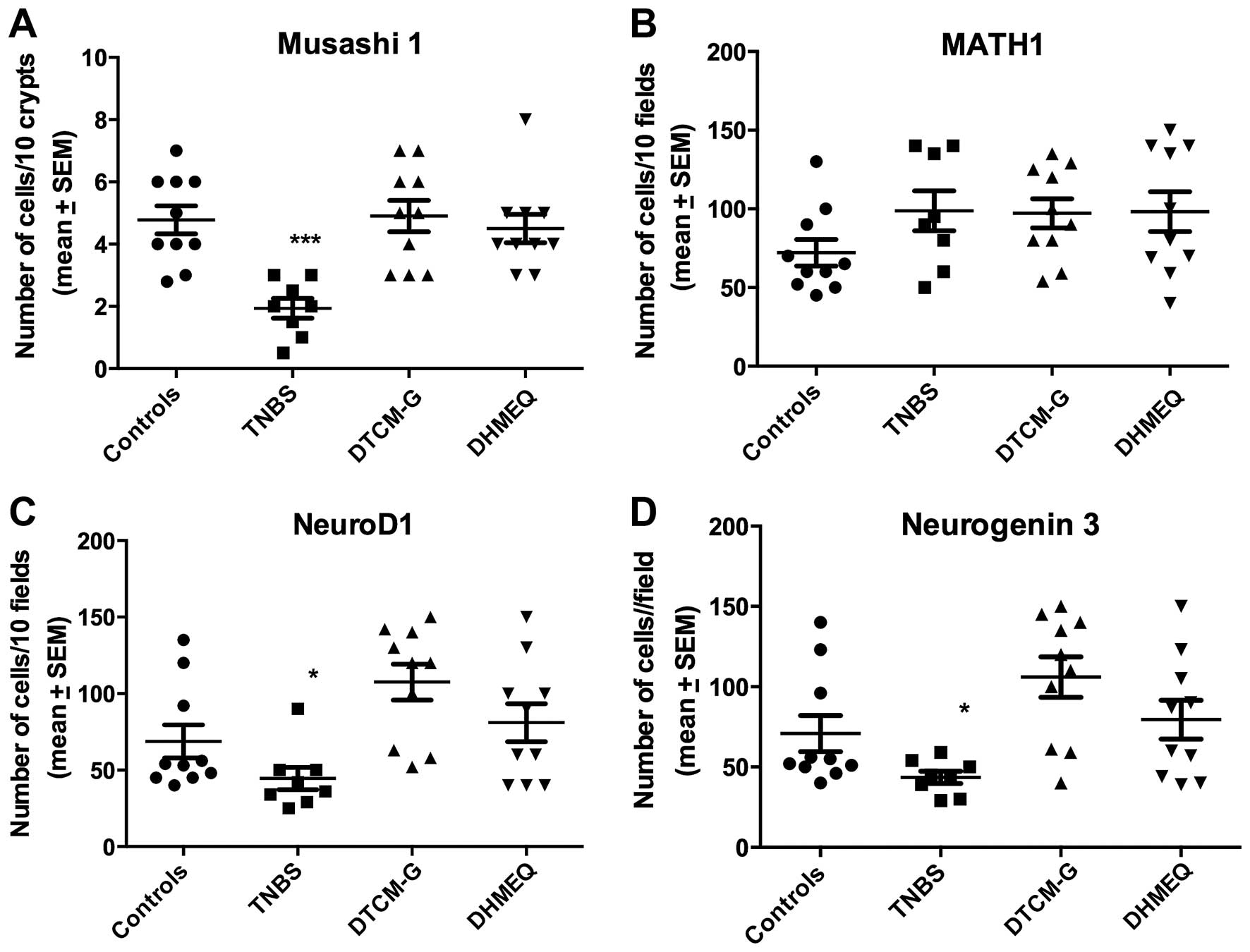
The Kruskal-Wallis test showed that there were
signifi-cant differences in the densities of Msi1-, Neurog3- and
NeuroD1-positive cells, but not in those of Math1-positive cells
(P=0.0008, 0.006, 0.003 and 0.2, respectively). The densities of
Msi1-, Neurog3- and NeuroD1-positive cells were reduced relative to
the controls (P=0.0004, 0.04 and 0.03, respectively), whereas the
density of Math1-positive cells was not (P=0.1) (Figs. 5 and 6).
Discussion
The interaction between enteroendocrine cells and
immune cells has been recently debated, and it is believed that
such an interaction plays an important role in the pathophysiology
of IBD (3,36–40). Enteroendocrine cells in the same
animal model for human CD studied herein have previously been
reported to be abnormal (27).
The mechanisms underlying these abnormalities however, are
unknown.
It is well known that two hormones can be localized
in the same enteroendocrine cell, namely glucagon-like peptide-1
(GLP-1) and gastric inhibitory peptide (GIP) in the small
intestine, and PYY and oxyntomodulin in the distal small and large
intestines (41–44). Recent studies have further
demonstrated that mature enteroendocrine cells are capable of
expressing up to 7 different hormones (45–47). In the present study, the increase
in the oxyntomodulin-positive cell density was accompanied by a
decrease in the PYY-positive cell density, with a significant
inverse correlation. Similar observations were found concerning the
CgA/serotonin-positive and PP/somatostatin-positive cell densities.
It is reasonable to assume that the inflammatory process affects
enteroendocrine cells so that they 'switch off' the expression of a
certain hormone and 'switch on' the expression of another.
The intestine contains 4 to 6 stem cells per crypt,
which either divide into new stem cells (self-renewal; clonogeny)
or differentiate into all types of epithelial cells
(differentiation progeny) (48–59). The differentiation progeny
includes two lineages: secretory and absorptive. The secretory
lineage gives rise to goblet, endocrine and Paneth cells, while the
absorptive lineage gives rise to absorptive enterocytes (48–59). Msi1 is a transcription factor
expressed by both intestinal stem cells and their early progeny
(60–63). Math1 is expressed in the secretory
lineage by an early progenitor, and mutant (Math1−/−)
mice have no secretory cells (64). Neurog3 is expressed by an early
progenitor in the secretory lineage, which directs the
differentiation of secretory progenitors into endocrine cells
(65). Transgenic mice
(Neurog3−/−) express normal densities of goblet and
Paneth cells, but no enteroendocrine cells at all (65–67). NeuroD1 is expressed by progenitors
derived from Neurog3 progenitors (68,69). Mice deficient in NeuroD1 do not
have a certain subgroup of enteroendocrine cells (66,70).
In this study, the density of Msi1-immunoreactive
cells in TNBS-induced colitis was reduced relative to the controls,
indicating that the clonogenic activity of the stem cells is
affected by inflammation. On the other hand, the density of
Math1-immunoreactive cells did not differ between the group with
TNBS-induced colitis and the controls, suggesting that inflammation
does not interfere with the early secretory lineage
differentiation. The present observation that the densities of both
Neurog3- and NeuroD1-positive cells were lower in rats with
TNBS-induced colitis than in the controls may indicate a decease in
the differentiation of stem cells into enteroendocrine cells.
The reduction in enteroendocrine cells observed in
this study following the induction of colitis by TNBS seems to be
caused by i) the 'switching on' and 'switching off' of the
expression of certain hormones by enteroendocrine cells, and ii)
decreases in the clonogenic activity of the stem cell and in the
differentiation into enteroendocrine cells from stem cell
progenitors. It may be speculated that the inflammatory processes
trigger certain signaling substances that cause certain
enteroendocrine cells to change their hormone expression. These
substances may also affect the colonogenic activity and the
differentiation of the stem cell secretory lineage into mature
enteroendocrine cells.
The 'switching on and off' of the expression of
hormones of enteroendocrine cells must occur on a timescale of
minutes or hours, and stem cells differentiate into mature
intestinal cells in 2–3 days (61). This explains why changes in the
densities of enteroendocrine cells, stem cells and differentiation
progeny to enteroendocrine cells could be detected 3 days after the
induction of colitis using TNBS, and that the treatment of colitis
for 5 days with anti-inflammatory agents restored their densities
to normal levels.
Acknowledgments
This study was supported by grants from Helse-Vest
(grant no. 911978) and Helse-Fonna (grant no. 40515).
References
|
1
|
El-Salhy M, Mazzawi T, Hausken T and
Hatlebakk JG: Interaction between diet and gastrointestinal
endocrine cells. Biomed Rep. 4:651–656. 2016.PubMed/NCBI
|
|
2
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Duodenal chromogranin a cell density as
a biomarker for the diagnosis of irritable bowel syndrome.
Gastroenterol Res Pract. 2014:4628562014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Salhy M and Hausken T: The role of the
neuropeptide Y (NPY) family in the pathophysiology of inflammatory
bowel disease (IBD). Neuropeptides. 55:137–144. 2016. View Article : Google Scholar
|
|
4
|
El-Salhy M, Danielsson A, Stenling R and
Grimelius L: Colonic endocrine cells in inflammatory bowel disease.
J Intern Med. 242:413–419. 1997. View Article : Google Scholar
|
|
5
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Chromogranin A cell density as a diagnostic marker for
lymphocytic colitis. Dig Dis Sci. 57:3154–3159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: High densities of serotonin and peptide YY cells in the
colon of patients with lymphocytic colitis. World J Gastroenterol.
18:6070–6075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Salhy M, Lomholt-Beck B and Gundersen
TD: High chromogranin A cell density in the colon of patients with
lymphocytic colitis. Mol Med Rep. 4:603–605. 2011.PubMed/NCBI
|
|
8
|
Moran GW, Pennock J and McLaughlin JT:
Enteroendocrine cells in terminal ileal Crohn's disease. J Crohn's
Colitis. 6:871–880. 2012. View Article : Google Scholar
|
|
9
|
Moran GW, Leslie FC and McLaughlin JT:
Crohn's disease affecting the small bowel is associated with
reduced appetite and elevated levels of circulating gut peptides.
Clin Nutr. 32:404–411. 2013. View Article : Google Scholar
|
|
10
|
Besterman HS, Mallinson CN, Modigliani R,
Christofides ND, Pera A, Ponti V, Sarson DL and Bloom SR: Gut
hormones in inflammatory bowel disease. Scand J Gastroenterol.
18:845–852. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Salhy M, Mazzawi T, Gundersen D,
Hatlebakk JG and Hausken T: The role of peptide YY in
gastrointestinal diseases and disorders (Review). Int J Mol Med.
31:275–282. 2013.PubMed/NCBI
|
|
12
|
Hirotani Y, Mikajiri K, Ikeda K, Myotoku M
and Kurokawa N: Changes of the peptide YY levels in the intestinal
tissue of rats with experimental colitis following oral
administration of mesalazine and prednisolone. Yakugaku Zasshi.
128:1347–1353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vona-Davis LC and McFadden DW: NPY family
of hormones: Clinical relevance and potential use in
gastrointestinal disease. Curr Top Med Chem. 7:1710–1720. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Salhy M, Suhr O and Danielsson A:
Peptide YY in gastrointestinal disorders. Peptides. 23:397–402.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tari A, Teshima H, Sumii K, Haruma K,
Ohgoshi H, Yoshihara M, Kajiyama G and Miyachi Y: Peptide YY
abnormalities in patients with ulcerative colitis. Jpn J Med.
27:49–55. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sciola V, Massironi S, Conte D, Caprioli
F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT and Piodi L:
Plasma chromogranin a in patients with inflammatory bowel disease.
Inflamm Bowel Dis. 15:867–871. 2009. View Article : Google Scholar
|
|
17
|
Bishop AE, Pietroletti R, Taat CW,
Brummelkamp WH and Polak JM: Increased populations of endocrine
cells in Crohn's ileitis. Virchows Arch A Pathol Anat Histopathol.
410:391–396. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manocha M and Khan WI: Serotonin and GI
Disorders: An update on clinical and experimental studies. Clin
Transl Gastroenterol. 3:e132012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stoyanova II and Gulubova MV: Mast cells
and inflammatory mediators in chronic ulcerative colitis. Acta
Histochem. 104:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamamoto H, Morise K, Kusugami K, Furusawa
A, Konagaya T, Nishio Y, Kaneko H, Uchida K, Nagai H, Mitsuma T and
Nagura H: Abnormal neuropeptide concentration in rectal mucosa of
patients with inflammatory bowel disease. J Gastroenterol.
31:525–532. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Payer J, Huorka M, Duris I, Mikulecky M,
Kratochvílová H, Ondrejka P and Lukác L: Plasma somatostatin levels
in ulcerative colitis. Hepatogastroenterology. 41:552–553.
1994.PubMed/NCBI
|
|
22
|
Watanabe T, Kubota Y, Sawada T and Muto T:
Distribution and quantification of somatostatin in inflammatory
disease. Dis Colon Rectum. 35:488–494. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koch TR, Carney JA, Morris VA and Go VL:
Somatostatin in the idiopathic inflammatory bowel diseases. Dis
Colon Rectum. 31:198–203. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mazzawi T and El-Salhy M: Changes in small
intestinal chromogranin A-immunoreactive cell densities in patients
with irritable bowel syndrome after receiving dietary guidance. Int
J Mol Med. 37:1247–1253. 2016.PubMed/NCBI
|
|
25
|
El-Salhy M, Umezawa K, Hatlebakk JG and
Gilja OH: Abnormal differentiation of stem cells into
enteroendocrine cells in rats with DSS-induced colitis. Mol Med
Rep. In press.
|
|
26
|
Elson CO, Sartor RB, Tennyson GS and
Riddell RH: Experimental models of inflammatory bowel disease.
Gastroenterology. 109:1344–1367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Salhy M and Umezawa K: Treatment with
novel AP-1 and NF-κB inhibitors restores the colonic endocrine
cells to normal levels in rats with DSS-induced colitis. Int J Mol
Med. 37:556–564. 2016.PubMed/NCBI
|
|
28
|
El-Salhy M, Wendelbo IH, Gundersen D,
Hatlebakk JG and Hausken T: Evaluation of the usefulness of
colonoscopy with mucosal biopsies in the follow-up of TNBS-induced
colitis in rats. Mol Med Rep. 8:446–450. 2013.PubMed/NCBI
|
|
29
|
Ota E, Takeiri M, Tachibana M, Ishikawa Y,
Umezawa K and Nishiyama S: Synthesis and biological evaluation of
molecular probes based on the 9-methylstreptimidone derivative
DTCM-glutarimide. Bioorg Med Chem Lett. 22:164–167. 2012.
View Article : Google Scholar
|
|
30
|
Takeiri M, Tachibana M, Kaneda A, Ito A,
Ishikawa Y, Nishiyama S, Goto R, Yamashita K, Shibasaki S, Hirokata
G, et al: Inhibition of macrophage activation and suppression of
graft rejection by DTCM-glutarimide, a novel piperidine derived
from the antibiotic 9-methylstreptimidone. Inflamm Res. 60:879–888.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishikawa Y, Tachibana M, Matsui C, Obata
R, Umezawa K and Nishiyama S: Synthesis and biological evaluation
on novel analogs of 9-methylstreptimidone, an inhibitor of
NF-kappaB. Bioorg Med Chem Lett. 19:1726–1728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ueki S, Yamashita K, Aoyagi T, Haga S,
Suzuki T, Itoh T, Taniguchi M, Shimamura T, Furukawa H, Ozaki M, et
al: Control of allograft rejection by applying a novel nuclear
factor-kappaB inhibitor, dehydroxymethylepoxyquinomicin.
Transplantation. 82:1720–1727. 2006. View Article : Google Scholar
|
|
33
|
Matsumoto N, Ariga A, To-e S, Nakamura H,
Agata N, Hirano S, Inoue J and Umezawa K: Synthesis of NF-kappaB
activation inhibitors derived from epoxyquinomicin C. Bioorg Med
Chem Lett. 10:865–869. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Umezawa N, Matsumoto N, Iwama S, Kato N
and Higuchi T: Facile synthesis of peptide-porphyrin conjugates:
Towards artificial catalase. Bioorg Med Chem. 18:6340–6350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hunter MM, Wang A, Hirota CL and McKay DM:
Neutralizing anti-IL-10 antibody blocks the protective effect of
tapeworm infection in a murine model of chemically induced colitis.
J Immunol. 174:7368–7375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khan WI and Ghia JE: Gut hormones:
Emerging role in immune activation and inflammation. Clin Exp
Immunol. 161:19–27. 2010.PubMed/NCBI
|
|
37
|
Margolis KG and Gershon MD: Neuropeptides
and inflammatory bowel disease. Curr Opin Gastroenterol.
25:503–511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bampton PA and Dinning PG: High resolution
colonic manometry - what have we learnt? - A review of the
literature 2012. Curr Gastroenterol Rep. 15:3282013. View Article : Google Scholar
|
|
39
|
Ameri P and Ferone D: Diffuse endocrine
system, neuroendocrine tumors and immunity: What's new?
Neuroendocrinology. 95:267–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Farzi A, Reichmann F and Holzer P: The
homeostatic role of neuropeptide Y in immune function and its
impact on mood and behaviour. Acta Physiol (Oxf). 213:603–627.
2015. View Article : Google Scholar
|
|
41
|
Spångéus A, Forsgren S and el-Salhy M:
Does diabetic state affect co-localization of peptide YY and
enteroglucagon in colonic endocrine cells? Histol Histopathol.
15:37–41. 2000.PubMed/NCBI
|
|
42
|
Pyarokhil AH, Ishihara M, Sasaki M and
Kitamura N: The developmental plasticity of colocalization pattern
of peptide YY and glucagon-like peptide-1 in the endocrine cells of
bovine rectum. Biomed Res. 33:35–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mortensen K, Christensen LL, Holst JJ and
Orskov C: GLP-1 and GIP are colocalized in a subset of endocrine
cells in the small intestine. Regul Pept. 114:189–196. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
El-Salhy M, Wilander E and Grimelius L:
Immunocytochemical localization of gastric inhibitory peptide (GIP)
in the human foetal pancreas. Ups J Med Sci. 87:81–85. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Svendsen B and Holst JJ: Regulation of gut
hormone secretion. Studies using isolated perfused intestines.
Peptides. 77:47–53. 2016. View Article : Google Scholar
|
|
46
|
Svendsen B, Pedersen J, Albrechtsen NJ,
Hartmann B, Toräng S, Rehfeld JF, Poulsen SS and Holst JJ: An
analysis of cosecretion and coexpression of gut hormones from male
rat proximal and distal small intestine. Endocrinology.
156:847–857. 2015. View Article : Google Scholar
|
|
47
|
Egerod KL, Engelstoft MS, Grunddal KV,
Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer
EM, Olsen J, et al: A major lineage of enteroendocrine cells
coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not
somatostatin. Endocrinology. 153:5782–5795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cardoso WV and Lü J: Regulation of early
lung morphogenesis: Questions, facts and controversies.
Development. 133:1611–1624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Darlington GJ: Molecular mechanisms of
liver development and differentiation. Curr Opin Cell Biol.
11:678–682. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fausto N, Campbell JS and Riehle KJ: Liver
regeneration. Hepatology. 43(Suppl 1): S45–S53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rawlins EL and Hogan BL: Ciliated
epithelial cell lifespan in the mouse trachea and lung. Am J
Physiol Lung Cell Mol Physiol. 295:L231–L234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Barker N and Clevers H: Tracking down the
stem cells of the intestine: Strategies to identify adult stem
cells. Gastroenterology. 133:1755–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barker N, van de Wetering M and Clevers H:
The intestinal stem cell. Genes Dev. 22:1856–1864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ and Clevers H: Identification of stem cells in small
intestine and colon by marker gene Lgr5. Nature. 449:1003–1007.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Barker N, van Oudenaarden A and Clevers H:
Identifying the stem cell of the intestinal crypt: Strategies and
pitfalls. Cell Stem Cell. 11:452–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cheng C, Chan AO, Hui WM and Lam SK:
Coping strategies, illness perception, anxiety and depression of
patients with idiopathic constipation: A population-based study.
Aliment Pharmacol Ther. 18:319–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rawdon BB and Andrew A: Origin and
differentiation of gut endocrine cells. Histol Histopathol.
8:567–580. 1993.PubMed/NCBI
|
|
58
|
Hoffman J, Kuhnert F, Davis CR and Kuo CJ:
Wnts as essential growth factors for the adult small intestine and
colon. Cell Cycle. 3:554–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Korinek V, Barker N, Moerer P, van
Donselaar E, Huls G, Peters PJ and Clevers H: Depletion of
epithelial stem-cell compartments in the small intestine of mice
lacking Tcf-4. Nat Genet. 19:379–383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Montgomery RK and Breault DT: Small
intestinal stem cell markers. J Anat. 213:52–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Potten CS1, Booth C, Tudor GL, Booth D,
Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S and Okano H:
Identification of a putative intestinal stem cell and early lineage
marker; musashi-1. Differentiation. 71:28–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kayahara T, Sawada M, Takaishi S, Fukui H,
Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H and Chiba
T: Candidate markers for stem and early progenitor cells, Musashi-1
and Hes1, are expressed in crypt base columnar cells of mouse small
intestine. FEBS Lett. 535:131–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He XC, Yin T, Grindley JC, Tian Q, Sato T,
Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et
al: PTEN-deficient intestinal stem cells initiate intestinal
polyposis. Nat Genet. 39:189–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang Q, Bermingham NA, Finegold MJ and
Zoghbi HY: Requirement of Math1 for secretory cell lineage
commitment in the mouse intestine. Science. 294:2155–2158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jenny M, Uhl C, Roche C, Duluc I,
Guillermin V, Guillemot F, Jensen J, Kedinger M and Gradwohl G:
Neurogenin3 is differentially required for endocrine cell fate
specification in the intestinal and gastric epithelium. EMBO J.
21:6338–6347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang J, Cortina G, Wu SV, Tran R, Cho JH,
Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, et al: Mutant
neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med.
355:270–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lee CS, Perreault N, Brestelli JE and
Kaestner KH: Neurogenin 3 is essential for the proper specification
of gastric enteroendocrine cells and the maintenance of gastric
epithelial cell identity. Genes Dev. 16:1488–1497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo
FJ, Leiter AB and Tsai MJ: Diabetes, defective pancreatic
morphogenesis, and abnormal enteroendocrine differentiation in
BETA2-neuroD-deficient mice. Genes Dev. 11:2323–2334. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ahlgren U, Jonsson J and Edlund H: The
morphogenesis of the pancreatic mesenchyme is uncoupled from that
of the pancreatic epithelium in IPF1/PDX1-deficient mice.
Development. 122:1409–1416. 1996.PubMed/NCBI
|
|
70
|
Schonhoff SE, Giel-Moloney M and Leiter
AB: Minireview: Development and differentiation of gut endocrine
cells. Endocrinology. 145:2639–2644. 2004. View Article : Google Scholar : PubMed/NCBI
|