Introduction
In central nervous system (CNS) neurons, calcium
metabolism, cell energy metabolism secondary to altered cerebral
blood flow, mitochondrial content and oxidative metabolism play
important roles in the regulation of the neurological status at the
cellular and organismal levels (1). Furthermore, age-related impairments
ultimately converge to negatively impact cell respiration, which
manifests as the overproduction of reactive oxygen species (ROS).
Oxidative stress is defined as an imbalance between the production
of ROS or reactive nitrogen species (RNS) and the production of
antioxidants (2). For this
reason, oxidative stress has become the one of the main targets for
the treatment of neurological diseases (3).
Ginger (the rhizome of Zingiber officinale)
is a popular spice and food supplement, and has been reputed to
have medicinal effects for centuries (4,5).
Ginger extract is still used traditionally as an herbal medicine
due to its analeptic properties, which include antioxidant,
anti-lipidemic, anti-microbial, anti-inflammatory,
anti-hyperglycemic, anti-emetic and anticancer effects (6–8).
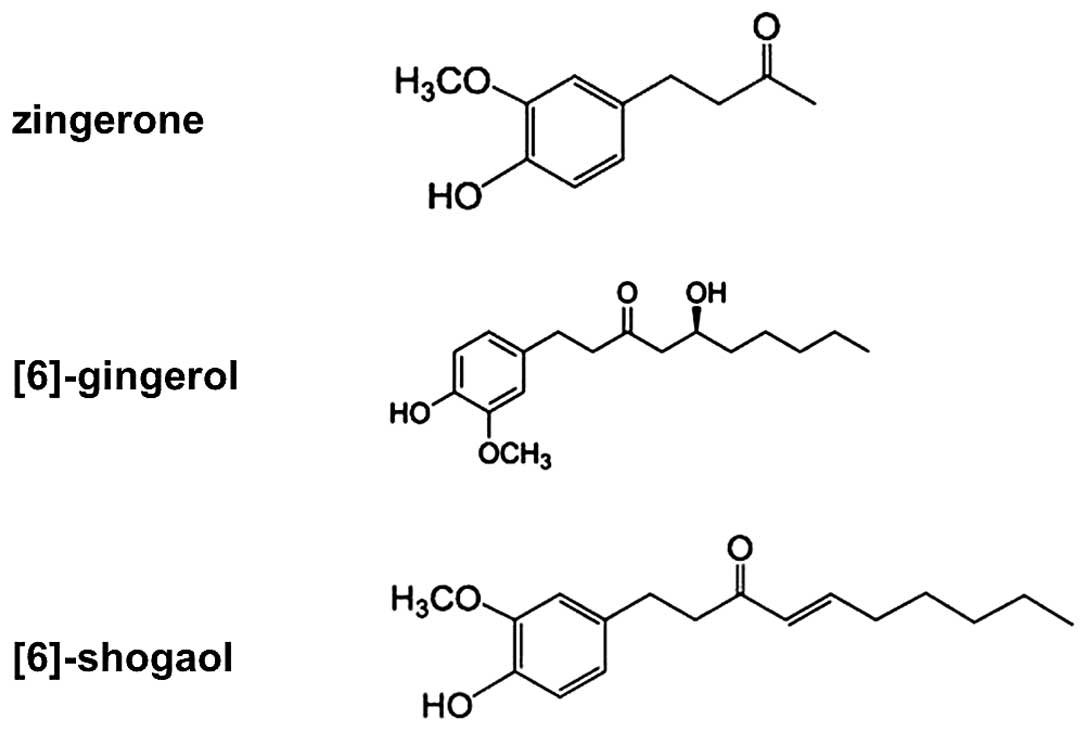
The pungent phytochemicals of ginger consist of [6]-gingerols,
zingerone, and [6]-shogaols and these constituents have also been
reported to be responsible for most of its pharmacological effects
(9,10). Many researchers have studied the
antioxidant properties of ginger. It has been reported to exert
antioxidant protective effects against Pb-induced hepatotoxicity
(11), and to exert significant
beneficial effects on sperm viability, motility and serum total
testosterone levels due to its antioxidant effects (12). [6]-Shogaol is a novel small
molecule activator of nuclear factor (erythroid-derived 2)-like 2
(Nrf2) in PC12 cells, and therefore, it is a potential candidate
for the prevention of oxidative stress-mediated neurodegenerative
disorders (2). Furthermore,
cysteine-conjugated shogaols in ginger have been found to induce
apoptosis via an oxidative stress-mediated p53 pathway in human
colon cancer cells (13).
Transient receptor potential (TRP) channels were
first cloned from Drosophila species and constitute a
superfamily of proteins that encode a diverse group of
Ca2+-permeable non-selective cation channels (NSCCs)
(14). Based on their amino acid
sequences, the TRP family can be divided into 7 subfamilies, namely
TRPC (canonical), TRPM (melastatin), transient receptor potential
cation channel, subfamily A, member 1 (TRPA; ankyrin 1), TRPV
(vanilloid), TRPP (polycistin), TRPN (NOMP-C homologues) and TRPML
(mucolipin), which are activated by different physical and chemical
stimuli (14). In particular, a
class of TRP channels has been found to be modulated by ROS/RNS and
to control various cellular processes. TRPM2, the first
ROS-sensitive TRP channel identified, is activated by
H2O2 (15)
and H2O2-activated Ca2+ influx
through TRPM2 mediates several cellular responses, including cell
death (15) and chemokine
production in monocytes (16).
TRPM7 is activated by ROS/RNS and is an essential mediator of
anoxic death (17). In addition
to TRPM channels, TRPC5 and TRPV1 are also activated by ROS and
nitric oxide (NO) (18). The
activation of TRPC5 and TRPV1 involves the oxidative modification
of free cystein sulfhydryl groups (18), and the activation of the TRPA1
channel has been shown to occur following oxidative cysteine
modification by ROS or RNS (19).
TRPA1 is also activated by environmental electrophiles and
endogenous electrophilic products of oxidative stress (20,21). However, the effects of ginger and
its pungent constituents on TRP channels and the modulatory effects
of oxidative stress have not yet been fully elucidated. Therefore,
in the present study, we investigated the effects of ginger and its
pungent constituents (Fig. 1) on
TRPC5, TRPM7 and TRPA1 channels.
Materials and methods
Materials
Ginger extract (W252108), zingerone (W312401),
[6]-gingerol (G1046), [6]-shogaol (SMB00311) and all other agents
were purchased from Sigma-Aldrich (St. Louis, MO, USA) and
dissolved in distilled water or dimethylsulfoxide (DMSO) to produce
stock solutions, which were stored at −20°C. The final
concentrations of DMSO in bath solution were always <0.1%, and
we confirmed that at a concentration below 0.1% DMSO did not affect
the results. Furthermore, the addition of the above-mentioned
chemicals did not alter bath solution pH values. A-967079
(Sigma-Aldrich) was dissolved in DMSO to produce a 10 mmol/l stock
solution, which was added to the extracellular bath solution at a
final concentration of 10 μM on the day of the experiment
for 2 min. Both allyl isothiocyanate (AITC) and niflumic acid (both
from Sigma-Aldrich) were dissolved in DMSO to produce a 30 mmol/l
stock solution, which was added to the extracellular bath solution
at a final concentration of 10 μM or 30 μM on the day of the
experiment for 4–5 min.
Cell culture
293 cells (ATCC, Manassas, VA, USA) were maintained
according to the supplier's recommendations. For transient
transfection, the cells were seeded in 12-well plates. 293 cells
stably transfected with the mouse TRPC5 (mTRPC5), TRPM7 or human
TRPA1 (hTRPA1) expression vectors were maintained in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), 1% penicillin-streptomycin, 0.02% hygromycin B (for
TRPA1), 5 μg/ml blasticidin (for TRPM7), and 0.4 mg/ml
zeocin (for TRPM7) (all reagents from Life Technologies, Carlsbad,
CA, USA) in a humidified 20% O2/10% CO2
atmosphere at 37°C. The cells were sub-cultured every 2–3 days.
TRPM7 expression was induced by the addition of 1 μg/ml
tetracycline to the culture medium.
Transfection of cells with TRPC5, TRPM7
and TRPA1 expression vectors
For patch clamp experiments, the cells were
transferred to 25-cm2 cell culture flasks (Life
Technologies) 1 day prior to transfection. The 293 cells were
transiently transfected with mammalian expression vectors carrying
TRPC5 (pcDNA5), TRPA1 (pcDNA5/FRT) or TRPM7 (pCDNA4-TO) (alll
vectors were obtained from Thermo Fisher Scientific, Waltham, MA,
USA) using Lipofectamine Plus reagent (Life Technologies) according
to the manufacturer's instructions. In all transfection
experimetns, the 293 cells were co-transfected with the pEGFP-N1
plasmid (from Clontech, Mountain view, CA, USA) to enable
visualization. Experiments were performed within 24–36 h of
transfection.
Electrophysiology
The stably 293 cells stably transfected with the
TRPC5, TRPM7 or TRPA1 expression vectors were detached from the
25-cm2 filtered culture flask by incubatiion with TrypLE
express (Life Technologies) for 2 min. The cells were collected and
maintained in a 35-mm2 Petri dish (BD Biosciences,
Bedford, MA, USA) in DMEM supplemented with 10% FBS at 37°C and 20%
O2/10% CO2. An aliquot of cells was allowed
to settle in a recording chamber for 5 min before perfusion was
initiated. Whole-cell patch clamp recordings were obtained using an
Axopatch 700B amplifier and pClamp v. 10.4 software, and signals
were digitized at 5 kHz using Digidata 1422A (all from Molecular
Devices, Sunnyvale, CA, USA). The RC-13 bath chamber (Warner
Instrument, Hamden, CT, USA) containing cells was perfused with an
extracellular bath solution composed of TRPC5 [Tyrode's solution:
135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM
MgCl2, 5 mM glucose, 10 mM HEPES adjusted to pH 7.4 with
NaOH (Cs+-rich external solution was made by replacing
NaCl and KCl with equimolar CsCl)], TRPA1 (150 mM NaCl, 2 mM
CaCl2, 1 mM MgCl2, 10 mM HEPES adjusted to pH
7.4 with NaOH) and TRPM7 (145 mM NaCl, 2.8 mM KCl, 2 mM
CaCl2, 10 mM glucose, 1.2 mM MgCl2, 10 mM
HEPES adjusted to pH 7.4 with NaOH). The pipette solution contained
TRPC5 (140 mM CsCl, 10 mM HEPES, 0.5 mM Tris-GTP, 0.5 mM EGTA, 3 mM
Mg-ATP adjusted to pH 7.3 with CsOH), TRPM7 [145 mM Cs-glutamate, 8
mM NaCl, 10 mM
Cs-2-bis(2-aminophenoxy)-ethane-N,N,N=,N=-tetraacetic
acid (BAPTA), 10 mM HEPES-CsOH adjusted to pH 7.2 with CsOH] and
TRPA1 (150 mM CsCl, 1 mM MgCl2, 10 mM HEPES, 10 mM BAPTA
adjusted to pH 7.2 with CsOH). Bath solutions were perfused at 3
ml/min. Patch pipettes were pulled from thin-walled borosilicate
glass (World Precision Instruments, Sarasota, FL, USA) using a
horizontal Flaming Brown P-1000 micropipette puller (Shutter
Instruments, Novato, CA, USA). Pipette tips were fire-polished to a
resistance of 2–3 MΩ to facilitate gigaseal formation (Narishige,
Tokyo, Japan). Junction potentials were adjusted prior to and
pipette capacitances were compensated for electronically following
gigaseal formation. Data were saved on a desktop computer and
analyzed using Clampfit v. 10.4 (Molecular Devices), Prism v. 6.0
(GraphPad, La Jolla, CA, USA), and Origin v.8.0 (Microcal,
Northampton, MA, USA) software. GTPγS was dissolved in DMSO to
produce a 1 mol/l stock solution, which was added to the pipette
solution at a final concentration of 0.2 mmol/l on the day of the
experiment to activate G-proteins.
Statistical analysis
Results are expressed as the means ± standard errors
of mean (SEM). N values refer to the number of separate cells
examined. Multiple comparison testing was performed by one-way
ANOVA with Bonferroni's post hoc comparison. P-values of <0.05
were considered to indicate statistically significant
differences.
Results
Effects of ginger extract and its pungent
constituents on TRPC5 channels
TRPC5 is activated by the stimulation of endogenous
G-proteins by G protein coupled receptor (GPCR, e.g., muscarinic
receptor hnd histamine receptor) or GTPγS. To determine whether
activated G-proteins activate TRPC5 in 293 cells stably expressing
mTRPC5, GTPγS (0.2 mM) was added to the intracellular solution.
Whole cell currents were recorded using patch clamp techniques.
Initially, whole cell currents were recorded under normal Tyrode's
solution. In order to obtain current-voltage (I-V) relationships,
we applied a ramp pulse from +100 to −100 mV for 500 msec. In 293
cells stably expressing mTRPC5, TRPC5 currents (ITRPC5) were
activated by applying GTPγS intracellularly. When the external
solution was changed from normal Tyrode's solution to 140 mM
extracellular bath Cs+ solution, ITRPC5 at −100 mV was
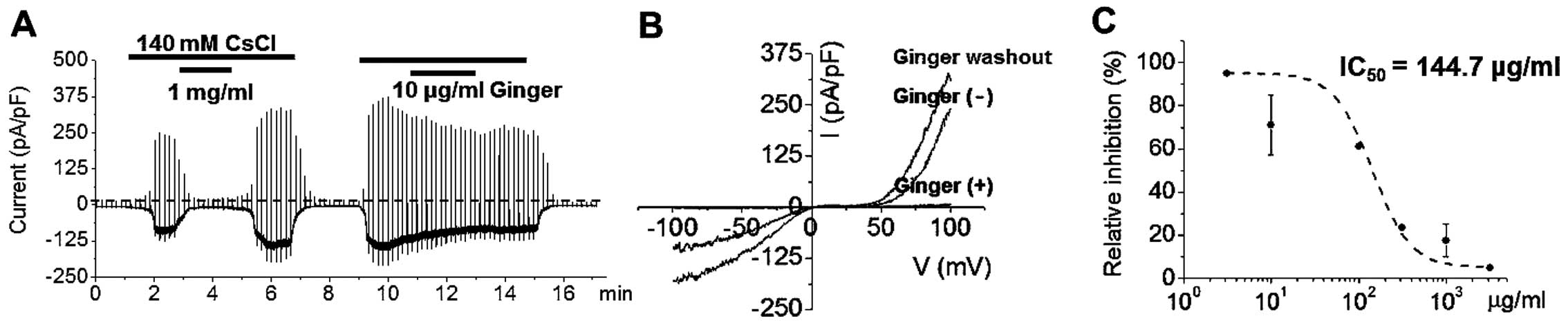
increased (Fig. 2A). Therefore,
ITRPC5 currents were induced repeatedly by applying external
Cs+ in the presence of 0.2 mM GTPγS in the pipette
solution (Fig. 2A). The I-V
relationship in the presence of 140 mM external Cs+
exhibited a typical doubly rectifying shape (Fig. 2B), and the second
Cs+-induced ITRPC5 was of similar amplitude to the first
(Fig. 2A). Ginger extract
inhibited ITRPC5 currents in a concentration-dependent manner
(Fig. 2A). After washing out the
ginger extract, Cs+ activated ITRPC5 currents, which
showed a typical doubly rectifying shape (Fig. 2B). Ginger extract effectively
inhibited ITRPC5 currents with an IC50 value of 144.7
μg/ml (Fig. 2C). In order
to identify the components of ginger extract responsible for ITRPC5
inhibition, we investigated the effects of the 3 major components
of ginger extract, that is, [6]-gingerol, zingerone and
[6]-shogaol, using whole-cell patch clamp experiments. Treatment
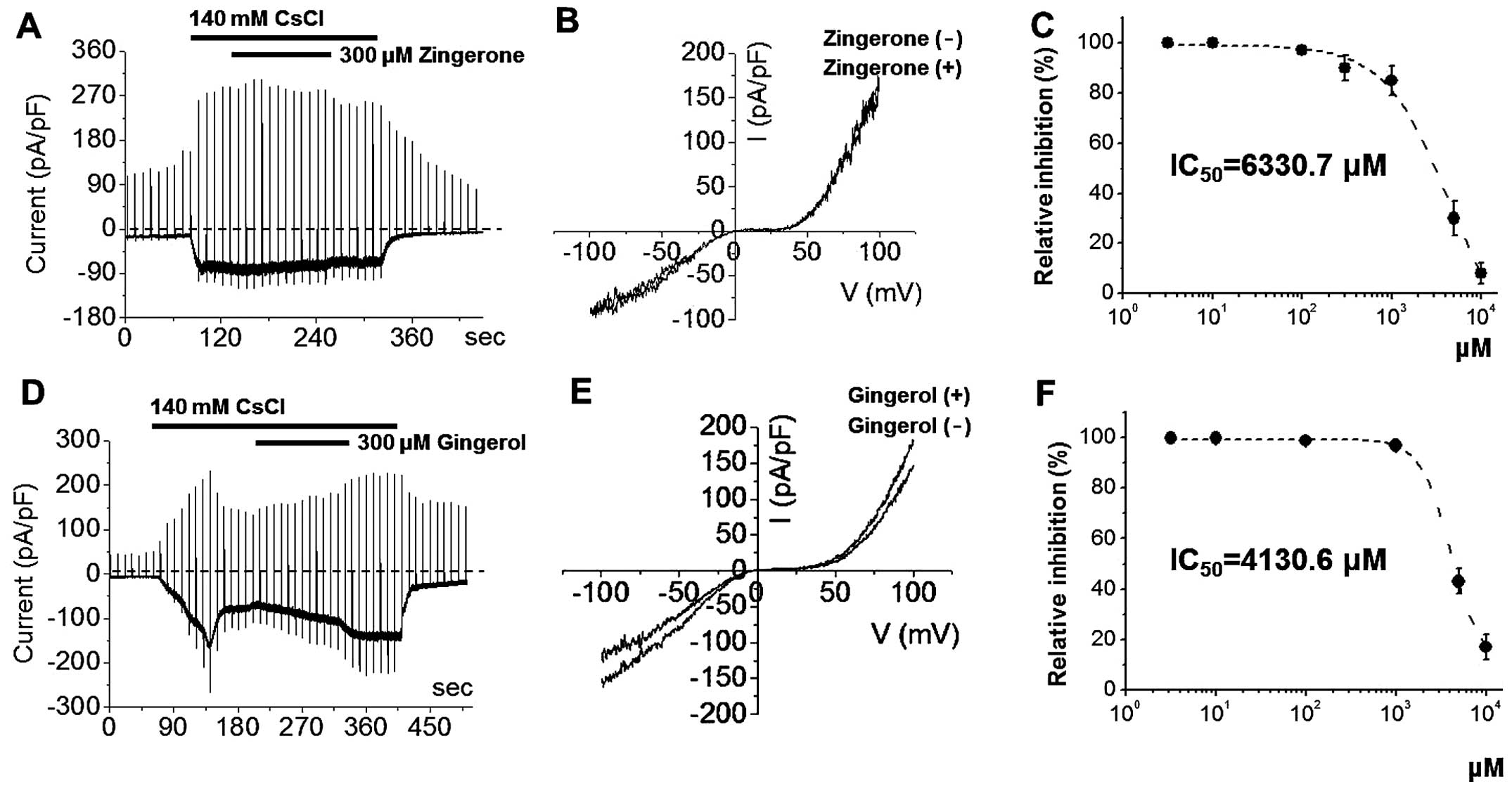
with zingerone or [6]-gingerol at 300 μM (Fig. 3) did not inhibit ITRPC5. The I-V
relationships showed a typical doubly rectifying shape (Fig. 3). However, at high concentrations,
zingerone and [6]-gingerol inhibited ITRPC5 with IC50
values of 6330.7 μM and 4130.6 μM, respectively
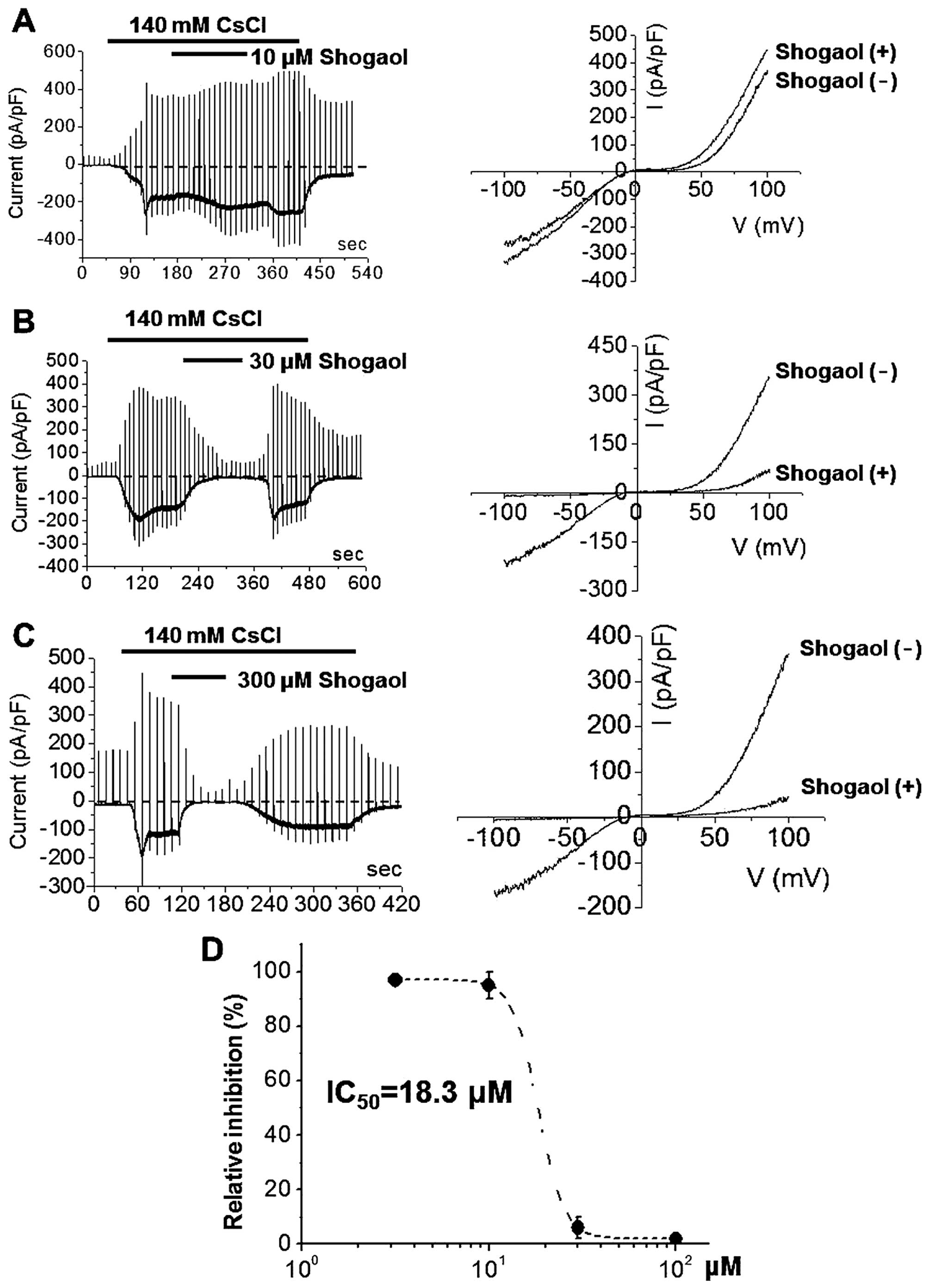
(Fig. 3). By contrast, ITRPC5 was
strongly and dose-dependently inhibited by [6]-shogaol at
concentrations of 10 μM (Fig.
4A), 30 μM (Fig. 4B)
or 300 μM (Fig. 4C). The
I-V relationships showed a typical doubly rectifying shape
(Fig. 4). [6]-shogaol effectively
inhibited ITRPC5 with an IC50 value of ~18.3 μM
(Fig. 4D). These results suggest
that [6]-shogaol is the main factor in ginger extract responsible
for ITRPC5 inhibition. However, ginger extract (≤1 mg/ml) had no
effects on TRPM7 currents (data not shown). Similarly, treatment
with zingerone, [6]-gingerol, or [6]-shogaol at 300 μM had
no effect on TRPM7 currents (data not shown). These results
indicate that TRPM7 currents are not the main target of ginger
extract.
Effects of ginger extract and its pungent
constituents on TRPA1 channels
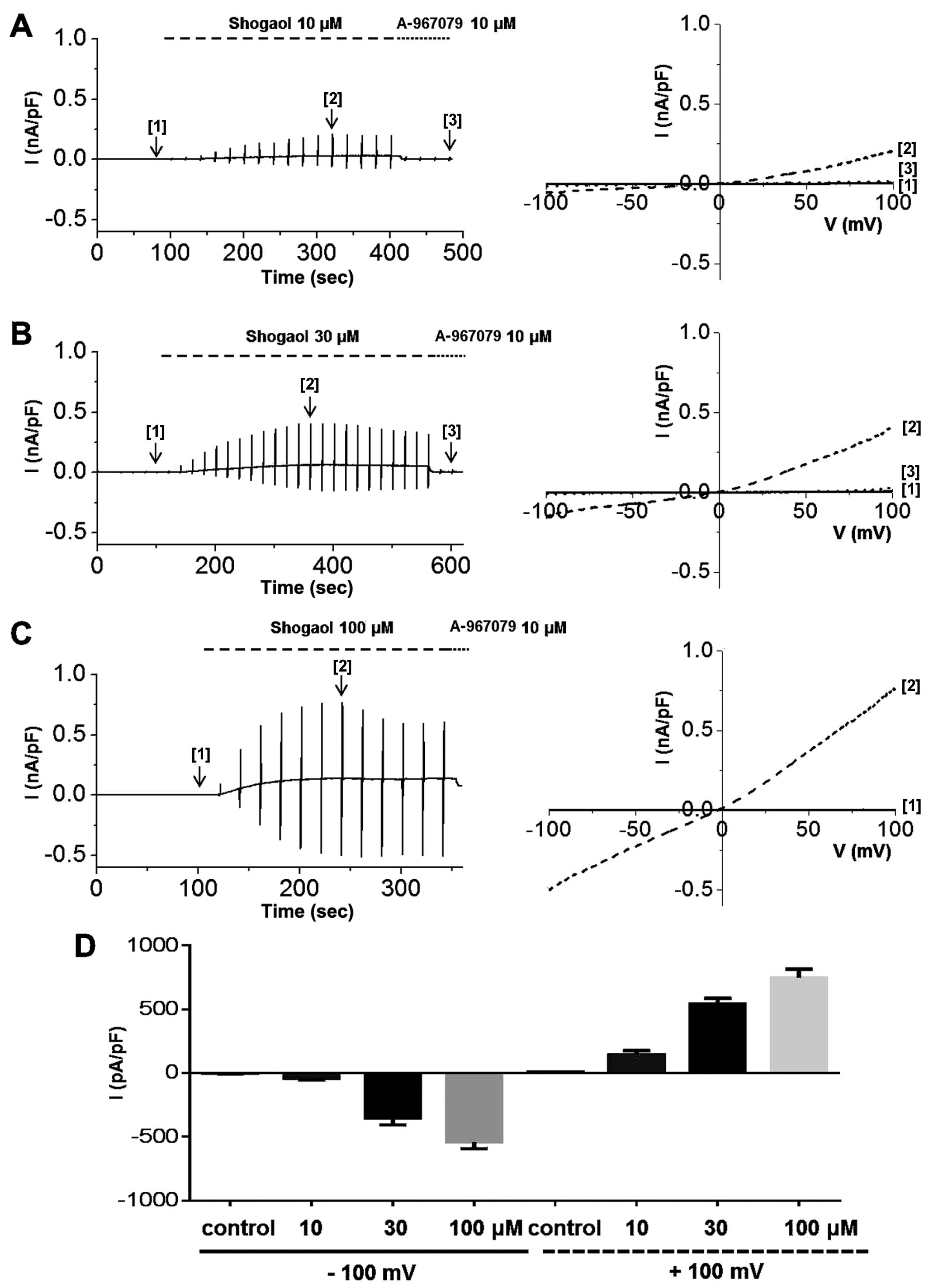
To determine whether ginger extract activates hTRPA1
channels, we obtained whole-cell patch clamp recordings of 293
cells stably expressing TRPA1. After confirming whole-cell patch
formation, we applied a ramp-like pulse protocol from −100 to +100
mV to obtain a current-voltage (I-V) curve from representative
traces obtained at each steady-state time-point before [1] and
after [2] ginger extract treatment and following treatment with the
selective TRPA1 inhibitor, A-967079 [3]. To determine whether TRPA1
channels are functioning well in our transfection system, we
performed whole-cell patch clamp with known agonists. The TRPA1
current (ITRPA1) has been shown to be activated by AITC, which is
the ingredient of mustard, and non-electrophilic non-steroidal
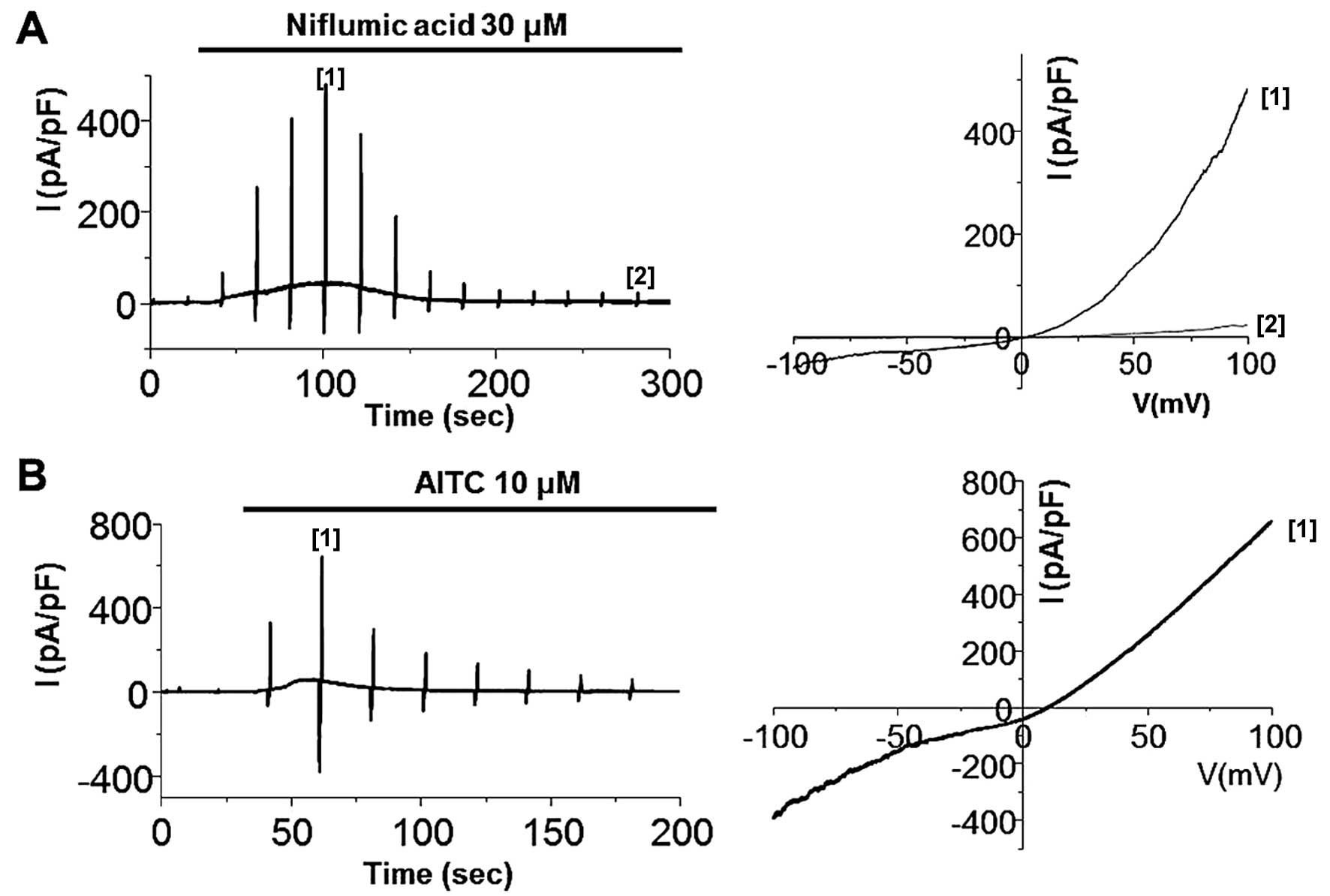
anti-inflammatory drugs (NSAIDs), such as niflumic acid (19,20). As shown in Fig. 5, the bath application of 30
μM niflumic acid and 10 μM AITC evoked transient
current development, demonstrating that the TRPA1 channel is
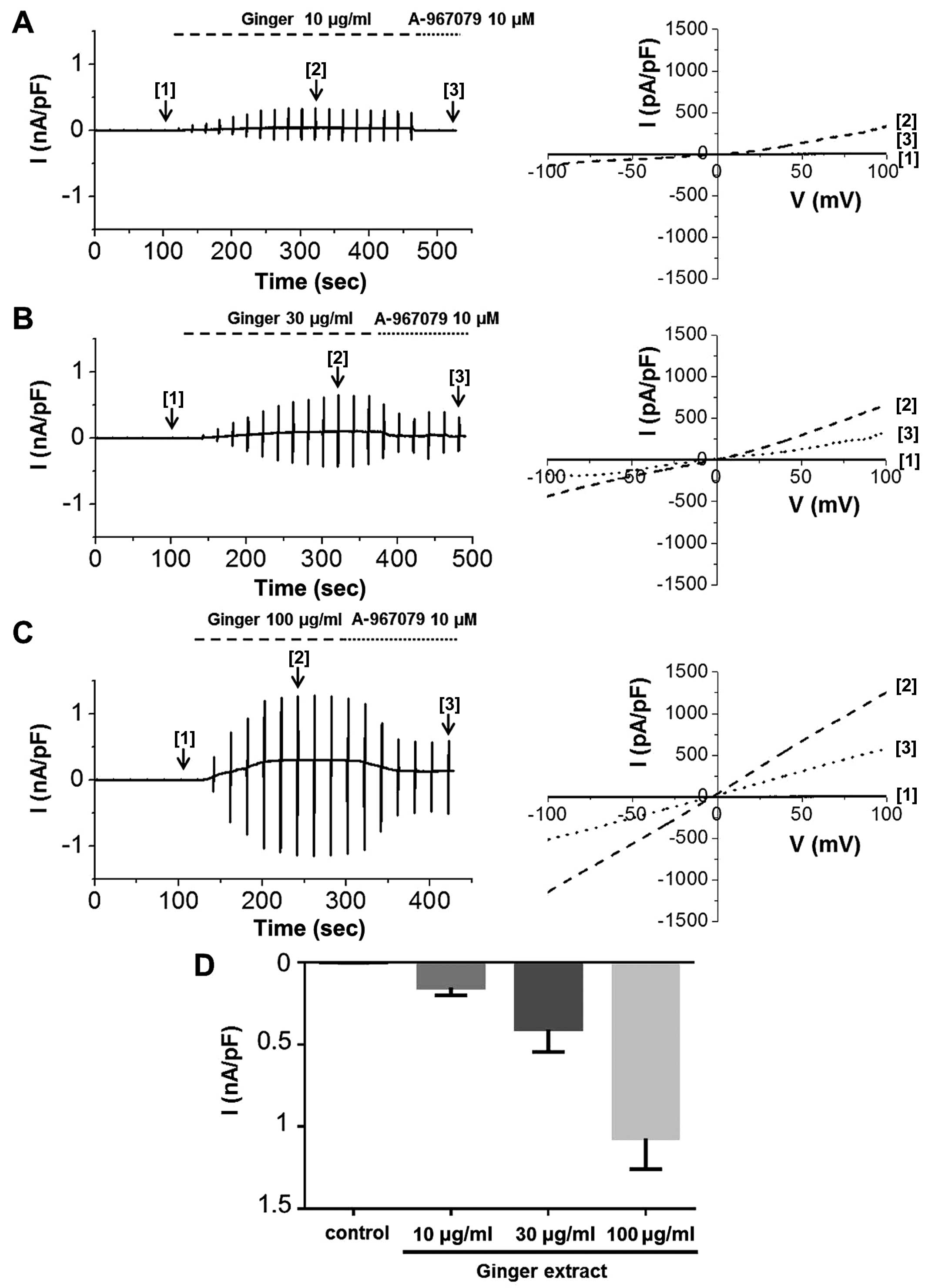
expressed and functional. We then experimented with ginger. ITRPA1
was evoked immediately after the application of ginger extract to
the bath, demonstrating that the TRPA1 channel was activated by the
extract (Fig. 6). The
applications of 10 μg/ml (Fig.
6A), 30 μg/ml (Fig.
6B), or 100 μg/ml (Fig.
6C) of ginger extract produced concentration-dependent
increases in ITRPA1 at both positive and negative potentials. To
confirm the stimulatory effects of the extract on TRPA1, the cells
were concomitantly treated with A-967079, a selective TRPA1
inhibitor. In the presence of 10 μg/ml of ginger extract, 10
μM A-967079 completely abolished ITRPA1 (Fig. 6A [3]); however, the currents
induced by 30 or 100 μg/ml of extract were not fully
inhibited by A-967079 at this concentration (Fig. 6B and C [3]) or even at 30
μM (data not shown). The mean current amplitudes evoked at
each concentration at −100 mV are shown in Fig. 6D. On the other hand, pre-treatment
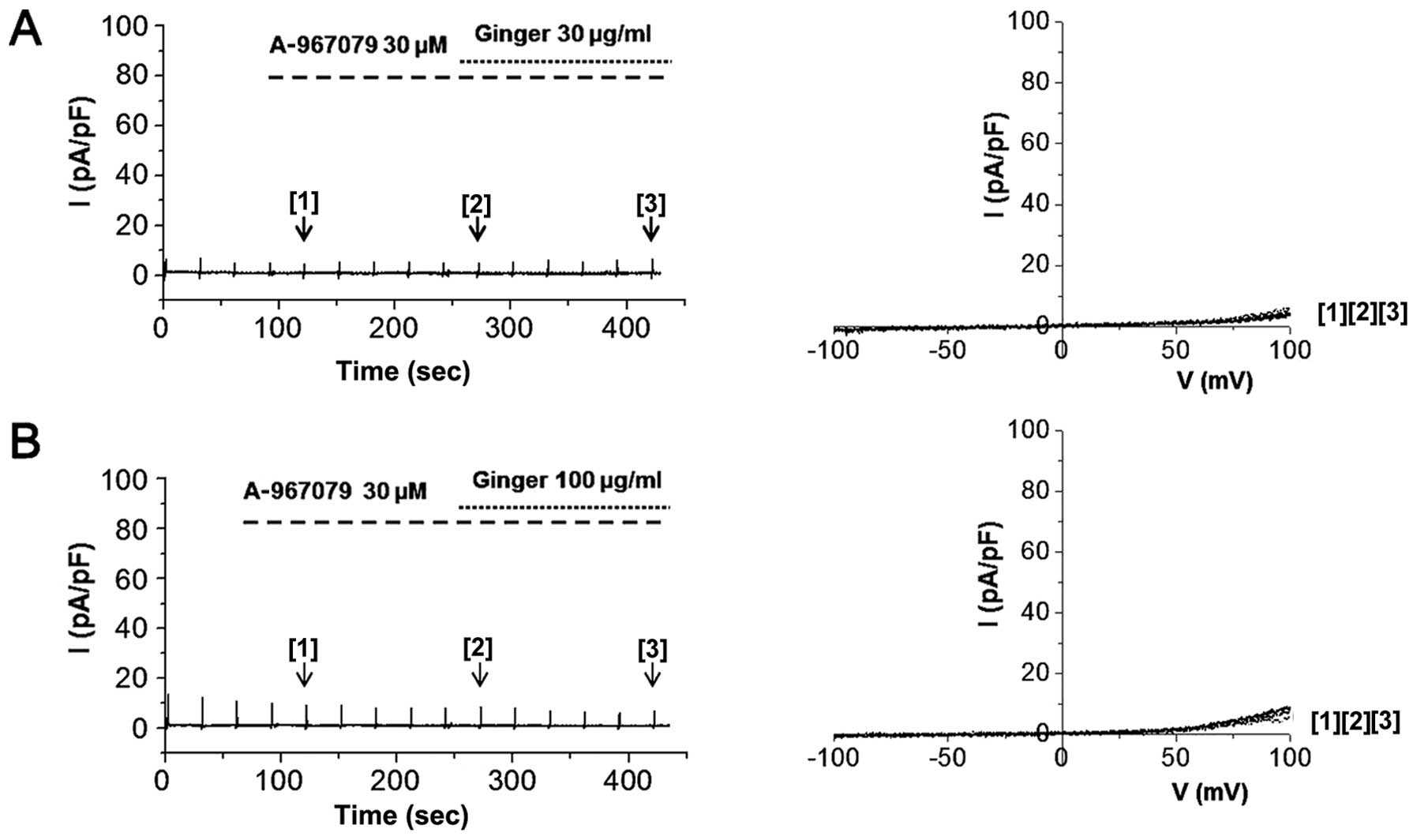
of the cells with 30 μM A-967079 completely blocked hTRPA1
activation by 30 or 100 μg/ml of ginger extract (Fig. 7). In order to identify the
components of ginger extract responsible for the stimulation of
ITRPA1, we examined the effects of 3 major components of the
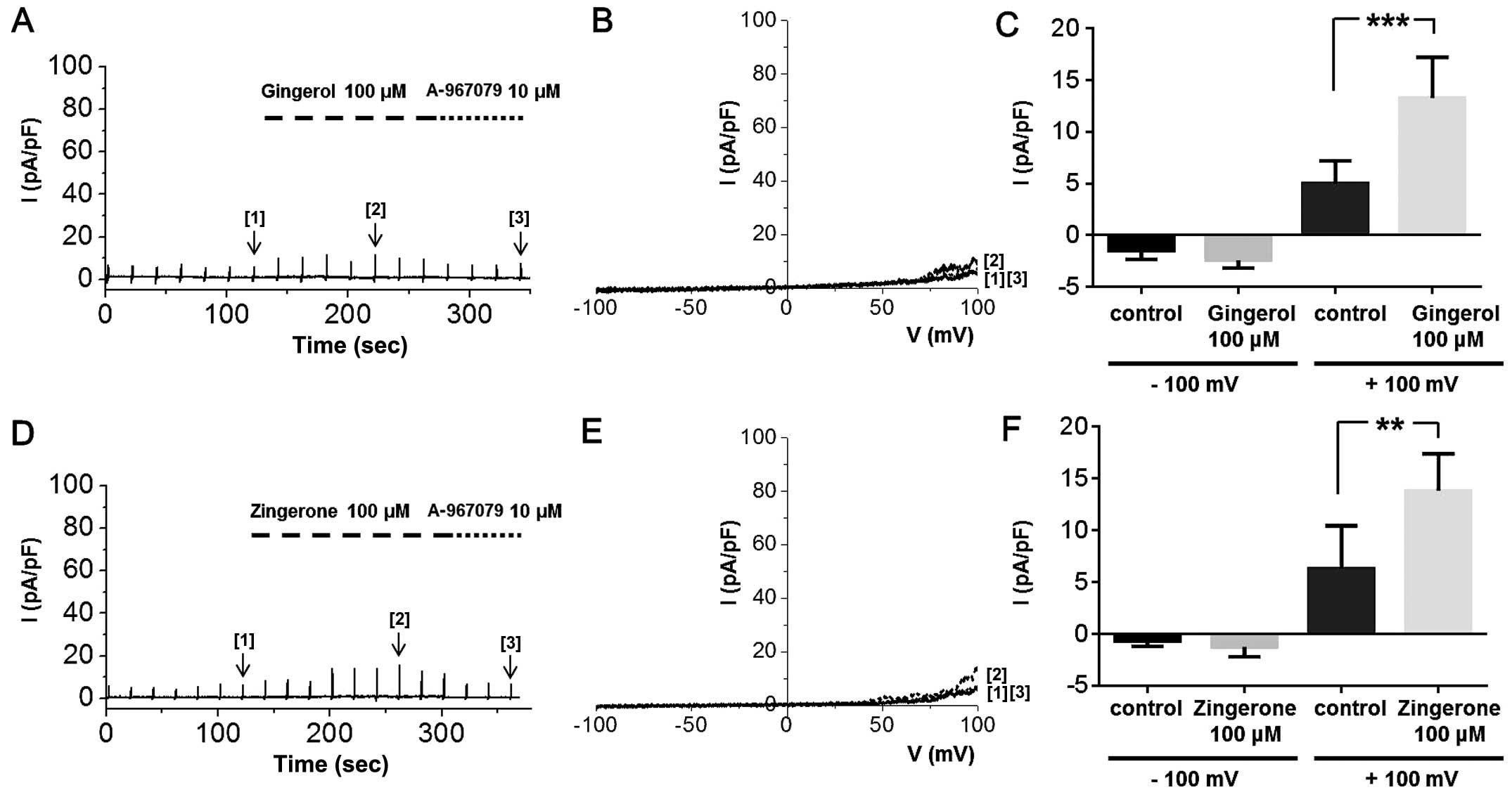
extract, that is, [6]-gingerol, zingerone and [6]-shogaol, on
ITRPA1 in whole-cell patch clamp experiments. Treatment of the
cells with zingerone or [6]-gingerol at a strong depolarizing
potential induced ITRPA1; at a concentration of 100 μM,
neither of these compounds produced an inward current, but both
induced a slight outward current at potentials over +50 mV
(Fig. 8), which was inhibited by
10 μM A-967079. The summarized data of ITRPA1 activated by
[6]-gingerol or zingerone at −100 and +100 mV is shown in Fig. 8C and F, respectively. Although the
ITRPA1 produced at +100 mV by these two compounds were significant,
their effects were weak compared to those of whole ginger extract.
By contrast, hTRPA1 was strongly activated in a dose-dependent
manner by 10 μM (Fig. 9A),
30 μM (Fig. 9B), or 100
μM (Fig. 9C) of
[6]-shogaol, and this effect was abolished by the addition of
A-967079 to the bath solution. The mean current amplitudes evoked
at the different [6]-shogaol concentrations at −100 and +100 mV are
shown in Fig. 9D. These results
suggest that [6]-shogaol is the main factor in ginger extract
responsible for ITRPA1 activation.
Discussion
Ginger is a medicinal plant that has been widely
used in traditional medicine for the treatment of arthritis,
catarrh, rheumatism, nervous diseases, gingivitis, toothache,
asthma, stroke, constipation, indigestion, vomiting, hypertension,
dementia and diabetes (8,22–24). Ginger is also one of the most
commonly used pungents and aromatic spices and adds a special
flavor and zest to food (6,8,25).
Ginger contains numerous active compounds which vary significantly
between plant varieties and geographic regions. More than 60 active
constituents are known to be present in ginger. Phytochemical
reports have demonstrated that the main constituents of ginger are
gingerols, zingerone and shogaols (26,27). [6]-Gingerol and [6]-shogaol are
the major gingerol and shogaol present in ginger (27,28). Zingerone is produced during the
drying of ginger and by the thermal degradation of gingerols or
shogaols (29). The
pharmacological actions of ginger and of its component compounds
include immunomodulatory, anti-tumorigenic, anti-inflammatory,
anti-apoptotic, anti-hyperglycemic, anti-lipidemic and anti-emetic
effects (6–8). In addition, ginger is a potent
antioxidant and may mitigate or prevent free radical generation
(2,8,11–13).
Several authors have demonstrated that ginger has
strong in vitro and in vivo antioxidant properties.
The antioxidant action of ginger has been proposed to underlie its
protective effects against radiation (8,30,31) and a number of toxic agents, such
as carbon tetrachloride and cisplatin (8,32,33), and its efficacy as an anti-ulcer
treatment (34). Among its
compounds, 6-gingerol is an effective agent at preventing ultra
violet B (UVB)-induced ROS production and cyclooxygenase (COX)-2
expression, and therefore, 6-gingerol may have antioxidant effects
in vivo, in addition to potent anti-inflammatory and
anti-apoptotic effects (8,35).
6-Shogaol effectively scavenges various free radicals in
vitro, and displays marked cytoprotective effects against
oxidative stress-induced cell damage by activating the Nrf2 gene in
a neuron-like rat pheochromocytoma cell line (PC12 cells) (2).
Ion channels play critical roles during essential
physiological functions, such as muscle contraction, hormone
secretion and neuroprotection (36), and ion channel defects lead to a
variety of diseases, including cancer (37,38). Previous studies have revealed that
TRP channels are associated with cell proliferation, apoptosis and
cancer development (39,40). These channels are NSCCs, and were
initially cloned from Drosophila melanogaster. All TRP
channels possess putative six-transmembrane spanning domains. The
various gating mechanisms of TRP channels play crucial roles in
their pathologic and physiologic functions (41–44). Recent studies have revealed that
multiple TRP channels sense reactive species and induce diverse
physiological and pathological responses, such as cell death,
chemokine production and pain transduction (45). TRP channels sense reactive species
either indirectly through second messengers or directly via
oxidative modification of cysteine residues. The redox-sensitive
TRP channels are TRPC5, TRPM7, TRPA1, TRPV1 and TRPM2 (45). TRPC5 is directly activated by
H2O2 and the NO donor,
S-nitroso-N-acetyl-DL-penicillamine, via cysteine modification
(46). In endothelial cells,
native TRPC5 is likely to be activated by NO generated by
endothelial-type NO synthase (18). The redox modification of cysteine
residue sulfhydryl groups has emerged as an important elementary
step in the signal transduction cascades that underlie many
physiological responses (47). In
a previous study, NO-activated TRPC5 channels were significantly,
but not entirely suppressed by ascorbate, which reduces
S-nitrosothiols (but not disulfides) to thiols, but dithiothreitol
(DTT), which reduces both S-nitrosothiols and disulfides to thiols,
fully suppressed NO-activated TRPC5 channel activity (45). The authors suggested that both
nitrosylation and disulfide bond formation are likely to be
involved in NO-induced TRPC5 activation (45). ROS and RNS can serve as activators
of cation conductance through TRPM7, and thus contribute to anoxic
neuronal death (17). It has been
proposed that during the oxygen or glucose deprivation of primary
cortical neurons, a Ca2+-permeable non-selective cation
conductance mediated by TRPM7 is activated by ROS/RNS and is
primarily responsible for neuronal death (17). This is supported by the
observation that ROS/RNS are able to enhance TRPM7-mediated inward
currents in TRPM7-transfected 293 cells. In addition, in primary
neurons, it appears that the electrophysiological properties of
currents activated by oxygen- and glucose deprivation, including
currents enhanced by low Mg2+ and inhibited by high
Mg2+, are characteristic of TRPM7 and are not shared by
TRPM2 (17). Furthermore, the
suppression of TRPM7 expression in primary cortical neurons blocked
TRPM7 currents, Ca2+ influx and ROS production,
protecting cells from anoxic cell death (17,45). TRPA1 is also modified via
oxidative cysteine modification by ROS and RNS. TRPA1 is activated
by ROS/RNS, such as, hypo-chlorite (OCl−) (48), H2O2
(19), NO (19), ozone (O3) (49) and ONOO− (50). Redox-sensitive TRP channels are
ubiquitous and participate in ROS-dependent cellular functions,
including cell death, chemokine production and ROS detection, but
novel roles are emerging in the contexts of ischemia/reperfusion,
neurodegeneration, mental illness, vascular hyperpermeability and
itch sensation (51–54). Considering the ubiquitous nature
of TRP channels and ROS/RNS production, it is conceivable that TRP
channel redox sensitivity may also participate in other as yet
unidentified biological phenomena. Thus, the characterization of
the in vivo functions of ROS-sensitive TRP channels under
physiological and pathological conditions is a fertile field for
exploration (45).
In the present study, we investigated the effects of
ginger and its pungent constituents on the redox-sensitive TRP
channels, TRPC5, TRPM7 and TRPA1 and, to the best of our knowledge,
present the results of the first electrophysiological study
undertaken to explore the modulatory effects of ginger and some of
its constituents on these TRP channels. Using whole-cell
patch-clamp recordings, we found that TRPC5 and TRPA1 currents were
modulated by ginger extract and by [6]-gingerols, zingerone and
[6]-shogaols. Above all, [6]-shogaol markedly inhibited TRPC5
currents in a dose-dependent manner with an IC50 value
of ~18.3 μM. On the other hand, [6]-shogaol strongly
activated TRPA1 currents in a dose-dependent manner and its effect
was abolished by the addition of A-967079 (a selective TRPA1
inhibitor). However, ginger extract, [6]-gingerols, zingerone and
[6]-shogaols, had no effects on TRPM7 currents. Therefore, it seems
that [6]-shogaol plays an important role in the regulation of TRPC5
and TRPA1 currents. However, there was no direct investigation of
the effects of the ginger extract components on the oxidant-induced
ion channel current changes and therefore, these results are only
the pharmacological effects of ginger and its pungent constituents
on the variety of TRP channels. In the future in further
investigations, we aim to apply these results on oxidant-induced
ion channels on single cells from tissues.
[6]-Shogaol is one of the phenolic alkanones
isolated from ginger that exhibits significant anti-proliferative
activity in various cancer cell lines (55,56). In addition, [6]-shogaol induces
cell death through oxidative stress-mediated caspase activation
(57). In particular, [6]-shogaol
has been reported to induce the apoptosis of human colorectal
carcinoma cells via ROS production and caspase activation (58). In addition, [6]-shogaol has been
shown to induce autophagy in human non-small cell lung cancer A549
cells by inhibiting the Akt/mTOR pathway (55). Although a number of studies have
addressed the activity of [6]-shogaol, in this study, we suggest
that [6]-shogaol has potent antioxidant effects on TRP channels. In
addition, [6,8,10]-shogaols have differences in the length of the
alkyl carbon chain and increased intracellular Ca2+
concentration in rat TRPV1-expressing 293 cells. In this regard,
shogaols are more potent than gingerols (59). Both [6]-gingerol and [8]-gingerol
evoke capsaicin-like Ca2+ transients and ion currents in
DRG neurons, with both effects being sensitive to the action of
capsazepine (60). Aversive
responses were shown to be induced by [6]- and [10]-gingerol, and
[6]-shogaol in rats when these compounds were applied to the eyes;
however, no response was observed in response to [10]-shogaol.
[10]-Shogaol induced nociceptive responses via TRPV1 in rats
following its subcutaneous injection into the hindpaw (61, and refs. therein). In addition,
[6]-shogaol induces Ca2+ signals in β-cells by
activating the TRPV1 channels, and it sensitizes β-cells to
stimulation by glucose (62).
Therefore, gingerols and shogaols function as activators of the
TRPV1 channel. Furthermore, the effects of shogaol may be the
electrophilic conjugation to the thiol residues of the tested TRP
channels. The chemical structure of shogaol (Fig. 1) shows an electrophilic
α-β-unsaturated carbonyl group, which may produce Michael adduct
formation with the channels. As the same mechanism responses on ion
channels as shogaol, there are curcumin and caffeic acid phenethyl
ester (CAPE). Curcumin and CAPE have been shown to inhibit
Ca2+ release-activated Ca2+ channel (CRAC)
current in Orai1/STIM1-co-expressing 293 cells (63,64) and the electrophilic addition to
the Orai1 195Cys was responsible for the inhibitory effect of
Ca2+ release-activated Ca2+ current by
curcumin and CAPE (65).
Taken together, the findings of our study suggest
that ginger extract exerts antioxidant effects on TRPC5 and TRPA1
channels, and that its pungent constituent, [6]-shogaol is
primarily responsible for the regulation of TRPC5 and TRPA1
currents. In view of the effects of the redox sensitivity of TRP
channels on various pathological and biological phenomena, future
studies are warranted to confirm that TRP channel regulation by
ginger and its pungent constituents is of pharmacological
importance in vivo.
Acknowledgments
The present study was supported by the a Korean
National Research Foundation (NRF) grant funded by the Korea
Government (MSIP) (no. 2014R1A5A2009936)
References
|
1
|
Stoll EA, Cheung W, Mikheev AM, Sweet IR,
Bielas JH, Zhang J, Rostomily RC and Horner PJ: Aging neural
progenitor cells have decreased mitochondrial content and lower
oxidative metabolism. J Biol Chem. 286:38592–38601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peng S, Yao J, Liu Y, Duan D, Zhang X and
Fang J: Activation of Nrf2 target enzymes conferring protection
against oxidative stress in PC12 cells by ginger principal
constituent 6-shogaol. Food Funct. 6:2813–2823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campos PB, Paulsen BS and Rehen SK:
Accelerating neuronal aging in in vitro model brain disorders: A
focus on reactive oxygen species. Front Aging Neurosci. 6:2922014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palatty PL, Haniadka R, Valder B, Arora R
and Baliga MS: Ginger in the prevention of nausea and vomiting: A
review. Crit Rev Food Sci Nutr. 53:659–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haniadka R, Saldanha E, Sunita V, Palatty
PL, Fayad R and Baliga MS: A review of the gastroprotective effects
of ginger (Zingiber officinale Roscoe). Food Funct. 4:845–855.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chrubasik S, Pittler MH and Roufogalis BD:
Zingiberis rhizoma: A comprehensive review on the ginger effect and
efficacy profiles. Phytomedicine. 12:684–701. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shukla Y and Singh M: Cancer preventive
properties of ginger: A brief review. Food Chem Toxicol.
45:683–690. 2007. View Article : Google Scholar
|
|
8
|
Ali BH, Blunden G, Tanira MO and Nemmar A:
Some phytochemical, pharmacological and toxicological properties of
ginger (Zingiber officinale Roscoe): A review of recent research.
Food Chem Toxicol. 46:409–420. 2008. View Article : Google Scholar
|
|
9
|
Govindarajan VS and Connell DW: Ginger -
chemistry, technology, and quality evaluation: Part 1. Crit Rev
Food Sci Nutr. 17:1–96. 1982. View Article : Google Scholar
|
|
10
|
Govindarajan VS and Connell DW:
Ginger-chemistry, technology, and quality evaluation: Part 2. Crit
Rev Food Sci Nutr. 17:189–258. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohamed OI, El-Nahas AF, El-Sayed YS and
Ashry KM: Ginger extract modulates Pb-induced hepatic oxidative
stress and expression of antioxidant gene transcripts in rat liver.
Pharm Biol. 16:1–9. 2015. View Article : Google Scholar
|
|
12
|
Khaki A, Khaki AA, Hajhosseini L, Golzar
FS and Ainehchi N: The anti-oxidant effects of ginger and cinnamon
on spermato-genesis dys-function of diabetes rats. Afr J Tradit
Complement Altern Medicines. 11:1–8. 2014. View Article : Google Scholar
|
|
13
|
Fu J, Chen H, Soroka DN, Warin RF and Sang
S: Cysteine-conjugated metabolites of ginger components, shogaols,
induce apoptosis through oxidative stress-mediated p53 pathway in
human colon cancer cells. J Agric Food Chem. 62:4632–4642. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park HS, Hong C, Kim BJ and So I: The
pathophysiologic roles of TRPM7 channel. Korean J Physiol
Pharmacol. 18:15–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hara Y, Wakamori M, Ishii M, Maeno E,
Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, et al:
LTRPC2 Ca2+-permeable channel activated by changes in
redox status confers susceptibility to cell death. Mol Cell.
9:163–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamoto S, Shimizu S, Kiyonaka S,
Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada
T, et al: TRPM2-mediated Ca2+ influx induces chemokine
production in monocytes that aggravates inflammatory neutrophil
infiltration. Nat Med. 14:738–747. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aarts M, Iihara K, Wei WL, Xiong ZG,
Arundine M, Cerwinski W, MacDonald JF and Tymianski M: A key role
for TRPM7 channels in anoxic neuronal death. Cell. 115:863–877.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshida T, Inoue R, Morii T, Takahashi N,
Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y and Mori Y:
Nitric oxide activates TRP channels by cysteine S-nitrosylation.
Nat Chem Biol. 2:596–607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi N, Mizuno Y, Kozai D, Yamamoto
S, Kiyonaka S, Shibata T, Uchida K and Mori Y: Molecular
characterization of TRPA1 channel activation by cysteine-reactive
inflammatory mediators. Channels (Austin). 2:287–298. 2008.
View Article : Google Scholar
|
|
20
|
Andersson DA, Gentry C, Moss S and Bevan
S: Transient receptor potential A1 is a sensory receptor for
multiple products of oxidative stress. J Neurosci. 28:2485–2494.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Macpherson LJ, Dubin AE, Evans MJ, Marr F,
Schultz PG, Cravatt BF and Patapoutian A: Noxious compounds
activate TRPA1 ion channels through covalent modification of
cysteines. Nature. 445:541–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Awang DVC: Ginger. Can Pharm J.
125:309–311. 1992.
|
|
23
|
Wang WH and Wang ZM: Studies of commonly
used traditional medicine-ginger. Zhongguo Zhong Yao Za Zhi.
30:1569–1573. 2005.In Chinese.
|
|
24
|
Tapsell LC, Hemphill I, Cobiac L, Patch
CS, Sullivan DR, Fenech M, Roodenrys S, Keogh JB, Clifton PM,
Williams PG, et al: Health benefits of herbs and spices: The past,
the present, the future. Med J Aust. 185(Suppl): S4–S24.
2006.PubMed/NCBI
|
|
25
|
Afzal M, Al-Hadidi D, Menon M, Pesek J and
Dhami MS: Ginger: An ethnomedical, chemical and pharmacological
review. Drug Metabol Drug Interact. 18:159–190. 2001. View Article : Google Scholar
|
|
26
|
Langner E, Greifenberg S and Gruenwald J:
Ginger: History and use. Adv Ther. 15:25–44. 1998.
|
|
27
|
Ghayur MN, Gilani AH, Afridi MB and
Houghton PJ: Cardiovascular effects of ginger aqueous extract and
its phenolic constituents are mediated through multiple pathways.
Vascul Pharmacol. 43:234–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Connell DW and McLachlan R: Natural
pungent compounds: Examination of gingerols, shogaols, paradols and
related compounds by thin-layer and gas chromatography. J
Chromatogr A. 67:29–35. 1972. View Article : Google Scholar
|
|
29
|
Ahmad B, Rehman MU, Amin I, Arif A, Rasool
S, Bhat SA, Afzal I, Hussain I, Bilal S and Mir Mu: A review on
pharmacological properties of zingerone
(4-(4-hydroxy-3-methoxyphenyl)-2-butanone). Scientific World
Journal. 2015:8163642015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jagetia GC, Baliga MS, Venkatesh P and
Ulloor JN: Influence of ginger rhizome (Zingiber officinale Rosc)
on survival, glutathione and lipid peroxidation in mice after
whole-body exposure to gamma radiation. Radiat Res. 160:584–592.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haksar A, Sharma A, Chawla R, Kumar R,
Arora R, Singh S, Prasad J, Gupta M, Tripathi RP, Arora MP, et al:
Zingiber officinale exhibits behavioral radioprotection against
radiation-induced CTA in a gender-specific manner. Pharmacol
Biochem Behav. 84:179–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amin A and Hamza AA: Effects of Roselle
and Ginger on cisplatin-induced reproductive toxicity in rats.
Asian J Androl. 8:607–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yemitan OK and Izegbu MC: Protective
effects of Zingiber officinale (Zingiberaceae) against carbon
tetrachloride and acetaminophen-induced hepatotoxicity in rats.
Phytother Res. 20:997–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siddaraju MN and Dharmesh SM: Inhibition
of gastric H+, K+-ATPase and Helicobacter
pylori growth by phenolic antioxidants of Zingiber officinale. Mol
Nutr Food Res. 51:324–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JK, Kim Y, Na KM, Surh YJ and Kim TY:
[6]-Gingerol prevents UVB-induced ROS production and COX-2
expression in vitro and in vivo. Free Radic Res. 41:603–614. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Doyle JL and Stubbs L: Ataxia, arrhythmia
and ion-channel gene defects. Trends Genet. 14:92–98. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kunzelmann K: Ion channels and cancer. J
Membr Biol. 205:159–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pardo LA: Voltage-gated potassium channels
in cell proliferation. Physiology (Bethesda). 19:285–292. 2004.
View Article : Google Scholar
|
|
39
|
Schwarz EC, Wissenbach U, Niemeyer BA,
Strauss B, Philipp SE, Flockerzi V and Hoth M: TRPV6 potentiates
calcium-dependent cell proliferation. Cell Calcium. 39:163–173.
2006. View Article : Google Scholar
|
|
40
|
Bödding M: TRP proteins and cancer. Cell
Signal. 19:617–624. 2007. View Article : Google Scholar
|
|
41
|
Clapham DE: TRP channels as cellular
sensors. Nature. 426:517–524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pedersen SF, Owsianik G and Nilius B: TRP
channels: An overview. Cell Calcium. 38:233–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ramsey IS, Delling M and Clapham DE: An
introduction to TRP channels. Annu Rev Physiol. 68:619–647. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nilius B and Owsianik G: The transient
receptor potential family of ion channels. Genome Biol. 12:2182011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kozai D, Ogawa N and Mori Y: Redox
regulation of transient receptor potential channels. Antioxid Redox
Signal. 21:971–986. 2014. View Article : Google Scholar
|
|
46
|
Takahashi N and Mori Y: TRP channels as
sensors and signal integrators of redox status changes. Front
Pharmacol. 2:582011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bindoli A and Rigobello MP: Principles in
redox signaling: From chemistry to functional significance.
Antioxid Redox Signal. 18:1557–1593. 2013. View Article : Google Scholar
|
|
48
|
Bessac BF, Sivula M, von Hehn CA, Escalera
J, Cohn L and Jordt SE: TRPA1 is a major oxidant sensor in murine
airway sensory neurons. J Clin Invest. 118:1899–1910. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Taylor-Clark TE and Undem BJ: Ozone
activates airway nerves via the selective stimulation of TRPA1 ion
channels. J Physiol. 588:423–433. 2010. View Article : Google Scholar :
|
|
50
|
Sawada Y, Hosokawa H, Matsumura K and
Kobayashi S: Activation of transient receptor potential ankyrin 1
by hydrogen peroxide. Eur J Neurosci. 27:1131–1142. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hecquet CM, Ahmmed GU, Vogel SM and Malik
AB: Role of TRPM2 channel in mediating
H2O2-induced Ca2+ entry and
endothelial hyperpermeability. Circ Res. 102:347–355. 2008.
View Article : Google Scholar
|
|
52
|
Hiroi T, Wajima T, Negoro T, Ishii M,
Nakano Y, Kiuchi Y, Mori Y and Shimizu S: Neutrophil TRPM2 channels
are implicated in the exacerbation of myocardial
ischaemia/reperfusion injury. Cardiovasc Res. 97:271–281. 2013.
View Article : Google Scholar
|
|
53
|
Liu T and Ji RR: Oxidative stress induces
itch via activation of transient receptor potential subtype ankyrin
1 in mice. Neurosci Bull. 28:145–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Roedding AS, Gao AF, Au-Yeung W, Scarcelli
T, Li PP and Warsh JJ: Effect of oxidative stress on TRPM2 and
TRPC3 channels in B lymphoblast cells in bipolar disorder. Bipolar
Disord. 14:151–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hung JY, Hsu YL, Li CT, Ko YC, Ni WC,
Huang MS and Kuo PL: 6-Shogaol, an active constituent of dietary
ginger, induces autophagy by inhibiting the AKT/mTOR pathway in
human non-small cell lung cancer A549 cells. J Agric Food Chem.
57:9809–9816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Weng CJ, Wu CF, Huang HW, Ho CT and Yen
GC: Anti-invasion effects of 6-shogaol and 6-gingerol, two active
components in ginger, on human hepatocarcinoma cells. Mol Nutr Food
Res. 54:1618–1627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen CY, Liu TZ, Liu YW, Tseng WC, Liu RH,
Lu FJ, Lin YS, Kuo SH and Chen CH: 6-shogaol (alkanone from ginger)
induces apoptotic cell death of human hepatoma p53 mutant Mahlavu
subline via an oxidative stress-mediated caspase-dependent
mechanism. J Agric Food Chem. 55:948–954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hu R, Zhou P, Peng YB, Xu X, Ma J, Liu Q,
Zhang L, Wen XD, Qi LW, Gao N and Li P: 6-Shogaol induces apoptosis
in human hepatocellular carcinoma cells and exhibits anti-tumor
activity in vivo through endoplasmic reticulum stress. PLoS One.
7:e396642012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Iwasaki Y, Morita A, Iwasawa T, Kobata K,
Sekiwa Y, Morimitsu Y, Kubota K and Watanabe T: A nonpungent
component of steamed ginger - [10]-shogaol - increases adrenaline
secretion via the activation of TRPV1. Nutr Neurosci. 9:169–178.
2006.PubMed/NCBI
|
|
60
|
Dedov VN, Tran VH, Duke CC, Connor M,
Christie MJ, Mandadi S and Roufogalis BD: Gingerols: A novel class
of vanilloid receptor (VR1) agonists. Br J Pharmacol. 137:793–798.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vriens J, Nilius B and Vennekens R: Herbal
compounds and toxins modulating TRP channels. Curr Neuropharmacol.
6:79–96. 2008. View Article : Google Scholar
|
|
62
|
Rebellato P and Islam MS: [6]-shogaol
induces Ca2+ signals by activating the TRPV1
channels in the rat insulinoma INS-1E cells. JOP. 15:33–37.
2014.PubMed/NCBI
|
|
63
|
Nam JH, Shin DH, Zheng H, Kang JS, Kim WK
and Kim SJ: Inhibition of store-operated Ca2+ entry
channels and K+ channels by caffeic acid phenethylester
in T lymphocytes. Eur J Pharmacol. 612:153–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shin DH, Seo EY, Pang B, Nam JH, Kim HS,
Kim WK and Kim SJ: Inhibition of Ca2+-release-activated
Ca2+ channel (CRAC) and K+ channels by
curcumin in Jurkat-T cells. J Pharmacol Sci. 115:144–154. 2011.
View Article : Google Scholar
|
|
65
|
Shin DH, Nam JH, Lee ES, Zhang Y and Kim
SJ: Inhibition of Ca(2+) release-activated Ca(2+) channel (CRAC) by
curcumin and caffeic acid phenethyl ester (CAPE) via electrophilic
addition to a cysteine residue of Orai1. Biochem Biophys Res
Commun. 428:56–61. 2012. View Article : Google Scholar : PubMed/NCBI
|