Introduction
Malignant glioma is the most common subtype of
primary brain tumor, and is characterized by rapid progression,
high resistance to traditional and newer targeted therapeutic
approaches and a poor clinical outcome (1–4).
Surgical resection is the primary treatment for patients suffering
from glioma. However, surgery may also increase the likelihood of
cancer dissemination and metastasis due to the release of tumor
cells into the circulation at the time of surgery. Therefore, the
prevention of tumor cell dissemination during surgery is an
important strategy with which to reduce cancer recurrence and
improve the overall survival following tumor resection.
Tumor cell migration and invasion are considered to
be primary features of the metastatic process (5). Anesthetics and anesthesia techniques
have been shown to have an impact on tumor cell migration and
invasion that can possibly influence the long-term outcome in
patients undergoing cancer surgery (6). Thus, it is important to select
appropriate anesthetics and anesthesia techniques that have
inhibitory properties against the migration and invasion of tumor
cells in order to eliminate the risk of tumor cell dissemination
during the surgical removal of tumors. Sevoflurane, a volatile
anesthetic agent, is widely used in neurosurgery. It has been
demonstrated that sevoflurane exerts anti-proliferative effects on
SW620 colon cancer cells and in Caco-2 laryngeal cancer cells
(7,8), and prevents the migration and
invasion of lung cancer cells (9). However, the effects of sevoflurane
on the migration and invasion of glioma cells are unclear.
MicroRNAs (miRNAs or miRs) are small non-coding RNA
molecules (approximately 21–25 nucleotides in length) that play
important roles in virtually all biological pathways in mammals and
other multicellular organisms (10). miRNAs have been shown to influence
numerous cancer-relevant processes, such as proliferation, cell
cycle control, apoptosis, differentiation, migration and metabolism
(10–12). A recent in vivo and in
vitro study demonstrated that the expression of miRNA-637 was
significantly lower in clinical glioma tissues than in normal brain
tissues, whereas the introduction of miRNA-637 prevented glioma
cell growth, migration and invasion (13). Sevoflurane has been reported to
modulate multiple miRNAs in the brain and in peripheral tissues
(14–16).
In the present study, we examined whether
sevoflurane inhibits glioma cell migration and invasion and, if so,
whether these beneficial effects are mediated by miRNA-637.
Materials and methods
Cell line and cell culture
The human glioma cell line, U251, was obtained from
the Chinese Academy of Sciences (Shanghai, China) and maintained in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf
serum (both Hyclone, Logan, UT, USA). The cells were incubated at
37°C in a humidified atmosphere of 5% CO2.
Protocol of U251 cell exposure to
sevoflurane
The U251 cells were divided into 4 groups as
follows: cells treated without (control, 95% air/5% CO2)
or with sevoflurane (Maruishi Pharmaceutical Co., Osaka, Japan) at
low (1.7% sevoflurane mixed with 95% air/5% CO2),
moderate (3.4% sevoflurane mixed with 95% air/5% CO2)
and high (5.1% sevoflurane mixed with 95% air/5% CO2)
concentrations. To examine whether sevoflurane inhibits glioma cell
migration and invasion by the modulation of miRNA-637, an
additional group of U251 cells was treated with an miRNA-637
inhibitor for 48 h prior to exposure to a high concentration (5.1%)
of sevoflurane. The protocol for the treatment of cells with
sevoflurane was in accordance with that of previous studies
(9,17). Briefly, the U251 cells in the
exponential growth phase were seeded onto plates and incubated in a
CO2 incubator (Thermo Fisher Scientific, Waltham, MA,
USA) for 24 h. The cell culture plates were then placed in an
airtight glass chamber connected to an anesthesia machine Cicero-EM
8060; Drager, Lübeck, Germany). An anesthetic vaporizer (Sevorane;
Abbott, Abbot Park, IL, USA) attached to the anesthesia machine was
used to supply sevoflurane into the chamber. The concentrations of
sevoflurane in the chamber were monitored by a gas monitor (PM
8060; Drager). After being exposed to various concentrations of
sevoflurane for 6 h, the cells were grown at 37°C in a
CO2 incubator for an additional 24 h and then used for
cell migration and invasion assays or molecular analyses.
Transfection of U251 cells with miRNA-637
inhibitor
The transfection of the U251 cells with miRNA-637
inhibitor was performed according to a previous study (13). siRNA targeting miR-637 (miR-637
inhibitor) was synthesized by Guangzhou RiboBio (Guangzhou, China).
The sequence of the miRNA-637 inhibitor was:
5′-ACGCAGAGCCCGAAAGCCCCAGU-3′. Twelve hours prior to transfection,
the cells were seeded in a 6-well plate (Nest Biotech, Shanghai,
China) at 30–50% confluence. Transfection with the siRNA was
performed using TurboFect siRNA transfection reagent (Fermentas,
Vilnius, Lithuania) according to the manufacturer's instructions.
Forty-eight hours following transfection with siRNA, the cells were
collected for further analyses.
Cell migration and invasion assays
In vitro cell migration and invasion
measurements were examined as previously described (13). For the determination of cell
migration, 1×104 cells in 100 µl DMEM medium
without fetal bovine serum were seeded on a fibronectin-coated
polycarbonate membrane insert in a Transwell apparatus (Costar,
Corning, NY, USA). A total of 500 µl DMEM with 10% fetal
bovine serum was added to the lower chamber as a chemoattractant.
Following 6 h of incubation at 37°C in a 5% CO2
atmosphere, the insert was washed twice with phosphate-buffered
saline, and the cells on the top surface of the insert were wiped
off with a cotton swab. The cells on the lower surface of the
insert were fixed with methanol, stained using crystal violet
solution (Sigma-Aldrich, St. Louis, MO, USA) and counted under a
microscope (Nikon Eclipse TS100; Nikon, Tokyo, Japan) in 5
predetermined fields (×200 magnification). All measurements were
repeated at least 3 times. The procedure for the measurement of
cell invasion was similar to the measurement of cell migration,
except that Matrigel (24 µg/µl; R&D Systems,
Inc., Minneapolis, MN, USA) was added to the Transwell membranes
and the cells were incubated for 8 h at 37°C in a 5% CO2
atmosphere. The cells on the lower surface were counted in the same
manner as those for the determination of cell migration.
RNA isolation and real-time polymerase
chain reaction (PCR)
RNA was isolated from the U251 cells using
TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) and
purification using an RNeasy mini kit (Qiagen, Valencia, CA, USA)
as previously described (18).
For the analysis of miRNA-637 expression, the RNA was transcribed
into cDNA and amplified with specific sense primer,
5′-ACTGGGGGCTTTCGGGCTCTGCGT-3′, and antisense general primer, using
the miRNA PrimeScript RT Enzyme Mix kit according to the
manufacturer's instructions (Takara Bio, Inc., Otsu, Japan). For
the analysis of Akt1 gene expression, the sense primer was
5′-CTGAGATTGTGTCAGCCCTGGA-3′, and the antisense primer was
5′-CACAGCCCGAAGTCTGTGATCTTA-3′. The expression of U6 (sense primer,
5′-CTCGCTTCGGCAGCACATATA-3′) and ADP-ribosylation factor 5 (ARF5)
(sense primer, 5′-ATCTGTTTCACAGTCTGGGACG-3′ and antisense primer,
5′-CCTGCTTGTTGGCAAATACC-3′) was also measured and used as miRNA and
gene internal controls, respectively. The thermocycling conditions
for PCR were as follows: 95°C for 10 min to activate DNA
polymerase, followed by 45 cycles of 95°C for 15 sec, 58°C (for
miR-637) or 60°C (for Akt1) for 15 sec and 72°C for 10 sec. Melting
curve analysis was used to confirm the specificity of
amplification. For each gene, PCR reactions were repeated 3
times.
Western blot analysis
Cell homogenates were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto a polyvinylidene fluoride membrane. The membrane
was blocked in Tris-buffered saline Tween-20 (TBST) using 5%
skimmed milk and incubated overnight with rabbit polyclonal Akt
(#4691) and phosphorylated (p-) Akt (#4058) antibodies (1:1,000;
Cell Signaling Technology). Mouse monoclonal β-actin antibody
(sc-47778; 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) was used as an internal control. The membrane was then
incubated for 1 h with a horseradish peroxidase-conjugated
anti-rabbit (sc-2030) or anti-mouse (sc-2005) immunoglobulin-G
secondary antibody (1:5,000; Santa Cruz Biotechnology, Inc.)
diluted with TBST. Signals were detected using an enhanced
chemiluminescence (ECL) reaction system (Millipore, Billerica, MA,
USA) and quantified using ImageJ software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
All quantified data are presented as an average of
at least triplicate samples. Values are expressed as the means ±
SE. The significance of differences in mean values was analyzed by
one-way ANOVA followed by Tukey's multiple comparison tests. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Sevoflurane inhibits the migratory and
invasive abilities of glioma cells
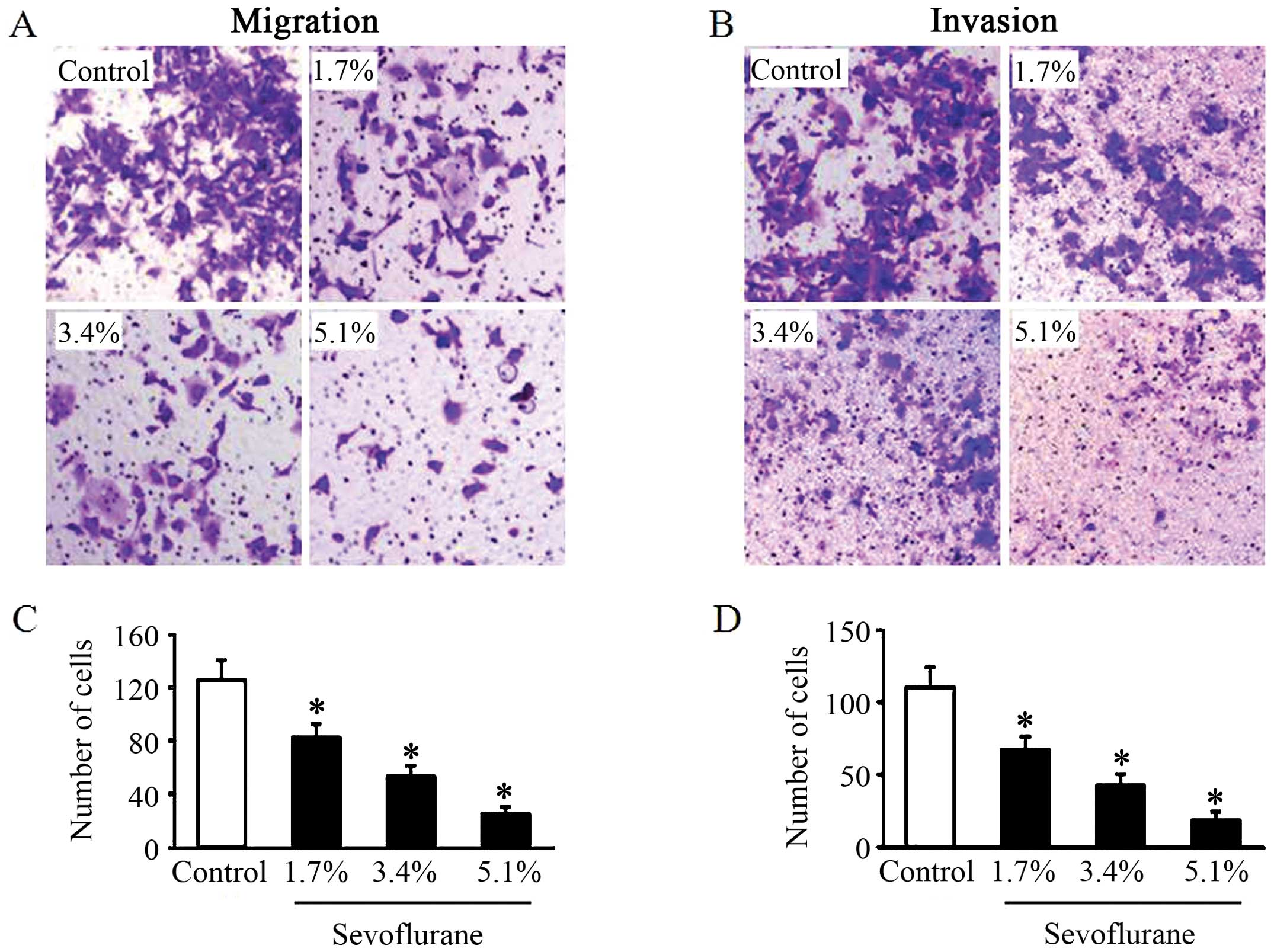
To examine the effects of sevoflurane on glioma cell
migration and invasion, the U251 cells, following treatment without
or with various concentrations of sevoflurane, were cultured in a
Transwell chamber. The alteration of cell migration following 6 h
of incubation was determined. Compared with the control group, the
U251 cells treated with sevoflurane exhibited a significantly
decreased migratory ability in a dose-dependent manner (Fig. 1A and C). For cell invasion assay,
following 8 h of incubation, the U251 cells in all groups invaded
through the matrigel. However, treatment with sevoflurane
attenuated the cell invasive ability compared with the control
group in a dose-dependent manner (Fig. 1B and D).
Sevoflurane induces the upregulation of
miRNA-637 expression in glioma cells
To determine whether sevoflurane induces changes in
miRNA-637 expression in glioma cells, we analyzed the expression
level of miRNA-637 by real-time PCR following treatment of the
cells with sevoflurane. The U251 cells treated with sevoflurane had
significantly increased levels of miRNA-637 as compared to those of
the control group, and the increases in the levels of miRNA-637
were dose-dependent (Fig. 2).
Sevoflurane suppresses Akt1 expression
and activity in glioma cells
As Akt1 is a crucial component of the Akt pathway
involved in the promotion of cell proliferation, migration and
invasion in several types of tumors (19) and is regulated by miRNA-637
(13), we then measured the
expression of Akt1 and that of p-Akt1 in the U251 cells using
real-time PCR and western blot analysis following treastment with
sevoflurane. Compared with the control group, the U251 cells
treated with sevoflurane exhibited a dose-dependent decrease in the
mRNA expression of Akt1 (Fig. 3A)
and in the protein levels of Akt1 and p-Akt1 (Fig. 3B–D). Thus, the expression of Akt1
and p-Akt1 negatively correlate with the expression of miRNA-637 in
glioma cells.
The sevoflurane-induced inhibition of the
migration and invasion of glioma cells is mediated by
miRNA-637
To further confirm whether the inhibitory effects of
sevoflurane on glioma cell migration and invasion are mediated by
miRNA-637, we treated the U251 cells with a miRNA-637 inhibitor
prior to exposure to a high concentration of sevoflurane. We found
that pre-treatment of the U251 cells with miRNA-637 inhibitor
completely abolished the inhibitory effects of sevoflurane on U251
cell migration and invasion (Fig.
4).
The sevoflurane-induced upregulation of
miRNA-637 inhibits glioma cell migration and invasion via the
suppression of Akt1
To confirm that the sevoflurane-induced upregulation
of miRNA-637 inhibits glioma cell migration and invasion via the
suppression of Akt1, we measured the expression of Akt1 and p-Akt1
in U251 cells pre-treated with miRNA-637 inhibitor followed by
exposure to a high concentration of sevoflurane. Our results
revealed that the decreases in the mRNA expression of Akt1 and
protein levels of Akt1 and p-Akt1 in the U251 cells induced by
sevoflurane were reversed by pre-treatment with miRNA-637 inhibitor
(Fig. 5).
Discussion
The novel findings of this study are the following:
i) sevoflurane inhibits the migratory and invasive abilities of
glioma cells; ii) the inhibitory effects of sevoflurane on glioma
cell migration and invasion are mediated by the upregulation of
miRNA-637, which suppresses Akt1 expression and activity. To the
best of our knowledge, this is the first study to demonstrate the
inhibitory effects of sevoflurane on glioma cell migration and
invasion, and to demonstrate the possible underlying
mechanisms.
Metastasis, the leading cause for the resultant
mortality of patients with cancer, is receiving increasing
attention in both scientific and clinical research. Metastasis is
an exceedingly complex process, which includes the detachment of
the tumor cells from the primary site, the degradation of the
extracellular matrix and the penetration of the tumor cells into
the blood vessel walls (20). All
of these processes are associated with the invasive and migration
properties of tumor cells (20).
It has been reported that surgical procedures may induce the
release of tumor cells into the circulation, resulting in the
invasive and migratory potential of tumor cells (21), and promoting the ability of tumor
metastasis (22). Thus,
preventing the metastatic potential of tumor cells during surgery
to remove the tumor is a challenging topic. The volatile
anesthetic, sevoflurane, has been shown to attenuate the
proliferation of SW620 colon cancer cells and Caco-2 laryngeal
cancer cells (7,8), and to inhibit the migration and
invasion of lung cancer cells (9). The present study extended these
findings by showing that sevoflurane significantly prevented the
migration and invasion of glioma cells.
Emerging evidence has suggested that alterations of
a group of miRNAs are involved in the pathogenesis of many types of
cancer, including glioma (23–26). Several deregulated miRNAs target
key gene products to regulate cell proliferation, invasion and
migration (27–29). The deregulation of miRNA-637,
which functions as a tumor suppressor, has been shown to be
associated with the initiation and progression of several types of
human cancers, such as hepatocellular carcinoma [Zhang et al
(30)], breast cancer (31) and follicular thyroid carcinoma
(32). A recent study
demonstrated that the expression level of miRNA-637 was
significantly reduced in clinical glioma tissues compared with
normal brain tissues (13);
moreover, the overexpression of miRNA-637 markedly suppressed
glioma cell growth, migration and invasion in vitro and
in vivo, whereas the inhibition of miRNA-637 resulted in a
significant increase in glioma cell invasion and migration
(13). These results suggest that
miRNA-637 exerts significant inhibitory effects on the migration,
invasion and tumorigenesis of glioma cells. A number of
anesthetics, including sevoflurane, have been reported to cause
alterations in the expression of many miRNAs in both brain and
peripheral tissues (14–16,33). In the present study, we found that
the inhibitory effects of sevoflurane on glioma cell migration and
invasion were dose-dependently associated with an increase in the
expression of miRNA-637. Importantly, the inhibition of miRNA-637
with an inhibitor (with siRNA transfection) completely abolished
the inhibitory effects of sevoflurane on glioma cell migration and
invasion. These results clearly indicate that sevoflurane prevents
the migration and invasion of glioma cells by upregulating
miRNA-637.
Akt1 is an essential component of the Akt pathway
that regulates cell proliferation, migration and invasion in
several types of tumors (19).
The silencing of Akt1 inhibits the growth and invasion of glioma
cells by decreasing phosphorylated Akt, β-catenin, phosphorylated
Foxo1 and cyclin D1 and inducing the expression of Foxo1, whereas
an increase in Akt1 protein levels is associated with the enhanced
migration and invasion of glioma cells (13,34,35). Akt1 is a direct target gene of
miRNA-637 (13). The
overexpression of miRNA-637 has been shown to prevent the
proliferation, migration and invasion of glioma cells through the
direct targeting of Akt1 (13).
Our results revealed that the upregulation of miRNA-637 in glioma
cells by sevoflurane suppressed both the Akt1 mRNA and protein
levels in a dose-dependent manner, and subsequently inhibited the
p-Akt1 levels. Moreover, the inhibition of miRNA-637 with an
inhibitor (by siRNA transfection) completely reversed the
sevoflurane-induced decrease in the expression of Akt1 and p-Akt1.
These findings suggest that the upregulation of miRNA-637 by
sevoflurane inhibits glioma cell migration and invasion by
suppressing Akt1 expression and activity.
In conclusion, the present study demonstrates that
the volatile anesthetic, sevoflurane, inhibits glioma cell
migration and invasion, and that these beneficial effects are
mediated by the upregulation of miRNA-637, which suppresses Akt1
expression and activity (Fig. 6).
These findings may have significant clinical implications for
anesthesiologists regarding the choice of volatile anesthetic
agents for the surgical resection of gliomas in order to prevent
metastases and improve patient outcomes.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81572487 and
81172404 to X. Li), the Major Project of Science and Technology of
Shandong Province (grant no. 2016GSF201070 to D. Li) and the
Shandong Provincial Outstanding Medical Academic Professional
Program and Special foundation for Taishan Scholars (no.
ts20110814).
References
|
1
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: Genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Emdad L, Dent P, Sarkar D and Fisher PB:
Future approaches for the therapy of malignant glioma: Targeting
genes mediating invasion. Future Oncol. 8:343–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group: Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lefranc F, Brotchi J and Kiss R: Possible
future issues in the treatment of glioblastomas: Special emphasis
on cell migration and the resistance of migrating glioblastoma
cells to apoptosis. J Clin Oncol. 23:2411–2422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Snyder GL and Greenberg S: Effect of
anaesthetic technique and other perioperative factors on cancer
recurrence. Br J Anaesth. 105:106–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kvolik S, Glavas-Obrovac L, Bares V and
Karner I: Effects of inhalation anesthetics halothane, sevoflurane,
and isoflurane on human cell lines. Life Sci. 77:2369–2383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kvolik S, Dobrosevic B, Marczi S, Prlic L
and Glavas-Obrovac L: Different apoptosis ratios and gene
expressions in two human cell lines after sevoflurane anaesthesia.
Acta Anaesthesiol Scand. 53:1192–1199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang H, Gu M, Yang C, Wang H, Wen X and
Zhou Q: Sevoflurane inhibits invasion and migration of lung cancer
cells by inactivating the p38 MAPK signaling pathway. J Anesth.
26:381–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Zhou Y, Feng X, An P, Quan X, Wang
H, Ye S, Yu C, He Y and Luo H: MicroRNA-126 functions as a tumor
suppressor in colorectal cancer cells by targeting CXCR4 via the
AKT and ERK1/2 signaling pathways. Int J Oncol. 44:203–210.
2014.
|
|
12
|
Gu JJ, Gao GZ and Zhang SM: miR-218
inhibits the migration and invasion of glioma U87 cells through the
Slit2-Robo1 pathway. Oncol Lett. 9:1561–1566. 2015.PubMed/NCBI
|
|
13
|
Que T, Song Y, Liu Z, Zheng S, Long H, Li
Z, Liu Y, Wang G, Liu Y, Zhou J, et al: Decreased miRNA-637 is an
unfavorable prognosis marker and promotes glioma cell growth,
migration and invasion via direct targeting Akt1. Oncogene.
34:4952–4963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeuchi J, Sakamoto A and Takizawa T:
Sevoflurane anesthesia persistently downregulates muscle-specific
microRNAs in rat plasma. Int J Mol Med. 34:291–298. 2014.PubMed/NCBI
|
|
15
|
Goto G, Hori Y, Ishikawa M, Tanaka S and
Sakamoto A: Changes in the gene expression levels of microRNAs in
the rat hippocampus by sevoflurane and propofol anesthesia. Mol Med
Rep. 9:1715–1722. 2014.PubMed/NCBI
|
|
16
|
Otsuki T, Ishikawa M, Hori Y, Goto G and
Sakamoto A: Volatile anesthetic sevoflurane ameliorates
endotoxin-induced acute lung injury via microRNA modulation in
rats. Biomed Rep. 3:408–412. 2015.PubMed/NCBI
|
|
17
|
Roesslein M1, Frick M, Auwaerter V, Humar
M, Goebel U, Schwer C, Geiger KK, Pahl HL, Pannen BH and Loop T:
Sevoflurane-mediated activation of p38-mitogen-activated
stresskinase is independent of apoptosis in Jurkat T-cells. Anesth
Analg. 106:1150–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li D, Wang C, Li N and Zhang L: Propofol
selectively inhibits nuclear factor-κB activity by suppressing p38
mitogen-activated protein kinase signaling in human EA.hy926
endothelial cells during intermittent hypoxia/reoxygenation. Mol
Med Rep. 9:1460–1466. 2014.PubMed/NCBI
|
|
19
|
Li GQ, Zhang Y, Liu D, Qian YY, Zhang H,
Guo SY, Sunagawa M, Hisamitsu T and Liu YQ: PI3 kinase/Akt/HIF-1α
pathway is associated with hypoxia-induced epithelial-mesenchymal
transition in fibroblast-like synoviocytes of rheumatoid arthritis.
Mol Cell Biochem. 372:221–231. 2013. View Article : Google Scholar
|
|
20
|
Bozzuto G, Ruggieri P and Molinari A:
Molecular aspects of tumor cell migration and invasion. Ann Ist
Super Sanita. 46:66–80. 2010.PubMed/NCBI
|
|
21
|
Coffey JC, Wang JH, Smith MJ,
Bouchier-Hayes D, Cotter TG and Redmond HP: Excisional surgery for
cancer cure: Therapy at a cost. Lancet Oncol. 4:760–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamaguchi K, Takagi Y, Aoki S, Futamura M
and Saji S: Significant detection of circulating cancer cells in
the blood by reverse transcriptase-polymerase chain reaction during
colorectal cancer resection. Ann Surg. 232:58–65. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hobert O: miRNAs play a tune. Cell.
131:22–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin D, Ogawa S, Kawamata N, Leiter A, Ham
M, Li D, Doan NB, Said JW, Black KL and Phillip Koeffler H: miR-34a
functions as a tumor suppressor modulating EGFR in glioblastoma
multiforme. Oncogene. 32:1155–1163. 2013. View Article : Google Scholar
|
|
26
|
Chen Z, Li D, Cheng Q, Ma Z, Jiang B, Peng
R, Chen R, Cao Y and Wan X: MicroRNA-203 inhibits the proliferation
and invasion of U251 glioblastoma cells by directly targeting PLD2.
Mol Med Rep. 9:503–508. 2014.
|
|
27
|
Fang L, Deng Z, Shatseva T, Yang J, Peng
C, Du WW, Yee AJ, Ang LC, He C, Shan SW and Yang BB: MicroRNA
miR-93 promotes tumor growth and angiogenesis by targeting
integrin-β8. Oncogene. 30:806–821. 2011. View Article : Google Scholar
|
|
28
|
Dontula R, Dinasarapu A, Chetty C, Pannuru
P, Herbert E, Ozer H and Lakka SS: MicroRNA 203 modulates glioma
cell migration via Robo1/ERK/MMP-9 signaling. Genes Cancer.
4:285–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu S, Lin Y, Xu D, Chen J, Shu M, Zhou Y,
Zhu W, Su X, Zhou Y, Qiu P, et al: MiR-135a functions as a
selective killer of malignant glioma. Oncogene. 31:3866–3874. 2012.
View Article : Google Scholar
|
|
30
|
Zhang JF, He ML, Fu WM, Wang H, Chen LZ,
Zhu X, Chen Y, Xie D, Lai P, Chen G, et al: Primate-specific
microRNA-637 inhibits tumorigenesis in hepatocellular carcinoma by
disrupting signal transducer and activator of transcription 3
signaling. Hepatology. 54:2137–2148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leivonen SK, Sahlberg KK, Mäkelä R, Due
EU, Kallioniemi O, Børresen-Dale AL and Perälä M: High-throughput
screens identify microRNAs essential for HER2 positive breast
cancer cell growth. Mol Oncol. 8:93–104. 2014. View Article : Google Scholar
|
|
32
|
Stokowy T, Wojtaś B, Fujarewicz K, Jarząb
B, Eszlinger M and Paschke R: miRNAs with the potential to
distinguish follicular thyroid carcinomas from benign follicular
thyroid tumors: Results of a meta-analysis. Horm Metab Res.
46:171–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishikawa M, Tanaka S, Arai M, Genda Y and
Sakamoto A: Differences in microRNA changes of healthy rat liver
between sevoflurane and propofol anesthesia. Anesthesiology.
117:1245–1252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Włodarski P, Grajkowska W, Łojek M, Rainko
K and Jóźwiak J: Activation of Akt and Erk pathways in
medulloblastoma. Folia Neuropathol. 44:214–220. 2006.
|
|
35
|
Schlegel J, Piontek G, Budde B, Neff F and
Kraus A: The Akt/protein kinase B-dependent anti-apoptotic pathway
and the mitogen-activated protein kinase cascade are alternatively
activated in human glioblastoma multiforme. Cancer Lett.
158:103–108. 2000. View Article : Google Scholar : PubMed/NCBI
|