Introduction
In recent years, the average human lifespan has
increased due to economic growth and advancements in modern
medicine. As a result, people have begun to pay more attention to
skin health and beauty (1). Many
research efforts have focused on the identification of strategies
with which to inhibit and delay skin aging, and many cosmetic and
food products related to skin anti-aging have been developed
(2).
Skin aging is driven by intrinsic and extrinsic
factors that cause structural degradation and alterations.
Intrinsic aging occurs naturally with aging, while extrinsic aging
is caused by external factors, such as ultraviolet (UV) radiation,
gravity and air pollution (3).
The characteristics of intrinsic aging include reduced levels of
extracellular matrix components, such as collagen and elastin,
which decrease skin elasticity and tension (4). Therefore, maintaining collagen
levels in the dermis is important for maintaining healthy skin.
Skin is composed of various layers, including the epidermis
composed of epithelial tissue and the dermis composed of connective
tissue and subcutaneous layer. The epidermis protects the skin from
microbial pathogens, UV light and chemical compounds. The dermis is
composed of fibrous proteins, including ground substance, collagen
and elastin. It is an important layer that constitutes >90% of
the skin (5). A variety of
substrates, including collagen and elastin in the extracellular
matrix (ECM), are made by dermal fibroblasts. There are several
types of collagen, which accounts for 80-90% of the dermis. Type 1
collagen constitutes approximately 85% of total collagen and
provides tension, elasticity and flexibility to the skin as it is
entangled in elastic fibers (6).
Its structure is maintained by several enzymes; matrix
metalloproteinases (MMPs) secreted by fibroblasts degrade collagen,
while MMP activity is inhibited by tissue inhibitor of tissue
inhibitor of metalloproteinases (TIMPs). During the aging process,
MMP expression gradually increases and TIMP expression decreases,
promoting collagen degradation and reducing skin elasticity
(7,8).
Transforming growth factor-β (TGF-β) is a
multifunctional cytokine with three isoforms, TGF-β1, TGF-β2 and
TGF-β3. TGF-β helps regulate cellular processes, such as cell
growth, differentiation, migration, apoptosis and the production of
various ECM components, including collagen, elastin and fibronectin
(9,10). TGF-β interacts with two types of
receptors containing type 1 and 2 receptors (serine/threonine
kinase receptors). To activate the TGF-β/Smad signaling pathway,
the TGF-β1 ligand first binds to type 2 receptors (TGF-βRII) at the
cell surface, which allows for the phosphorylation of the GS domain
of TGF-βRI, activating downstream signaling. The complex then
enters the nucleus from the cytoplasm, where it can regulate the
expression of target genes by binding to promoters and co-factors
to activate transcription (11,12).
Recent studies have found that marine algae,
including red, brown and green algae, are rich in nutrients with a
variety of bioactive functions. For example, Pyropia yezoensis
(P. yezoensis), a red alga, is cultivated abundantly in East
Asian countries, including China, Japan and Korea (13). P. yezoensis is composed of
25–40% carbohydrates and 25–50% proteins based on its dry weight,
and is a good source of physiologically active substances (14). P. yezoensis has numerous
biological functions, including antioxidant, antitumor,
anti-fatigue and anti-inflammatory activities, and has been shown
to reduce blood pressure and protect against UVA-induced
photo-aging (15–18). Although a number of studies are in
progress to examine the biological effects of P. yezoensis,
no studies have yet examined its effects against skin aging using
human dermal fibroblasts, at least to the best of our knowledge. In
this study, we found that the P. yezoensis peptide, PYP1-5,
affected collagen synthesis in Hs27 cells. Furthermore, we
determined the intracellular mechanisms responsible for PYP1-5
induced-collagen synthesis, focusing on the TGF-β/Smad signaling
pathway and enzymes related to collagen expression.
Materials and methods
Preparation of P. yezoensis peptide
PYP1-5
PYP1-5 (D-P-K-G-K-Q-Q-A-I-H-V-A-P-S-F) was prepared
as described previously (19).
The 15 N-terminal residues of PYP1-5 were synthesized by Peptron
(Daejeon, Korea). PYP1-5 was purified using a Shimadzu Prominence
high-performance liquid chromatography (HPLC) apparatus and the
software package Class-VP version 6.14 (Shimadzu, Kyoto, Japan),
with a C18 column (Capcell Pak; Shiseido, Tokyo, Japan) in 0.1%
trifluoroacetic acid (TFA)/water, a gradient of 10–70% acetonitrile
(0–20% acetonitrile for 2 min, 20–50% acetonitrile for 10 min, and
50–80% acetonitrile for 2 min) in 0.1% TFA, a flow rate of 1.0
ml/min, and UV detection at 220 nm. The molecular mass of PYP1-5
was confirmed to be 1,622 kDa based on mass spectrometry (HP 110
Series LC/MSD).
Cell culture
The human skin fibroblast cell line Hs27, was
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). The cells were maintained in complete
Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine
serum (FBS; HyClone, Logan, UT, USA), 100 U/ml penicillin, and 100
mg/ml streptomycin in a humidified 5% CO2 incubator at
37°C. The Hs27 cells were cultured to 70–80% confluence in a 100-mm
diameter plate and were used between passage numbers 5 and 15.
MTS assay
Hs27 cell viability was estimated using a CellTiter
96 AQueous Nonradioactive Cell Proliferation assay (Promega,
Madison, WI, USA). The cells were plated in 48-well plates at a
density of 2×104 cells/well, and subsequently treated
with PYP1-5 (250, 500 and 1,000 ng/ml) in serum-free medium (SFM)
for 24 h. The cells were then incubated with 10 µl of MTS
solution for 30 min at 37°C, and the absorbance was quantified
spectroscopically at 490 nm using a microplate reader (Benchmark
microplate reader; Bio-Rad Laboratories, Hercules, CA, USA). Cell
viability was calculated as the ratio of absorbance of treated
cells to that of untreated cells.
Procollagen type I C-peptide (PIP) EIA
assay
Procollagen was measured with a PIP EIA assay kit
(Takara Bio Inc., Tokyo, Japan). The cells were inoculated in
6-well plates at a density of 1×105 cells/well and
incubated for 24 h in SFM containing PYP1-5 (250, 500 and 1,000
ng/ml). After 24 h, the supernatant was collected from each well
and tested with the PIP EIA kit following the manufacturer's
instructions.
Treatment with TGF-βRI inhibitor
(SB431542)
The cells were incubated for 2 h with 10 µM
SB431542 (Tocris, Bristol, UK) prior to treatment with PYP1-5.
Whole-cell protein lysate extraction
The Hs27 cells were seeded in 100-mm dishes and
cultured to 80% confluence. The cells were treated for 24 h with
PYP1-5 (250, 500 and 1,000 ng/ml), washed 2 times in
phosphate-buffered saline, and scraped on ice in lysis buffer (50
mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1%
Triton X-100, 0.1% SDS, 2 mM EDTA) containing protease inhibitor (1
mg/ml aprotinin, 1 mg/ml leupeptin, 1 mg/ml pepstatin A, 200 mM
Na3VO4, 500 mM NaF and 100 mM
phenylmethylsulfonyl fluoride). The cell extracts were centrifuged
at 14,000 rpm for 10 min, and the supernatant was collected for use
in western blot analysis.
Western blot analysis
Sample proteins (30 µg) were separated with
5–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), and then transferred onto polyvinylidene fluoride
(PVDF) membranes (Millipore, Billerica, MA, USA). The membranes
were blocked with 1% bovine serum albumin in TBS-T (10 mM Tris-HCl,
pH 7.5, 150 mM NaCl, 0.1% Tween-20), probed with specific primary
antibodies, and then probed with the secondary antibodies. Signals
were detected using SuperSignal West Pico Chemiluminescent
Substrate (Thermo Fisher Scientific, Inc., Rockford, IL, USA). The
following primary and secondary antibodies were used: anti-collagen
I (COL-1; sc-59772, anti-mouse, 1:1,000), anti-elastin (sc-166543,
anti-mouse, 1:1,000), anti-MMP-1 (sc-21731, anti-mouse, 1:1,000),
anti-TIMP-1 (sc-5538, anti-rabbit, 1:2,000), anti-TIMP-2 (sc-5539,
anti-rabbit, 1:2,000), anti-TGF-β1 (sc-146, anti-rabbit, 1:1,000),
anti-p-Smad2 (sc-135644, anti-rabbit, 1:500), anti-Smad2 (sc-6200,
anti-goat, 1:1,000), anti-p-Smad3 (sc-130218, anti-rabbit, 1:500),
anti-Smad7 (sc-11392, anti-rabbit, 1:2,000) and anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH) (sc-25778, anti-rabbit, 1:2,000)
(all from Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA),
anti-Smad3 (#9513, anti-rabbit, 1:1,000; Cell Signaling Technology,
Danvers, MA, USA), donkey anti-goat IgG (A50-101P, 1:10,000; Bethyl
Laboratories, Inc., Montgomery, TX, USA), goat anti-mouse IgG-HRP
(sc-2031, 1:10,000; Santa Cruz Biotechnology, Inc) and goat
anti-rabbit IgG (#7074, 1:10,000; Cell Signaling Technology).
Quantitative PCR (qPCR)
The Hs27 cells seeded in 6-well plates were cultured
to 80% confluence and incubated in SFM containing PYP1-5 (250, 500
and 1,000 ng/ml) for 24 h. Total RNA was isolated from the cells
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and the
extracted RNA was used as a template for cDNA synthesis using
oligo(dT) (Intron Biotechnology Inc., Seongnam, Korea). The PCR
amplification mixtures (total volume, 20 µl) contained 10
µl of TOPreal qPCR 2X PreMIX SYBR-Green (Enzynomics Inc.,
Daejeon, Korea), 1 µl of sense primer, 1 µl of
anti-sense primer, 2 µl of cDNA template, and 6 µl of
RNase-free water. qPCR was performed using the Eco Real-Time PCR
system (Illumina Inc., San Diego, CA, USA) using the following
amplification conditions: pre-incubation at 95°C for 10 min, 40
cycles of 95°C for 10 sec, annealing at 60°C for 15 sec, and
elongation at 72°C for 15 sec. Gene expression levels were
normalized to those of GAPDH and calculated using the comparative
ΔΔCT method, as previously described (20). The oligonucleotide primers used
for PCR are listed in Table
I.
 | Table IThe oligonucleotide primer sequences
used in the real-time PCR. |
Table I
The oligonucleotide primer sequences
used in the real-time PCR.
| Gene | Accession no. | Sequence
(5′-3′) | Amplicon (bp) |
|---|
| COL1A1 | NM_000088.3 | F:
AGGGCCAAGACGAAGACATC
R: AGATCACGTCATCGCACAACA | 138 |
| COL1A2 | NM_000089.3 | F:
TCTGGATGGATTGAAGGGACA
R: CCAACACGTCCTCTCTCACC | 126 |
| Elastin | XM_011515875.1 | F:
ATGCACACTGGTGCAGAGAG
R: TGTAAGCACACAGGCAGGTC | 98 |
| TGF-β1 | NM_000660.5 | F:
AGCGACTCGCCAGAGTGGTTA
R: GCAGTGTGTTATCCCTGCTGTCA | 130 |
| MMP-1 | NM_002421.3 | F:
CCCAAAAGCGTGTGACAGTAAG
R: CTTCCGGGTAGAAGGGATTTG | 113 |
| TIMP-1 | NM_003254.2 | F:
TGACATCCGGTTCGTCTACA
R: TGCAGTTTTCCAGCAATGAG | 102 |
| TIMP-2 | NM_003255.4 | F:
GCGGTCAGTGAGAAGGAAGTGGA
R: GAGGAGGGGGCCGTGTAGATAAAC | 140 |
| GAPDH | NM_001256799.2 | F:
ACCCACTCCTCCACCTTTGA
R: TGGTGGTCCAGGGGTCTTAC | 157 |
Statistical analysis
All samples were analyzed in triplicate. The results
are expressed as the means ± standard deviation (SD). To determine
statistical significance, analysis of variance (ANOVA) was
conducted using SPSS software (SPSS, Inc., Chicago, IL, USA). A
value of p<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of PYP1-5 on Hs27 cell
viability
We used MTS assay to determine PYP1-5 cytotoxicity
to Hs27 cells. Fig. 1 shows the
effects of various concentrations (250, 500 and 1,000 ng/ml) of
PYP1-5 on Hs27 cell viability. The PYP1-5-treated cells exhibited
similar effects on viability at concentrations of 250, 500 and
1,000 ng/ml as the untreated cells and had no significant
cytotoxicity.
PYP1-5 promotes type I collagen
synthesis
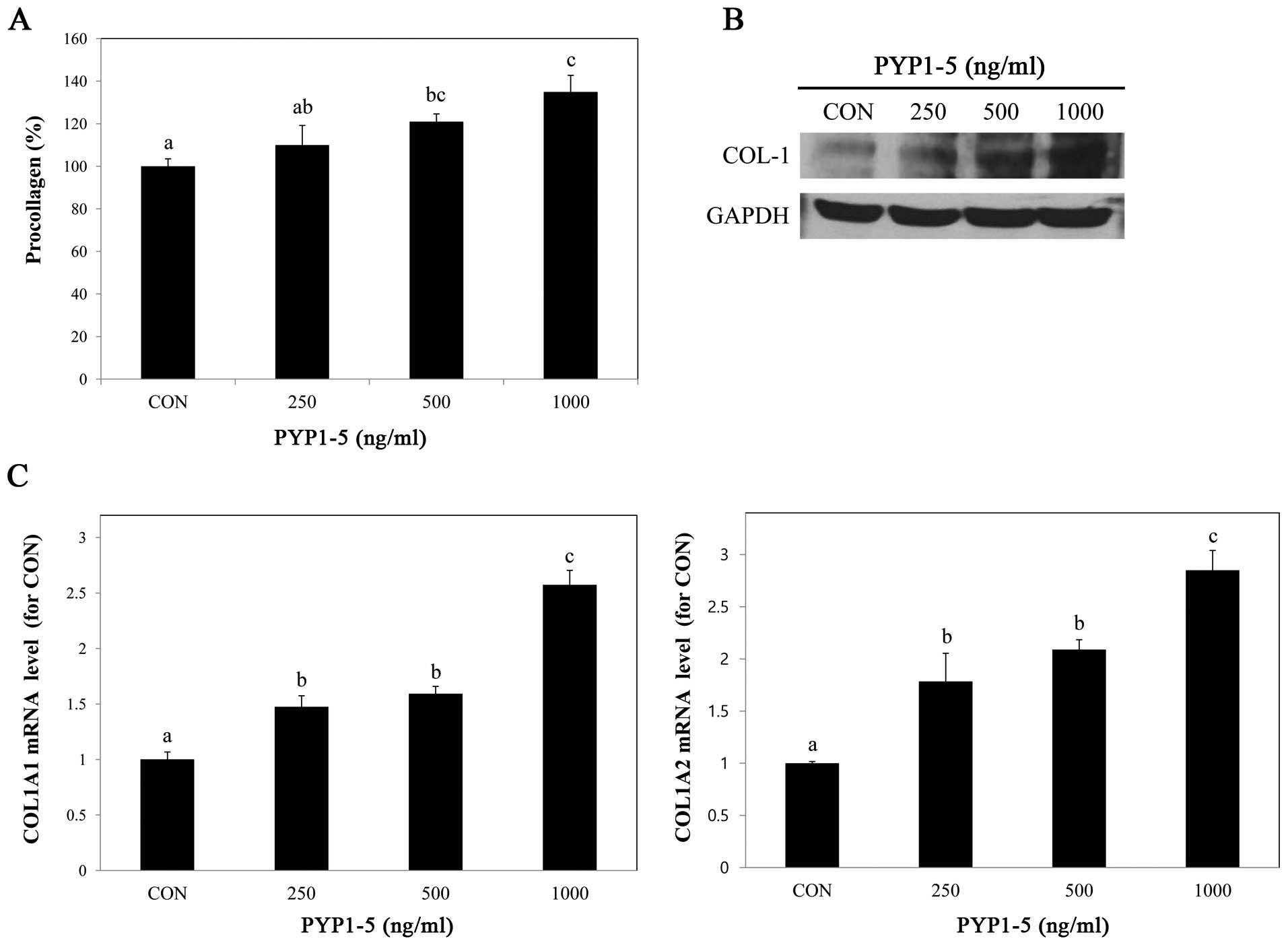
To determine whether PYP1-5 affects collagen
synthesis, we performed a PIP EIA assay. After treating the Hs27
cells with various concentrations of PYP1-5, we measured
procollagen products in the cells and found that procollagen
product levels increased in a dose-dependent manner (Fig. 2A). Using western blot analysis, we
confirmed that the type 1 collagen protein expression levels were
increased (Fig. 2B). Type 1
collagen is composed of two α1(I) chains and one α2(I) chain, which
are encoded by two genes, COL1A1 and COL1A2,
respectively (21). We further
investigated the above-mentioned by qPCR and found that both the
COL1A1 and COL1A2 mRNA expression levels increased in
a dose-dependent manner (Fig.
2C).
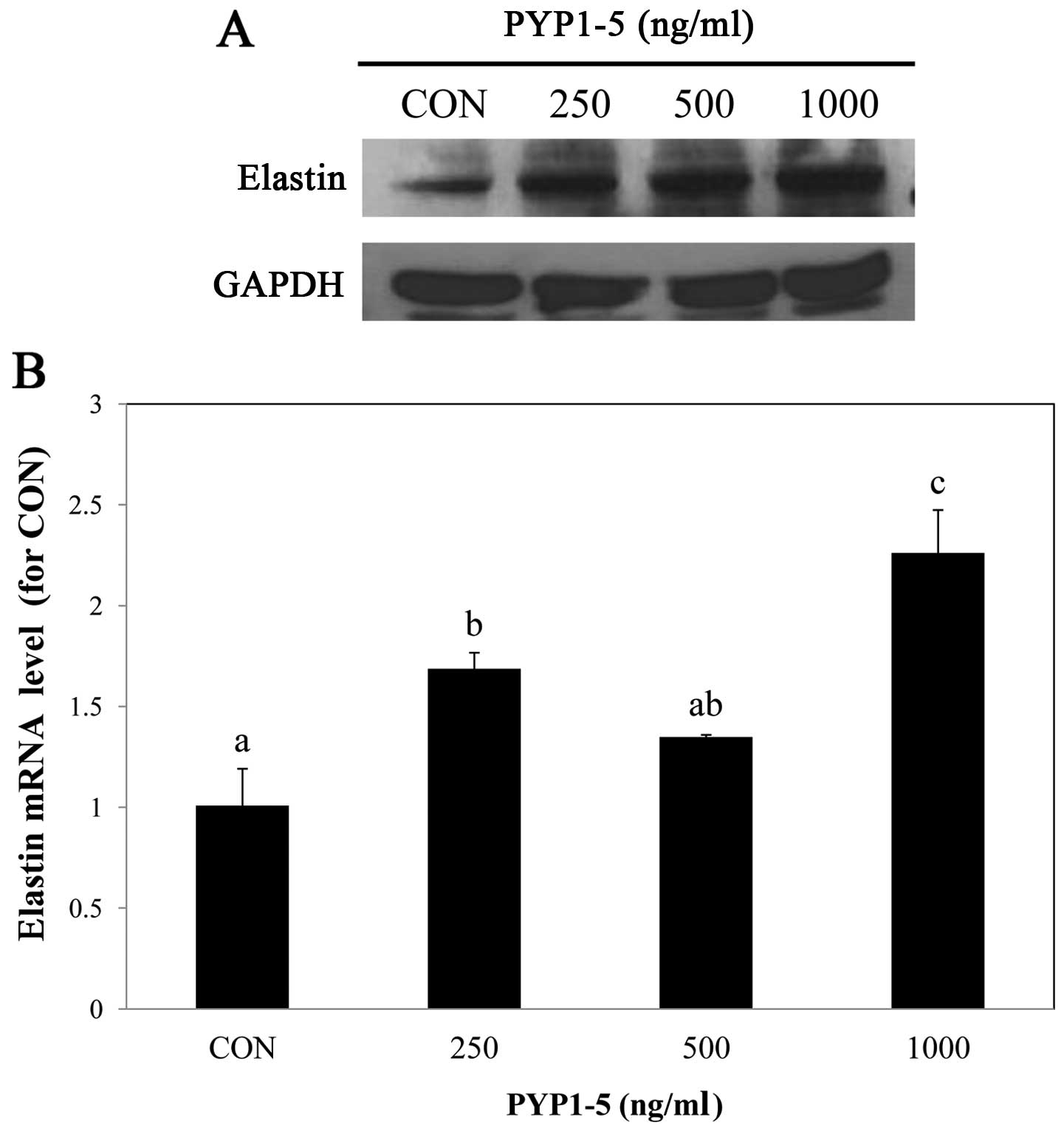
PYP1-5 increases elastin expression
Elastin is an important component of connective
tissue, such as collagen and is located between collagen in the
dermis, providing elasticity and flexibility to the skin. With age,
elastin decomposes, resulting in reduced skin elasticity and
increased skin aging (22,23).
In this study, to examine the effect of PYP1-5 on elastin in Hs27
cells, we performed western blot analysis and qPCR and found that
the elastin protein and mRNA expression levels increased following
treatment with PYP1-5 (Fig.
3).
PYP1-5 decreases MMP-1 expression and
increases TIMP-1 and -2 expression
To confirm the regulatory effects of PYP1-5 on ECM
synthesis enzymes, we examined MMP-1 and TIMP-1 and -2 protein and
mRNA expression by western blot analysis and qPCR, respectively.
Following treatment of the cells with PYP1-5 for 24 h, the MMP-1
protein and mRNA expression levels decreased in a dose-dependent
manner (Fig. 4A and B), while the
TIMP-1 and -2 protein and mRNA expression levels increased
(Fig. 4A and C). These results
indicate that PYP1-5 regulates collagen synthesis by reducing MMP-1
expression and inducing TIMP-1 and -2 expression.
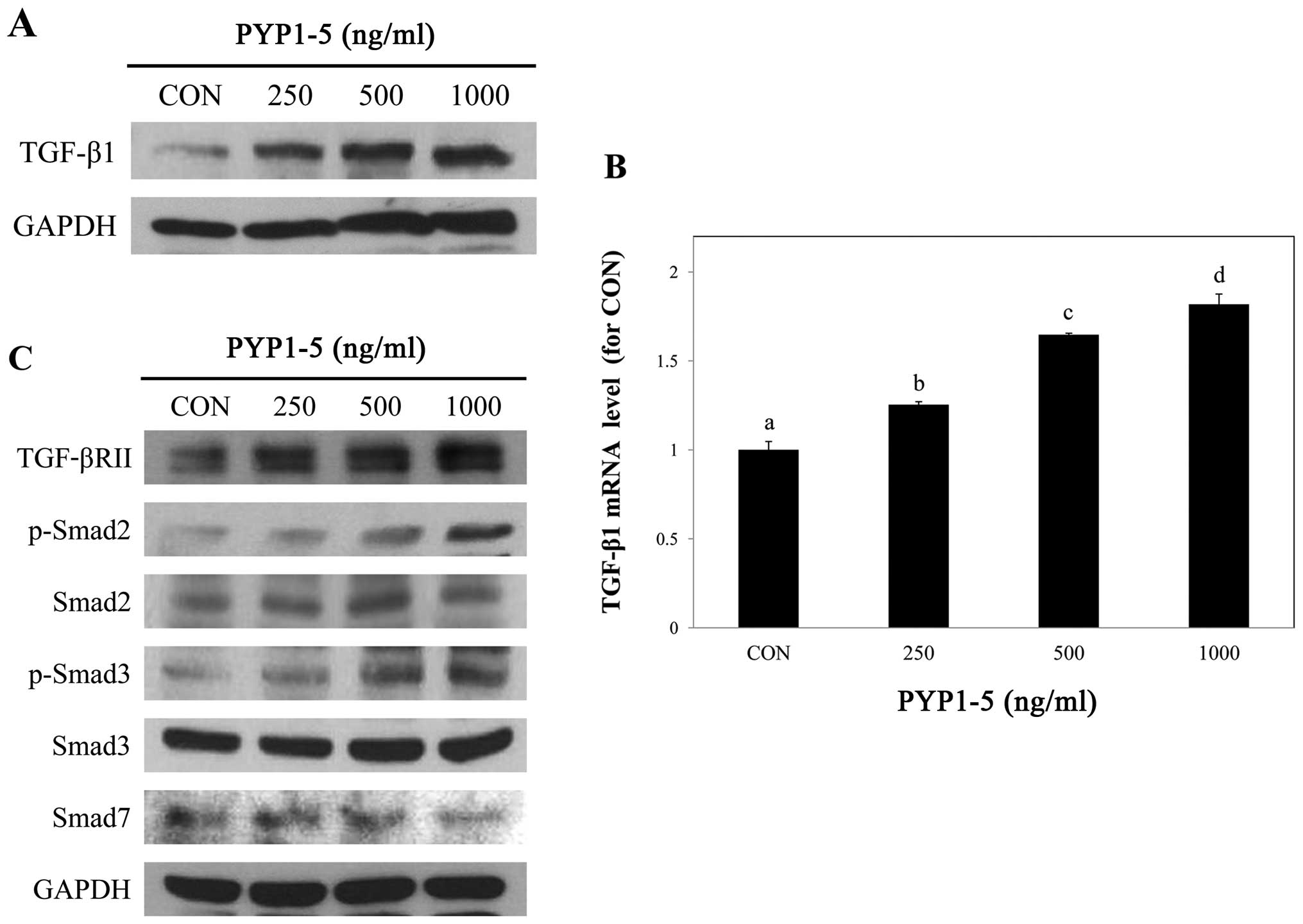
PYP1-5 activates the TGF-β/Smad signaling
pathway
We then investigated the mechanisms through which
PYP1-5 promotes type 1 collagen expression in Hs27 cells. Since the
TGF-β/Smad pathway is the major signaling pathway of collagen
synthesis in the dermis (24), we
assessed whether it is involved in PYP1-5-induced type 1 collagen
expression.
To investigate the regulatory effects of PYP1-5 on
the TGF-β/Smad signaling pathway, we measured downstream protein
and mRNA expression levels in Hs27 cells using by western blot
analysis and qPCR, respectively. We confirmed that PYP1-5 increased
TGF-β1 ligand protein and mRNA expression levels in a
dose-dependent manner (Fig. 5A and
B). In addition, treatment with PYP1-5 increased the TGF-βRII
and p-Smad2/3 protein levels. Treatment of the Hs27 cells with
PYP1-5 also decreased the Smad7 protein levels, an inhibitor of the
TGF-β/Smad signaling pathway (Fig.
5C). These results indicate that treatment of the Hs27 cells
with PYP1-5 activates the TGF-β/Smad signaling pathway. To further
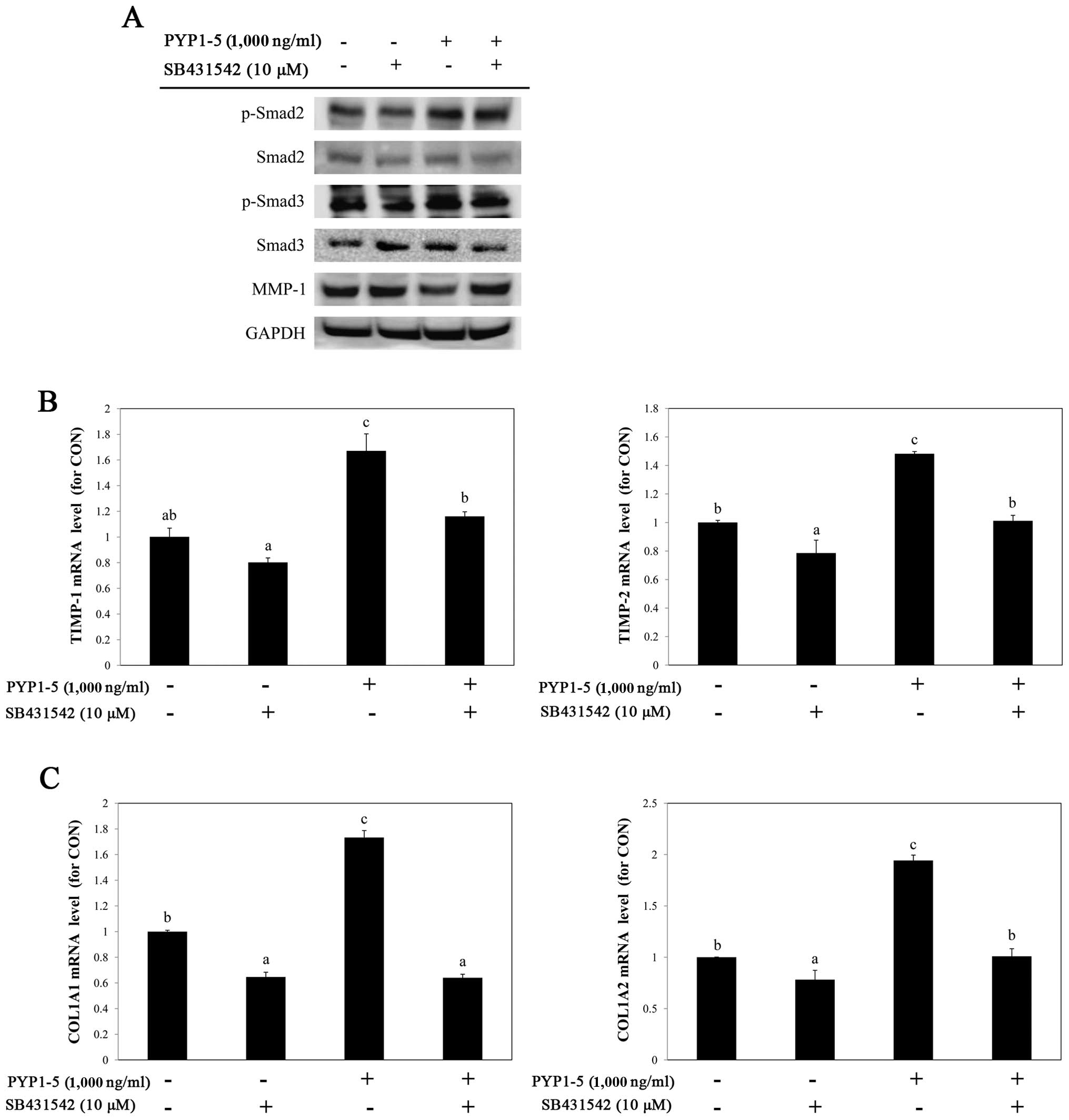
investigate the involvement of the TGF-β/Smad pathway in
PYP1-5-induced collagen synthesis, we used a specific inhibitor of
TGF-βRI, SB431542 (10 µM). Treatment with SB431542
downregulated the PYP1-5-induced upregulation of p-Smad2/3, a
downstream target of TGF-β/Smad signaling (Fig. 6A) and attenuated the effects of
PYP1-5 on the MMP-1 protein, and TIMP-1 and TIMP-2
mRNA levels (Fig. 6A and B). In
addition, treatment with SB431542 reduced the PYP1-5-induced
COL1A1 and COL1A2 mRNA levels (Fig. 6C). These data suggest that PYP1-5
regulates collagen synthesis via the TGF-β/Smad signaling
pathway.
PYP1-5 increases the expression of
specificity protein 1 (Sp1) transcription factor via the TGF-β/Smad
signaling pathway
Sp1 is an essential zinc finger transcription factor
of Smad- dependent positive regulation of collagen synthesis that
is induced by TGF-β. The Sp1 transcription factor regulates
multiple biological processes, such as tumorigenesis, apoptosis,
cell cycle and angiogenesis, and induces type I procollagen
synthesis in fibroblasts (25).
The Smad3/4 complex induces the trans-activation of the human
COL1A1 and COL1A2 promoter in normal skin fibroblasts
(26,27). In this study, we confirmed the
effect of PYP1-5 on Sp1 protein levels induced by the the
TGF-β/Smad signaling pathway and found that treatment with PYP1-5
increased Sp1 protein expression (Fig. 7).
Discussion
Interest in anti-aging has grown with the increase
in life expectancy. In particular, many efforts have been made to
prevent skin aging (28) and much
research has been dedicated to skin health and beauty. Skin aging
is classified as either intrinsic or extrinsic. Intrinsic aging
occurs due to a reduction in the levels of ECM components, such as
collagen and elastin, with age (29). Since collagen, the predominant
component of the dermis, must be maintained to prevent the skin
aging process, it is important to prevent its decomposition.
Various bioactive ingredients have been identified
in many marine algae species, several of which have been studied
for their anti-aging effects on skin, including anti-photoaging,
anti-free radical activity, moisturization, and collagen
biosynthesis (30). It has been
demonstrated that P. yezoensis extract exerts a protective
effect against UVA radiation on skin fibroblasts (18). However, the effects of P.
yezoensis on collagen synthesis in human dermal fibroblasts
remain unclear. In this study, we examined the P. yezoensis
peptide, PYP1-5, for its anti-aging function by promoting collagen
synthesis in human dermal fibroblasts. First, we investigated
whether PYP1-5 induced cytotoxicity in Hs27 cells. We found that
the PYP1-5-treated cells had a similar viability as the untreated
cells (Fig. 1), suggesting that
PYP1-5 was non-toxic to Hs27 cells. In the dermis, collagen
gradually decomposes due to extrinsic and intrinsic factors, which
promotes wrinkle formation and skin aging (31). PYP1-5 increased procollagen
synthesis in a dose-dependent manner, with a maximum increase of
35% more than the untreated cells (Fig. 2A). In addition, PYP1-5 upregulated
type 1 collagen protein levels and COL1A1 and COL1A2
mRNA expression (Fig. 2B and C).
These results indicate that PYP1-5 promotes collagen synthesis.
Elastin is another vital component of the skin, as
it is a protein that is located between collagen in the dermis that
provides elasticity and flexibility to the skin (23). Following the treatment of the Hs27
cells with PYP1-5, the elastin protein and mRNA expression levels
increased (Fig. 3), suggesting
that PYP1-5 promotes the synthesis of other ECM products. Further
research is required to elucidate its effects on other ECM
components, such as hyaluronan and fibronectin. In the dermis,
various MMP enzymes degrade collagen, while TIMP enzymes inhibit
MMP activity (8). We confirmed
that PYP1-5 suppressed the MMP-1 protein and mRNA expression levels
(Fig. 4A and B), and enhanced the
TIMP-1 and -2 protein and mRNA expression levels (Fig. 4A and C).
TGF-β is the major activator of the collagen
synthesis process in skin fibroblasts. The process is also related
to the TGF-β/Smad signaling pathway (32). PYP1-5 upregulated the TGF-β1
protein and mRNA levels in a dose-dependent manner (Fig. 5A and B). Moreover, we confirmed
that PYP1-5 increased TGF-βRII, which resulted in Smad2 and Smad3
phosphorylation (Fig. 5C). This
forms a complex with Smad4, which can translocate to the nucleus.
To confirm that PYP1-5-induced collagen synthesis was caused by
TGF-β/Smad signaling pathway activation, we treated the Hs27 cells
with SB431542, a specific inhibitor of TGF-βRI. Treatment with
SB431542 decreased TGF-β/Smad signaling pathway activation by
PYP1-5 (Fig. 6A). In addition,
PYP1-5-induced Smad2/3 activation was inhibited by SB431542,
suggesting that the phosphorylation of Smad2/3 is mediated by
PYP1-5 treatment. The effects of PYP1-5 on MMP-1 and TIMP-1 and -2
regulation were attenuated by treatment with SB431542 (Fig. 6A and B). Moreover, the
PYP1-5-induced increase in COL1A1 and COL1A2 mRNA
expression was decreased by treatment with SB431542 (Fig. 6C), indicating that PYP1-5-induced
collagen synthesis is regulated by TGF-β/Smad signaling pathway
activation. The Sp1 transcription factor, which is induced by
TGF-β/Smad signaling pathway activation, regulates type I
procollagen expression (26). In
this study, following treatment with PYP1-5, Sp1 protein expression
increased (Fig. 7). This
demonstrates that the treatment of Hs27 cells with PYP1-5 affects
transcription.
In conclusion, the findings of this study
demonstrate that PYP1-5 promotes type 1 collagen synthesis in Hs27
cells. Moreover, the TGF-β/Smad signaling pathway may be important
in mediating the effects of PYP1-5 on collagen synthesis. Our
results suggest that PYP1-5 may have beneficial effects on
intrinsic skin aging.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
2012R1A6A1028677).
References
|
1
|
Kim SH, Yong HJ, Shin C and Ko SG:
Research of traditional herbal medicines for anti-aging, inhibition
effect of wrinkle and whitening effect in the skin. Kor J Ori
Physiol Pathol. 22:691–698. 2008.
|
|
2
|
Mukherjee PK, Maity N, Nema NK and Sarkar
BK: Bioactive compounds from natural resources against skin aging.
Phytomedicine. 19:64–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farage MA, Miller KW, Elsner P and Maibach
HI: Intrinsic and extrinsic factors in skin ageing: A review. Int J
Cosmet Sci. 30:87–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naylor EC, Watson RE and Sherratt MJ:
Molecular aspects of skin ageing. Maturitas. 69:249–256. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Costin GE and Hearing VJ: Human skin
pigmentation: Melanocytes modulate skin color in response to
stress. FASEB J. 21:976–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon JH, Kim J, Lee H, Kim SY, Jang HH,
Ryu SH, Kim BJ and Lee TG: Laminin peptide YIGSR induces collagen
synthesis in Hs27 human dermal fibroblasts. Biochem Biophys Res
Commun. 428:416–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim EJ, Kim MK, Jin XJ, Oh JH, Kim JE and
Chung JH: Skin aging and photoaging alter fatty acids composition,
including 11,14,17-eicosatrienoic acid, in the epidermis of human
skin. J Korean Med Sci. 25:980–983. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kähäri VM and Saarialho-Kere U: Matrix
metalloproteinases in skin. Exp Dermatol. 6:199–213. 1997.
View Article : Google Scholar
|
|
9
|
Dennler S, Goumans MJ and ten Dijke P:
Transforming growth factor β signal transduction. J Leukoc Biol.
71:731–740. 2002.PubMed/NCBI
|
|
10
|
Amento EP and Beck LS: TGF-β and wound
healing In Ciba Foundation Symposium 157 - Clinical Applications of
TGF-β. John Wiley & Sons Ltd; Chichester: pp. 115–136. 1991
|
|
11
|
Czuwara-Ladykowska J, Sementchenko VI,
Watson DK and Trojanowska M: Ets1 is an effector of the
transforming growth factor-β (TGF-β) signaling pathway and an
antagonist of the profibrotic effects of TGF-β. J Biol Chem.
277:20399–20408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verrecchia F and Mauviel A: Transforming
growth factor-β signaling through the Smad pathway: Role in
extracellular matrix gene expression and regulation. J Invest
Dermatol. 118:211–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitano Y, Murazumi K, Duan J, Kurose K,
Kobayashi S, Sugawara T and Hirata T: Effect of dietary porphyran
from the red alga, Porphyra yezoensis, on glucose metabolism in
diabetic KK-Ay mice. J Nutr Sci Vitaminol (Tokyo). 58:14–19. 2012.
View Article : Google Scholar
|
|
14
|
Jiang LF: The polysaccharides from
porphyra yezoensis suppress the denaturation of bighead carp
myofibrillar protein. Int J Biol Macromol. 68:18–20. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwon MJ and Nam TJ: Porphyran induces
apoptosis related signal pathway in AGS gastric cancer cell lines.
Life Sci. 79:1956–1962. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shin ES, Hwang HJ, Kim IH and Nam TJ: A
glycoprotein from Porphyra yezoensis produces anti-inflammatory
effects in liposaccharide-stimulated macrophages via the TLR4
signaling pathway. Int J Mol Med. 28:809–815. 2011.PubMed/NCBI
|
|
17
|
Qian L, Zhou Y and Ma JX: Hypolipidemic
effect of the polysaccharides from Porphyra yezoensis. Int J Biol
Macromol. 68:48–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryu J, Park SJ, Kim IH, Choi YH and Nam
TJ: Protective effect of porphyra-334 on UVA-induced photoaging in
human skin fibroblasts. Int J Mol Med. 34:796–803. 2014.PubMed/NCBI
|
|
19
|
Lee MK, Kim YM, Kim IH, Choi YH and Nam
TJ: Pyropia yezoensis peptide protects against
dexamethasone-induced muscle atrophy through the down-regulation
Atrogin1/MAFbx and MuRF1 in mouse C2C12 myotubes. Mol Med Rep. In
press.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
21
|
Shoulders MD and Raines RT: Collagen
structure and stability. Annu Rev Biochem. 78:929–958. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryhanen L and Uitto J: Elastic fibers of
the connective tissue. Biochem Physiol Skin. 1:433–447. 1983.
|
|
23
|
Sephel GC and Davidson JM: Elastin
production in human skin fibroblast cultures and its decline with
age. J Invest Dermatol. 86:279–285. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi MS, Yoo MS, Son DJ, Jung HY, Lee SH,
Jung JK, Lee BC, Yun YP, Pyo HB and Hong JT: Increase of collagen
synthesis by obovatol through stimulation of the TGF-beta signaling
and inhibition of matrix metalloproteinase in UVB-irradiated human
fibroblast. J Dermatol Sci. 46:127–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wierstra I: Sp1: emerging roles - beyond
constitutive activation of TATA-less housekeeping genes. Biochem
Biophys Res Commun. 372:1–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park JH, Kim SR, An HJ, Kim WJ, Choe M and
Han JA: Esculetin promotes type I procollagen expression in human
dermal fibroblasts through MAPK and PI3K/Akt pathways. Mol Cell
Biochem. 368:61–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang W, Ou J, Inagaki Y, Greenwel P and
Ramirez F: Synergistic cooperation between Sp1 and Smad3/Smad4
mediates transforming growth factor β1 stimulation of α
2(I)-collagen (COL1A2) transcription. J Biol Chem. 275:39237–39245.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim YM, Jung HJ, Choi JS and Nam TJ:
Anti-wrinkle effects of a tuna heart H2O fraction on
Hs27 human fibroblasts. Int J Mol Med. 37:92–98. 2016.
|
|
29
|
El-Domyati M, Attia S, Saleh F, Brown D,
Birk DE, Gasparro F, Ahmad H and Uitto J: Intrinsic aging vs.
photoaging: A comparative histopathological, immunohistochemical,
and ultra-structural study of skin. Exp Dermatol. 11:398–405. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samarakoon K and Jeon YJ:
Bio-functionalities of proteins derived from marine algae - A
review. Food Res Int. 48:948–960. 2012. View Article : Google Scholar
|
|
31
|
Varani J, Warner RL, Gharaee-Kermani M,
Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ and
Voorhees JJ: Vitamin A antagonizes decreased cell growth and
elevated collagen-degrading matrix metalloproteinases and
stimulates collagen accumulation in naturally aged human skin. J
Invest Dermatol. 114:480–486. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leask A and Abraham DJ: TGF-β signaling
and the fibrotic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|