Introduction
Organ transplantation is the most effective
treatment for end-stage organ failure (1). The surgical methods of organ
transplantation are now widely used; however, allograft rejection
limits its further development. A major task in transplantation
research is to rapidly achieve a state of immunological tolerance
to alloantigens (2). T cells play
a key role in regulating transplantation immunology. Allospecific T
cell tolerance implies that T cells do not mount pathogenic immune
reactions towards allogeneic organs, but preserve protective
activity towards environmental pathogens. Thus, allospecific
tolerized T cells are critical to accomplishing transplantation
tolerance (3,4).
Costimulation blockade is an emerging therapeutic
strategy in transplantation medicine that circumvents the need for
lifelong chronic immunosuppression by inhibiting the activation of
the immune system at the time of transplantation (5). OX40 is a costimulatory receptor
expressed primarily on activated CD4+ and
CD8+ T cells. OX40 ligand (OX40L) is expressed on
activated antigen-presenting cells and binds to OX40, inducing an
agonistic response in T lymphocytes, that results in cell
proliferation, increased cytokine production and the long-term
survival of T lymphocytes (6). As
a costimulator, OX40 is a promising drug target for T cell-mediated
inflammatory diseases (7). The
blockade of the OX40 and OX40L pathway has been shown to inhibit
graft rejection and graft-versus-host disease (GVHD), and to
ameliorate autoimmune diseases (8,9).
In addition, we previously demonstrated that the blockade of the
OX40-OX40L pathway by OX40-Ig fusion protein (OX40Ig) may induce
antigen-specific T cell anergy in vitro in patients with
acute renal allograft rejection (10).
Cell therapies applied to solid organ
transplantation have gained much attention over the past years, and
among these therapies, mesenchymal stem/stromal cell (MSC) therapy
has strongly emerged as one of the main therapies. In addition to
their potential role in therapies for renal repair, the
immunomodulatory properties of MSCs offer promise as a novel
cellular therapy for the long-term protection of kidney allografts
(11). Although the most common
and well-characterized source of MSCs is the bone marrow, adipose
tissue is the most promising source of MSCs suitable for autologous
stem cell therapy. Adipose tissue has several advantages as a
tissue stem cell source, including the richest source, easy
accessibility, less invasive collection procedures and safe,
autologous cell transplantation without immune rejection (12–14). Although MSC-based therapies have
been shown to be safe and effective to a certain degree, the
efficacy of MSCs in vivo remains low in most cases when MSCs
are applied alone.
MSC monotherapy and costimulation blockade modulate
many of the same components of the immune system and can induce the
peripheral conversion of T cells into regulatory T cells (Tregs).
These two treatment strategies are being tested independently in
clinical organ transplantation and in autoimmune diseases. Since
these strategies share common goals and converge on some of the
same target cells, it seems imperative to study their ability to
synergize in downmodulating immune responses. For example,
Takahashi et al (15)
demonstrated that the combination of MSCs and costimulation
blockade yielded superior islet graft survival and function.
However, the half-time of an injected Ig fusion protein is reduced
and the patient needs more of the biological agent to achieve the
same effect in vivo. To enhance their therapeutic efficacy,
genetic engineering is one approach to improve the in vivo
performance of MSCs. As MSCs migrate to the target tissue, the
therapeutic agent can be released in a local and sustained
manner.
The aim of the present study was to clone OX40Ig to
generate a recombinant pcDNA3.1(−)OX40Ig vector and trans-duce the
vector into Lewis rat recipient adipose tissue-derived mesenchymal
stem cells (ADSCs). We also investigated the anti-proliferative
activity in vitro, as well as the prevention of graft
rejection following allogeneic renal transplantation.
Materials and methods
Animals
A total of 60 age-matched inbred male Brown Norway
(BN, RT1n) and 84 Lewis (LEW, RT1) rats
weighing 200 to 250 g were obtained from the Academy of Military
Medical Science [Beijing, China; certificate no. SCXK (JUN)
2007-004]. These animals were maintained in a standard animal
laboratory with free activity and free access to water and rodent
chow. They were maintained in a temperature controlled environment
at 22–24°C with a 12 h light/dark cycle. The rats were fasted for
12 h prior to surgery, and were provided with free access to 10%
glucose water following surgery. All the surgical procedures were
performed under sanitary conditions, and all the experiments were
performed according to the National Institutes of Health Guide for
Care and Use of Laboratory Animals (16). The present study was approved
(approval ID no. E2013008K) by the Ethics Committee of Tianjin
First Central Hospital (Tianjin, China).
Isolation and characterization of MSCs
from rat adipose tissue
ADSCs were isolated from LEW rats (n=72) using a
previously described method (17). Briefly, the rats were anesthetized
with isoflurane inhalation (LUNAN Pharmaceutical Co., Ltd.,
Shandong, China) 14 days before mixed lymphocyte reaction (MLR)
assay and autologous cell transplantation, and the subcutaneous
adipose tissue was then obtained from the hemi-inguinal regions of
these rats, which were carefully excised and minced into ~1
mm3 sections. The adipose tissue was digested in
collagenase type I solution (Sigma-Aldrich, St. Louis, MO, USA) for
60 min at 37°C with constant agitation (100 rpm). The stromal cells
were separated from the floating adipocytes by centrifugation at
200 × g for 5 min at room temperature. The cells released were then
resuspended in DMEM/F12 medium (Gibco, Waltham, MA, USA), then
sieved through a 70 µm mesh (BD Falcon, Bedford, MA, USA).
The resulting ADSCs were cultivated in DMEM/F12 medium containing
10% FBS (Gibco). When the cells reached confluence, the adherent
cells were detached with trypsin/ethylenediaminetetraacetic acid
(EDTA) (Sigma-Aldrich) and reseeded for expansion. Following in
vitro culture for 14 days at 37°C, 5% CO2 and 95%
humidity, we obtained a sufficient amount of ADSCs for autologous
transplantation. The cultured ADSCs (3×106) from each
experimental rat were respectively labeled and cryopreserved in
liquid nitrogen [Air Products and Chemicals (Tianjin) Co., Ltd.,
Tianjin, China] prior to injection.
The cultured ADSCs were characterized for the
expression of hematopoietic markers, CD34 and CD45, and mesenchymal
cell markers, CD90, CD73 and CD105 by fluorescence-activated cell
sorting (FACS) analysis using a flow cytometer (FACSCalibur flow
cytometer; Becton-Dickinson, Franklin Lakes, NJ, USA), and data
were analyzed using the CellQuest software program.
Multi-differentiation ability of
ADSCs
DSCs were also confirmed by their capacity to
differentiate into adipogenic, islet and osteogenic lineages as
previously described (17).
Briefly, the ADSCs were seeded in medium at 2×104
cells/cm2 in 6-well tissue culture plates. When the
cells reached 100% confluency, DMEM/F12 was subsequently replaced
with specific inducer medium. Adipogenic inducer medium is DMEM/F12
containing 1 µmol/l dexamethasone, 0.5 mmol/l
3-isobutyl-1-methylxanthine (Sigma-Aldrich), 5 mg/l insulin
(Sigma-Aldrich), 100 µmol/l indomethacin (Sigma-Aldrich).
Islet inducer medium is DMEM (high glucose; Gibco) containing 10
mmol/l nicotinamide (Sigma-Aldrich), 100 µg/l conophylline
(BioBioPha, Yunnan, China), 10 µg/l betacellulin (PeproTech,
Rocky Hill, NJ, USA), 10 µg/l hepatocyte growth factor (HGF)
(PeproTech), 2% B27 supplement (Gibco), 1% N2 supplement (Gibco).
Osteogenic inducer medium is DMEM/F12 containing 100 nmol/l
dexamethasone (Sigma-Aldrich), 10 mmol/l β-sodium glycerophosphate
(Sigma-Aldrich) and 50 µg/ml vitamin C (Sigma-Aldrich).
Following a 21-day induction period, adipocytes, islet-like cells,
and osteoblasts were identified by Oil Red O staining
(Sigma-Aldrich), dithizone staining (Sigma-Aldrich), and Alizarin
Red S staining (Genmed, Shanghai, China), respectively.
Eukaryotic expression vector
construction
Briefly, the NCBI database was screened for coding
sequences of rat OX40 extracellular domains. RNA was extracted from
rat peripheral blood mononuclear cells (PBMCs) using TRIzol reagent
(Life Technologies, Carlsbad, CA, USA). cDNA was synthesized using
a reverse transcription system (Promega, Madison, WI, USA). The
cycling conditions for polymerase chain reaction (PCR) were 94°C
for 4 min; followed by 32 cycles of 94°C for 60 sec, 58°C for 60
sec, and 72°C for 60 sec; and then 72°C for 10 min. OX40 primers,
designed with Primer Premier 5.0 software (Premier Biosoft, Palo
Alto, CA, USA), were added into the cDNA mixture for PCR
amplification (Promega). The primers used were designed as follows
(forward and reverse, respectively):
5′-ACGGGATCCACCACCATGGTTACAGTGAAGCTCAAC-3′ and
5′-CGGAATTCAGGGCCCTCAGGAGCCACC-3′ (underlined letters denote
restriction endo-nuclease recognition sequences). These PCR
products were inserted into the pcDNA3.1(+)/linker (Life
Technologies) plasmid using BamHI and EcoRI
restriction sites to generate the recombinant plasmid,
pcDNA3.1(+)/OX40-linker. The plasmid was double-checked by
BamHI-EcoRI digestion with electrophoresis on agarose
gel and sequencing (Sangon Biotech Co., Ltd., Shanghai, China).
A PCR-amplified cDNA fragment encoding the human IgG
Fc fragment was inserted into the XhoI and XbaI sites
of the pcDNA3.1(+)/OX40-linker vector to obtain the eukaryotic
expression vector (plasmids) pcDNA3.1(+)/OX40-IgG Fc
(pcDNA3.1/OX40Ig). The human IgG Fc primers used were designed as
follows (forward and reverse, respectively): 5′-GTCAGCTCGAGGCAAGCTTCAAGGGCC-3′ and
5′-GCTCTAGACTATTTACCCGGAGACAGGGAGAG-3′
(the underlined letters denote restriction endonuclease recognition
sequences). The constructed plasmids were verified by double
restriction endonuclease digestion and DNA sequence analysis to
confirm the sequence accuracy. The cytomegalovirus (CMV) promoter
pcDNA3.1(+)/GFP (Invitrogen, Carlsbad, CA, USA) was used to gauge
the transfection efficiency.
ADSC nucleofection
The nucleofection of the ADSCs was performed
according to the optimized protocols provided by the manufacturer
(Amaxa Biosystems, Cologne, Germany). Briefly, prior to
nucleofection, a Petri dish culture containing 1 ml of DMEM was
incubated in the CO2 incubator at 37°C. The ADSCs were
digested and centrifuged following the adjustment of the density of
the suspended cells to 2×106/ml. The cells
(5×105) and plasmid (2 µg) were suspended in 100
µl prewarmed nucleofector solution (Amaxa Biosystems). For
nucleofection, the program U-23 was selected. Immediately,
following nucleofection, the cells were transferred into prewarmed
fresh medium in six-well plates, and incubated in a CO2
incubator at 37°C and monitored daily. The cells were analyzed 24 h
post-nucleofection for transfection efficiency by FACS (FACSCalibur
flow cytometer; Becton-Dickinson) and a Nikon Ts100 fluorescence
microscope (Nikon Corp., Tokyo, Japan) for GFP expression. The
ADSCs transduced with pcDNA3.1/OX40Ig or pcDNA3.1/GFP are referred
to as ADSCsOX40Ig or ADSCsGFP,
respectively.
Western blot analysis
The transduced OX40Ig was confirmed by western blot
analysis, as previously described (18). Briefly, the transduced ADSCs
(ADSCsOX40Ig or ADSCsGFP) were fractionated
on a 12% SDS-polyacrylamide gel, and the fractionated proteins were
electrophoretically transferred onto nitrocellulose membranes
(Millipore, Billerica, MA, USA). The protein samples were then
incubated with primary anti-OX40 rabbit polyclonal antibodies (Cat
no. ab203220) and thereafter with a horseradish
peroxidase-conjugated goat anti-rabbit IgG antibody (Cat no.
ab6721) (both from Abcam, Cambridge, MA, USA). Protein bands were
visualized using an ECL western blotting kit (Amersham Pharmacia
Biotech, Piscataway, NJ, USA).
MLR assay
To test the allostimulatory activity of the
gene-transduced ADSCs, one-way MLR were performed. Splenocytes from
the 24 BN and 24 LEW rats were filtered through nylon cell
strainers (BD Falcon). The lymphocyte population was purified by
density gradient centrifugation using a commercially lymphocyte
separation medium (Sigma-Aldrich) and centrifuged at 400 × g for 25
min. The LEW lymphocytes (1×105, responders) and BN
lymphocytes (2×105, stimulator) were co-cultured with
untransduced ADSCs (ADSCsnative), ADSCsGFP,
or ADSCsOX40Ig (1×104) in 0.2 ml of culture medium. The
stimulator was mitotically inactivated with 50 µg/ml
mitomycin C (Sigma-Aldrich) at 37°C for 30 min prior to MLR
co-culture, while the responder was not treated. The mitomycin C
pre-treated ADSCsnative, ADSCsGFP, or
ADSCsOX40Ig were allowed to adhere to the plate for 2 h
before the lymphocytes were added to allow attachment. The cultures
were maintained at 37°C in a humidified atmosphere of 5%
CO2. Following routine culture for 4 days, MTT
colorimetry was used to measure the absorbance values at 570 nm in
each well. The inhibitory rate was calculated according to the
following formula: inhibitory rate (%) = (1 − average absorbance
for experimental group/average absorbance for control group)
×100.
Flow cytometric assessment of
CD4+CD25+ Tregs
The cells were analyzed for Treg markers using mouse
anti-rat monoclonal antibody to CD4 (Cat no. 554843) and CD25 (Cat
no. 554866) (both from BD Biosciences, San Diego, CA, USA). A
FACSCalibur flow cytometer was used to determine the
CD4+CD25+ cells, and data analysis was
performed using CellQuest software (both from BD Biosciences,
Franklin Lakes, NJ, USA).
Rat renal transplantation model
For orthotopic renal transplantation, the 36 LEW
rats received a kidney from the 36 BN rats using a previously
described technique (19). In
brief, following humane animal sacrifice by deep anesthesia with
isoflurane inhalation (LUNAN Pharmaceutical Co., Ltd.), the kidneys
from the BN rats were harvested, perfused with hypertonic
citrate-adenine preservation (HC-A) solution (provide by the Long
March Hospital of Shanghai, China) to remove blood from the
vascular beds and maintained at 4°C until implantation. The kidney
grafts were transplanted orthotopically into left nephrectomized
LEW rat recipients, and the blood flow was restored using standard
microsurgical techniques. The contralateral kidneys were removed
immediately following the implantation of the left kidney graft.
After the effect of graft reperfusion was observed, the abdomen was
closed. No immunosuppressive agents were provided. All
microsurgical procedures were performed by one surgeon. Five days
post-transplantation, the rats were euthanized by deep anesthesia
as described above, the grafts were removed and subjected to
morphological, reverse transcription-quantitative PCR (RT-qPCR) and
biochemical analysis.
Cell transplantation procedures
In the BN-LEW allograft models, the LEW recipient
rats were injected with autologous 2.0×106 ADSCs diluted
in 1 ml PBS or 1 ml PBS alone via the penile vein 4 days prior to
transplantation, and intrarenal injection performed immediately
following reperfusion, followed by an intravenous injection 6 h
after the ischemia/reperfusion (I/R) procedure through the penile
vein. The LEW-LEW syngeneic transplant models (n=12) were used as a
control group.
The animals were randomly divided into 4 groups as
follows: the isografts control group (n=12), the PBS control group
(n=12), the ADSCsnative-treated group (n=12), and the
ADSCsOX40Ig-treated group (n=12).
Renal function analysis
The 2 ml of blood was collected from the inferior
vena cava at days 5 post-transplantation. Serum creatinine (SCr)
levels were determined as a measure of renal function via an
enzymatic colorimetric method using an automatic biochemistry
analyzer (Hitachi High-Technologies Corp., Tokyo, Japan).
Renal histopathological analysis
The excised kidneys were fixed in formalin overnight
and embedded in paraffin wax. Five-micrometer-thick kidney sections
were deparaffinized and fixed. For histological analysis, the
sections were stained with hematoxylin and eosin (H&E;
Sigma-Aldrich), and observed using a Nikon Ni-U fluorescence
microscope (Nikon Corp.).
Examination of gene expression by
RT-qPCR
Total RNA was extracted from the frozen kidney
tissue samples using TRIzol reagent (Invitrogen) and then subjected
to reverse transcription using a High Capacity cDNA Reverse
Transcription kit (Applied Biosystems Life Technologies, Foster
City, CA, USA). qPCR was performed using an ABI 7500 Sequence
Detection System (Applied Biosystems Life Technologies) with
SYBR-Green (Takara, Tokyo, Japan). Rat primer sequences for
intragraft interferon-γ (IFN-γ), interleukin (IL)-4, IL-10,
transforming growth factor-β (TGF-β), forkhead box protein 3
(Foxp3) and β-actin were as follows: IFN-γ sense,
5′-AGGCCATCAGCAACAACATAAGTG-3′ and antisense,
5′-GACAGCTTTGTGCTGGATCTGTG-3′; IL-4 sense,
5′-AGAAGCTGCACCGTGAATGA-3′ and antisense,
5′-TCGTAGGATGCTTTTTAGGCTTTC-3′; IL-10 sense,
5′-AAGGCCATGAATGAGTTTGACAT-3′ and antisense,
5′-CGGGTGGTTCAATTTTTCATTT-3′; TGF-β sense,
5′-CAAGGGCTACCATGCCAACT-3′ and antisense,
5′-CCGGGTTGTGTTGGTTGTAGA-3′; Foxp3 sense,
5′-ACCGTATCTCCTGAGTTCCAT-3′ and antisense,
5′-GTCCAGCTTGACCACAGTTTAT-3′; and β-actin sense,
5′-CGTTGACATCCGTAAAGACCTC-3′ and antisense,
5′-TAGGAGCCAGGGCAGTAATCT-3′. The level of expression was calculated
using the 2−ΔCt method, in which ΔCt was calculated as
the Ct value of the target molecule - the Ct value of β-actin.
Statistical analysis
Data are expressed as the means ± standard
deviation. Comparisons among treatment groups were analyzed by
one-way analysis of variance (ANOVA). Animal survival analysis was
performed using Kaplan-Meier survival estimates, and statistical
significance was analyzed by the log-rank test. Statistical
analyses were performed using SPSS 16.0 software (SPSS Inc.,
Chicago, IL, USA). A value of P <0.05 was considered to indicate
a statistically significant difference.
Results
Recombinant eukaryotic expression
plasmid
The recombinant vector, pcDNA3.1/OX40Ig, was
identified by restrict enzyme digestion assay (Fig. 1). The DNA sequencing data of the
pcDNA3.1/OX40Ig was consistent with DNA sequences of OX40 (extra)
and IgG Fc listed in GenBank (data not shown).
Phenotypic and functional
characterization of rat ADSCs
The adherent ADSCs had a spindle-shaped fibroblastic
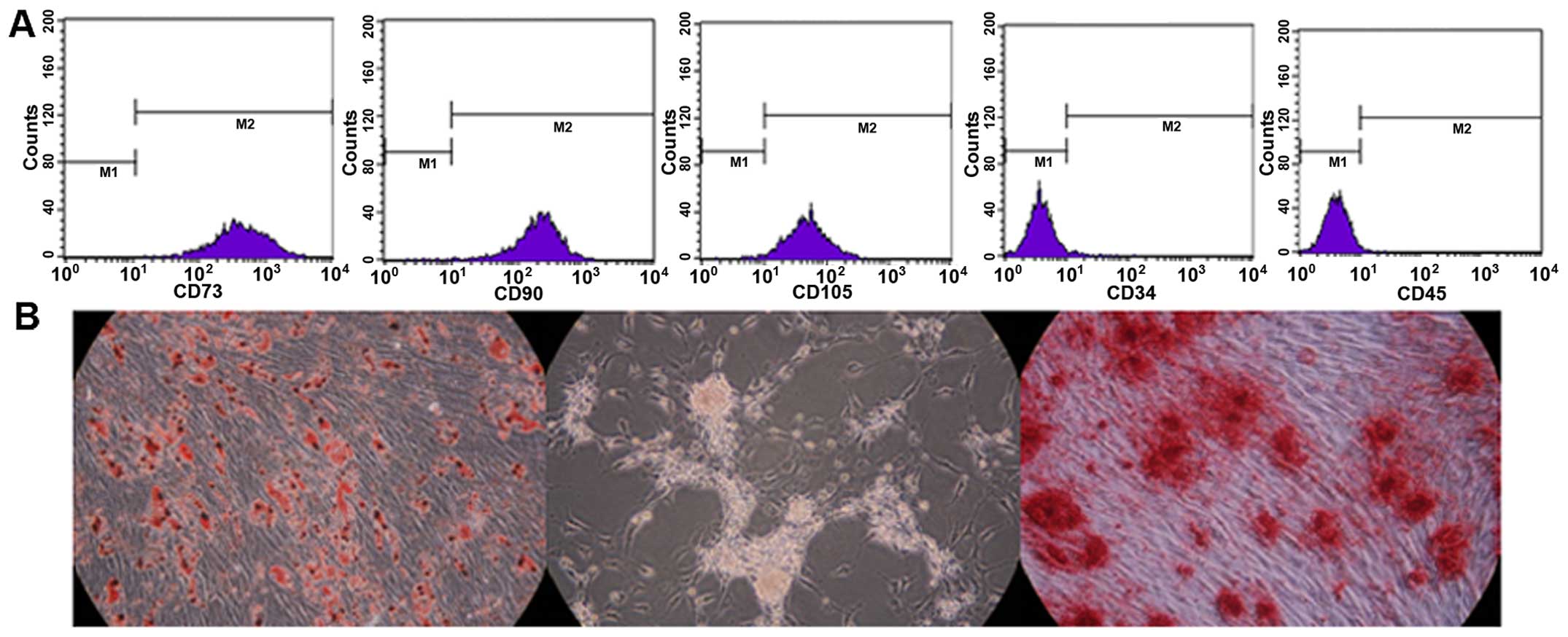
morphology following expansion. The rat ADSCs expressed typical
markers and differentiation profiles. They strongly expressed the
stem cell markers, CD90, CD73 and CD105, but were negative for the
hematopoietic markers, CD34 and CD45, as shown by flow cytometric
analysis (Fig. 2A). In addition,
the culture-expanded ADSCs were also functionally capable of
differentiating into adipocytes, islet-like cells and osteoblasts
under inductive culture conditions, and this was confirmed using
Oil Red O, dithizone and Alizarin Red S staining (Fig. 2B).
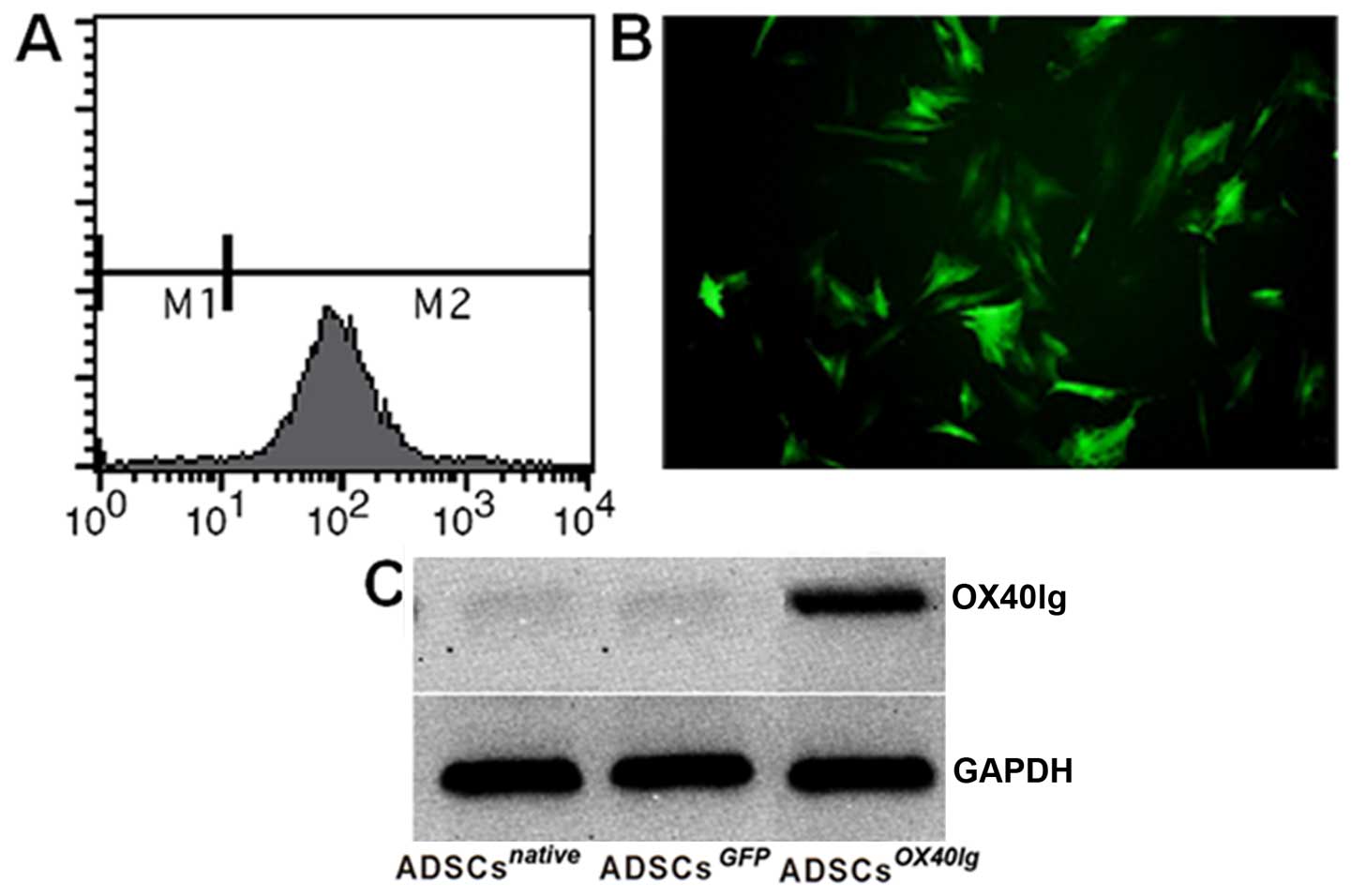
Analysis of nucleofection
To confirm the nucleofection efficiency, the vector
pcDNA3.1/green fluorescent protein (GFP) encoding for GFP was used
and observed with fluorescence microscopy and flow cytometry. Green
fluorescent ADSCsGFP could be observed (Fig. 3A and B). The expression of OX40Ig
in the ADSCs was examined at the protein level. At 48 h
post-transduction, OX40Ig protein was specifically detected in the
ADSCsOX40Ig, but not in the ADSCsGFP and
ADSCsnative (Fig.
3C).
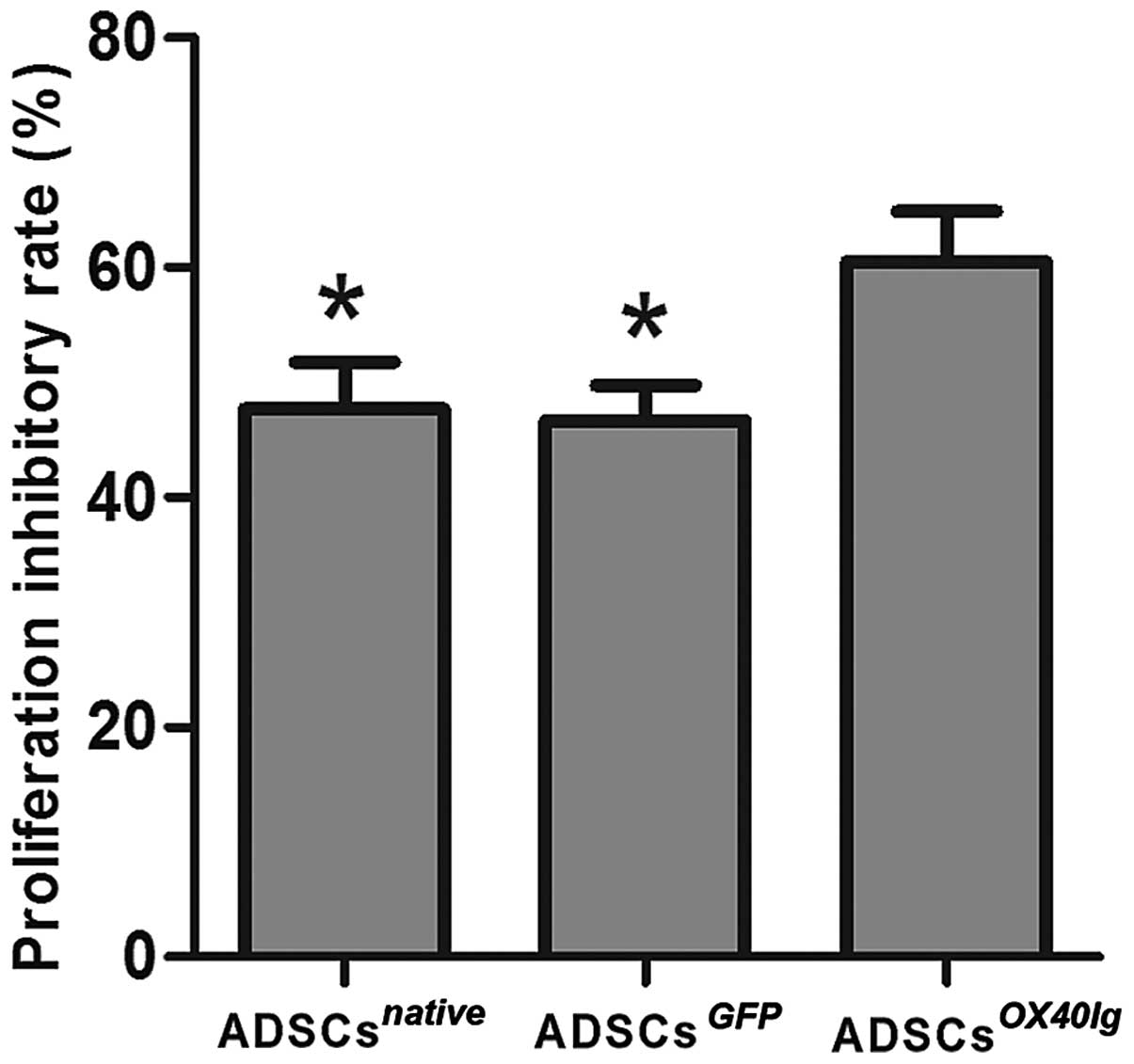
ADSCsOX40Ig suppresses the
proliferation of allostimulated T cells and modulated T cell
subsets in vitro
The data indicated that the ADSCsOX40Ig
group markedly inhibited the allostimulatory T cell proliferation
compared with the ADSCsGFP and ADSCsnative
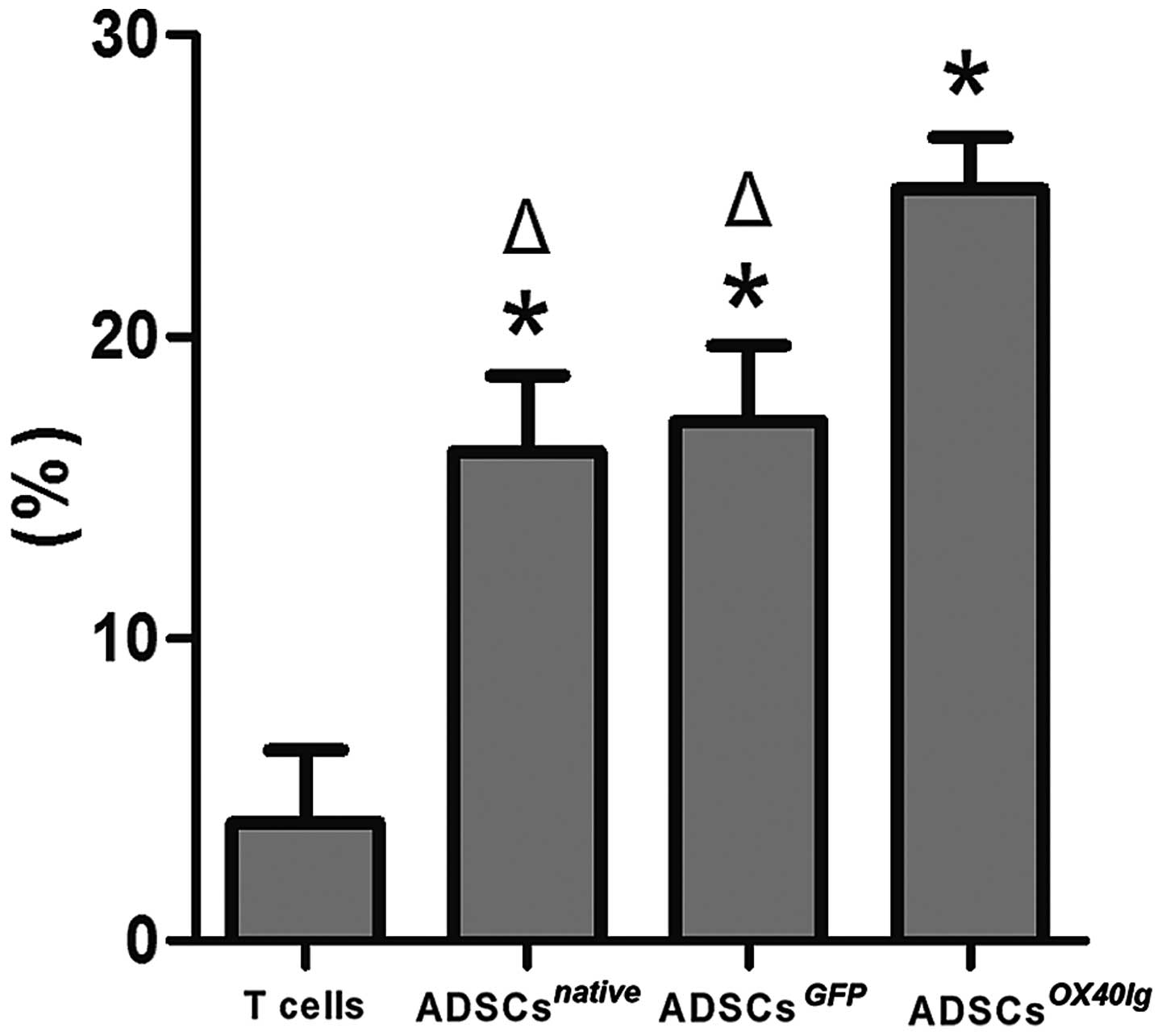
groups (P<0.01) (Fig. 4). Flow
cytometric analysis of CD4+CD25+ Tregs
revealed that this population was significantly increased in the
ADSCsnative, ADSCsGFP and
ADSCsOX40Ig groups as compared with allogeneic T cells
cultured alone (P<0.01). The percentage of
CD4+CD25+ Tregs significantly increased in
the ADSCsOX40Ig group as compared with
ADSCsnative and ADSCsGFP groups (P<0.01)
(Fig. 5). These results indicate
that OX40Ig genetic modification may substantially enhance the
immunosuppressive ability of ADSCs to allogeneic T cell
proliferation and may increase the number of Tregs.
Administration of ADSCsOX40Ig
ameliorates transplanted renal failure and slightly prolongs graft
survival
Renal transplantation induced a substantial increase
in the SCr levels in the PBS control group than in the isografts
control group (P<0.01). The administration of
ADSCsOX40Ig and ADSCsnative significantly
attenuated the increase in the SCr levels compared with the PBS
control group (P<0.01). However, the ADSCsOX40Ig
treated group had significantly lower SCr levels compared with the
ADSCsnative treated group (P<0.05) (Fig. 6). Our results indicated that the
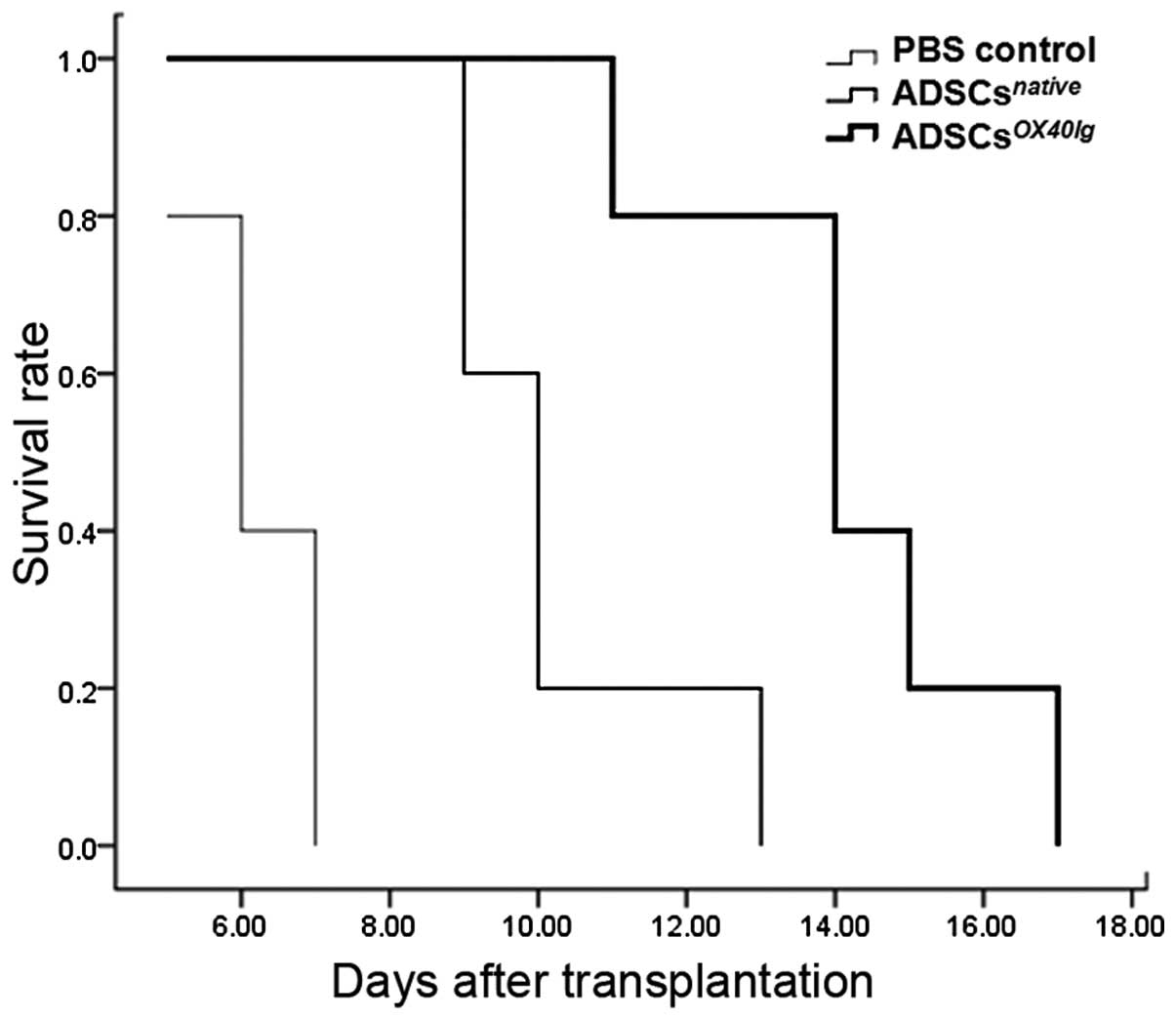
isograft survival was >90 days. The survival of the allografts
in the ADSCsnative treated group (10.2 ± 1.6 days) was
slightly, but significantly prolonged compared to that of the PBS
control group (6.2±0.8 days) (P<0.05). The administration of
ADSCsOX40Ig markeldy improved allograft survival,
increasing the mean graft survival time to 14.2±2.2 days. The
survival time of all recipients is shown in Fig. 7.
Renal histopathological evaluation
To determine the reason for graft failure, we then
evaluated renal morphologies. The allogeneic PBS control group
exhibited the histological characteristics of acute rejection, as
shown by dense parenchymal mononuclear cell infiltration, extensive
tubulitis and interstitial edema. By contrast, these changes were
significantly attenuated (the signs of acute rejection) in the
kidneys from the ADSCsnative- and
ADSCsOX40Ig-treated groups, although mild interstitial
edema and a small amount of inflammatory cell infiltration was
observed, and some tubular dilatation, or some epithelial swelling
and degeneration were present. The kidneys from the isografts
control group lacked the histological signs of rejection.
Consistent with the functional analysis data, the histological
examination also confirmed the beneficial effects of the
administration of ADSCsOX40Ig. Representative light
microscopic findings are shown in Fig. 8.
Production of cytokines related to
rejection or tolerance in allografts
Cytokines are key mediators in the induction and
effector phases of the immune and inflammatory responses in kidney
transplantation. In order to detect the effects of ADSCs on the
level of rejection or tolerance-associated cytokines in allograft
kidneys, we prepared total allograft mRNA for the measurement of
cytokine transcript expression by RT-qPCR. Compared with the
isografts control group, the allogeneic PBS control group exhibited
a significantly increased mRNA expression of IFN-γ, IL-4, IL-10,
TGF-β and Foxp3 in the allografts. Compared with the allogeneic PBS
control group, the ADSCsnative- and
ADSCsOX40Ig-treated groups exhibited a significantly
decreased expression of IFN-γ in the allografts, and there was a
marked downregulation in the IFN-γ mRNA level in the
ADSCsOX40Ig-treated group. Furthermore, compared with
the allogeneic PBS control group, the mRNA expression levels of
IL-10, TGF-β and Foxp3 were significantly increased in the
ADSCsnative- and ADSCsOX40Ig-treated groups
(Fig. 9). A significantly higher
mRNA level of the Treg marker, Foxp3, was observed in the
ADSCsOX40Ig-treated group compared with the
ADSCsnative-treated group (Fig. 9).
Discussion
The main approach designed to reduce graft rejection
has been focused on the development of immunosuppressive agents at
present. In the present study, we investigated the potential
benefits of treatment with ADSCs and OX40Ig-expressing ADSCs on the
modulation of the rejection response in an acute rat renal
transplantation model. Our results indicated that combination of
autologous ADSCs infusion and OX40-OX40L costimulation blockade may
be an intriguing strategy with which to exert a synergistic
immunosuppressive effect and thereby lead to the attenuation of
histological damage caused by acute rejection.
The ease of isolation, the absence of costimulatory
receptors, and their immunomodulatory and anti-inflammatory
properties of MSCs have led to the profound idea of developing
genetically engineered MSCs expressing the desired therapeutic
factors as a cell based vector system (20,21). MSCs can be readily transduced with
all the known viral and non-viral vectors and can effectively
overexpress the transgene (22,23). However, the cytogenetic stability
of MSCs following viral transduction needs to be established to the
allay safety issues of malignant transformation. The nucleofection
technology is a safe non-viral electroporation-based transfection
system (24,25). In this study, we modified ADSCs
with the plasmid pcDNA3.1 that can expressed the OX40Ig fusion
protein in eukaryotic expressiion systems by nucleofection. The
transient expression of OX40Ig in ADSCs was obtained. It was proven
that the OX40Ig fusion protein was expressed in ADSCs, and both of
the ADSCsnative and ADSCsOX40Ig significantly
suppressed T cell proliferation, and increased the proportion of
CD4+CD25+ Tregs in allogeneic MLR assays
in vitro, with the ADSCsOX40Ig being more
effective. This indicated that the ADSCsOX40Ig expressed
OX40Ig and had biological function, and exerted synergistic effects
on ADSC-mediated antigen-specific T cell anergy through blockade
OX40/OX40L costimulation signals.
MSCs as a cell therapy have demonstrated efficacy
for GVHD, systemic lupus erythematosus (SLE), rheumatoid arthritis
(RA), multiple sclerosis (MS), type 1 diabetes, myocardial
infarction, thyroditis, different types of neurological disorders
and organ transplantation (26–32). ADSCs were used in clinical trials
as soon as 5 years after their description (14,33) and more than 100 clinical trials
have been reported at http://www.clinicaltrials.gov. The first clinical
study in kidney transplantation with autologous MSC treatment was
reported by Perico et al (34) as a safety and feasibility study,
but with limited success. Other very limited studies set up
clinical trials using autologous or even allogeneic MSCs in kidney
transplantation (35,36). Tan et al (37) demonstrated that the use of
autologous MSCs as a replacement for induction therapy resulted in
a lower incidence of acute rejection, decreased risk of
opportunistic infection, and better estimated renal function at 1
year in living related kindey transplantation. In a rat organ
transplant model, Casiraghi et al (38) observed that, in contrast to
post-transplant MSC infusion, pre-transplant MSC infusion induced a
significant prolongation of kidney graft survival by a
Treg-dependent mechanism. Contrary data have also been published
for the rat heart transplantation model, with either accelerated
rejection (39) or prolonged
graft survival (40) obtained
depending on the experimental approach. Therefore, the time point
of injection and the number of cells applied seem to be important
parameters influencing the success of MSC therapy. Our observations
that pre-transplant, intro-transplant, and post-transplant ADSC
administration significantly improved renal function compared with
the PBS control group. This result is in agreement with our
previous study that autologous ADSC ameliorated acute renal damage
undergoing cold I/R injury and improved renal function (17).
However, the single administration of ADSCs is only
practical for a limited number of applications, and is insufficient
to overcome the alloreactive T cell response totally and to achieve
a long-term positive allograft outcome (41). Thus, as gene delivery vehicles,
the localization of ADSCs combined with costimulation blockade may
provide a new opportunity for successful therapy (15,42). In this study, our results revealed
that the administration of OX40Ig gene-modified ADSCs resulted in a
modest, but greater prolongation in the survival time of renal
grafts compared with ADSC monotherapy. Furthermore, renal function
and histological examination revealed that ADSCsOX40Ig
therapy significantly improved renal function and lessened tissue
damage. The present results also indicated that
ADSCsOX40Ig therapy effectively prevented T lymphocyte
infiltration to the grafts. This indicated that acute rejection was
effectively prevented by the intrarenal ADSC immunomodulatory
effect in combination with simultaneous local OX40-OX40L pathway
blockade, which may contribute to a tolerogenic environment.
Infiltrated T cells produce effector cytokines in
situ to recruit additional immune cells that mediate early
graft tissue damage. Blocking the expression of pro-inflammatory
cytokines is a rational approach to the immunosuppressive therapy
of graft rejection. Cytokine transcription profiles of intragraft
tissues give us a more precise insight of the immune regulation. In
this study, one mechanism by with ADSCsOX40Ig led to the
suppression of immunity and prolongation of survival involved the
induction of Tregs. Consistent with previous data (41,43), our study demonstrated that the
gene expression profiles in grafts from the PBS group exhibited an
increased expression of genes associated with Th1 cells (IFN-γ),
Th2 cells (IL-4 and IL-10), Th3 cells (TGF-β) and Tregs (Foxp3).
The present study demonstrated that graft survival in the
ADSCsOX40Ig group was enhanced, along with the
significantly decreased mRNA expression of IFN-γ, and the increased
mRNA expression of IL-10, TGF-β and Foxp3. The upregulation of
IL-10 and TGF-β is important for the differentiation and
proliferation of Tregs. Tregs have very important immunoregulatory
effects and play a significant role in the induction of
immunotolerance or the maintenance of immunosuppressive activity
(44). These data provide
evidence that increased allograft survival following
ADSCsOX40Ig administration, at least in part, occurs in
association with the increased intragraft expression of genes
associated with altered T cell differentiation, as well as a shift
from a pro-inflammatory to an anti-inflammatory state. Further
studies are warranted in order to elucidate the signal transduction
pathways through which ADSCsOX40Ig modulate T cells and
prolong allotransplant survival.
Taken together, the results of the present study
demonstrated that the administration of ADSCsOX40Ig was
able to alleviate acute renal allograft rejection and prolong graft
survival by combining the immunomodulatory effects of ADSCs and
ADSCs-mediated intrarenal OX40/OX40L pathway blockade. More
functional assessments are still required if tolerogenic strategy
utilizing ADSCsOX40Ig is to be developed in the near
future.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81470982); the National
Clinical Key Specialty Project Foundation of the Ministry of Health
(grant no. 2013544); and the Tianjin Research Program of
Application Foundation and Advanced Technology (grant nos.
13JCYBJC23000 and 12ZCZDSY02600).
References
|
1
|
Shrestha B, Haylor J and Raftery A:
Historical perspectives in kidney transplantation: an updated
review. Prog Transplant. 25:64–69. 762015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Newell KA: Clinical transplantation
tolerance. Semin Immunopathol. 33:91–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thorp EB, Stehlik C and Ansari MJ: T-cell
exhaustion in allograft rejection and tolerance. Curr Opin Organ
Transplant. 20:37–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waldmann H, Hilbrands R, Howie D and
Cobbold S: Harnessing FOXP3+ regulatory T cells for
transplantation tolerance. J Clin Invest. 124:1439–1445. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kinnear G, Jones ND and Wood KJ:
Costimulation blockade: current perspectives and implications for
therapy. Transplantation. 95:527–535. 2013. View Article : Google Scholar :
|
|
6
|
Kaur D and Brightling C: OX40/OX40 ligand
interactions in T-cell regulation and asthma. Chest. 141:494–499.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jensen SM, Maston LD, Gough MJ, Ruby CE,
Redmond WL, Crittenden M, Li Y, Puri S, Poehlein CH, Morris N, et
al: Signaling through OX40 enhances antitumor immunity. Semin
Oncol. 37:524–532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kotani A, Hori T, Fujita T, Kambe N,
Matsumura Y, Ishikawa T, Miyachi Y, Nagai K, Tanaka Y and Uchiyama
T: Involvement of OX40 ligand+ mast cells in chronic
GVHD after allogeneic hematopoietic stem cell transplantation. Bone
Marrow Transplant. 39:373–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou YB, Ye RG, Li YJ and Xie CM:
Targeting the CD134−CD134L interaction using anti-CD134
and/or rhCD134 fusion protein as a possible strategy to prevent
lupus nephritis. Rheumatol Int. 29:417–425. 2009. View Article : Google Scholar
|
|
10
|
Wang YL, Li G, Fu YX, Wang H and Shen ZY:
Blockade of OX40/OX40 ligand to decrease cytokine messenger RNA
expression in acute renal allograft rejection in vitro. Transplant
Proc. 45:2565–2568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Casiraghi F, Remuzzi G and Perico N:
Mesenchymal stromal cells to promote kidney transplantation
tolerance. Curr Opin Organ Transplant. 19:47–53. 2014. View Article : Google Scholar
|
|
12
|
Marx C, Silveira MD and Beyer Nardi N:
Adipose-derived stem cells in veterinary medicine: characterization
and therapeutic applications. Stem Cells Dev. 24:803–813. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alipour F, Parham A, Kazemi Mehrjerdi H
and Dehghani H: Equine adipose-derived mesenchymal stem cells:
phenotype and growth characteristics, gene expression profile and
differentiation potentials. Cell J. 16:456–465. 2015.PubMed/NCBI
|
|
14
|
Minteer DM, Marra KG and Rubin JP: Adipose
stem cells: biology, safety, regulation, and regenerative
potential. Clin Plast Surg. 42:169–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takahashi T, Tibell A, Ljung K, Saito Y,
Gronlund A, Osterholm C, Holgersson J, Lundgren T, Ericzon BG,
Corbascio M, Kumagai-Braesch M, et al: Multipotent mesenchymal
stromal cells synergize with costimulation blockade in the
inhibition of immune responses and the induction of
Foxp3+ regulatory T cells. Stem Cells Transl Med.
3:1484–1494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clark JD, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: Special Report: the 1996 guide for the care and use
of laboratory animals. ILAR J. 38:41–48. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang YL, Li G, Zou XF, Chen XB, Liu T and
Shen ZY: Effect of autologous adipose-derived stem cells in renal
cold ischemia and reperfusion injury. Transplant Proc.
45:3198–3202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Redmond WL, Triplett T, Floyd K and
Weinberg AD: Dual anti-OX40/IL-2 therapy augments tumor
immunotherapy via IL-2R-mediated regulation of OX40 expression.
PLoS One. 7:e344672012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seifert M, Stolk M, Polenz D and Volk HD:
Detrimental effects of rat mesenchymal stromal cell pre-treatment
in a model of acute kidney rejection. Front Immunol. 3:2022012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu H, Ye Z and Mahato RI: Genetically
modified mesenchymal stem cells for improved islet transplantation.
Mol Pharm. 8:1458–1470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Ezzelarab MB, Ayares D and Cooper
DK: The potential role of genetically-modified pig mesenchymal
stromal cells in xenotransplantation. Stem Cell Rev. 10:79–85.
2014. View Article : Google Scholar :
|
|
22
|
Stiehler M, Duch M, Mygind T, Li H,
Ulrich-Vinther M, Modin C, Baatrup A, Lind M, Pedersen FS and
Bünger CE: Optimizing viral and non-viral gene transfer methods for
genetic modification of porcine mesenchymal stem cells. Adv Exp Med
Biol. 585:31–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fülbier A, Schnabel R, Michael S, Vogt PM,
Strauß S, Reimers K and Radtke C: Successful nucleofection of rat
adipose-derived stroma cells with ambystoma mexicanum epidermal
lipoxygenase (AmbLOXe). Stem Cell Res Ther. 5:1132014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fakiruddin KS, Baharuddin P, Lim MN,
Fakharuzi NA, Yusof NA and Zakaria Z: Nucleofection optimization
and in vitro anti-tumourigenic effect of TRAIL-expressing human
adipose-derived mesenchymal stromal cells. Cancer Cell Int.
14:1222014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Copland IB, Qayed M, Garcia MA, Galipeau J
and Waller EK: Bone marrow mesenchymal stromal cells from patients
with acute and chronic graft-versus-host disease deploy normal
phenotype, differentiation plasticity, and immune-suppressive
activity. Biol Blood Marrow Transplant. 21:934–940. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Q, Qian S, Li J, Che N, Gu L, Wang Q,
Liu Y and Mei H: Combined transplantation of autologous
hematopoietic stem cells and allogenic mesenchymal stem cells
increases T regulatory cells in systemic lupus erythematosus with
refractory lupus nephritis and leukopenia. Lupus. 24:1221–1226.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Bari C: Are mesenchymal stem cells in
rheumatoid arthritis the good or bad guys? Arthritis Res Ther.
17:1132015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao J, Yang R, Biswas S, Qin X, Zhang M
and Deng W: Mesenchymal stem cells and induced pluripotent stem
cells as therapies for multiple sclerosis. Int J Mol Sci.
16:9283–9302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong D, Zhuang X, Wang D, Qu H, Jiang Y,
Li X, Wu W, Xiao J, Liu X, Liu J, et al: Umbilical cord mesenchymal
stem cell transfusion ameliorated hyperglycemia in patients with
type 2 diabetes mellitus. Clin Lab. 60:1969–1976. 2014.
|
|
30
|
Chullikana A, Majumdar AS, Gottipamula S,
Krishnamurthy S, Kumar AS, Prakash VS and Gupta PK: Randomized,
double-blind, phase I/II study of intravenous allogeneic
mesenchymal stromal cells in acute myocardial infarction.
Cytotherapy. 17:250–261. 2015. View Article : Google Scholar
|
|
31
|
Suksuphew S and Noisa P: Neural stem cells
could serve as a therapeutic material for age-related
neurodegenerative diseases. World J Stem Cells. 7:502–511. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim MH, Ong WK and Sugii S: The current
landscape of adipose-derived stem cells in clinical applications.
Expert Rev Mol Med. 16:e82014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perico N, Casiraghi F, Introna M, Gotti E,
Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo
P, et al: Autologous mesenchymal stromal cells and kidney
transplantation: a pilot study of safety and clinical feasibility.
Clin J Am Soc Nephrol. 6:412–422. 2011. View Article : Google Scholar :
|
|
34
|
Perico N, Casiraghi F, Gotti E, Introna M,
Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo
P, et al: Mesenchymal stromal cells and kidney transplantation:
pretransplant infusion protects from graft dysfunction while
fostering immunoregulation. Transpl Int. 26:867–878. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng Y, Ke M, Xu L, Liu L, Chen X, Xia W,
Li X, Chen Z, Ma J, Liao D, et al: Donor-derived mesenchymal stem
cells combined with low-dose tacrolimus prevent acute rejection
after renal transplantation: a clinical pilot study.
Transplantation. 95:161–168. 2013. View Article : Google Scholar
|
|
36
|
Reinders ME, de Fijter JW, Roelofs H,
Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP,
Roelen DL, van Kooten C, et al: Autologous bone marrow-derived
mesenchymal stromal cells for the treatment of allograft rejection
after renal transplantation: results of a phase I study. Stem Cells
Transl Med. 2:107–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tan J, Wu W, Xu X, Liao L, Zheng F,
Messinger S, Sun X, Chen J, Yang S, Cai J, et al: Induction therapy
with autologous mesenchymal stem cells in living-related kidney
transplants: a randomized controlled trial. JAMA. 307:1169–1177.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Casiraghi F, Azzollini N, Todeschini M,
Cavinato RA, Cassis P, Solini S, Rota C, Morigi M, Introna M,
Maranta R, et al: Localization of mesenchymal stromal cells
dictates their immune or proinflammatory effects in kidney
transplantation. Am J Transplant. 12:2373–2383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Inoue S, Popp FC, Koehl GE, Piso P,
Schlitt HJ, Geissler EK and Dahlke MH: Immunomodulatory effects of
mesenchymal stem cells in a rat organ transplant model.
Transplantation. 81:1589–1595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Popp FC, Eggenhofer E, Renner P, Slowik P,
Lang SA, Kaspar H, Geissler EK, Piso P, Schlitt HJ and Dahlke MH:
Mesenchymal stem cells can induce long-term acceptance of solid
organ allografts in synergy with low-dose mycophenolate. Transpl
Immunol. 20:55–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kato T, Okumi M, Tanemura M, Yazawa K,
Kakuta Y, Yamanaka K, Tsutahara K, Doki Y, Mori M, Takahara S and
Nonomura N: Adipose tissue-derived stem cells suppress acute
cellular rejection by TSG-6 and CD44 interaction in rat kidney
transplantation. Transplantation. 98:277–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang D, Wang LP, Zhou H, Cheng H, Bao XC,
Xu S, Zhang WP and Wang JM: Inducible costimulator gene-transduced
bone marrow-derived mesenchymal stem cells attenuate the severity
of acute graft-versus-host disease in mouse models. Cell
Transplant. 24:1717–31. 2015. View Article : Google Scholar
|
|
43
|
Wang YL, Tang ZQ, Gao W, Jiang Y, Zhang XH
and Peng L: Influence of Th1, Th2, and Th3 cytokines during the
early phase after liver transplantation. Transplant Proc.
35:3024–3025. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee JH, Jeon EJ, Kim N, Nam YS, Im KI, Lim
JY, Kim EJ, Cho ML, Han KT and Cho SG: The synergistic
immunoregulatory effects of culture-expanded mesenchymal stromal
cells and CD4(+)25(+)Foxp3+ regulatory T cells on skin allograft
rejection. PLoS One. 8:e709682013. View Article : Google Scholar : PubMed/NCBI
|