Introduction
Keloid scars are lesions of unknown etiology,
characterized by fibroblastic proliferation and excessive collagen
deposition. They develop as a result of abnormal wound healing
(1). Keloid fibroblasts express
α-smooth muscle actin (α-SMA) and over-secrete collagen proteins
such as collagen I and III (2).
The process of transition from fibroblasts to myofibroblasts is
mainly regulated by transforming growth factor (TGF)-β1 (3). In response to TGF-β1, fibroblasts
differentiate into myofibroblasts, which contract the wound and aid
in the remodeling of the extracellular matrix (ECM) (4). The major pathway of TGF-β1-induced
myofibroblast differentiation is mediated via Smad activation by
the TGF-β1 receptor complex, leading to Smad2 and Smad3 complex
association with Smad4 and translocation into the nucleus. Smad3
binding to Smad binding elements in the promoter region regulates
α-SMA transcription in conjunction with a variety of transcription
factors, to further enhance the deposition of ECM proteins
(5). The imbalance of the
synthesis and degradation of ECM results in scarring (6). Currently, there is no ideal
treatment to reverse or reduce such dermal scarring.
Hypoxia is a common environmental stress factor and
is associated with various physiological and pathological
conditions, such as hepatic diseases and cancer (7,8).
Hypoxia inducible factors (HIFs) are a group of transcription
factors rapidly activated in hypoxic cells (9). Once activated, these transcription
factors regulate the expression of genes that allow cells to adapt
to a hypoxic environment. HIFs are composed of an α subunit (either
HIF-1α or HIF-2α) and a β subunit (HIF-1β). HIF-1α and HIF-2α
protein subunits are constitutively produced in cells (10). In normoxic cells, these subunits
are immediately targeted for proteasomal degradation. In hypoxic
cells however, the mechanisms that target HIFs for degradation are
inhibited, allowing HIF-1α and HIF-2α to translocate to the
nucleus. In the nucleus, both HIF-1α and HIF-2α heterodimerize with
HIF-1β and regulate the expression of genes involved in oxygen
homeostasis (11). Accumulating
evidence suggests that a hypoxic microenvironment is associated
with keloids due to an abnormally large number of occluded
microvessels, and that hypoxia plays a crucial role in keloid
pathogenesis (12,13). Hypoxia has been found to increase
the expression of vascular endothelial growth factor (VEGF) in
keloid fibroblasts (14). The
level of HIF-1α is consistently higher in freshly biopsied keloid
tissues than in their associated normal skin borders, which
provides direct evidence of a local hypoxic state in keloids
(9). However, whether hypoxia
drives the differentiation of human dermal fibroblasts into
myofibroblasts has not yet been reported, and the way this can
influence human scarring is not clear. Thus, the aim of this study
was to examine the effects of hypoxia on the transition of dermal
fibroblasts and to clarify the potential transduction mechanisms
involved.
Materials and methods
All experimental procedures were conducted under the
instructions reviewed and approved by the Ethics Committee of
Xijing Hospital, Xi'an, China. Keloid scar tissue and paired normal
skin tissues were surgically obtained from 5 Chinese patients
(male, 21 years old; male, 27 years old; male, 24 years old; male,
19 years old; male, 36 years old) with an average age of 25 years.
All patients provided written informed consent prior to obtaining
the samples. The diagnosis of keloid scarring was confirmed by
routine pathological examination.
Cell culture and treatment
Human adult dermal fibroblasts (lot no. 61447289)
were obtained from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and seeded at a density of 10,000
cells/cm2 in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% heat-inactivated fetal bovine serum (FBS) and
1% antibiotic-antimycotic in a humidified incubator at 37°C with 5%
CO2. To induce hypoxia, the cells were placed in
three-gas incubator (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) that maintains a sub-ambient O2 level (1%, 5% or
10%) with or without 10 ng/ml of TGF-β1 (Peprotech, Rocky Hill, NJ,
USA) by the regulated injection of N2 for 48 h. The
control cells were placed in a similar incubator which was
maintained at 5% CO2 and 21% oxygen level. All reagents
were purchased from Invitrogen (Carlsbad, CA, USA) unless otherwise
stated.
Immunofluorescence staining
The cells were pre-incubated in PBS and fixed with
4% formaldehyde for 30 min, followed by incubation with rabbit
anti-human p-Smad3 (9520; Cell Signaling Technology, Inc., Danvers,
MA, USA) overnight at 4°C. After washing with PBS, goat anti-rabbit
IgG-CFL 555 secondary antibody (sc-362272; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) was added in 1% BSA followed by
incubation for 1 h at room temperature in the dark. Images were
obtained using a FSX100 microscope (Olympus Corp., Tokyo, Japan).
Nuclei were counterstained using DAPI (Sigma-Aldrich, St. Louis,
MO, USA).
Cell apoptosis and viability
Flow cytometry (BD FACSAria; BD Biosciences,
Franklin Lakes, NJ, USA) was performed to detect cell apoptosis.
The following 2 groups were under investigation: i) the control
group and ii) the 1% oxygen group. We observed the apoptotic rates
at 48 h post-treatment. In accordance with the Annexin V/propidium
iodide (PI) apoptosis kit (BioVision, San Francisco, CA, USA),
5×105 cells were collected in each tube and 1 ml Annexin
V binding buffer was added followed by thorough mixing.
Subsequently, 5 µl Annexin V-fluorescein isothiocyanate and
10 µl PI were added. After mixing, the tube was incubated in
the dark at 37°C for 15 min. For the early apoptotic cells,
membrane phosphatidylserine was exposed and combined with Annexin V
but no PI. For the late apoptotic cells, the membranes were
permeable to PI and the cells were stained with Annexin V and PI.
The dead cells were stained only with PI.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The TRIzol reagent kit (Invitrogen) was used for RNA
extraction. The isolated RNA was reverse transcribed into
complementary DNA using the PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). Primers were obtained from
Beijing AuGCT DNA-SYN Biotechnology Co., Ltd., (Beijing, China).
Quantitative PCR (qPCR) was performed using the iQ5 real-time PCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
using SYBR Premix Ex Taq II (obtained from Takara Biotechnology
Co., Ltd.) in a 20 ml volume of the PCR reaction solution. The
sequences for primers are listed as follows: HIF-1α forward,
5′-AGCCGAGGAAGAACTATGAAC-3′ and reverse,
5′-ATTTGATGGGTGAGGAATGGG-3′; and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
The results were normalized against the mean Ct-values of GAPDH
using the ΔCt method as follows: ΔCt = Ctgene of
interest − Ctmean (GAPDH). The fold increase was
calculated as 2−ΔΔCt.
Western blot analysis
Total protein lysates were generated using RIPA
lysis buffer supplemented with protease and phosphatase inhibitor
mixtures (KC-440; Shanghai KangChen Biological Technology Co.,
Shanghai, China). Nuclear protein extracts were obtained using the
NE-PER nuclear and cytoplasmic extraction reagents (Pierce
Biotechnology, Inc., Rockford, IL, USA), according to the
manufacturer's instructions. Proteins (40 µg) were loaded
onto a 5–10% polyacrylamide gel, separated by electrophoresis and
transferred onto a polyvinylidene difluoride (PVDF) membrane. After
blocking with 5% non-fat milk, the PVDF membrane was incubated with
rabbit polyclonal antibodies to collagen I (ab96723) and III
(ab7778) (both from Abcam, Cambridge, MA, USA), p-Smad2 (3108),
Smad2 (3122), p-Smad3 (9520), Smad3 (9523) (all from Cell Signaling
Technology, Inc.), HIF-1α (ab51608; Abcam) and histone H3
(sc-8654-R; Santa Cruz Biotechnology) or mouse polyclonal α-SMA
antibody (BM0002; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) and β-actin antibody (4970; Cell Signaling Technology,
Inc.). Horseradish peroxidase-conjugated goat anti-rabbit (BA1054)
or anti-mouse (BA1050) antibody (Wuhan Boster Biological
Technology, Ltd.) was used as a secondary antibody. Proteins were
visualized by enhanced chemiluminescence system using FluorChem FC
system (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
means ± standard error of 3 independent experiments. Statistical
analysis was performed using the Student's t-test. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Keloids are a relatively hypoxic
tissue
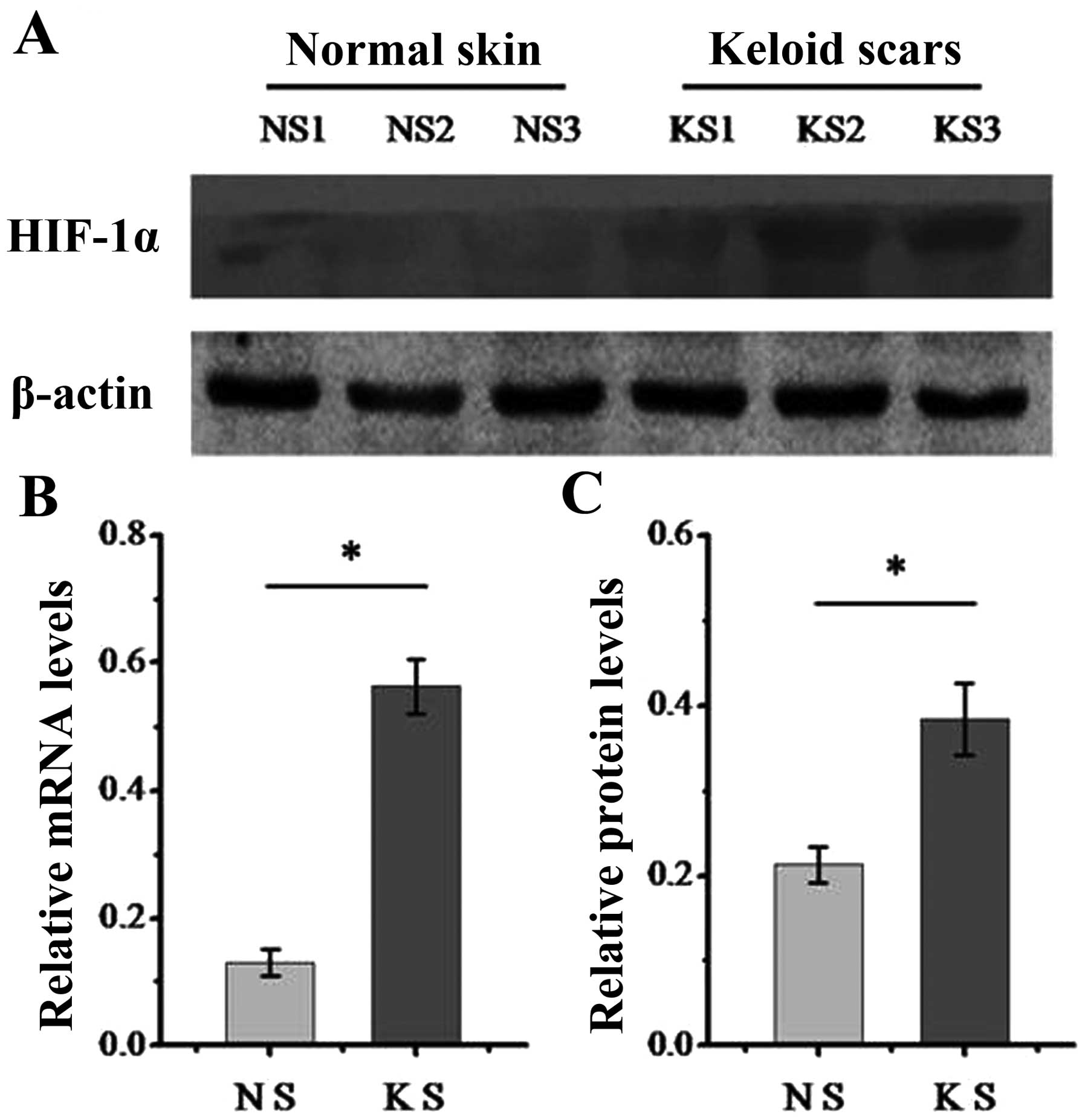
We first determined the expression of HIF-1α in
dermal normal and keloid tissue. As is known, HIF-1α functions as a
key transcription factor in response to hypoxia (15). The results from western blot
analysis (Fig. 1A) and RT-qPCR
(Fig. 1B) demonstrated that the
keloid tissue expressed higher levels of HIF-1α compared with
normal tissue, which indicates that keloids are a relatively
hypoxic tissue and that HIF signaling may play a role during the
formation of keloids.
Hypoxia induces a pro-fibrotic state in
dermal fibroblasts in vitro
Human adult dermal fibroblasts were cultured in 21,
10, 5 or 1% oxygen for 48 h. Culturing cells in 1% oxygen
significantly increased the expression of HIF-1α and stabilized
nuclear HIF-1α (Fig. 2).
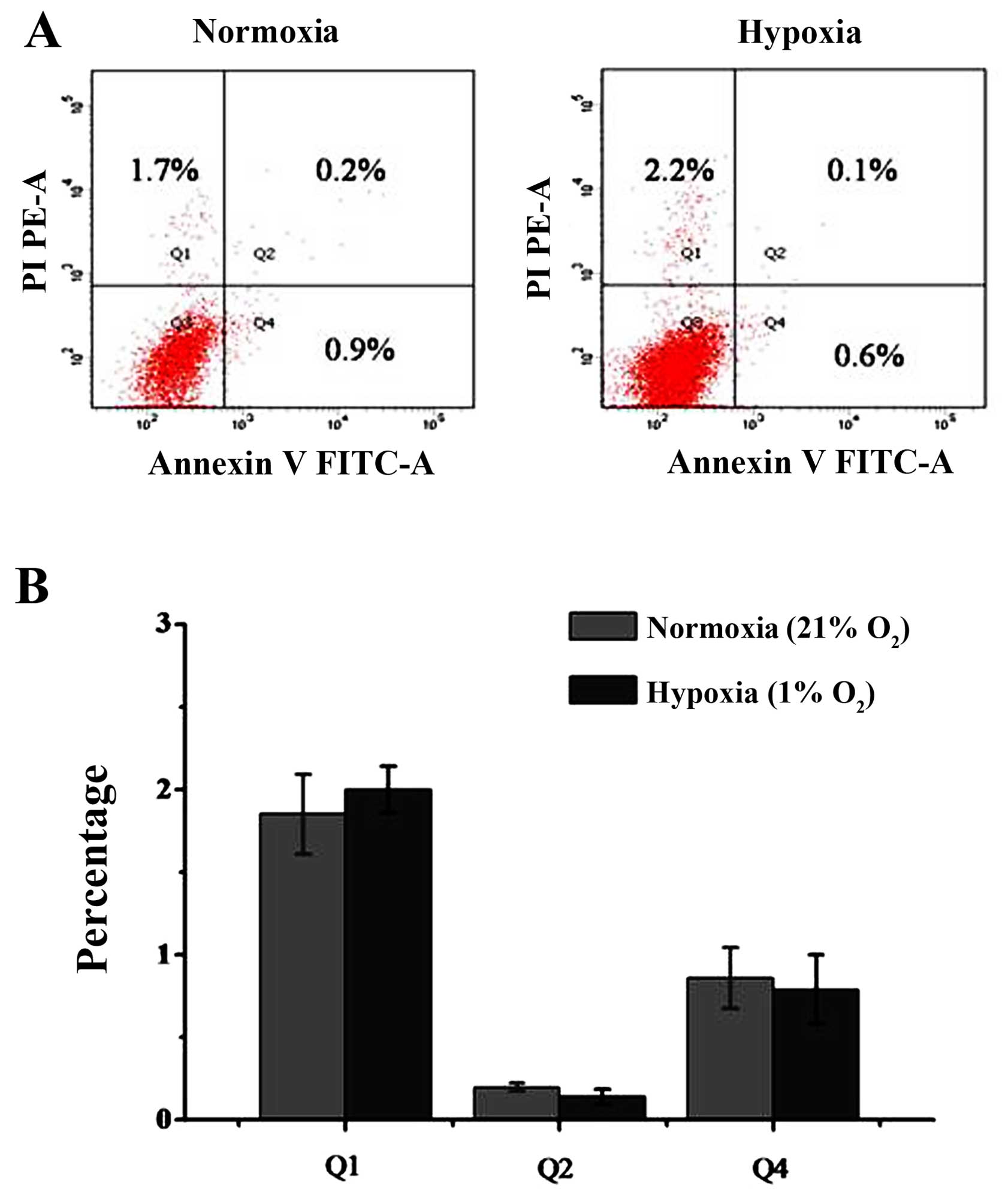
Moreover, under 1% oxygen conditions, most of the cells that were
negative in staining (Q3 area) were normal. Cells in the early
apoptotic phase were stained with Annexin V but no PI and are shown
in the Q4 area. Cells in the late apoptotic phase were stained with
Annexin V and PI, and are shown in the Q2 area, and dead cells were
stained with PI and are shown in the Q1 area. The percentage of
early apoptotic cells was 0.9±0.2% in the untreated group and
0.6±0.3% in the group treated with 1% oxygen. No significant
differences were observed between the cells. The percentage of late
apoptotic cells was 0.2±0.06% in the control group and 0.1±0.05% in
the 1% oxygen-treated group. There was also no significant
differences between the control group and the 1% oxygen-treated
group. There was a slight trend toward more dead cells with 1%
oxygen treatment, but this did not reach statistical significance
(Fig. 3). The percentage of dead
cells was 1.7±0.4% in the untreated group and 2.2±0.5% in the group
treated with 1% oxygen (P>0.05).
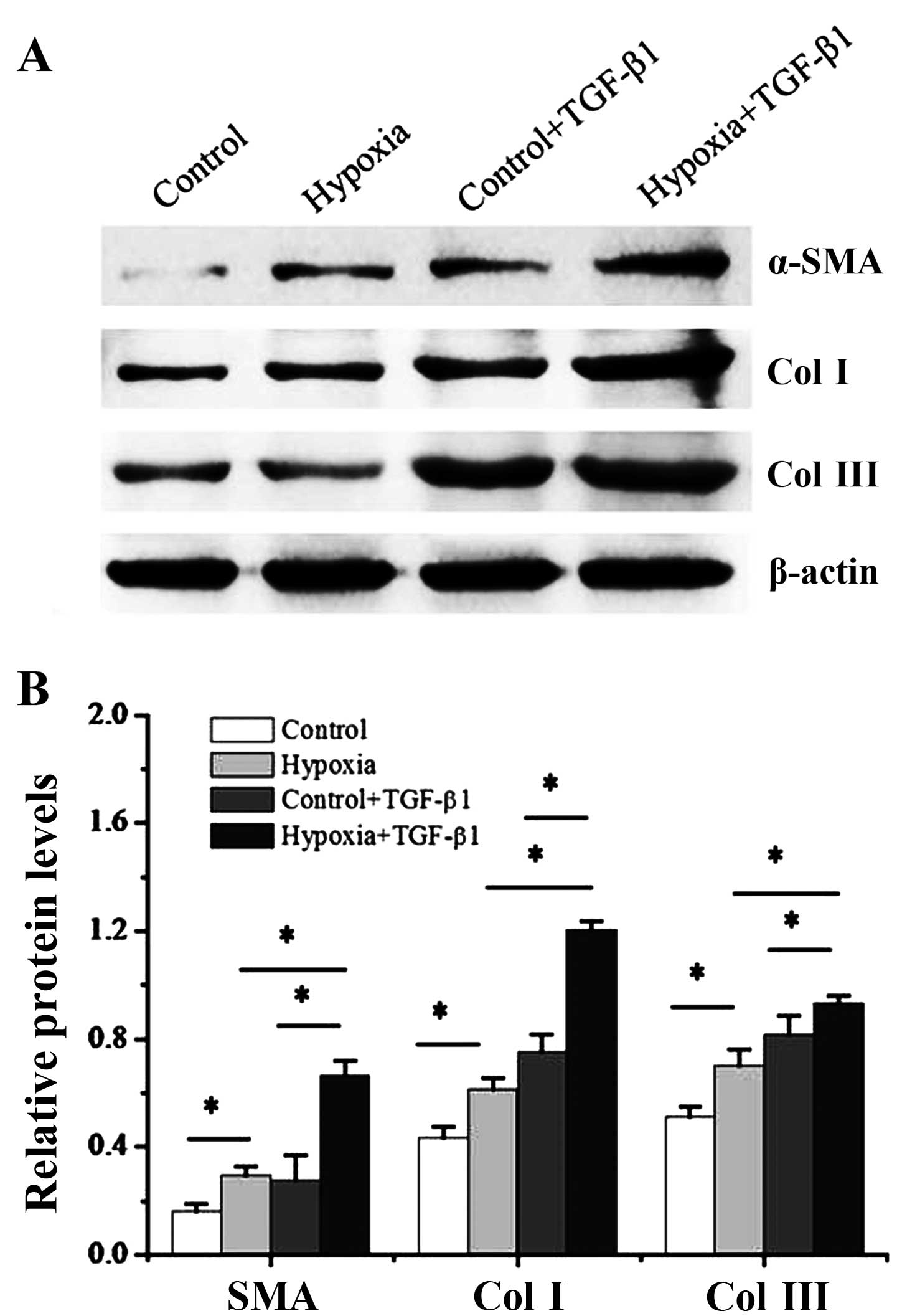
We then cultured the dermal fibroblasts with 1%
oxygen alone or, 10 ng/ml TGF-β1 alone or a combination of 1%
oxygen and 10 ng/ml TGF-β1 for 48 h. In addition, the levels of
myofibroblast makers, α-SMA and collagen I and III, were measured
by western blot analysis. As shown in Fig. 4, a significant increase in both
α-SMA, collagen I and III protein expression were detected at 2
days post-treatment iwth 1% oxygen compared with the controls
(P<0.05). Of note, the pro-fibrotic effects of treatment with
TGF-β1 were enhanced by hypoxia. Treatment of the dermal
fibroblasts with TGF-β1 significantly increased the expression of
α-SMA and collagen I and III (P<0.05), and this was further
enhanced when the cells were exposed to hypoxia (P<0.05).
The hypoxia-induced transition of dermal
fibroblasts to a myofibroblast-like phenotype is associated with
the activation of Smad3
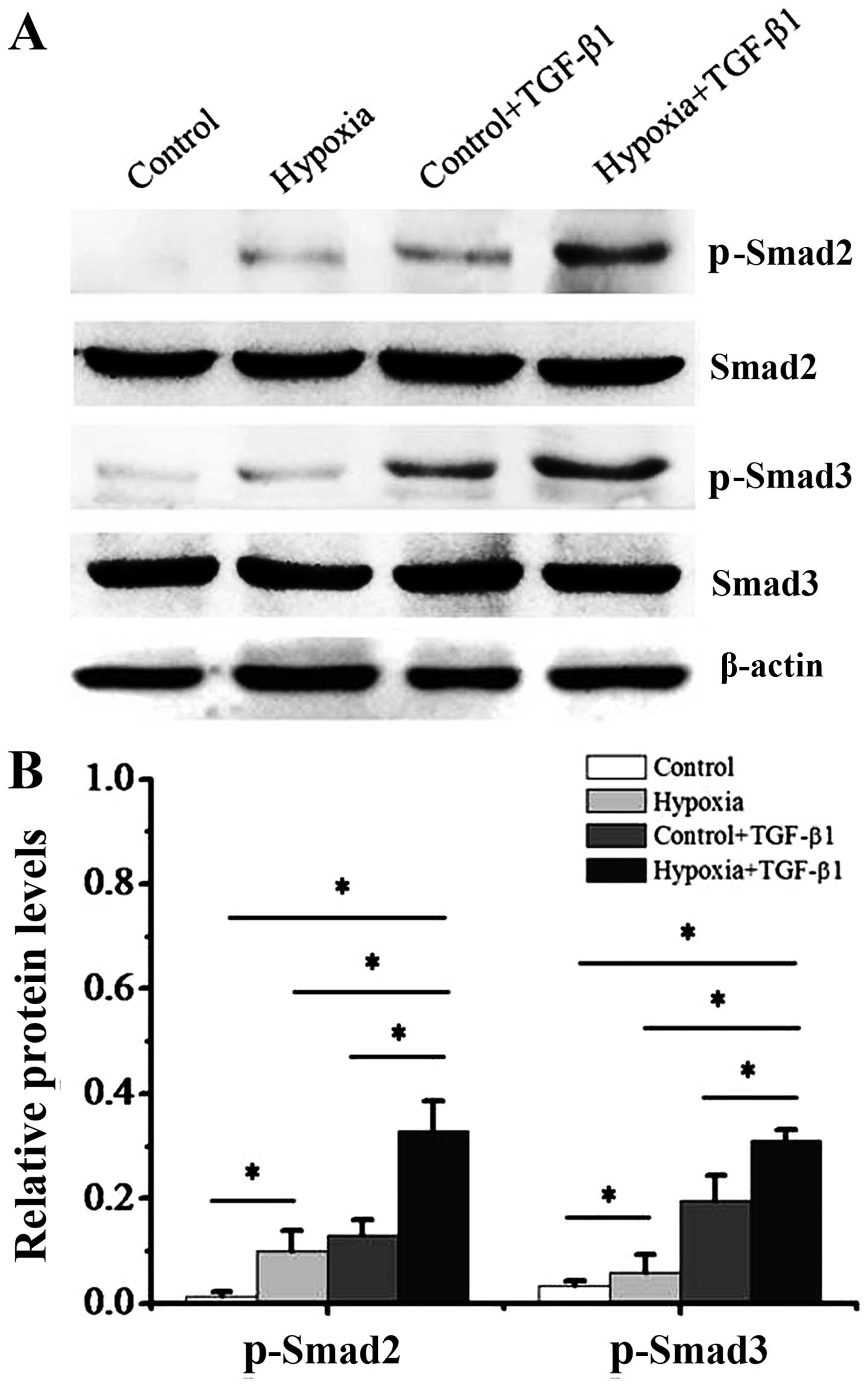
It is well known that Smad3 phosphorylation is
linked to the fibrotic process (16,17). Thus, in this study, we addressed
the question of whether Smad3 activation participates in the
hypoxia-induced transition of dermal fibroblasts to a
myofibroblast-like phenotype. First, we cultured dermal fibroblasts
with 1% oxygen alone or, 10 ng/ml TGF-β1 alone, or a combination of
1% oxygen and 10 ng/ml TGF-β1 for 48 h. In addition, the
phosphorylation of Smad2 and Smad3 was measured by western blot
analysis (Fig. 5) and
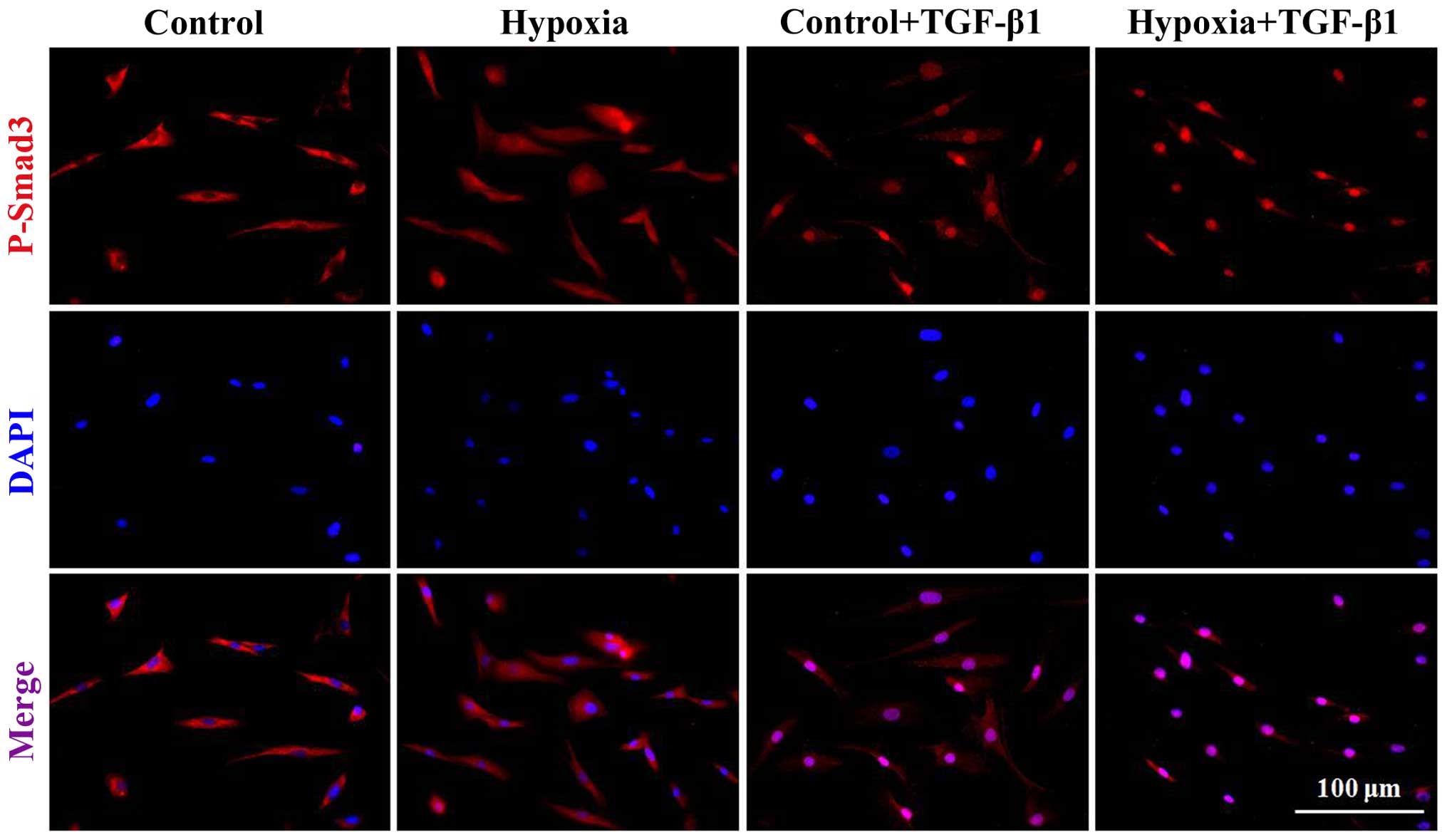
immunofluorescence staining (Fig.
6). Our results revealed that the dermal fibroblasts exposed to
1% oxygen alone or 10 ng/ml TGF-β1 alone exhibited an increase in
the activity of p-Smad2 and p-Smad3. Moreover, the levels of Smad2
and Smad3 phosphorylation were further significantly enhanced when
the cells were treated when a combination of TGF-β1 and 1% hypoxia
(P<0.01). Immunofluorescence staining indicated that following
treatment with 1% oxygen or TGF-β1 stimulation, the complex of
Smad2 and Smad3 was imported from the cytoplasm to the nucleus.
Furthermore, the imported complex of Smad2 and Smad3 in the nucleus
was further enhanced when the cells were treated with a combination
of TGF-β1 and 1% oxygen.
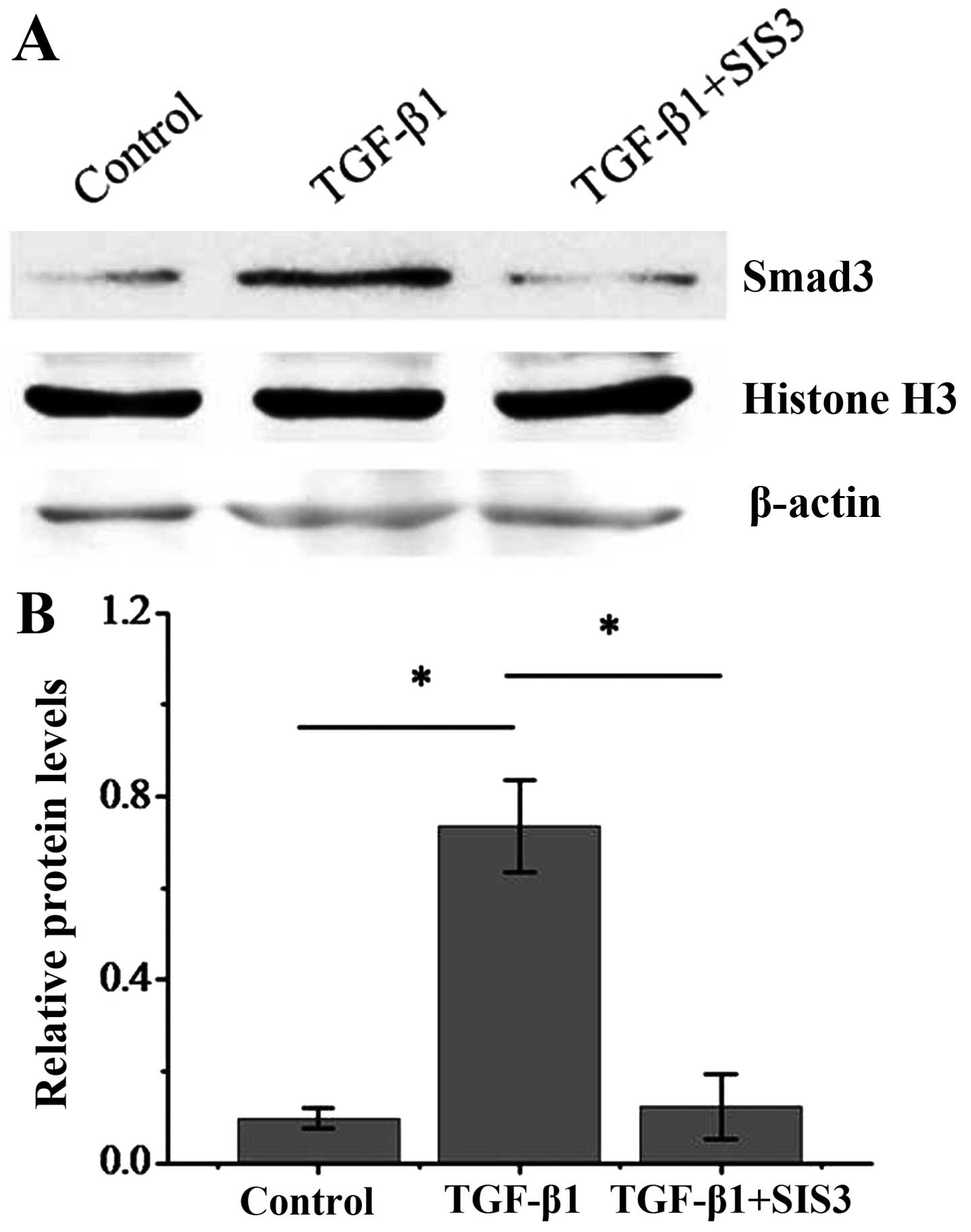
To further address the question of whether the Smad3
signaling pathway is required in the hypoxia-induced transition of
dermal fibroblasts to a myofibroblast-like phenotype, we used the
specific inhibitor of Smad3, SIS3 (18). To demonstrate that SIS3 is
effective in inhibiting the activation of Smad3 through
translocation to the nucleus, protein extracts from nuclear
fractions was obtained from dermal fibroblasts exposed TGF-β1 with
or without SIS3. The results revealed that in TGF-β1-treated dermal
fibroblasts, Smad3 expression was significantly enhanced in the
nucleus, suggesting nuclear translocation. However, in the
TGF-β1-treated dermal fibroblasts also treated with SIS3, the
expression of Smad3 was significantly impaired, indicating that
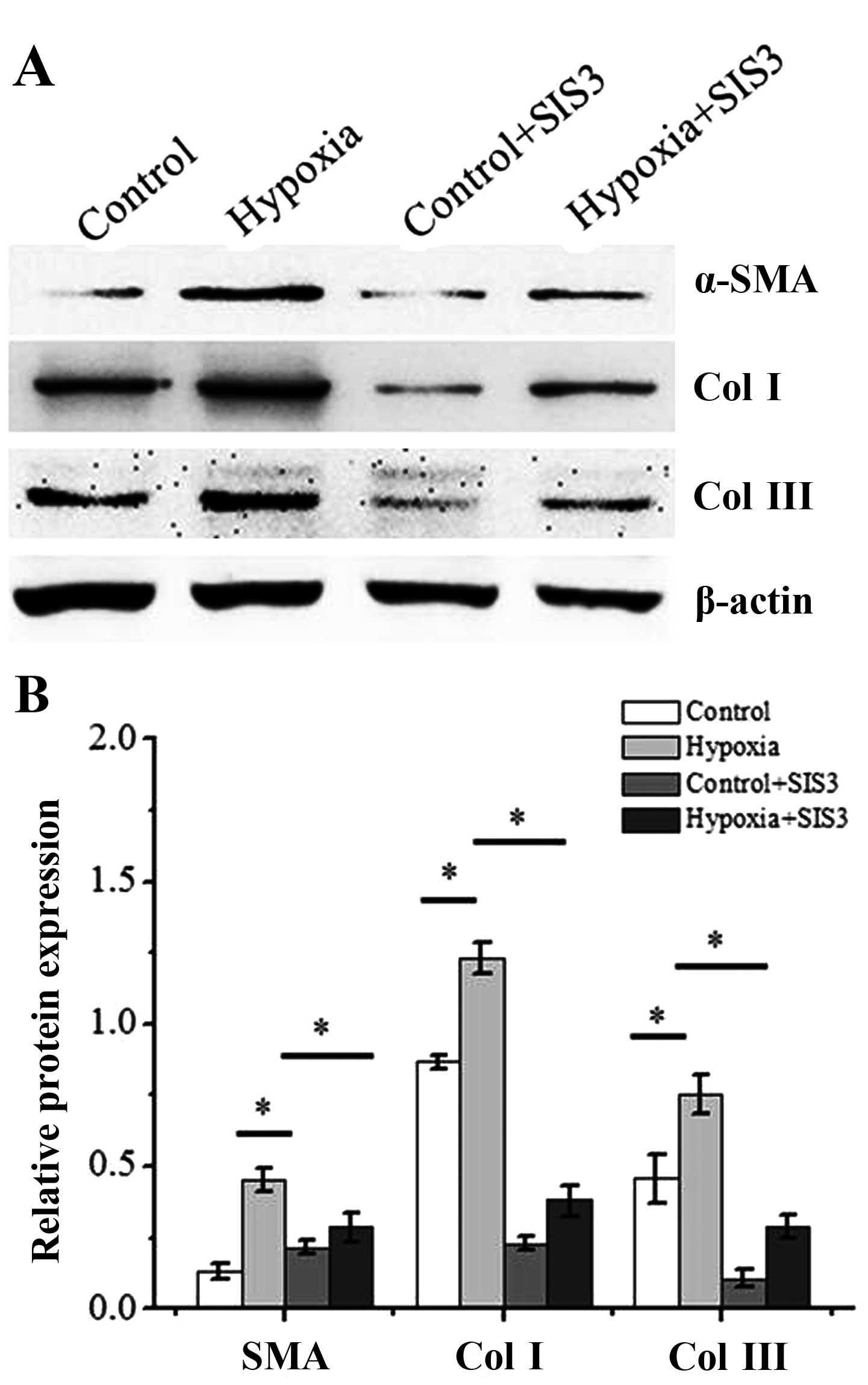
SIS3 inhibited the Smad3 nuclear translocation (Fig. 7). We then examined the effects of
SIS3 on the levels of fibrotic markers induced by hypoxia. SIS3
incubation inhibited the increase in the protein level of the
fibrotic markers, α-SMA and collagen I and III (Fig. 8). These results suggest that the
hypoxia-induced transition of dermal fibroblasts into
myofibroblasts is dependent on the TGF-β1/Smad3 pathway.
Discussion
The fibroblast to myofibroblast transition is a
crucial step in wound healing. Myofibroblasts contribute to tissue
repair mainly by the significant enhancement of contractile and ECM
synthesis (19). When the wound
heals, the myofibroblasts are removed by apoptosis (20). However, the persistence of
myofibroblasts in an otherwise healed wound leads to the formation
of scars (21). TGF-β1 is a key
fibrogenic cytokine both in vitro and in vivo. In
response to TGF-β1, fibroblasts differentiate into myofibroblasts,
which contract the wound and aid in the remodeling of the ECM
(22). Hence, the conversion of
fibroblasts into myofibroblasts by TGF-β1 is an important mechanism
in the development of fibrosis (23). In addition, the regulation of
cellular function by TGF-β1 is mediated by TGF-β/Smad3 signaling.
Smad proteins are thought to play an important role in regulating
intracellular responses to TGF-β1. Following the TGF-β1-induced
phosphorylation of Smad2 and Smad3, these proteins have been shown
to localize to the nucleus and form a complex with Smad4, which
mediates pro-fibrotic gene expression (24).
Oxygen has long been known to play a prominent role
in the healing process, re-epithelialization and other healing
processes (25–27). Hypoxia has been traditionally
regarded as an important stimulus for fibroblast growth and
angiogenesis through the activation of HIF-1α (28). HIF-1α, which functions as a key
transcription factor in response to hypoxic stress by regulating
genes involved in maintaining oxygen homeostasis, is critically
involved in virtually all wound healing and remodeling processes
(15). It is also associated with
cancer progression, metastasis and fibrotic disorders, and is
emerging as an important trigger and modulator of
epithelial-mesenchymal transition (EMT) (29). As has been previously reported,
the epidermis is a relatively hypoxic tissue, indicating that
hypoxia and HIF signaling may play a role during the formation of
keloids (26,30). Our results also suggest that human
keloid tissue is located in a local hypoxia environment.
It has been reported that hypoxia and HIF-1α
activation can modulate EMT via the TGF-β pathway and play a key
role during cancer progression and fibrotic disorders (31). It has also been demonstrated that
hypoxia stimulates hepatocyte EMT by TGF-β-dependent mechanisms
during the development of liver fibrosis (32). It has been indciated that
hypoxia-induced epigenetic modifications are associated with
cardiac fibrosis and the development of a myofibroblast-like
phenotype (33). The progression
of fibrosis is similar in most organs and involves pathogenic
processes of interstitial hypercellularity and matrix accumulation,
which lead to the loss of normal function and organ failure
(34). As expected, our results
revealed that hypoxia was able to drive the differentiation of
normal dermal fibroblasts though an EMT-like mechanism, and are in
accordance with the evidence indicated above. Moreover, the
expression of p-Smad2 and p-Smad3 was significantly increased in
the hypoxia-exposed cells compared with the controls, and this
effect was significantly inhibited by treatment with SIS3,
indicating that hypoxia is able to drive the transition of human
dermal fibroblasts into myofibroblasts by regulating the
TGF-β1/Smad3 pathway.
In conclusion, to the best of our knowledge, the
findings of this study demonstrate for the first time that hypoxia
is an important stimulus of the differentiation of human dermal
fibroblasts to a myofibroblast-like phenotype. Furthermore, we
demonstrate that Smad3 signaling contributes to the mechanism
through which hypoxia stimulates the differentiation of fibroblasts
into myofibroblasts. This information will be useful in designing
new and improved therapeutic strategies against hypoxia-mediated
fibrotic diseases.
Acknowledgments
This study was supported by the National Health and
Family Planning Commission of China (grant no. 2015SQ00060).
References
|
1
|
Bran GM, Goessler UR, Hormann K, Riedel F
and Sadick H: Keloids: current concepts of pathogenesis (Review).
Int J Mol Med. 24:283–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyer LJ, Russell SB, Russell JD, Trupin
JS, Egbert BM, Shuster S and Stern R: Reduced hyaluronan in keloid
tissue and cultured keloid fibroblasts. J Invest Dermatol.
114:953–959. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daian T, Ohtsuru A, Rogounovitch T,
Ishihara H, Hirano A, Akiyama-Uchida Y, Saenko V, Fujii T and
Yamashita S: Insulin-like growth factor-I enhances transforming
growth factor-beta-induced extracellular matrix protein production
through the P38/activating transcription factor-2 signaling pathway
in keloid fibroblasts. J Invest Dermatol. 120:956–962. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong X, Mao S and Wen H: Upregulation of
proinflammatory genes in skin lesions may be the cause of keloid
formation (Review). Biomed Rep. 1:833–836. 2013.
|
|
5
|
Olman MA: Beyond TGF-beta: a prostaglandin
promotes fibrosis. Nat Med. 15:1360–1361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ong CT, Khoo YT, Mukhopadhyay A, Do DV,
Lim IJ, Aalami O and Phan TT: mTOR as a potential therapeutic
target for treatment of keloids and excessive scars. Exp Dermatol.
16:394–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhan L, Huang C, Meng XM, Song Y, Wu XQ,
Yang Y and Li J: Hypoxia-inducible factor-1alpha in hepatic
fibrosis: a promising therapeutic target. Biochimie. 108:1–7. 2015.
View Article : Google Scholar
|
|
8
|
O'Connell MP and Weeraratna AT: Change is
in the air: the hypoxic induction of phenotype switching in
melanoma. J Invest Dermatol. 133:2316–2317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Nie F, Kang C, Chen B, Qin Z, Ma
J, Ma Y and Zhao X: Increased periostin expression affects the
proliferation, collagen synthesis, migration and invasion of keloid
fibroblasts under hypoxic conditions. Int J Mol Med. 34:253–261.
2014.PubMed/NCBI
|
|
10
|
Cash TP, Pan Y and Simon MC: Reactive
oxygen species and cellular oxygen sensing. Free Radic Biol Med.
43:1219–1225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gaber T, Dziurla R, Tripmacher R,
Burmester GR and Buttgereit F: Hypoxia inducible factor (HIF) in
rheumatology: low O2! See what HIF can do! Ann Rheum
Dis. 64:971–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Q, Wu Y, Ann DK, Messadi DV, Tuan
TL, Kelly AP, Bertolami CN and Le AD: Mechanisms of hypoxic
regulation of plasminogen activator inhibitor-1 gene expression in
keloid fibroblasts. J Invest Dermatol. 121:1005–1012. 2003.
View Article : Google Scholar
|
|
13
|
Ueda K, Yasuda Y, Furuya E and Oba S:
Inadequate blood supply persists in keloids. Scand J Plast Reconstr
Surg Hand Surg. 38:267–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steinbrech DS, Mehrara BJ, Chau D, Rowe
NM, Chin G, Lee T, Saadeh PB, Gittes GK and Longaker MT: Hypoxia
upregulates VEGF production in keloid fibroblasts. Ann Plast Surg.
42:514–519; discussion 519–520. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruthenborg RJ, Ban JJ, Wazir A, Takeda N
and Kim JW: Regulation of wound healing and fibrosis by hypoxia and
hypoxia-inducible factor-1. Mol Cells. 37:637–643. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lebrin F, Deckers M, Bertolino P and Ten
Dijke P: TGF-beta receptor function in the endothelium. Cardiovasc
Res. 65:599–608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santibañez JF, Quintanilla M and Bernabeu
C: TGF-β/TGF-β receptor system and its role in physiological and
pathological conditions. Clin Sci (Lond). 121:233–251. 2011.
View Article : Google Scholar
|
|
18
|
Jinnin M, Ihn H and Tamaki K:
Characterization of SIS3, a novel specific inhibitor of Smad3, and
its effect on transforming growth factor-beta1-induced
extracellular matrix expression. Mol Pharmacol. 69:597–607. 2006.
View Article : Google Scholar
|
|
19
|
Liu J, Wang Y, Pan Q, Su Y, Zhang Z, Han
J, Zhu X, Tang C and Hu D: Wnt/β-catenin pathway forms a negative
feedback loop during TGF-β1 induced human normal skin
fibroblast-to-myofibroblast transition. J Dermatol Sci. 65:38–49.
2012. View Article : Google Scholar
|
|
20
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Desmoulière A, Chaponnier C and Gabbiani
G: Tissue repair, contraction, and the myofibroblast. Wound Repair
Regen. 13:7–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hinz B, Celetta G, Tomasek JJ, Gabbiani G
and Chaponnier C: Alpha-smooth muscle actin expression upregulates
fibroblast contractile activity. Mol Biol Cell. 12:2730–2741. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Nie F, Chen X, Qin Z, Kang C,
Chen B, Ma J, Pan B and Ma Y: Upregulated periostin promotes
angiogenesis in keloids through activation of the ERK 1/2 and focal
adhesion kinase pathways, as well as the upregulated expression of
VEGF and angiopoietin 1. Mol Med Rep. 11:857–864. 2015.
|
|
24
|
Jiang HS, Zhu LL, Zhang Z, Chen H, Chen Y
and Dai YT: Estradiol attenuates the TGF-β1-induced conversion of
primary TAFs into myofibroblasts and inhibits collagen production
and myofibroblast contraction by modulating the Smad and Rho/Rock
signaling pathways. Int J Mol Med. 36:801–807. 2015.PubMed/NCBI
|
|
25
|
Bosco MC, Puppo M, Blengio F, Fraone T,
Cappello P, Giovarelli M and Varesio L: Monocytes and dendritic
cells in a hypoxic environment: spotlights on chemotaxis and
migration. Immunobiology. 213:733–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sen CK and Roy S: Oxygenation state as a
driver of myofibroblast differentiation and wound contraction:
hypoxia impairs wound closure. J Invest Dermatol. 130:2701–2703.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nauta TD, van Hinsbergh VW and Koolwijk P:
Hypoxic signaling during tissue repair and regenerative medicine.
Int J Mol Sci. 15:19791–19815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kelly BD, Hackett SF, Hirota K, Oshima Y,
Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA and Semenza GL:
Cell type-specific regulation of angiogenic growth factor gene
expression and induction of angiogenesis in nonischemic tissue by a
constitutively active form of hypoxia-inducible factor 1. Circ Res.
93:1074–1081. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haase VH: Oxygen regulates
epithelial-to-mesenchymal transition: insights into molecular
mechanisms and relevance to disease. Kidney Int. 76:492–499. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sloan DF, Brown RD, Wells CH and Hilton
JG: Tissue gases in human hypertrophic burn scars. Plast Reconstr
Surg. 61:431–436. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Copple BL: Hypoxia stimulates hepatocyte
epithelial to mesenchymal transition by hypoxia-inducible factor
and transforming growth factor-beta-dependent mechanisms. Liver
Int. 30:669–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watson CJ, Collier P, Tea I, Neary R,
Watson JA, Robinson C, Phelan D, Ledwidge MT, McDonald KM, McCann
A, et al: Hypoxia-induced epigenetic modifications are associated
with cardiac tissue fibrosis and the development of a
myofibroblast-like phenotype. Hum Mol Genet. 23:2176–2188. 2014.
View Article : Google Scholar
|
|
34
|
Henderson NC, Arnold TD, Katamura Y,
Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E,
Betsholtz C, Ruminski PG, et al: Targeting of αv integrin
identifies a core molecular pathway that regulates fibrosis in
several organs. Nat Med. 19:1617–1624. 2013. View Article : Google Scholar : PubMed/NCBI
|