Introduction
Spinal cord injury (SCI) is a serious and
debilitating disease that is characterized by axonal tissue
degeneration and neurological dysfunction (1). SCI consists of two pathological
phases, primary mechanical injury and secondary injury. Secondary
injury consists of neuronal inflammation, demyelination, axonal
degeneration and various degrees of oligodendrocyte and neuronal
cell death, and plays an important role in the physical and
functional deficits that occur after SCI (2). Therefore, a thorough elucidation of
the mechanisms responsible for secondary injury is important in
order to understand neurodegenerative disorders and to discover an
appropriate therapeutic method.
Autophagy is an important cellular pathway
characterized by the degradation of cytoplasmic proteins and
organelles during development and under stress conditions (3). Autophagy plays a vital role in
cellular homeostasis and has been shown to be involved in a number
of biological processes and diseases, including SCI. For example, a
previous study proved that vascular endothelial growth factor
(VEGF165) attenuated SCI by inhibiting inflammation and
increasing autophagic activity (4). Another study reported that exendin-4
(Ex-4) significantly enhanced motor function in rats following SCI
by promoting autophagy (5). In
addition, pollen typhae has been shown to increase autophagic
activity in damaged neural tissues following SCI (6).
Mammalian target of rapamycin (mTOR) is a
serine/threonine protein kinase belonging to the
phosphatidylinositol 3-kinase-related kinase protein (PIKK) family.
mTOR is formed by two different protein complexes: mTORC1 and
mTORC2 (7). mTOR1 regulates
protein growth, autophagy and ribosomal biogenesis through
integrating growth factor, whereas mTORC2 is involved in cell
survival and cytoskeletal regulation (8). It has been proven that transforming
growth factor-β1 (TGF-β1) inhibits autophagy by activating mTORC1
in fibroblasts (9). Consequently,
TGF-β/mTOR signaling may be a potential target for the treatment of
SCI.
BMP and activin membrane-bound inhibitor (BAMBI)
acts as a pseudo-receptor for the TGF-β type I receptor family and
as a negative modulator of TGF-β kinase signaling due to its lack
of the intracellular kinase domain (10). BAMBI elimination enhances
alternative TGF-β signaling in diabetic mice (10), whereas it has been reported that
the expression of TGF-β is markedly increased followng SCI
(11). The inhibition of TGF-β1
in injured neurons has been shown to enhance the growth of axons
and to eventually promote functional recovery in rat models of SCI
(12). However, whether BAMBI is
associated with autophagy in the progression of SCI remains
unknown.
Based on the above information, in the present
study, we aimed to determine whether BAMBI alleviates motor
function impairment following SCI and to explore the underlying
mechanisms. The findings of our study may shed light into the
molecular mechanisms responsible for the neuroprotective effects of
BAMBI, and provide a potential approach for the treatment of
SCI.
Materials and methods
Ethics statement
All animal procedures were approved by the
Institutional Animal Care and Use Committee of the Second
Affiliated Hospital, Xi'an Jiaotong University, Xi'an, China.
Following the conclusion of the experiment, the mice were
euthanized by an intraperitoneal injection of sodium pentobarbital
(50 mg/kg).
Animals
Adult male Sprague-Dawley rats (n=96; 250–300 g)
were obtained form Xi'an Jiaotong University Health Science Center
(Xi'an, China). The rats were randomly divided into the following 4
groups (24 rats/group): The sham-operated (sham) group, the SCI
group, the pLentiH1-BAMBI shRNA group (rats with SCI rats injected
with pLentiH1-BAMBI shRNA vector), and the pAd-BAMBI group (rats
with SCI were injected with pAd-BAMBI vector). Each group also
contained 4 subgroups (n=6) for use in the following experiments:
i) behavioral analysis of motor function; ii) western blot analysis
and reverse transcription-quantitative PCR (RT-qPCR); iii) Nissl
staining; and iv) enzyme-linked immunosorbent assay (ELISA).
Rat model of SCI
The rat model of SCI was developed according to the
method of Hu et al (13).
The rats were anesthetized with chloral hydrate (300 mg/kg). After
the skin was sterilized with 75% alcohol, the lamina was exposed by
cutting and separating the skin. A laminectomy was performed to
expose the spinal cord at T9-T11. A spinal contusion was created
using a 10 g weight impactor dropped from a height of 2 cm. The
skin was then closed in layers. The bladder of each rat was
expressed 3 times a day until reflex bladder emptying was
established. The sham-operated rats were subjected to the same
process, but underwent the laminectomy only. The successful SCI
model was verified as follows: flicking of the legs, spinal cord
ischemia, flaccid paralysis of the lower limbs and the formation of
tail sway reflex. All rats were housed individually in a room at
24°C under controlled light conditions.
Intraspinal microinjection
Lentiviral shRNA vectors, named pLentiH1-BAMBI
shRNA, containing the BAMBI interference fragment were constructed.
The recombinant adenoviral vector, named pAd-BAMBI, that
overexpresses BAMBI was constructed and high viral titres were
obtained. These two recombinant vectors were obtained from
Genomeditech (Shanghai, China). Intraspinal microinjection was
performed as previously described (14). The vectors, pLentiH1-BAMBI shRNA,
pAd-BAMBI, and their corresponding control vectors were bilaterally
injected into the gray and white matter of the spinal cord at T8 in
the rats with SCI only once. Injections were made into the lateral
funiculus 1.1 mm lateral to the midline at a depth of 1 mm. For
gray matter, bilateral injections were made at 0.5 mm lateral to
the midline at a depth of 1.3 mm to target motor neurons in the
ventral horn. Injections were made using a beveled glass
micropipette (60 µm tip diameter) and nano-injector
(Stoelting Co., Wood Dale, IL, USA). For each injection site, a
volume of up to 1.0 µl was slowly infused over a period of 4
min (100 nl/min).
Behavioral analysis
The recovery of behavioral function was conducted
using the Basso Beattie Bresnahan (BBB) locomotor rating scale at
1, 3, 7 and 14 days after each treatment. The score was assessed by
two independent observers. The score ranged from 0 to 21. A score
of 0 indicates complete hind limb paralysis and 21 indicates
completely normal locomotion. The rats were placed in an open
field. The 22-point scale included the monitoring of hind limb
movements, trunk position and stability, stepping, co-ordination,
paw placement, toe clearance and tail position.
Nissl staining
After the mice were anesthetized by an
intraperitoneal injection of 10% chloral hydrate (300 mg/kg), the
spinal cords from the T7–T10 level around the lesion epicenter were
obtained and were incubated at 60°C for 30 min. The spinal cord
slices were then cleared with xylene 3 times and dehydrated with
100, 90 and 85% ethanol for 5 min each, and then with 80 and 70%
ethanol for 3 min each. The slices were rinsed with distilled water
for 1 min, and stained with crystal violet dye (Sigma, St. Louis,
MO, USA) in an oven at 37°C for 30 min, rinsed with water for 8
min, and rapidly separated with 95% ethanol. The sections were then
incubated in anhydrous alcohol and xylene for 2×5 min. The slices
were mounted with neutral gum.
RT-qPCR
The spinal cord injury tissues were collected
following treatment and total RNA was extracted using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA
synthesis was carried out using the MMLV reverse transcriptase kit
(Takara, Dalian, China). Quantitative (real-time) PCR (qPCR) was
conducted using the SYBR Premix Ex Taq™ kit according to the
manufacturer's instructions (Takara). All of the primers used in
this study were synthesized by Sangon Biotech (Shanghai, China) and
were as follows: BAMBI 5′-CCG TGC TGC TCA CCA AAG GTG-3′, 5′-ATA
CCT GTT TCC TTG TCC TGA-3′. Each individual sample was run in
triplicate wells and the reactions were conducted in an ABI 7500
real-time PCR system Applied Biosystems, Carlsbad, CA, USA). The
reaction conditions were an initial denaturation at 95°C for 30 sec
followed by 40 cycles at 95°C for 10 sec and 60°C for 60 sec. The
relative expression levels of the genes were calculated using the
2−ΔΔCT method. The 18S RNA gene was selected as a
reference.
Western blot analysis
Spinal cords containing the injury site were
collected following treatment and lysed with RIPA buffer containing
PMSA (Sangon Biotech, Shanghai, China), and centrifuged at 12,000 ×
g for 20 min at 4°C. The protein concentration was measured using a
bicinchoninic acid (BCA) protein assay kit (Sangon Biotech). Total
proteins (30 µg) were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene difluoride (PVDF) membranes
(Millipore, Darmstadt, Germany). The membranes were then probed
with primary antibodies specific for rabbit polyclonal anti-rat
BAMBI (Cat. no. ab203070; 1:200 dilution; Abcam, Cambridge, UK),
rabbit monoclonal anti-rat Bim (Cat. no. ab7888; 1:1,000 dilution;
Abcam), rabbit polyclonal anti-rat Beclin 1 (Cat. no. NB500-249;
1:1,000 dilution; Novus Biologicals, Littleton, CO, USA), rabbit
polyclonal anti-rat levels light chain 3B (LC3B; Cat. no. a b48394;
1:1,000 dilution; Abcam), rabbit polyclonal anti-rat p62 (Cat. no.
ab91526; 1:1,000 dilution; Abcam), rabbit polyclonal anti-rat TGF-β
(Cat. no. ab155264; 1:1,000 dilution; Abcam), rabbit polyclonal
anti-rat mTOR (Cat. no. ab2833; 1:2,000 dilution; Abcam), rabbit
polyclonal anti-rat p-p70s6k (Cat. no. ab2571, 1:250 dilution;
Abcam). Following overnight incubation at 4°C, the goat anti-rabbit
IgG-HRP secondary antibody (Cat. no. ab6721; 1:2,000 dilution;
Abcam) was added followed by incubation for 1 h at room
temperature. The immunoreactive proteins were visualized using an
ECL detection system (Amersham Biosciences, Amersham, UK).
ELISA
The spinal cords that included the injury site were
collected and re-suspended in PBS. The tissues were then
centrifuged at 3,000 rpm. The supernatant were collected and added
to the appropriate wells for 2.5 h of incubation at room
temperature. After washing with wash buffer 4 times, 100 µl
of 1X prepared biotinylated detection antibody were added to each
well followed by 1 h of incubation. The prepared HRP-streptavidin
solution (100 µl) was added to each well followed by
incubation for 45 min. ELISA colorimetric TMB reagent (100
µl) was added followed by incubation for 30 min in the dark
with gentle shaking. Finally, stop solution (50 µl) was
added to each well and read at 450 nm immediately.
Statistical analysis
Statistical analysis was performed using the
Student's unpaired t-test (SPSS release 19.0; SPSS, Inc., Chicago,
IL, USA). Data are expressed as the means ± SD. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
The expression of BAMBI is decreased in
rats with SCI
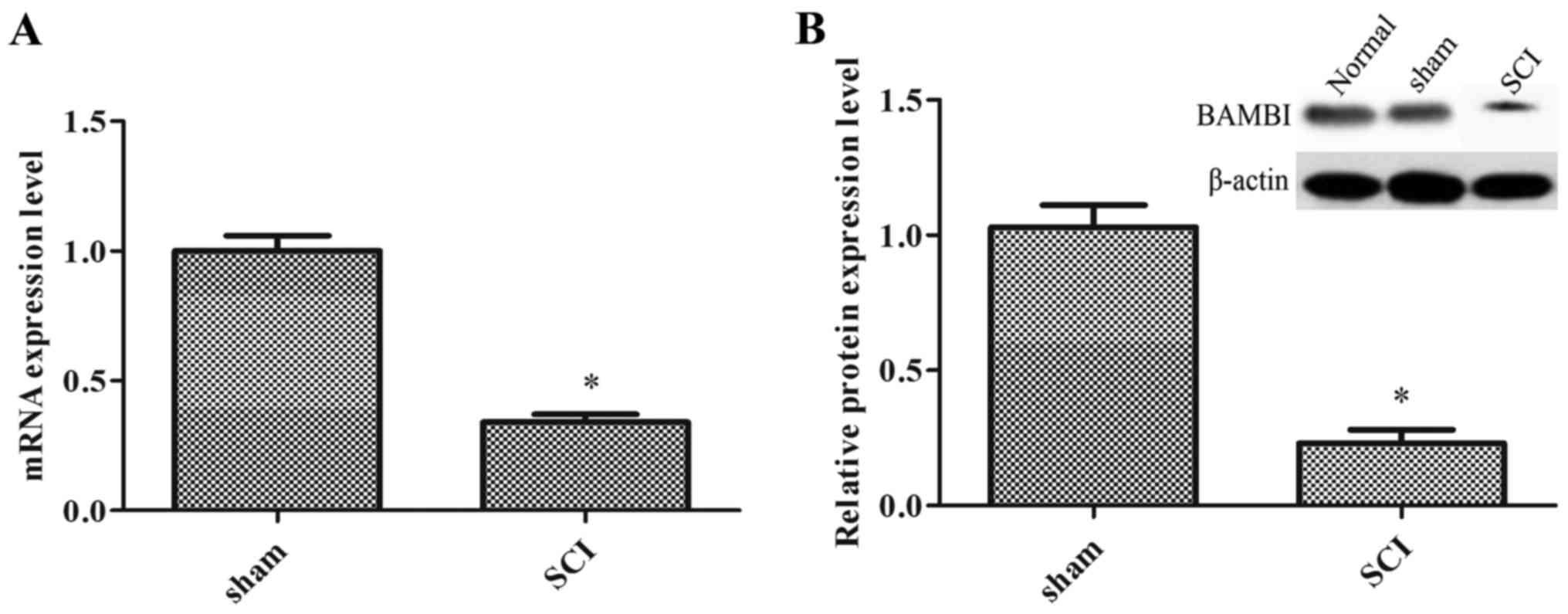
In order to confirm the role of BAMBI in SCI, a rat
model of SCI was established. Compared with the sham-operated
group, the mRNA and protein expression levels of BAMBI were
significantly decreased in the spinal cord tissue of the rats with
SCI (P<0.05) (Fig. 1).
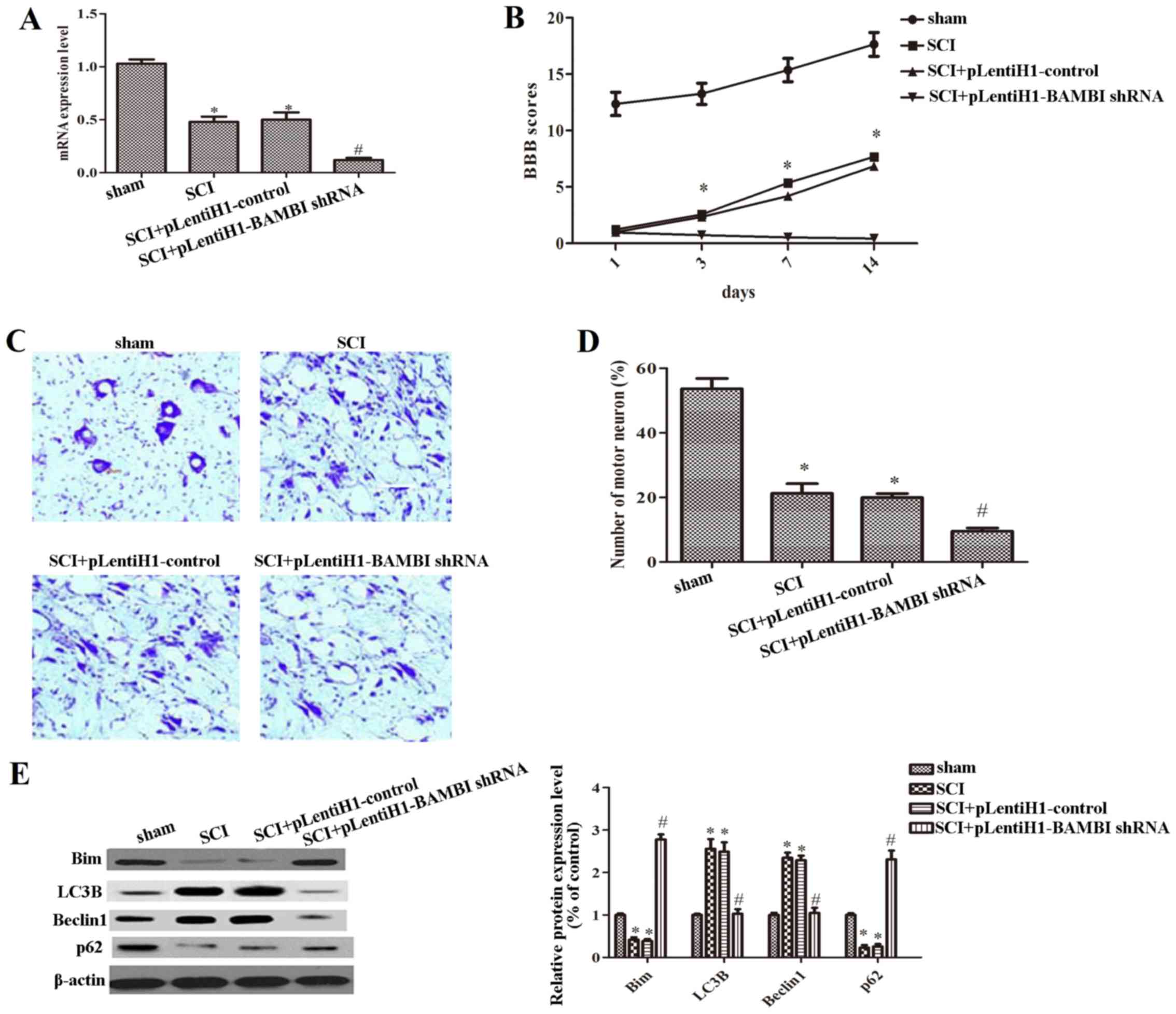
Knockdown of BAMBI expression aggravates
locomotor dysfunction and inhibits autophagy in rats with SCI
In order to examine the effects of BAMBI on SCI,
BAMBI was knocked down by injecting the rats with SCI with a
pLentiH1-BAMBI shRNA injection. The interference efficiency of
pLentiH1-BAMBI shRNA is shown in Fig.
2A. Compared with the sham-operated group, the expression of
BAMBI was significantly decreased in the SCI group (P<0.05).
When compared with the SCI group, the expression of BAMBI was
decreased even further in the group that was injected with
pLentiH1-BAMBI shRNA (P<0.05). The results of the BBB score
revealed that there was no locomotor dysfunction in the rats from
the sham-operated group, but that locomotor function was
significantly decreased in the rats with SCI (Fig. 2B). Even more severe hind limb
locomotor dysfunction was observed in the rats that were injected
with pLentiH1-BAMBI shRNA (Fig.
2B). As shown in Fig. 2C and
D, compared with the sham-operated group, the anterior horn
cells and the number of motor neurons were markedly decreased in
the SCI group, particularly in the group infected with
pLentiH1-BAMBI shRNA. Moreover, we also measured the expression
levels of LC3B, Beclin 1, Bim and p62, which are related to the
progression of autophagy. The results revealed that the expression
levels of Bim and p62 were notably decreased, whereas those of LC3B
and Beclin 1 were significantly increased in the SCI group
(P<0.05). Compared with the SCI group, the expression levels of
Bim and p62 were significantly increased, whereas those of LC3B and
Beclin 1 were decreased in the group that was injected with
pLentiH1 BAMBI shRNA (Fig. 2E).
From these results, it can be concluded that the silencing of BAMBI
expression leads to the aggravation of locomotor dysfunction and
the inhibition of autophagy.
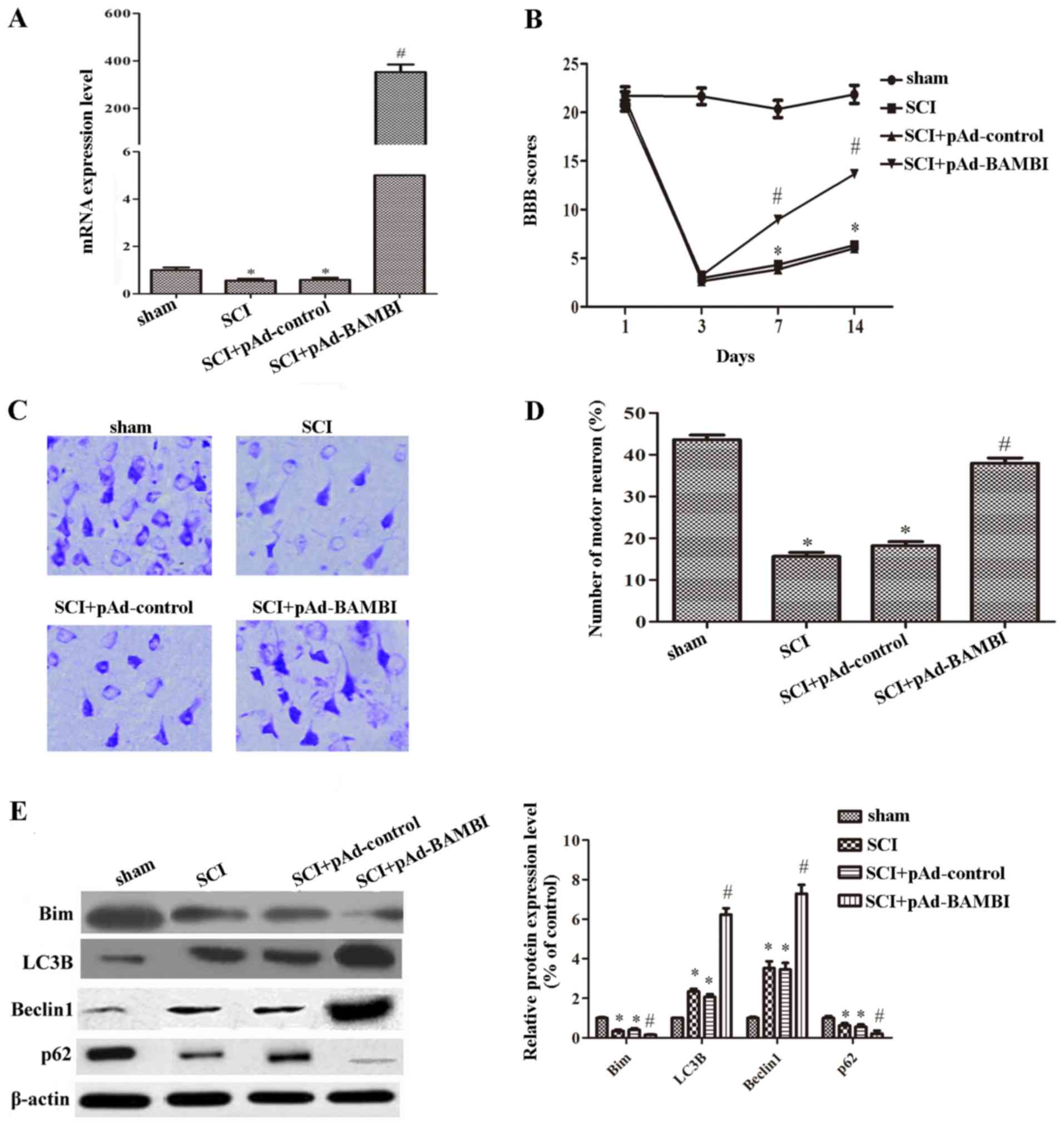
The overexpression of BAMBI attenuates
locomotor dysfunction and increases autophagy in rats with SCI
To further analyze the effects of BAMBI on SCI,
BAMBI was overexpressed by injecting the rats with SCI with a BAMBI
overexpression vector (pAd-BAMBI). The overexpression efficiency of
pAd-BAMBI is shown in Fig. 3A.
Compared with the SCI group, the expression of BAMBI was
significantly increased in the group injected with pAd-BAMBI
(Fig. 3A). As shown in Fig. 3B, we found that when the rats were
injected with pAd-BAMBI, the BBB score was significantly increased
on days 7 and 14 following the induction of SCI. As shown in
Fig. 3C and D, the number of
motor neurons in the anterior horn cells were markedly increased
when the rats were injected with pAd-BAMBI. These results suggested
that the overexpression of BAMBI attenuated motor dysfunction
caused by SCI. We also measured the expression of proteins related
to autophagy. The expression levels of Bim and p62 were
significantly decreased, whereas those of LC3B and Beclin 1 were
notably increased in the group injected with pAd-BAMBI (P<0.05)
(Fig. 3E). The above-mentioned
results indicate that BAMBI plays a positive role in SCI.
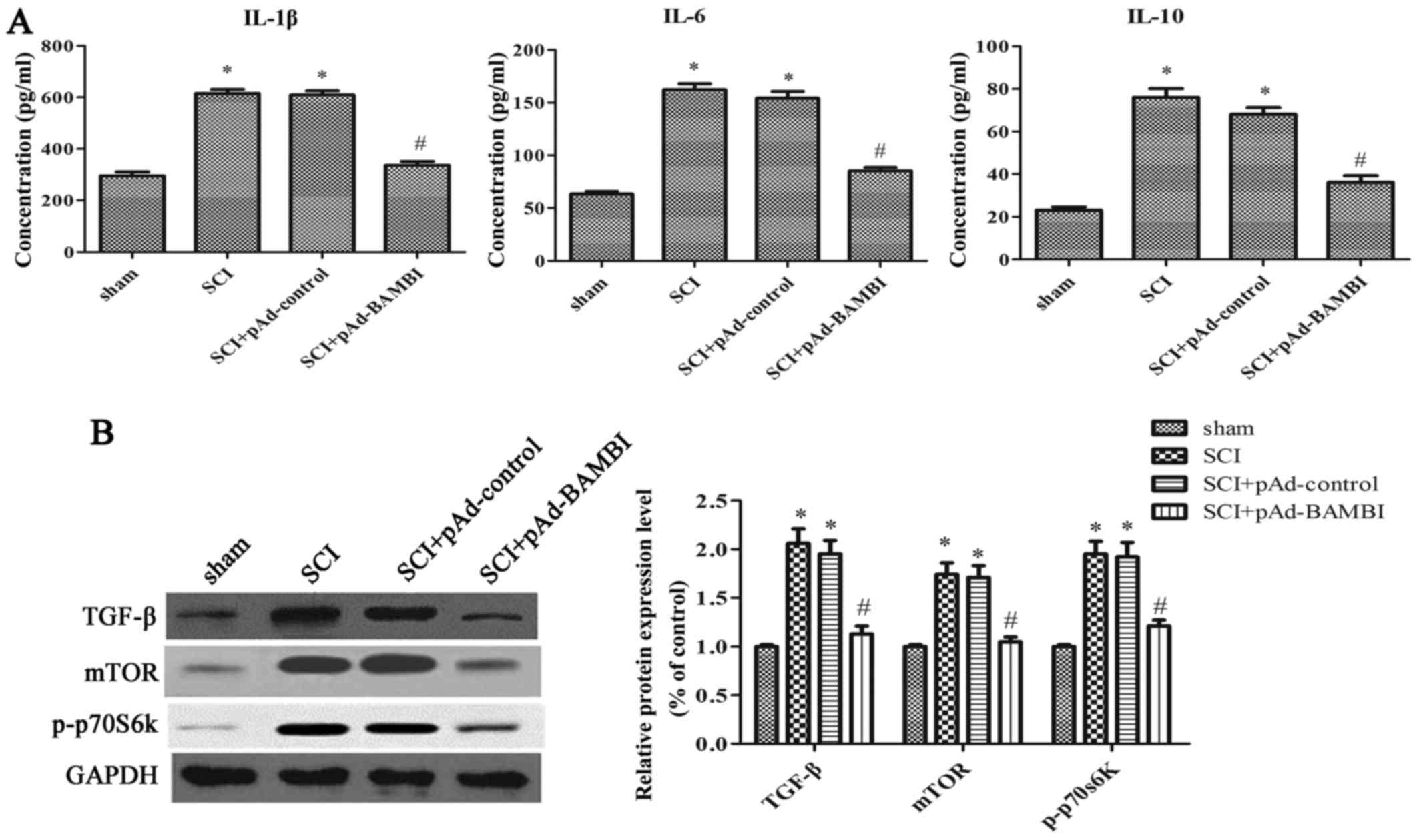
Overexpression of BAMBI inhibits
inflammation and the activation of the mTOR signaling pathway in
rats with SCI
To further elucidate the underlying mechanisms
responsible for the protective effects of BAMBI in rats with SCI,
we analyzed the levels of cytokines, including IL-1β, IL-6, IL-10,
and the protein expression levels in the mTOR signaling pathway,
which is related to autophagy. When compared with the SCI group,
the concentrations of IL-1β, IL-6 and IL-10 were significantly
decreased in the group injected with pAd-BAMBI (P<0.05)
(Fig. 4A). As shown in Fig. 4B, the expression levels of TGF-β,
mTOR and p-p70s6k were markedly decreased in the group injected
with pAd-BAMBI (P<0.05). These results indicate that the
overexpression of BAMBI inhibits inflammation and the activation of
the mTOR signaling pathway in rats with SCI.
Discussion
SCI is regarded as a major health concern and is a
frequent cause of disability and mortality worldwide (15). A number of studies have proven
that neuroprotection and neurorecovery play an important role in
the treatment of SCI. For example, apigenin may be a potential
agent for the treatment of SCI by promoting the recovery of rat
neuronal function (16). Also, in
another study, the inhibition of miR-20a expression was shown to
induce definitive motor neuron survival and neurogenesis, and
animals with SCI exhibited improved functional deficit (17). In the present study, our results
confirmed that the overexpression of BAMBI attenuated motor
dysfunction and decreased inflammation and induced autophagy in
rats with SCI.
An increasing number of studies have verified that
BAMBI plays a vital role in various diseases. BAMBI acts as a
negative modulator of myocardial remodeling under pressure overload
(18). The overexpression of
BAMBI has been shown to inhibit keloid growth through the
suppression of TGF-β1-induced fibroblast cell proliferation and the
excessive accumulation of collagen I (19). This study demonstrated that BAMBI
was downregulated in rats with SCI, and the overexpression of BAMBI
promoted functional neurobehavioral recovery, which was evidenced
by the increased BBB score and the number of motor neurons in rats
with SCI.
Autophagy has been shown to exert a neuroprotective
effect in rats with acute SCI (20). It has been demonstrated that LC3B
and Beclin 1 are two reliable markers for the progression of
autophagy (21). For example, the
expression of Beclin 1 has been shown to be markedly increased in
damaged neural tissue and this induces autophagic cell death
following SCI (22). Treatment
with rammycin has been shown to induce autophagy by increasing the
expression levels of LC3 and Beclin 1 following SCI (23). Bim and p62 are also two major
factors in autophagy. Bim inhibits autophagy by recruiting Beclin 1
to microtubules (24). The loss
of total Bim in IL-7-deprived T cells has beeb shown to cause a
delayed degradative phase of autophagy (25). p62 acts as a cellular metabolic
switch in autophagy (26) and
exerts a protective effect against polyQ-induced neurodegeneration
through the autophagic degradation of polyQ protein oligomers
(27). Our results also indicated
that the overexpression of BAMBI significantly increased the
expression levels of LC3B and Beclin 1, and decreased the
expression of Bim and p62 in rats with SCI. This result indicated
that the overexpression of BAMBI protected the rats from SCI by
activating autophagy.
A growing number of studies have demonstrated that
mTOR signaling is involved in autophagy (7). For example, TNF alpha induced
protein 3 (TNFAIP3) inhibits mTOR signaling and promotes autophagy
(28). Another study reported
that Che-1 induced autophagy by inhibiting the mTOR pathway
(29). mTOR has been proven to
promote compensatory neuronal sprouting important for recovery
following nerve injury (30). An
increased Rheb expression has been shown to contribute to mTOR
activation in SCI (31). Our
results also revealed that the overexpression of BAMBI inhibited
the expression of TGF-β, thus leading to the inhibition of mTOR
signaling in rats with SCI. Furthermore, we also found that the
overexpression of BAMBI decreased inflammation in rats with SCI. A
previous study also demonstrated that TGF-β activated the NF-κB
pathway to promote osteoclast survival (32). TGF-β is regarded as a
pro-inflammatory agent by recruiting and activating resting
monocytes (12). The inhibition
of TGF-β1 enhances the activation of macrophages in rat models of
SCI (12). Therefore, we
concluded that the overexpression of BAMBI causes the
downregulation of TGF-β, leading to the inhibition of the NF-κB
pathway, further resulting in the decrease in the levels of IL-1β,
IL-6 and IL-10. Taken together, our results demonstrate that the
overexpression of BAMBI decreases inflammation and induces
autophagy by inhibiting mTOR signaling in rats with SCI.
In conclusion, the present study suggests that the
overexpression of BAMBI exerts a neuroprotective and neurorecovery
effect on SCI. Furthermore, the upregulation of BAMBI decreases
inflammation and induces autophagy by inhibiting mTOR signaling in
rats with SCI. Our findings reveal the molecular mechanisms
responsible for the neuroprotective roles of BAMBI, and may provide
a potential therapy for SCI.
References
|
1
|
Chen B, He J, Yang H, Zhang Q, Zhang L,
Zhang X, Xie E, Liu C, Zhang R, Wang Y, et al: Repair of spinal
cord injury by implantation of bFGF-incorporated HEMA-MOETACL
hydrogel in rats. Sci Rep. 5:90172015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su M, Guan H, Zhang F, Gao Y, Teng X and
Yang W: HDAC6 regulates the chaperone-mediated autophagy to prevent
oxidative damage in injured neurons after experimental spinal cord
injury. Oxid Med Cell Longev. 2016:72637362016. View Article : Google Scholar
|
|
3
|
Siracusa R, Paterniti I, Bruschetta G,
Cordaro M, Impellizzeri D, Crupi R, Cuzzocrea S and Esposito E: The
association of palmitoylethanolamide with luteolin decreases
autophagy in spinal cord injury. Mol Neurobiol. 53:3783–3792. 2016.
View Article : Google Scholar :
|
|
4
|
Wang H, Wang Y, Li D, Liu Z, Zhao Z, Han
D, Yuan Y, Bi J and Mei X: VEGF inhibits the inflammation in spinal
cord injury through activation of autophagy. Biochem Biophys Res
Commun. 464:453–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li HT, Zhao XZ, Zhang XR, Li G, Jia ZQ,
Sun P, Wang JQ, Fan ZK and Lv G: Exendin-4 enhances motor function
recovery via promotion of autophagy and inhibition of neuronal
apoptosis after spinal cord injury in rats. Mol Neurobiol.
53:4073–4082. 2016. View Article : Google Scholar
|
|
6
|
Wang W, Guo Z, Xu Z, Meng Q, Chen C, Zhang
Y and Cao X: Effect of pollen typhae on inhibiting autophagy in
spinal cord injury of rats and its mechanisms. Int J Clin Exp
Pathol. 8:2375–2383. 2015.PubMed/NCBI
|
|
7
|
Perluigi M, Di Domenico F and Butterfield
DA: mTOR signaling in aging and neurodegeneration: At the crossroad
between metabolism dysfunction and impairment of autophagy.
Neurobiol Dis. 84:39–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Din FV, Valanciute A, Houde VP, Zibrova D,
Green KA, Sakamoto K, Alessi DR and Dunlop MG: Aspirin inhibits
mTOR signaling, activates AMP-activated protein kinase, and induces
autophagy in colorectal cancer cells. Gastroenterology.
142:1504–1515.e1503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel AS, Lin L, Geyer A, Haspel JA, An
CH, Cao J, Rosas IO and Morse D: Autophagy in idiopathic pulmonary
fibrosis. PLoS One. 7:e413942012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan Y, Li X, Xiao W, Fu J, Harris RC,
Lindenmeyer M, Cohen CD, Guillot N, Baron MH, Wang N, et al: BAMBI
elimination enhances alternative TGF-β signaling and glomerular
dysfunction in diabetic mice. Diabetes. 64:2220–2233. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hellal F, Hurtado A, Ruschel J, Flynn KC,
Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J,
et al: Microtubule stabilization reduces scarring and causes axon
regeneration after spinal cord injury. Science. 331:928–931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kohta M, Kohmura E and Yamashita T:
Inhibition of TGF-beta1 promotes function recovery after spinal
cord injury. Neurosci Res. 65:393–401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu JZ, Long H, Wu T-D, Zhou Y and Lu H-B:
The effect of estrogen-related receptor α on the regulation of
angiogenesis after spinal cord injury. Neuroscience. 290:570–580.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu CG, Yezierski RP, Joshi A, Raza K, Li Y
and Geddes JW: Involvement of ERK2 in traumatic spinal cord injury.
J Neurochem. 113:131–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Jiang HK, Li YP and Guo YP: Hydrogen
sulfide protects spinal cord and induces autophagy via miR-30c in a
rat model of spinal cord ischemia-reperfusion injury. J Biomed Sci.
22:502015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang F, Li F and Chen G: Neuroprotective
effect of apigenin in rats after contusive sipinal cord injury.
Neurol Sci. 35:583–588. 2014. View Article : Google Scholar
|
|
17
|
Jee MK, Jung JS, Im YB, Jung SJ and Kang
SK: Silencing of miR-20a is crucial for Ngn1-mediated
neuroprotection in injured spinal cord. Hum Gene Ther. 23:508–520.
2012. View Article : Google Scholar
|
|
18
|
Villar AV, García R, Llano M, Cobo M,
Merino D, Lantero A, Tramullas M, Hurlé JM, Hurlé MA and Nistal JF:
BAMBI (BMP and activin membrane-bound inhibitor) protects the
murine heart from pressure-overload biomechanical stress by
restraining TGF-β signaling. Biochim Biophys Acta. 1832:323–335.
2013. View Article : Google Scholar
|
|
19
|
Lin L, Wang Y, Liu W and Huang Y: BAMBI
inhibits skin fibrosis in keloid through suppressing TGF-β1-induced
hypernomic fibroblast cell proliferation and excessive accumulation
of collagen I. Int J Clin Exp Med. 8:13227–13234. 2015.PubMed/NCBI
|
|
20
|
Tang P, Hou H and Zhang L, Lan X, Mao Z,
Liu D, He C, Du H and Zhang L: Autophagy reduces neuronal damage
and promotes locomotor recovery via inhibition of apoptosis after
spinal cord injury in rats. Mol Neurobiol. 49:276–287. 2014.
View Article : Google Scholar
|
|
21
|
Barth S, Glick D and Macleod KF:
Autophagy: Assays and artifacts. J Pathol. 221:117–124. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanno H, Ozawa H, Sekiguchi A and Itoi E:
Spinal cord injury induces upregulation of Beclin 1 and promotes
autophagic cell death. Neurobiol Dis. 33:143–148. 2009. View Article : Google Scholar
|
|
23
|
Sekiguchi A, Kanno H, Ozawa H, Yamaya S
and Itoi E: Rapamycin promotes autophagy and reduces neural tissue
damage and locomotor impairment after spinal cord injury in mice. J
Neurotrauma. 29:946–956. 2012. View Article : Google Scholar
|
|
24
|
Luo S, Garcia-Arencibia M, Zhao R, Puri C,
Toh PP, Sadiq O and Rubinsztein DC: Bim inhibits autophagy by
recruiting Beclin 1 to microtubules. Mol Cell. 47:359–370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruppert SM, Li W, Zhang G, Carlson AL,
Limaye A, Durum SK and Khaled AR: The major isoforms of Bim
contribute to distinct biological activities that govern the
processes of autophagy and apoptosis in interleukin-7 dependent
lymphocytes. Biochim Biophys Acta. 1823:1877–1893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moscat J and Diaz-Meco MT: Feedback on
fat: p62-mTORC1-autophagy connections. Cell. 147:724–727. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saitoh Y, Fujikake N, Okamoto Y, Popiel
HA, Hatanaka Y, Ueyama M, Suzuki M, Gaumer S, Murata M, Wada K and
Nagai Y: p62 plays a protective role in the autophagic degradation
of polyglutamine protein oligomers in polyglutamine disease model
flies. J Biol Chem. 290:1442–1453. 2015. View Article : Google Scholar :
|
|
28
|
Matsuzawa Y, Oshima S, Takahara M,
Maeyashiki C, Nemoto Y, Kobayashi M, Nibe Y, Nozaki K, Nagaishi T,
Okamoto R, et al: TNFAIP3 promotes survival of CD4 T cells by
restricting MTOR and promoting autophagy. Autophagy. 11:1052–1062.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Desantis A, Bruno T, Catena V, De Nicola
F, Goeman F, Iezzi S, Sorino C, Ponzoni M, Bossi G, Federico V, et
al: Che-1-induced inhibition of mTOR pathway enables stress-induced
autophagy. EMBO J. 34:1214–1230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park KK, Liu K, Hu Y, Smith PD, Wang C,
Cai B, Xu B, Connolly L, Kramvis I, Sahin M and He Z: Promoting
axon regeneration in the adult CNS by modulation of the PTEN/mTOR
pathway. Science. 322:963–966. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Codeluppi S, Svensson CI, Hefferan MP,
Valencia F, Silldorff MD, Oshiro M, Marsala M and Pasquale EB: The
Rheb-mTOR pathway is upregulated in reactive astrocytes of the
injured spinal cord. J Neurosci. 29:1093–1104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gingery A, Bradley EW, Pederson L, Ruan M,
Horwood NJ and Oursler MJ: TGF-β coordinately activates
TAK1/MEK/AKT/NFκB and SMAD pathways to promote osteoclast survival.
Exp Cell Res. 314:2725–2738. 2008. View Article : Google Scholar : PubMed/NCBI
|