Introduction
Skin functions as a barrier to protect the internal
organs from environmental toxins. Skin consists of the epidermis,
dermis and subcutaneous tissue. The epidermis is the outermost
layer composed mainly of keratinocytes that secrete keratin protein
and lipids to form the extracellular matrix (ECM), melanocytes to
produce pigment, and Langerhans cells to present antigen (1). The dermis is the layer of skin
beneath the epidermis. It is composed of connective tissues to
provide tensile force and elasticity to skin through the ECM
composed of collagen fibrils, microfibrils and elastic fibers
(2). The subcutaneous tissue
below the dermis is composed of fibroblasts to produce ECM
proteins, macrophages to eliminate pathogens and adipocytes to
conserve body fat (3–8).
The aging of the skin is induced by complex
processes, including intrinsic (e.g., genetic mutation, cellular
metabolism and hormonal changes) and extrinsic factors [e.g.,
chemicals, toxins, pollutants and ultraviolet (UV) radiation]
(5,9–11).
Aging skin is mainly associated with the general atrophy of ECM
components with a decrease in the number of fibroblasts, reduced
levels of collagen and elastin, and the disorganization of collagen
fibrils and elastin fibers (12–14). Alterations in the levels of
collagen and elastin primarily cause clinical symptoms of aging
skin, such as wrinkles, sagging and laxity (15). The degradation of collagen and
elastin in aged or photodamaged (UV-irradiation) skin is associated
with matrix metalloproteinases (MMPs) released from epidermal
keratinocytes and dermal fibroblasts (10,14–17).
The root of the ginseng plant (Panax ginseng
Meyer, Araliaceae) is traditionally used as an herbal medicine in
East Asian countries, including Korea, Japan and China. It is known
to possess the ability to enhance physical performance, as well as
to exert neuroprotective effects, enhance sexual function, and to
exert anti-cancer effects (18–22). In addition, the antioxidant and/or
anti-inflammatory effects of ginseng are considered to be relevant
to its anti-aging effects on skin (23,24). Pharmacologically active components
in ginseng include polysaccharides, polyacetylenes and gisenosides,
with ginsenosides being considered as the most important component
(25). To date, about 50 types of
ginsenosides have been identified from the ginseng root. These
natural ginsenosides from raw ginseng are converted to more stable,
bioavailable and bioactive forms through the processes of drying
and/or steaming (26).
Raw ginseng is processed into white ginseng by a
simple drying process or into red ginseng by steaming and drying
processes to preserve or improve the efficacy (27). Black ginseng is made from raw
ginseng by repetitive steaming and drying processes. After being
subjected to steaming and drying processes (9 times), raw ginseng
will become black ginseng (28).
The fermentaion of black ginseng using Saccharomyces
cerevisiae can produce more active gisenosides (29,30). Fermented black ginseng (FBG) has
different ratios of bioactive ingredients and contents of
ginsenosides compared to white or red ginseng (31,32). However, the effects of FBG on skin
remain unclear. Thus, the aim of this study was to determine the
in vitro toxicity and anti-aging effect of FBG as a cosmetic
ingredient on skin to provide safety and efficacy data to support
the use of FBG as a comsmetic ingredient.
Materials and methods
Reagents
FBG was obtained from (Ginseng By Pharm Co., Ltd.,
Wonju, Korea). Its detailed composition information has been
previously described (26). All
media required for cell growth, such as Dulbecco's modified Eagle's
medium (DMEM) high glucose, fetal bovine serum (FBS),
penicillin-streptomycin, and trypsin-EDTA (0.25%) were purchased
from (Gibco-BRL Inc., Franklin Lakes, NJ, USA).
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazoliumbromide (MTT),
retinoic acid, Dulbeco's phosphate-buffered saline (D-PBS) were
purchased from (Sigma-Aldrich, St. Louis, MO, USA). Cell lysis
buffer, phenylmethylsulfonyl fluoride (PMSF), antibodies against
MMP-9 (sc-3852S), β-actin (sc-1616), horseradish
peroxidase-conjugated anti-rabbit IgG (7074) and anti-mouse IgG
(7076) were purchased from (Cell Signaling Technology, Inc.,
Danvers, MA, USA). Polyvinylidene fluoride (PVDF) and antibody
against tissue inhibitor of metalloproteinase (TIMP)-2 (MAB 3310)
were purchased from Millipore (Billerica, MA, USA). All other
reagents were of the highest quality available.
Cell culture
Human skin fibroblasts (HS68) were obtained from the
American Type Culture Collection (ATCC, Manassas, VA, USA). The
cells were cultured in DMEM containing 10% FBS and 1% of
penicillin-streptomycin at 37°C in a humidified atmosphere
containing 5% CO2.
Cell viability assay
The HS68 human fibroblasts were seeded onto 96-well
plates at a density of 5×104 cells/well and incubated
for 48 h, as previously described (10). After 48 h of incubation, various
concentrations of FBG (10, 25, 50, 100, and 200 μg/ml in
fresh medium) or distilled water (DW; control) were used to treat
the cells. The cells were further cultured for 48 h. Subsequently,
200 μl of MTT (0.5 mg/ml MTT in fresh medium) was added to
each well followed by incubation at 37°C for 3 h. The MTT medium
was removed by aspiration and 200 μl of dimethyl sulfoxide
was added to each well. After reacting for 10 min at room
temperature, formazan production was detected by measuring the
optical density at 570 nm on a PowerWave XS microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA). Data were then
expressed as a percentage of viable cells compared to viable cells
in the DW-treated control.
Eye irritation test
The ocular irritation potential of FBG was examined
using the EpiOcular Eye Irritation Test (OCL-200-EIT; MatTek Corp.,
Ashland, MA, USA). Following incubation at 37°C in 5%
CO2 overnight, OCL-200-EIT was pre-wet with 20 μl
Ca++- and Mg++-free D-PBS (Sigma-Aldrich) for
30±2 min. After pre-wetting, 50 μl of FBG (10 and 100
μg/ml) was topically applied to the pre-wet OCL-200-EIT and
incubated for 30±2 min. Tissues included in OCL-200-EIT kit were
then rinsed with 300 ml D-PBS, post-soaked with 5 ml fresh medium,
and incubated for 2±0.4 h at 37°C with 5% CO2. To
measure cell viability, OCL-200-EIT was incubated with MTT solution
(1 g/ml) for 3 h. After extracting isopropanol, the absorbance of
formazan was measured at 570 nm on a PowerWave XS microplate reader
(BioTek Instruments, Inc.). The mean value for each test substance
was calculated from 2 wells. D-PBS was used as a control. Data were
expressed as a percentage of the viability compared to that of the
D-PBS-treated control.
Quantification of type I procollagen and
MMP-1 levels
The levels of type I procollagen and MMP-1 in the
human fibroblasts were quantified using the procollagen Type I
C-peptide (PIP) EIA kit (Takara Bio, Inc., Otsu, Japan) and the
Human MMP-1 ELISA kit (Young In Frontier, Seoul, Korea),
respectively. The human fibroblasts were seeded at a density of
5×104 cells/well. After 48 h, the cells were treated
with DW (control), various concentrations of FBG (0.3, 1, 3 and 10
μg/ml), or 0.03 μg/ml retinoic acid for 48 h. The
levels of type I procollagen and MMP-1 from the cultured media were
measured using the Type I PIP EIA kit and Human MMP-1 ELISA kit
according to the manufacturer's instructions. Data were expressed
as the percentage of expression compared to that of the DW-treated
control.
Quantification of MMP-2, MMP-9 and TIMP-2
levels
The expresssion levels of MMP-2, MMP-9 and TIMP-2
were measured by western blot analysis. The human fibroblasts were
treated with DW (control), various concentrations of FBG (0.3, 1, 3
and 10 μg/ml), or 0.03 μg/ml retinoic acid for 48 h.
To extract total protein, the treated cells were incubated with
cell lysis buffer (Cell Signaling Technology, Inc.) containing 1 mM
PMSF (Cell Signaling Technology, Inc.) for 5 min on ice. Following
incubation, whole cell lysates were briefly sonicated and
centrifuged at 12,000 rpm for 20 min at 4°C. The supernatant was
stored at −80°C until use. Before running on a gel, the protein
concentration was determined by Bradford assay. Total protein (25
μg) was separated by 10% SDS-PAGE and transferred onto PVDF
membranes (Merck Millipore, Darmstadt, Germany) using a transfer
apparatus (Bio-Rad Laboratories Inc., Hercules, CA, USA) according
to the manufacturer's instructions. The membranes were incubated in
blocking buffer (5% w/v skim milk in TBST) for 3 h. Primary
antibody (1:1,000 for MMP-2, 1:500 for MMP-9, and 1:500 for TIMP-2)
was incubated with the transferred membranes at 4°C overnight.
After washing the membranes with TBST, the membranes were incubated
with the secondary antibody (anti-rabbit for MMP-2 and MMP-9,
anti-mouse IgG for TIMP-2 at 1:1,000 dilution) for 2 h at room
temperature for MMP-2 or overnight at 4°C for MMP-9 and TIMP-2.
After washing the membranes with TBST, protein signal was detected
by horseradish peroxidase detection system (Sigma-Aldrich). Data
were expressed as a percentage of expression compared to that of
the DW-treated control.
Statistical analysis
The means ± standard deviations of the expression
values were calculated using Microsoft Excel. The statistical
significance (P<0.05 or P<0.01) of apparent differences in
protein expression among pre-dosing and treatments were assessed
using analysis of variance followed by Bonferroni's test in Prism
5.01 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
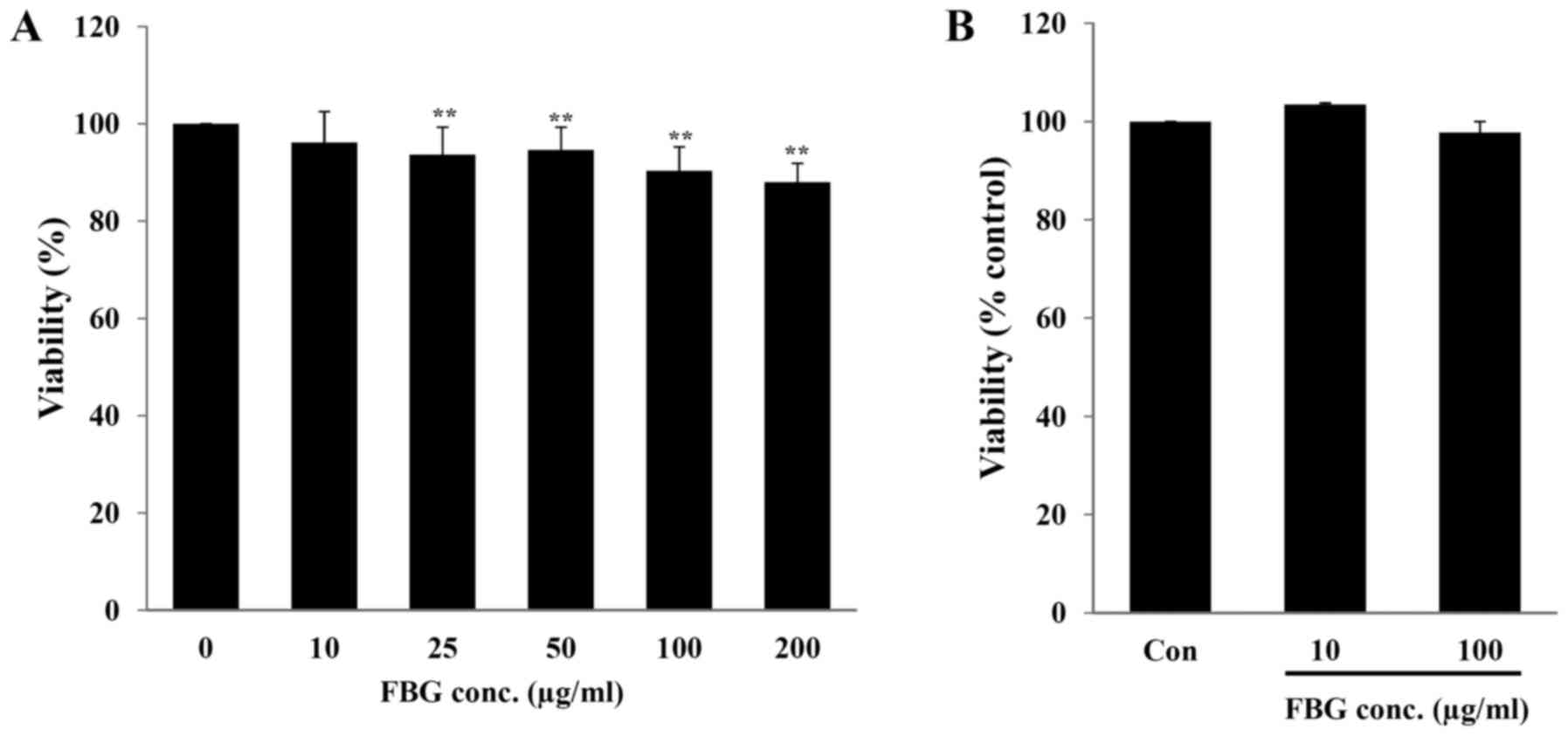
In vitro toxicity of FBG
The in vitro toxicity of FBG on the skin and
eyes, the major exposure routes of cosmetic ingredients, was
evaluated using human skin fibroblasts (HS68) and the EpiOcular Eye
Irritation test (OCL-200-EIT), respectively. When the HS68 cells
were treated with 10, 25, 50, 100, and 200 μg/ml FBG for 48
h, cell viability was 96.17±6.36, 93.68±5.64, 94.64±4.66,
90.31±4.97 and 88.01±3.87%, respectively. Only FBG at 10
μg/ml did not exhibit any significant difference in cell
viability (P>0.05) compared to the control (without FBG
treatment) (Fig. 1A). The
EpiOcular-EIT results revealed that FBG at 10 and 100 μg/ml
caused no potential eye irritation compared to the control (PBS
treatment) (Fig. 1B). These
results suggest that FBG at a concentration of <10 μg/ml
is not cytotoxic. In addition, FBG does not cause eye irritation at
concentrations up to 100 μg/ml.
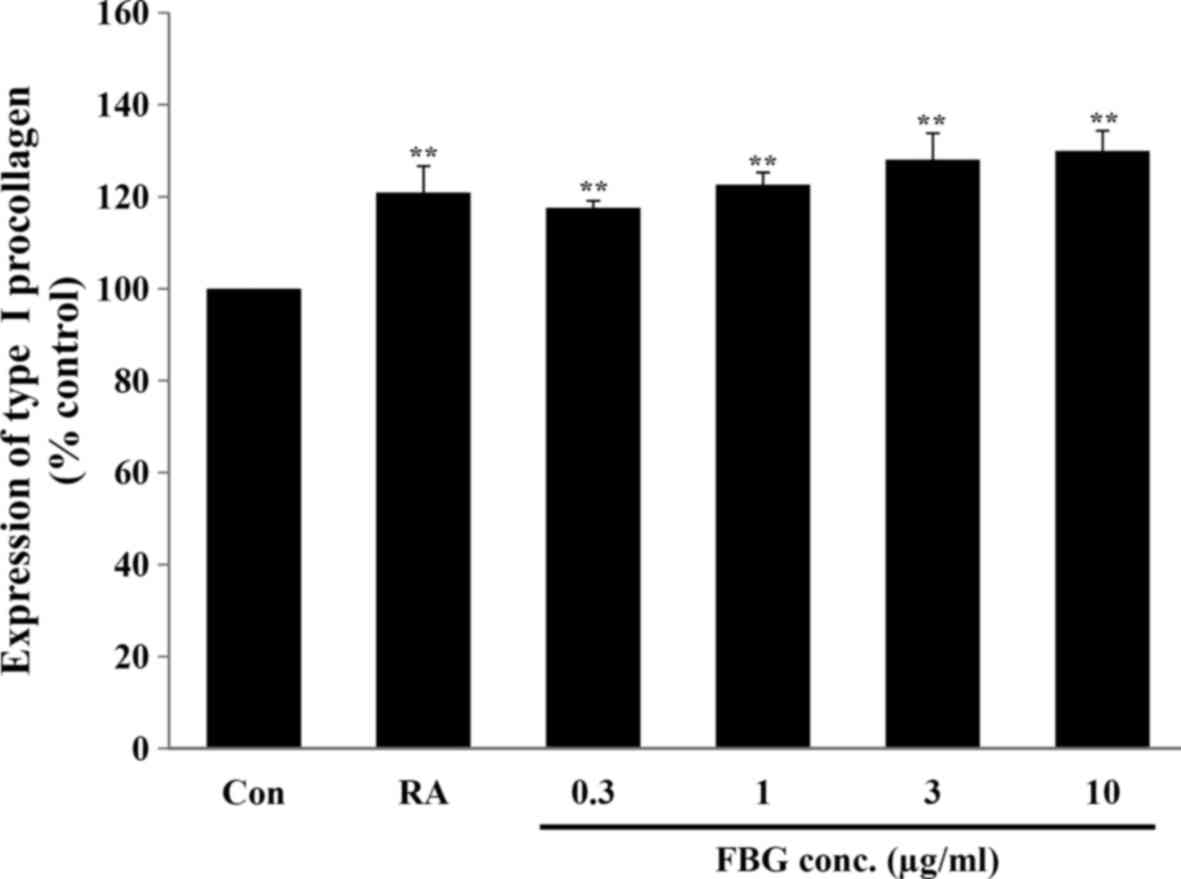
Effect of FBG on the production of
collagen
To examine the effect of FBG on collagen synthesis
in skin, the fibroblasts were treated with FBG at non-cytotoxic
concentrations. Our results revealed that FBG at 0.3, 1, 3, and 10
μg/ml significantly (P<0.05) increased type I procollagen
production to 117.61±1.51, 122.62±2.69, 128.07±5.76, and
129.95±4.47%, respectively, compared to the control (without FBG
treatment). The positive control, retinoic acid at 0.03
μg/ml, significantly increased (P<0.05) type I
procollagen production to 120.88±5.82% compared to the control
(without FBG treatment) (Fig.
2).
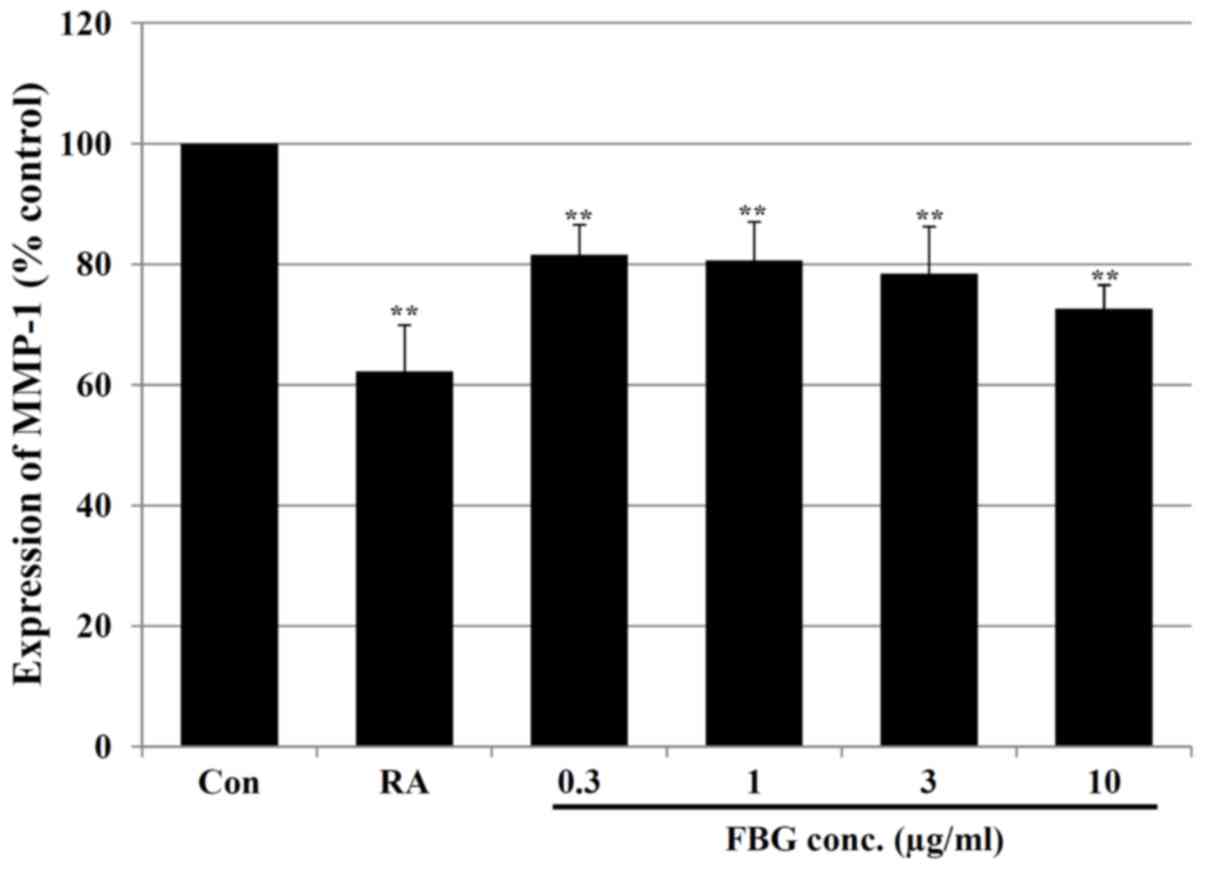
Effect of FBG on MMPs
Subsequently, we analyzed the levels of MMPs
associated with collagen degradation in FBG-treated HS68
fibroblasts. Our results revealed that FBG at concentrations of
0.3, 1, 3 and 10 μg/ml significantly (P<0.05) decreased
the MMP-1 levels by 18.41±4.96, 19.35±6.39, 21.53±7.81 and
27.41±3.96%, respectively compared to the levels of MMP-1 in the
control HS68 cells (without FBG treatment). The positive control,
retinoic acid at 0.03 μg/ml, also significantly (P<0.05)
decreased the MMP-1 level by 37.78±7.71% compared to the level of
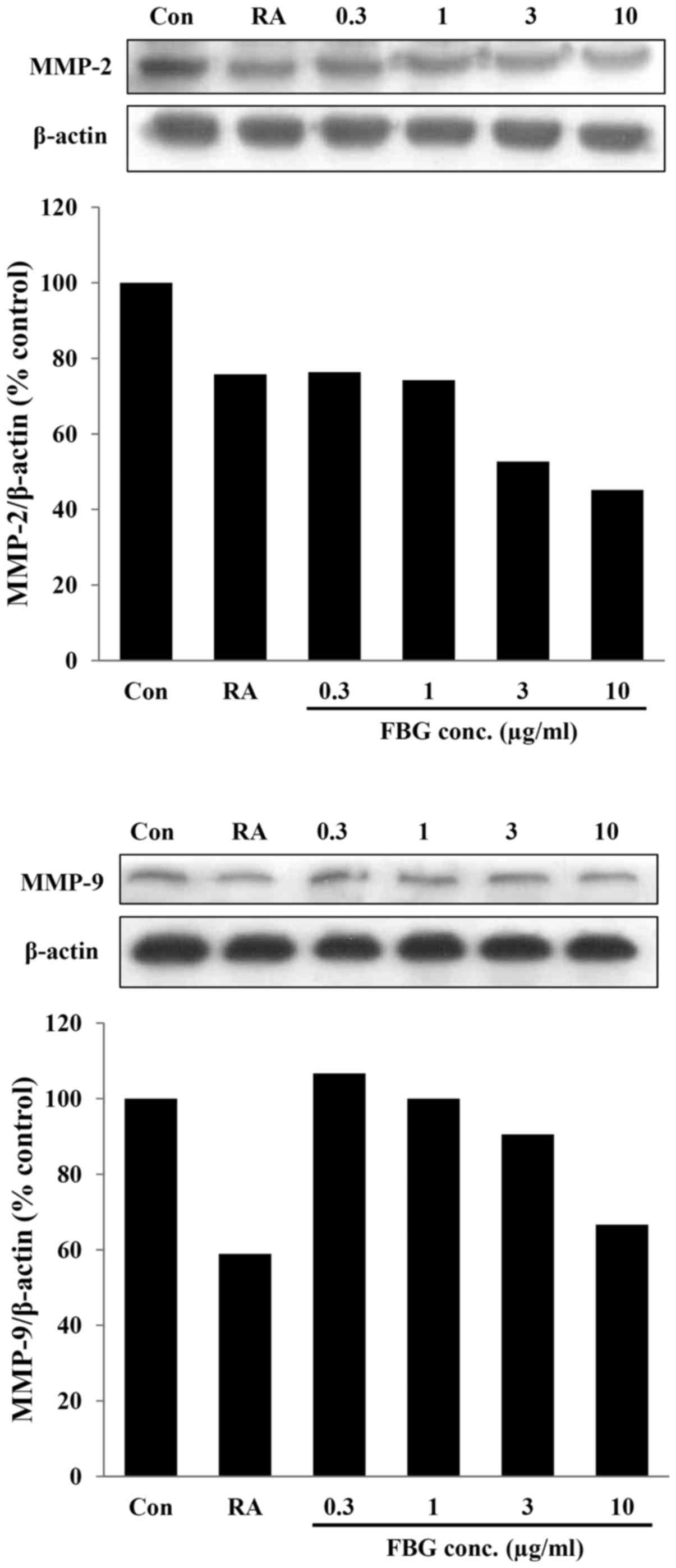
MMP-1 in the control HS68 cells not treated with FBG (Fig. 3). In addition, in the cells
treated with FBG at concentrations of 0.3, 1, 3, and 10
μg/ml, the expression levels of MMP-2 and MMP-9 were 76.32,
74.28, 52.71 and 45.15% and 106.66, 100.00, 90.53 and 66.65%
compared to those of the control, respectively. The positive
control, retinoic acid at 0.03 μg/ml, decreased the
expression of MMP-2 and MMP-9 by 24.18% and 41.07% compared to
control (Fig. 4). These results
suggest that the levels of these MMPs were inhibited by FBG in a
dose-dependent manner. The reduction in the levels of MMPs may be
associated with the increased in type I collagen production caused
by FBG treatment.
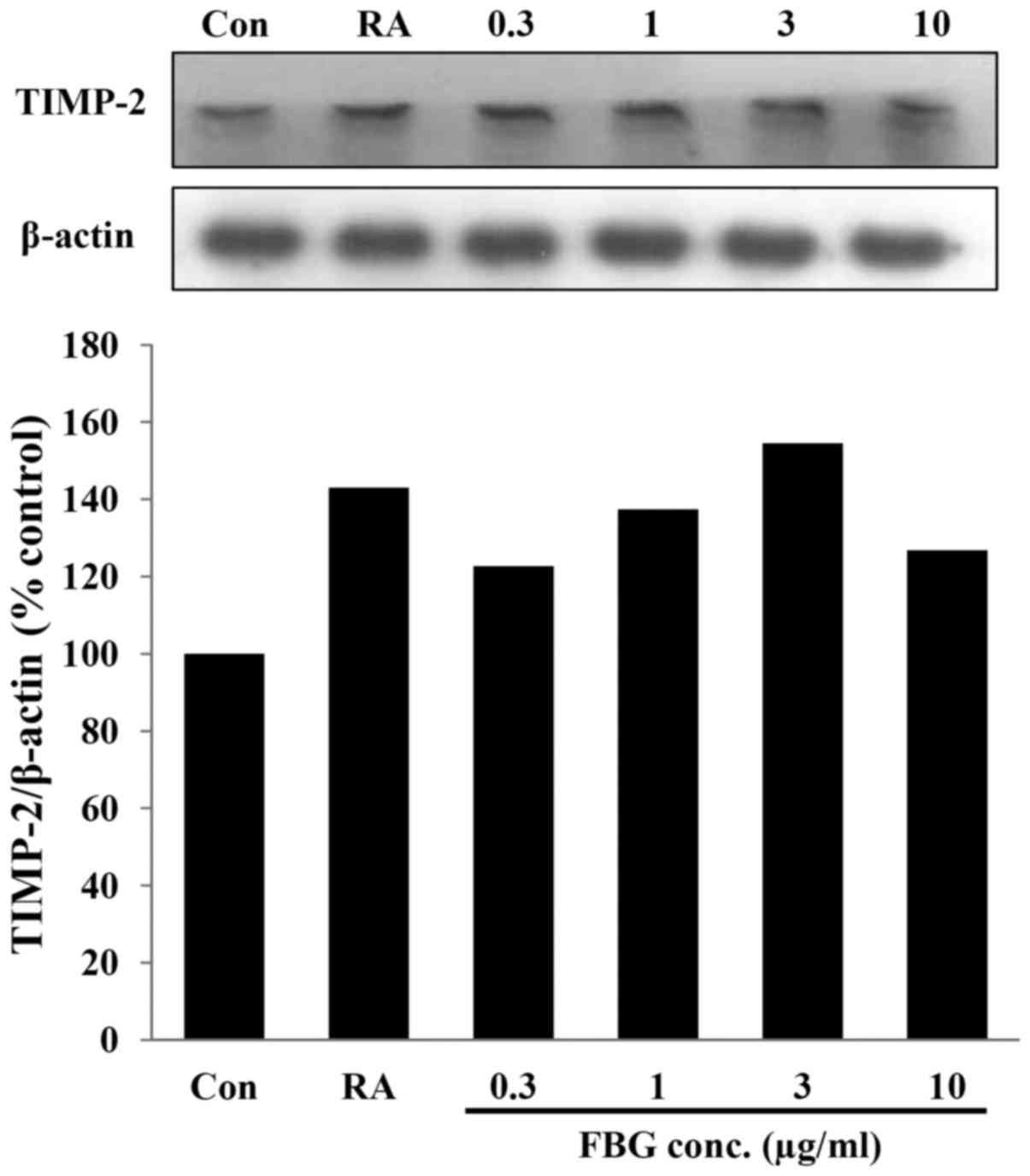
Effect of FBG on the expression of
TIMP-2
TIMP-2 expression was examined in the FBG-treated
HS68 cells. After the HS68 cells were treated with FBG at 0.3, 1, 3
and 10 μg/ml, the expression level of TIMP-2 was increased
to 122.66, 137.45, 154.55 and 126.76% compared to that of the
untreated control. The positive control, retinoic acid at 0.03
μg/ml, increased the level of TIPM-2 to 143.06 % compared to
that of the control (Fig. 5).
These results suggest that FBG increases the levels of TIMP-2, thus
causing the inhibition of MMPs.
Discussion
Ginsenosides are the major active components
responsible for the pharmacological properties of ginseng (25). Ginsenosides can be grouped into
protopanaxadiol, protopanaxatriol and oleanolic saponins based on
their chemical structures (33).
It has been previously reported that
20-O-b-d-glucopyranosyl-20(S)-protopanaxadiol
(compound K) of ginsenosides has anti-metastatic, anti-angiogenic
and anti-allergic activities in vivo (34,35). In particular, skin wrinkles and
xerosis can be ameliorated by the topical application of compound K
that induces hyaluronan synthase 2 in human keratinocytes and
increases hyaluronan content in aged hairless mouse skin (36). In addition, skin wrinkles in
humans can be improved by extracts from red ginseng roots with
increased levels of type I procollagen (37). Collectively, these data suggest
that the topical application of ginseng extracts can enhance its
anti-wrinkle effects on human skin.
FBG extracts display different compositions of
ginsenosides by more complex processes, such as repetitive steaming
and drying with fermentation using Saccharomyces cerevisiae
compared to fresh, white, or red ginseng (38,39). Such differences have been
considered to be able to enhance their antioxidant and free radical
scavenging activities (29,40–42). However, the anti-wrinkle effects
of FBG extracts have not been studied on human skin. In the present
study, FBG extracts significantly increased the expression of type
I procollagen in human fibroblasts. This result indicates that FBG
extracts have anti-wrinkle effects by increasing type I procollagen
level in human skin.
Type I collagen is the most abundant protein in skin
connective tissue. It maintains skin structure with other types of
collagen (III, V and VII), elastin, proteoglycans, fibronectin and
other ECM proteins (43). Type I
procollagen is synthesized in human dermal fibroblasts and secreted
into the dermal extracellular space where it undergoes proteolytic
processing. Finally, type I collagen forms collagen bundles (fibre
bundles) that are responsible for the elasticity associated with
other ECM proteins (5,13). Fibrillar (types I and III)
collagen can characteristically reduce chronologically aged and
photo-damaged skin (14,44,45). The degradation of type I collagen
is closely associated with MMPs, a family of zinc-requiring
endoprotease with the capacity to degrade all components of ECM
(46). In particular, MMP-1 of
the MMPs initiates the degradation of types I and III fibrillar
collagens, while MMP-9 further degrades collagen fragments
generated by collagenases (44).
MMP-2 and MMP-9 together can cleave elastin, type IV collagen, and
several other ECM molecules while MMP-2 can digest interstitial
collagen types I, II, and III (47). It has been previously suggested
that the function of MMP-1 is directly involved in the reduction of
type I procollagen. MMP-2 and MMP-9 also regulate the expression of
type I procollagen (47,48). All known MMPs are inhibited by 4
homologous TIMPs (49). TIMP-2 of
TIMPs inhibits ECM proteolysis in several tissues by directly
inhibiting metalloproteinases, including MMP-2 (50). TIMP-2 is also known to be required
for the activation of MMP-2 through association with MMP-14
(50,51). In this study, FBG extracts
significantly inhibited the expression of MMP-1 in human
fibroblasts. In addition, the expression levels of MMP-2 and MMP-9
were dose-dependently decreased by FBG. Moreover, the expresssion
of TIMP-2 exhibited a generally increased tendency by FBG
treatment. Collectively, these results suggest that the increase in
the level of type I procollagen caused by FBG may be induced by the
inhibition of MMP-1, MMP-2 and MMP-9. The inhibition of these MMPs
may correlated with the upregulation of TIMP-2 due to FBG
treatment.
Retinoic acid can effectively attenuate the clinical
symptoms of photodamaged skin. It can reverse the adverse
consequences of chronological aged skin (14,45,52). Treatment with retinoic acid can
stimulate fibroblast proliferation, new collagen synthesis and the
degradation of out-of-date or damaged collagen (53). The effects of retinoic acid are
mainly associated with the inhibition of MMPs (16,17). Additionally, retinoic acid results
in the selective downregulation of MMP-9 and the simultaneous
upregulation of TIMP-1 in human bronchoalveolar lavage cells
(54). It can also reduce the
expression of MMP-2 in human breast cancer cells, suggesting that
the inhibition of MMP-2 may be due to upregulated TIMP-2 (55). In this study, we demonstrated that
the treatment of human fibroblasts with retinoic acid exerted
similar effects with FBG treament as regards the expression
patterns of type I procollagen, MMP-1, MMP-2, MMP-9 and TIMP-2.
These results suggest that FBG and retinoic acid may share similar
mechanisms as regards their anti-wrikle effects on human skin.
In conclusion, our in vitro dermal study
demonstrated that FBG treatment increased type I procollagen
correlating with associated regulators. In addition, we assessed
the non-toxic concentration of FBG through cosmetic exposure routes
using human fibroblasts and ocular tissues. Conclusively, we
demonstrated that FBG may be used as a safe cosmetic ingredient
with anti-wrinkle effects.
Acknowledgments
This study was supported by the research fund of
Dankook University in 2014. We would like to thank Professor
Kyung-Min Lim (Ewha Womans University) for providing support to the
current study.
References
|
1
|
Tobin DJ: Biochemistry of human skin - our
brain on the outside. Chem Soc Rev. 35:52–67. 2006. View Article : Google Scholar
|
|
2
|
Farage MA, Miller KW, Elsner P and Maibach
HI: Structural characteristics of the aging skin: A review. Cutan
Ocul Toxicol. 26:343–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Breitkreutz D, Mirancea N and Nischt R:
Basement membranes in skin: Unique matrix structures with diverse
functions? Histochem Cell Biol. 132:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kruglikov IL and Scherer PE: Dermal
Adipocytes: From Irrelevance to Metabolic Targets? Trends
Endocrinol Metab. 27:1–10. 2016. View Article : Google Scholar :
|
|
5
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shiau CJ, Abi Daoud MS, Wong SM and
Crawford RI: Lymphocytic panniculitis: An algorithmic approach to
lymphocytes in subcutaneous tissue. J Clin Pathol. 68:954–962.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thangapazham RL, Darling TN and Meyerle J:
Alteration of skin properties with autologous dermal fibroblasts.
Int J Mol Sci. 15:8407–8427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tobin DJ: Introduction to skin aging. J
Tissue Viability. View Article : Google Scholar : 2016.Epub ahead of
print. PubMed/NCBI
|
|
9
|
Makrantonaki E and Zouboulis CC: Molecular
mechanisms of skin aging: State of the art. Ann N Y Acad Sci.
1119:40–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ha BG, Park MA, Lee CM and Kim YC:
Antioxidant activity and anti-wrinkle effects of Aceriphyllum
rossii leaf ethanol extract. Toxicol Res. 31:363–369. 2015.
View Article : Google Scholar
|
|
11
|
Lee KO, Kim SN and Kim YC: Anti-wrinkle
effects of water extracts of teas in hairless mouse. Toxicol Res.
30:283–289. 2014. View Article : Google Scholar
|
|
12
|
Braverman IM and Fonferko E: The elastic
fiber network: Studies in cutaneous aging: I. The elastic fiber
network. J Invest Dermatol. 78:434–443. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uitto J: Connective tissue biochemistry of
the aging dermis. Age-related alterations in collagen and elastin.
Dermatol Clin. 4:433–446. 1986.PubMed/NCBI
|
|
14
|
Varani J, Spearman D, Perone P, Fligiel
SE, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ and Voorhees JJ:
Inhibition of type I procollagen synthesis by damaged collagen in
photoaged skin and by collagenase-degraded collagen in vitro. Am J
Pathol. 158:931–942. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Philips N, Auler S, Hugo R and Gonzalez S:
Beneficial regulation of matrix metalloproteinases for skin health.
Enzyme Res. 2011:4272852011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fisher GJ, Datta SC, Talwar HS, Wang ZQ,
Varani J, Kang S and Voorhees JJ: Molecular basis of sun-induced
premature skin ageing and retinoid antagonism. Nature. 379:335–339.
1996. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fisher GJ, Wang ZQ, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–1428. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi J, Kim TH, Choi TY and Lee MS:
Ginseng for health care: A systematic review of randomized
controlled trials in Korean literature. PLoS One. 8:e599782013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Helms S: Cancer prevention and
therapeutics: Panax ginseng. Altern Med Rev. 9:259–274.
2004.PubMed/NCBI
|
|
20
|
Jang KJ, Choi SH, Yu GJ, Hong SH, Chung
YH, Kim CH, Yoon M, Kim GY, Kim BW and Choi YH: Anti-inflammatory
potential of total saponins derived from the roots of Panax ginseng
in lipopolysaccharide-activated RAW 264.7 macrophages. Exp Ther
Med. 11:1109–1115. 2016.PubMed/NCBI
|
|
21
|
Kim HJ, Kim P and Shin CY: A comprehensive
review of the therapeutic and pharmacological effects of ginseng
and ginsenosides in central nervous system. J Ginseng Res. 37:8–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kulaputana O, Thanakomsirichot S and
Anomasiri W: Ginseng supplementation does not change lactate
threshold and physical performances in physically active Thai men.
J Med Assoc Thai. 90:1172–1179. 2007.PubMed/NCBI
|
|
23
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung SH, Woo MS, Kim SY, Kim WK, Hyun JW,
Kim EJ, Kim DH and Kim HS: Ginseng saponin metabolite suppresses
phorbol ester-induced matrix metalloproteinase-9 expression through
inhibition of activator protein-1 and mitogen-activated protein
kinase signaling pathways in human astroglioma cells. Int J Cancer.
118:490–497. 2006. View Article : Google Scholar
|
|
25
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: Multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Christensen LP: Ginsenosides chemistry,
biosynthesis, analysis, and potential health effects. Adv Food Nutr
Res. 55:1–99. 2009. View Article : Google Scholar
|
|
27
|
Lee SM, Bae BS, Park HW, Ahn NG, Cho BG,
Cho YL and Kwak YS: Characterization of Korean Red Ginseng (Panax
ginseng Meyer): History, preparation method, and chemical
composition. J Ginseng Res. 39:384–391. 2015. View Article : Google Scholar
|
|
28
|
Lee MR, Yun BS, In OH and Sung CK:
Comparative study of korean white, red, and black ginseng extract
on cholinesterase inhibitory activity and cholinergic function. J
Ginseng Res. 35:421–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bak MJ, Jeong WS and Kim KB: Detoxifying
effect of fermented black ginseng on
H2O2-induced oxidative stress in HepG2 cells.
Int J Mol Med. 34:1516–1522. 2014.PubMed/NCBI
|
|
30
|
Kang KS, Yamabe N, Kim HY, Okamoto T, Sei
Y and Yokozawa T: Increase in the free radical scavenging
activities of American ginseng by heat processing and its safety
evaluation. J Ethnopharmacol. 113:225–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hasegawa H, Sung JH and Huh JH: Ginseng
intestinal bacterial metabolite IH901 as a new anti-metastatic
agent. Arch Pharm Res. 20:539–544. 1997. View Article : Google Scholar
|
|
32
|
Yun TK: Experimental and epidemiological
evidence on non-organ specific cancer preventive effect of Korean
ginseng and identification of active compounds. Mutat Res.
523–524:63–74. 2003. View Article : Google Scholar
|
|
33
|
Gillis CN: Panax ginseng pharmacology: A
nitric oxide link? Biochem Pharmacol. 54:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hasegawa H, Sung JH, Matsumiya S and
Uchiyama M: Main ginseng saponin metabolites formed by intestinal
bacteria. Planta Med. 62:453–457. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee PS, Han JY, Song TW, Sung JH, Kwon OS,
Song S and Chung YB: Physicochemical characteristics and
bioavailability of a novel intestinal metabolite of ginseng saponin
(IH901) complexed with beta-cyclodextrin. Int J Pharm. 316:29–36.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim S, Kang BY, Cho SY, Sung DS, Chang HK,
Yeom MH, Kim DH, Sim YC and Lee YS: Compound K induces expression
of hyaluronan synthase 2 gene in transformed human keratinocytes
and increases hyaluronan in hairless mouse skin. Biochem Biophys
Res Commun. 316:348–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho S, Won CH, Lee DH, Lee MJ, Lee S, So
SH, Lee SK, Koo BS, Kim NM and Chung JH: Red ginseng root extract
mixed with Torilus fructus and Corni fructus improves facial
wrinkles and increases type I procollagen synthesis in human skin:
A randomized, double-blind, placebo-controlled study. J Med Food.
12:1252–1259. 2009. View Article : Google Scholar
|
|
38
|
Lee HS, Kim MR, Park Y, Park HJ, Chang UJ,
Kim SY and Suh HJ: Fermenting red ginseng enhances its safety and
efficacy as a novel skin care anti-aging ingredient: In vitro and
animal study. J Med Food. 15:1015–1023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee SA, Jo HK, Im BO, Kim S, Whang WK and
Ko SK: Changes in the Contents of Prosapogenin in the Red Ginseng
(Panax ginseng) Depending on Steaming Batches. J Ginseng Res.
36:102–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee MR, Kim BC, Kim R, Oh HI, Kim HK, Choi
KJ and Sung CK: Anti-obesity effects of black ginseng extract in
high fat diet-fed mice. J Ginseng Res. 37:308–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park HJ, Shim HS, Kim KS and Shim I: The
protective effect of black ginseng against transient focal
ischemia-induced neuronal damage in rats. Korean J Physiol
Pharmacol. 15:333–338. 2011. View Article : Google Scholar
|
|
42
|
Sun BS, Gu LJ, Fang ZM, Wang CY, Wang Z,
Lee MR, Li Z, Li JJ and Sung CK: Simultaneous quantification of 19
ginsenosides in black ginseng developed from Panax ginseng by
HPLC-ELSD. J Pharm Biomed Anal. 50:15–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ivarsson M, McWhirter A, Borg TK and Rubin
K: Type I collagen synthesis in cultured human fibroblasts:
Regulation by cell spreading, platelet-derived growth factor and
interactions with collagen fibers. Matrix Biol. 16:409–425. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fligiel SE, Varani J, Datta SC, Kang S,
Fisher GJ and Voorhees JJ: Collagen degradation in
aged/photodamaged skin in vivo and after exposure to matrix
metalloproteinase-1 in vitro. J Invest Dermatol. 120:842–848. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Varani J, Dame MK, Rittie L, Fligiel SE,
Kang S, Fisher GJ and Voorhees JJ: Decreased collagen production in
chronologically aged skin: Roles of age-dependent alteration in
fibroblast function and defective mechanical stimulation. Am J
Pathol. 168:1861–1868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Inomata S, Matsunaga Y, Amano S, Takada K,
Kobayashi K, Tsunenaga M, Nishiyama T, Kohno Y and Fukuda M:
Possible involvement of gelatinases in basement membrane damage and
wrinkle formation in chronically ultraviolet B-exposed hairless
mouse. J Invest Dermatol. 120:128–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chakrabarti S and Patel KD: Matrix
metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp
Lung Res. 31:599–621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Arpino V, Brock M and Gill SE: The role of
TIMPs in regulation of extracellular matrix proteolysis. Matrix
Biol. 44–46:247–254. 2015. View Article : Google Scholar
|
|
50
|
Kandalam V, Basu R, Abraham T, Wang X,
Soloway PD, Jaworski DM, Oudit GY and Kassiri Z: TIMP2 deficiency
accelerates adverse post-myocardial infarction remodeling because
of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ
Res. 106:796–808. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: A
mini-review. Med Sci Monit. 15:RA32–RA40. 2009.PubMed/NCBI
|
|
52
|
Varani J, Warner RL, Gharaee-Kermani M,
Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ and
Voorhees JJ: Vitamin A antagonizes decreased cell growth and
elevated collagen-degrading matrix metalloproteinases and
stimulates collagen accumulation in naturally aged human skin. J
Invest Dermatol. 114:48–486. 2000. View Article : Google Scholar
|
|
53
|
Lateef H, Stevens MJ and Varani J:
All-trans-retinoic acid suppresses matrix metalloproteinase
activity and increases collagen synthesis in diabetic human skin in
organ culture. Am J Pathol. 165:167–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Frankenberger M, Hauck RW, Frankenberger
B, Häussinger K, Maier KL, Heyder J and Ziegler-Heitbrock HW: All
trans-retinoic acid selectively down-regulates matrix
metalloproteinase-9 (MMP-9) and up-regulates tissue inhibitor of
metalloproteinase-1 (TIMP-1) in human bronchoalveolar lavage cells.
Mol Med. 7:263–270. 2001.PubMed/NCBI
|
|
55
|
Dutta A, Sen T, Banerji A, Das S and
Chatterjee A: Studies on Multifunctional Effect of All-Trans
Retinoic Acid (ATRA) on Matrix Metalloproteinase-2 (MMP-2) and Its
Regulatory Molecules in Human Breast Cancer Cells (MCF-7). J Oncol.
2009:6278402009. View Article : Google Scholar : PubMed/NCBI
|