Introduction
Osteosarcoma, one of the malignant bone tumors,
predominantly occurs in children and adolescents (1–3).
Currently, the treatment for osteosarcoma mainly includes surgery
and combination chemotherapy (4,5).
Multidrug resistance (MDR) is a formidable obstacle of chemotherapy
in the treatment of osteosarcoma (6–8).
Tumor cells can develop resistance to a wide variety of anticancer
drugs, whose structure and function are usually unrelated, thus
limiting the curative effects of chemotherapeutic drugs (9,10).
Therefore, the understanding of the pathological mechanisms of MDR
is of great importance for developing effective therapies for
osteosarcoma.
It has been reported that there are several
mechanisms responsible for osteosarcoma becoming resistant to
chemotherapeutic agents (6). One
mechanism is the overexpression of ATP-binding cassette (ABC)
transporters in cells which develop MDR, which causes reduced drug
uptake and enhanced drug efflux (11–13). P-glycoprotein (Pgp), one of the
most important ABC transporters, is encoded by MDR gene 1 (MDR1)
(14). Pgp overexpression has
been detected in multiple osteosarcoma cell lines with MDR and
residual tumor cells in post-chemotherapy patients (14–17). Numerous drugs in osteosarcoma
chemotherapy are substrates of Pgp, including doxorubicin,
paclitaxel, vinblastine, vincristine and etoposide (18).
Pgp-mediated resistance to drugs can be reversed by
inhibiting MDR1 drug pump function or by preventing MDR1 expression
(11). A number of studies have
been carried out on Pgp-mediated MDR. Various Pgp inhibitors have
been developed, such as verapamil, PSC833, VX-710 and XR9576
(19), and curcumin has also been
shown to inhibit Pgp (20). Over
the past decade, preventing the initiation of MDR following
chemotherapy has gained much attention (21–24). A variety of drugs, including
verapamil, CsA, P85 and LGD1069 have been found to be preventers,
averting the emergence of MDR (21,22,24,25). Although clinical trials examining
these preventers have been initiated, notable therapeutic results
have not been acquired in these trials (26–29). Thus, exploring more potent and
selective MDR preventers is of utmost importance.
Tetrandrine (TET), a bis-benzylisoquinoline alkaloid
compound, was isolated from the root of Stephania tetrandra.
TET is utilized as an anti-rheumatic, anti-inflammatory and
anti-hypertensive agent with low toxicity in traditional Chinese
medicine (30). Moreover, TET
exhibits antitumor activity in both tumor cells and animal models
(31–34). It has been reported that TET
treatment can cause the notable downregulation of Pgp expression,
which significantly reverses drug resistance in leukemia cells
(35). Furthermore, TET has the
ability to stimulate Pgp ATPase activity, thereby reversing
Pgp-mediated MDR in cancer cells (36). Additionally, studies have
demonstrated that TET can inhibit the development of MDR by
preventing Pgp overexpression in the leukemia cell line, K562
(37). However, whether TET has
the ability to prevent the enmergence of MDR in osteosarcoma has
yet to be determined.
In the present study, we evaluated the effects of
TET on the prevention of MDR in the osteosarcoma cell line, U-2OS,
and investigated the underlying mechanisms.
Materials and methods
Drugs and cell line
TET was supplied from Sigma-Aldrich (St. Louis, MO,
USA). Paclitaxel, doxorubicin, vincristine, gemcitabine and
methotrexate were supplied from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Docetaxel and cisplatin were supplied from
Sigma-Aldrich. PM-00104 was purchased by PharmaMar (Madrid, Spain).
The human osteosarcoma cell line, U-2OS, was supplied from the
American Type Culture Collection (ATCC, Manassas, VA, USA),
cultured in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS) and 1% penicillin/streptomycin (both from Invitrogen Life
Technologies, Carlsbad, CA, USA) in a humidified atmosphere of 5%
CO2 at 37°C.
Development of resistant osteosarcoma
cell line
In our study, the establi shment of a resistant
osteosarcoma cell line followed similar previously described
protocols (21,23,38). A paclitaxel-resistant cell line
was established from the parental cell line, U-2OS. The culture
medium was supplemented with 0.0001 µM paclitaxel alone, 1
µM TET alone or a combination of 1 µM TET and 0.0001
µM paclitaxel. When the cells reached 90% confluence, they
were harvested and then reseeded, and cultured in medium with an
increased paclitaxel concentration. The cells were then treated
with increasing concentrations of paclitaxel over a period of 6
months. Cell sublines at different selection points were stored in
liquid nitrogen for further analyses.
Cell groups
The groups were divided into the control cells
(U-2OS/control), cells treated with TET alone (U-2OS/tetrandrine),
6 resistant U-2OS cell lines that developed resistance with final
paclitaxel concentrations of 0.003, 0.006, 0.03, 0.06, 0.1, 0.2
µM paclitaxel, respectively (paclitaxel0.003,
paclitaxel0.006, paclitaxel0.03,
paclitaxel0.06, paclitaxel0.1 and
paclitaxel0.2) and 2 non-resistant U-2OS cell lines that
were exposed to a combination of 0.003 or 0.006 µM
paclitaxel with 1 µM TET
(paclitaxel0.003/tetrandrine and
paclitaxel0.006/tetrandrine).
Cytotoxicity assay
The cytotoxicity of chemotherapeutic agents in the
different cell sublines was evaluated by MTT assay. In brief, the
cells were seeded in 96-well plates and treated with various
concentrations (0.0001, 0.001, 0.01, 0.1, 1, 10 and 100 µM)
of chemotherapeutic agents for 5 days, followed by the addition of
20 µl of MTT (Sigma-Aldrich) to each well for 4 h. The
crystals were dissolved in 100 µl DMSO. The absorbance at
490 nm was measured using a Bio-Rad microplate reader (Bio-Rad,
Hercules, CA, USA). Experiments were performed in triplicate. The
IC50 value was analyzed using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
The mRNA expression level of MDR1 was determined by
RT-qPCR. TRIzol reagent (Invitrogen) was used to extract total RNA
according to the manufacturer's instructions. The primeScript RT
reagent kit (Takara, Dalian, China) was used to reverse transcribe
the RNA into cDNA, then stored at −20°C. cDNA was amplified using a
SYBR Premix Ex Taq kit (Takara) and Mx3000P instrument (Agilent
Technologies, Inc., Santa Clara, CA, USA). PCR programs were
carried out as follows: 95°C for 5 min, followed by 30 cycles of
95°C for 30 sec, 56°C for 30 sec, 72°C for 30 sec, and a final
extension for 5 min at 72°C. PCR products were analyzed according
to the 2−ΔΔCt method with β-actin as the standard
gene.
Western blot analysis
The protein expression levels were determined by
western blot analysis. Each group of cells was collected and washed
with phosphate-buffered saline (PBS). The total protein extraction
kit (KeyGen Biotech Co., Ltd., Nanjing, China) was used to extract
the total protein according to the manufacturer's instructions. The
BCA protein assay kit (KeyGen Biotech Co., Ltd.) was used to
determine the protein concentrations. Briefly, an aliquot of total
protein was run on SDS-PAGE and transferred onto nitrocellulose
membranes (Millipore, Billerica, MA, USA), which were blocked with
5% BSA-PBS at room temperature for 1 h, and subsequently incubated
overnight with primary antibodies against Pgp (Cat. no. HPA002199,
Sigma-Aldrich), p-IκB-α (Cat. no. 2859S, Cell Signaling Technology,
Danvers, MA, USA) or GAPDH (Cat. no. sc-20357, Santa Cruz
Biotechnology) at 4°C, respectively. To determine the NF-κB
expression level, total nuclear protein was prepared using a
commercial kit (KeyGen Biotech Co., Ltd.) according to the
manufacturer's instructions. Following incubation with primary
antibodies, the membranes were rinsed 3 times with PBS and
anti-rabbit IgG (Cat. no. 4414, Cell Signaling Technology) and
anti-mouse IgG (Cat. no. 4408, Cell Signaling Technology) were then
added at a dilution of 1:10,000 followed by incubation at room
temperature for 1 h. Finally, the membranes were detected and
quantified using the LI-COR Odyssey infrared imaging system and
software (LI-COR Biosciences, Lincoln, NE, USA).
Rh123 accumulation assay
The different group cells were incubated at a
concentration of 0.5 mg/ml fluorescent dye, Rh123 (Sigma-Aldrich)
for 2 h. The cells were harvested and washed twice with PBS, then
suspended and kept in the dark. The intracellular Rh123 was
determined using a flow cytometer (Becton Dickinson, San Diego, CA,
USA). The data were analyzed using FlowJo 7.6.2 software (Tree Star
Inc., Ashland, OR, USA).
Dual-luciferase reporter assay
The promoter activity was determined by a
dual-luciferase reporter assay. Plasmid preparation was performed
as previously described (39).
The cells were plated in 24-well plates overnight. Using
Lipofectamine 2000 according to the instructions provided by the
manufacturer (Invitrogen), the cells were co-transfected
transiently with an hMDR1-Luc or NF-κB-Luc construct and pRL-SV
plasmid (Renilla luciferase expression for normalization)
(Promega, Madison, WI, USA). Luciferase activity in the cell
lysates was measured using a dual-luciferase reporter assay kit
(Promega).
NF-κB DNA-binding activity assay
The DNA-binding activity was determined by
electrophoretic mobility shift assay (EMSA), as previously
described (37). The
concentration of the nuclear protein of cells from different cell
sublines was quantified by BCA assay. Equal amounts of nuclear
protein (2 µl) were mixed with P-labeled NF-κB binding probe
(1 µl), nuclease-free water (5 µl) and EMSA/gel-shift
binding buffer (5X; 2 µl). The mixture was incubated at room
temperature for 20 min. The samples were separated by
non-denaturing PAGE. The gels were dried and kept in an exposure
cassette for 72 h at −70°C for autoradiography.
The binding ability of NF-κB to the MDR1
gene promoter
The binding ability was determined by chromatin
immunoprecipitation (ChIP) as described in a previous study
(37). A commercially available
ChIP assay kit (Upstate Biotechnology, Inc., Lake Placid, NY, USA)
was used according to the instructions provided by the
manufacturer. Briefly, 7×107 cells in 4 different groups
(U-2OS/control, U-2OS/tetrandrine, paclitaxel0.2 and
paclitaxel0.006/tetrandrine) were used. The chromatin
fraction was immunoprecipitated with an anti-NF-κB p65 antibody
(Cat. no. 06-418, Upstate Biotechnology, Inc.) overnight at 4°C and
finally examined by PCR.
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism 5 software (GraphPad Software, Inc.). The data in our study
are presented as the means ± SD. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Tetrandrine prevents the emergence of
paclitaxel resistance in the osteosarcoma cell line, U-2OS
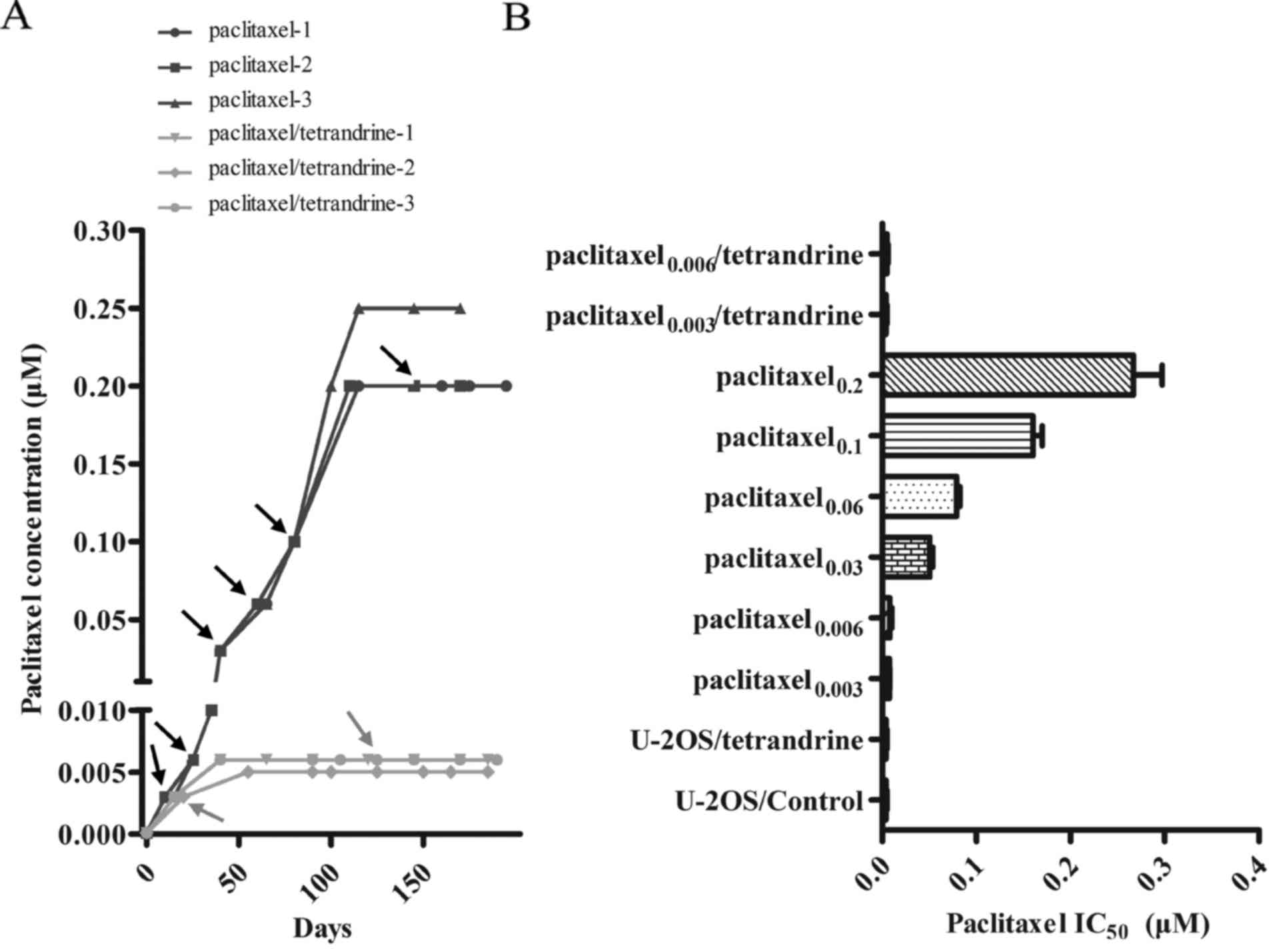
The U-2OS cells were treated with increasing
concentrations of paclitaxel alone or a combination of paclitaxel
with 1 µM TET. After 6 months of drug treatment, the cells
treated with paclitaxel alone exhibited stable growth in the
culture medium with 0.2 µM paclitaxel. By contrast, the
cells treated with the paclitaxel-TET combination were not able to
grow when treated with paclitaxel at concentrations >0.006
µM in the culture medium (Fig.
1A). The IC50 value of paclitaxel was then evaluated
to further confirm the effects of TET on paclitaxel resistance. As
shown in Fig. 1B, the
IC50 value of paclitaxel in the cells treated with
paclitaxel alone increased as the concentration of paclitaxel
increased. In addition, the IC50 value of the U-2OS
cells treated with ≥0.03 µM paclitaxel alone was
significantly increased compared with the control cells. An
enhancement of 64-fold in the IC50 value of paclitaxel
was observed in the cells treated with 0.2 µM paclitaxel
alone (paclitaxel0.2) compared with the control cells.
However, the cells treated with the 0.006 µM paclitaxel-TET
combination (paclitaxel0.006/TET) exhibited no obvious
increase in the IC50 value of paclitaxel compared with
the control cells. Notably, the IC50 value of paclitaxel
in the paclitaxel0.2 cells was 53.3-fold higher than
that of the paclitaxel0.006/TET cells, demonstrating
that TET inhibited the initiation of paclitaxel resistance in the
osteosarcoma cell line, U-2OS . There was no change in the
IC50 value of paclitaxel in the cells treated with 1
µM TET alone (Fig.
1B).
Tetrandrine inhibits the development of
MDR in cells treated with paclitaxel and different chemotherapeutic
agents
As shown above, TET inhibited the development of
paclitaxel resistance. Thus, we further investigated whether TET
inhibits resistance to other chemotherapeutic drugs. Compared with
the control cells, the IC50 value of doxorubicin,
docetaxel and vincristine increased 30-, 16.7- and 8-fold in the
paclitaxel0.2 cells, respectively. By contrast, thne
paclitaxel0.006/TET cells remained sensitive to these 3
agents and no significant differences in the IC50 values
were observed (Fig. 2B, C and D).
Apart from doxorubicin, docetaxel and vincristine, the
IC50 values of other Pgp substrate drugs, such as
PM-00104 and gemcitabine, were also significantly increased in the
paclitaxel0.2 cells. As expected, the
paclitaxel0.006/TET cells remained sensitive to PM-00104
and gemcitabine (Fig. 2E and F).
Thus, our data indicate that the cells treated with paclitaxel
alone naturally developed MDR, whereas the cells treated with the
paclitaxel-TET combination did not acquire MDR. Taken together,
these results demonstrate that TET was able to inhibit the
initiation of MDR in U-2OS cells during continued paclitaxel
treatment. Furthermore, the IC50 values of cisplatin and
methotrexate (Fig. 2G and H),
which are not Pgp substrates, exhibited no significant differences
between the paclitaxel0.006/TET and
paclitaxel0.2 cells, suggesting that TET may
specifically suppress the development of Pgp-mediated MDR during
paclitaxel treatment.
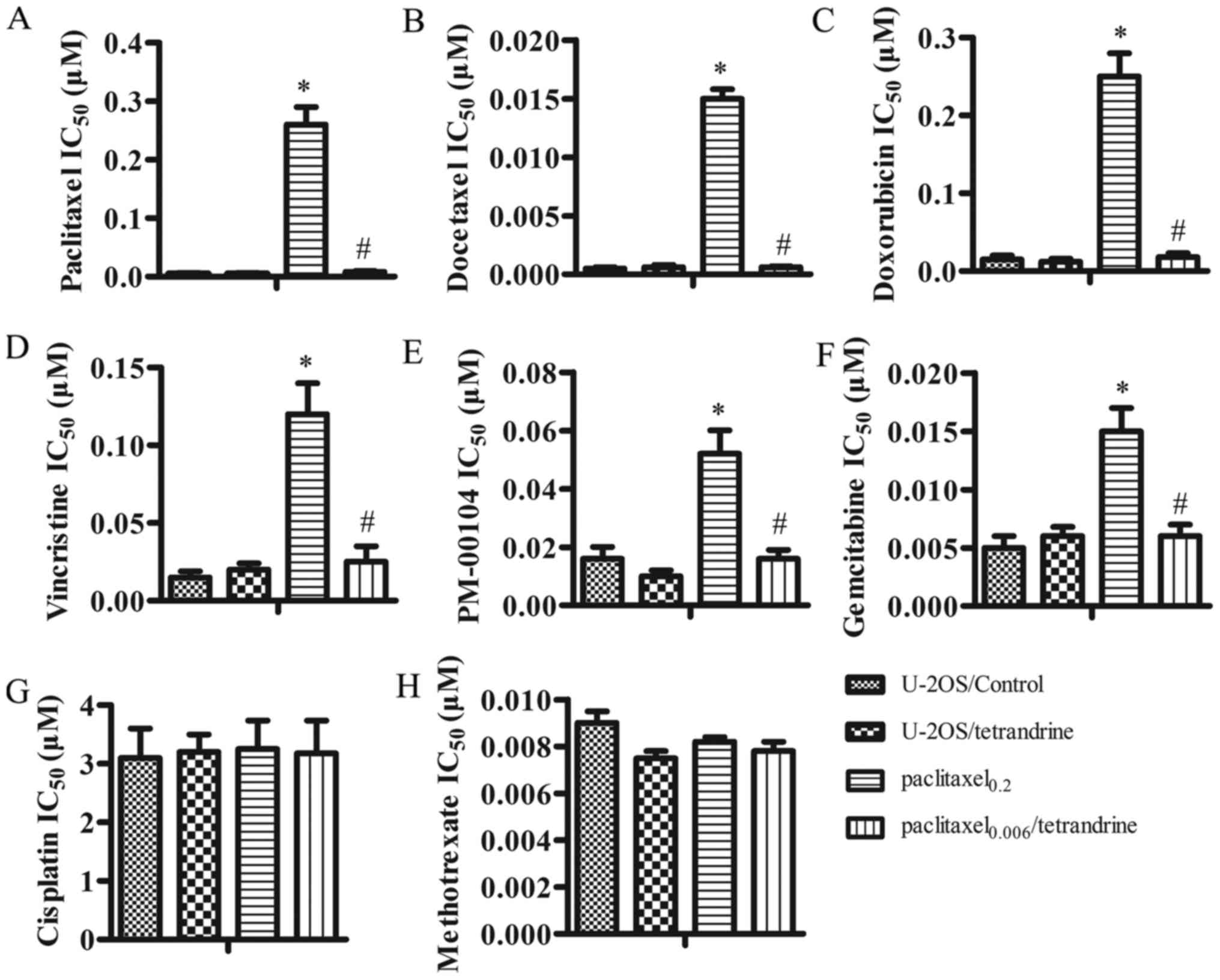 | Figure 2Tetrandrine (TET) inhibits the
introduction of multidrug resistance (MDR) during continued
paclitaxel treatment. MTT assay was performed in the U-2OS/control,
U-2OS/TET, paclitaxel0.2 and
paclitaxel0.006/TET cell sublines treated with different
chemotherapeutic agents, including (A) paclitaxel, (B) docetaxel,
(C) doxorubicin, (D) vincristine, (E) PM-00104, (F) gemcitabine,
(G) cisplatin and (H) methotrexate. Data are presented as the means
± SD. *P<0.05 vs. U-2OS/control group, #P<0.05 vs.
paclitaxel0.2 group. |
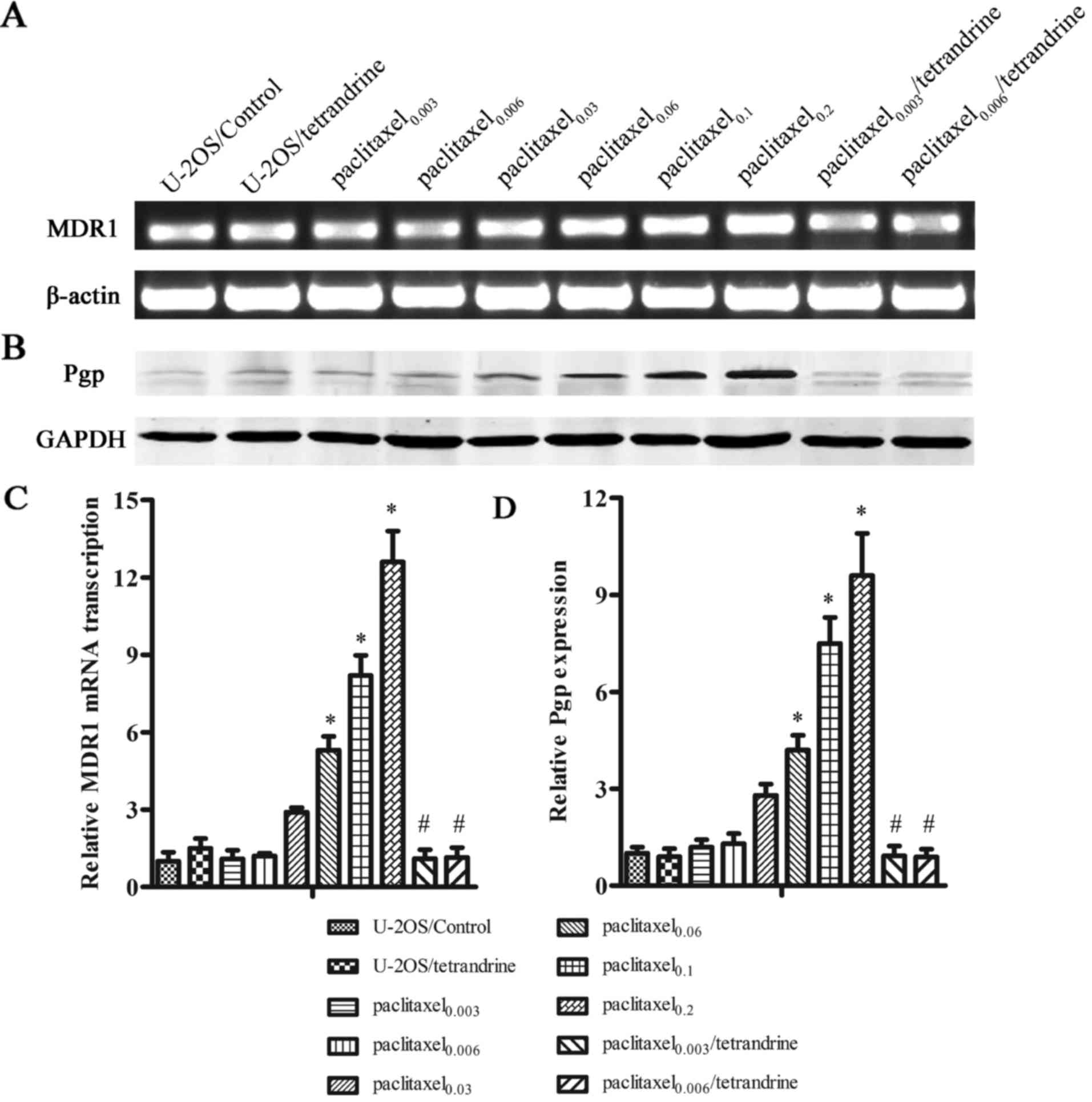
Tetrandrine prevents the development of
MDR by inhibiting MDR1 and Pgp
The expression levels of MDR1 and Pgp were examined
to investigate the underlying mechanisms responsible for the
inhibitory effects of TET on the development of MDR. The results of
RT-qPCR revealed that as paclitaxel treatment continued, an obvious
stepwise increase in MDR1 expression was observed in the cells
treated with paclitaxel alone. However, there was a marked
reduction in MDR1 expression in the paclitaxel0.006/TET
cells compared to the paclitaxel0.2 cells, which
indicated that TET prevented MDR1 overexpression during paclitaxel
treatment (Fig. 3A and C). The
results of western blot analysis revealed that the Pgp expression
levels in the cells treated with ≥0.03 µM paclitaxel alone
were significantly increased compared with those of the control
cells. Additionally, the increased Pgp expression levels exhibited
a strong correlation with the increased paclitaxel concentration.
However, Pgp overexpression was not detected in the cells treated
with the paclitaxel-TET combination (Fig. 3B and D), which suggested that TET
prevented the initiation of MDR in osteosarcoma by suppressing Pgp
overexpression. These results indicated that culture of the cells
with paclitaxel alone induced MDR1 and Pgp overexpression, which
were responsible for the development of MDR. No significant
differences in MDR1 and Pgp expression levels were detected in the
cells cultured with the paclitaxel-TET combination compared to the
control group. Taken together, our data indicated that TET
prevented the emergence of MDR in the U-2OS cells by preventing the
overexpression of MDR1 and Pgp during paclitaxel treatment.
Treatment of the cells with 1 µM TET alone had no effect on
the expression of MDR1 and Pgp.
TET decreases Pgp activity, characterized
by maintaining the intracellular retention of Rh123
The intracellular accumulation level of Rh123 was
examined by flow cytometry to identify the functional activity of
Pgp. Rh123 is a substrate of Pgp with yellow-green fluorophores.
The lower retention of fluorescence intensity inside cells
indicates a higher activity of the Pgp pump (40). As shown in Fig. 4, a considerable difference was
observed between the cells cultured with the paclitaxel-TET
combination and those cultured with paclitaxel alone. The
fluorescence intensity of Rh123 in the paclitaxel0.2
group was lower compared with that in the control cells and
increased significantly in the paclitaxel0.006/TET cells
as compared with the paclitaxel0.2 cells. In addition,
treatment with TET alone had no obvious effect on Pgp activity. The
accumulation activity of Pgp was related to the expression level of
Pgp, strongly indicating that TET maintained the intracellular
retention of a Pgp substrate by suppressing the overexpression of
Pgp.
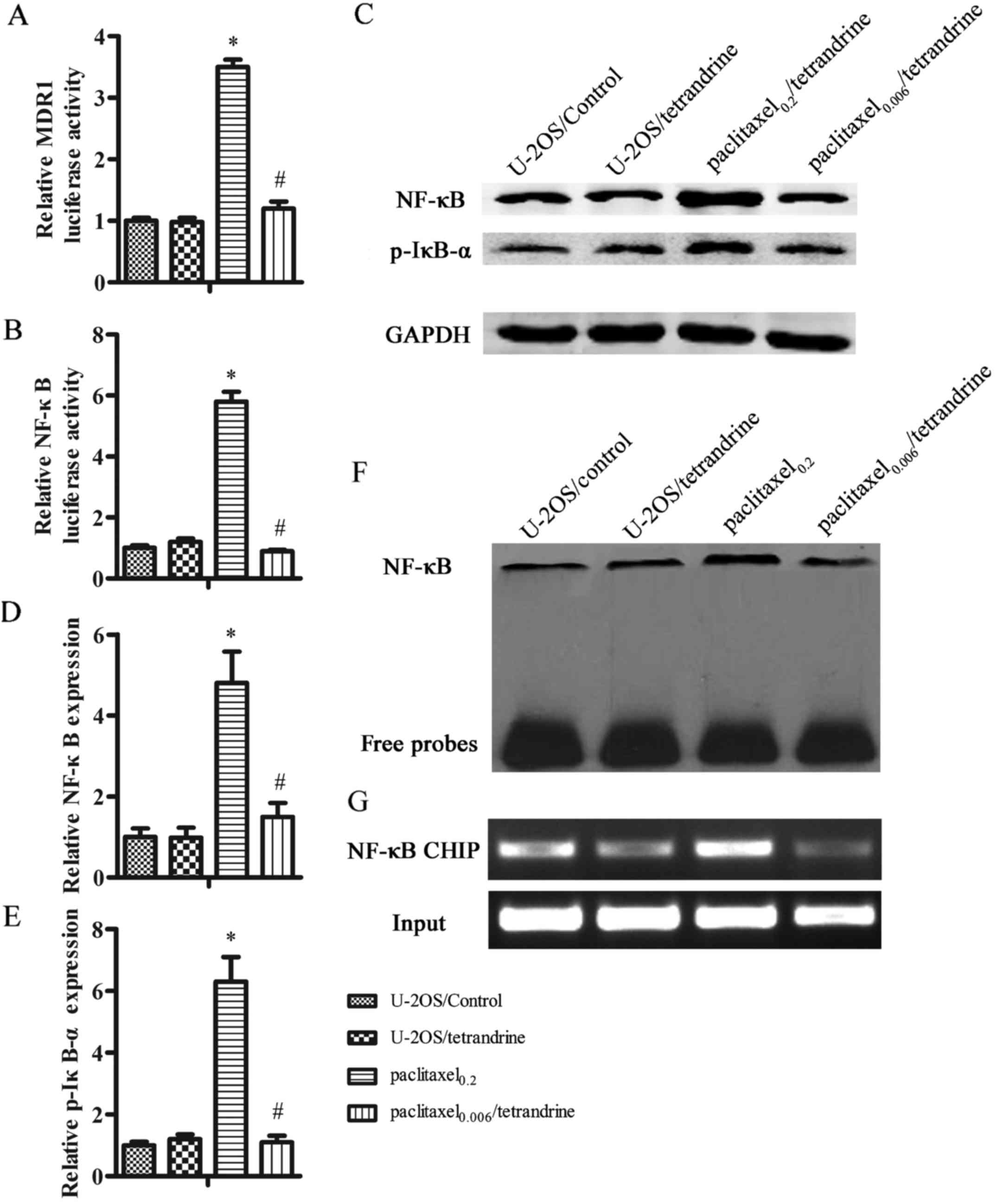
TET inhibits the overexpression of Pgp by
inhibiting the NF-κB signaling pathway
A recent study found a mutation of a NF-κB binding
site located to the MDR1 promoter (41). Furthermore, the overexpression of
Pgp is regulated by the NF-κB signaling pathway, and thus requires
a NF-κB binding site in the MDR1 promoter (42). To better understand the underlying
mechanisms responsible for the inhibitory effect of TET on MDR, the
promoter activities of MDR1 and NF-κB were detected by
dual-luciferase reporter assay. Both MDR1 and NF-κB transcriptional
activities were significantly increased in the
paclitaxel0.2 cells compared with the control cells.
However, in the cells treated with paclitaxel0.006/TET,
these transcriptional activities were significantly inhibited and
were similar to those of the control cells (Fig. 5A and B), implying that the
inhibition of MDR1 activity, at least in part, may be associated
with the downregulation of NF-κB activity. As a transcription
factor, NF-κB translocates to the nucleus to exhibit
transcriptional activity. Therefore, we detected the expression of
NF-κB in the nucleus by western blot analysis (Fig. 5C and D). Additionally, the
phosphorylation of IκB-α is required for the activation of NF-κB.
Thus, we also assessed the p-IκB-α protein levels (Fig. 5C and E). The level of nucleic
NF-κB was markedly upregulated in the paclitaxel0.2
cells compared to the control cells. In the
paclitaxel0.006/TET cells, however, NF-κB protein
expression was decreased compared with the paclitaxel0.2
cells. Similar to NF-κB, the expression of p-IκB-α in the
paclitaxel0.2 cells was 6.3-fold higher compared to that
of the control cells; however, there was no difference between
thepaclitaxel0.006/TET and the control cells.
To further investigate the inhibition of NF-κB
transcriptional activity by TET, NF-κB DNA-binding activity was
assessed by EMSA. This assay demonstrated that the NF-κB
DNA-binding activity in the paclitaxel0.2 cells was
notably enhanced as compared with that of the control cells, but
was reduced in the paclitaxel0.006/TET cells, suggesting
that TET inhibited NF-κB DNA-binding activity, which may prevent
Pgp overexpression in U-2OS cells (Fig. 5F). To verify that the inhibitory
effect of TET on Pgp is regulated by NF-κB signaling, a ChIP assay
was performed. As shown in Fig.
5G, the amplified PCR product was evident, implying that NF-κB
was bound to the MDR1 promoter. In the paclitaxel0.2
cells, the PCR product was markedly increased, which demonstrated
that the ability of NF-κB binding to the MDR1 promoter was enhanced
by paclitaxel treatment. By contrast, the PCR product was markedly
decreased in the paclitaxel0.006/TET cells, which
indicated that TET attenuated the ability of NF-κB binding to the
MDR1 promoter. On the whole, these data strongly indicated that TET
inhibited the overexpression of Pgp by inhibiting NF-κB
signaling.
Discussion
Overexpression of Pgp exhibits an important function
on the development of MDR (11,12,23) and correlates well with an overall
poor chemotherapy response and prognosis (43). Pgp acts as an energy-dependent
membrane transporter, rapidly pumping out functionally and
structurally unrelated chemotherapeutic drugs from cells.
Inhibiting the initiation of MDR at the onset of chemotherapy may
fundamentally assist in overcoming drug resistance. TET is an
alkaloid isolated from the tuberous root of Stephania
tetrandra. A previous study revealed that TET significantly
reversed MDR in different cancer cell lines by promoting Pgp ATPase
activity and suppressing Pgp function (44). Moreover, TET has been shown to
prevent the leukemia cell line, K562, from developing MDR through
the prevention of MDR1 transcription (37). In the present study, we
established an MDR osteosarcoma cell model and demonstrated that
the initiation of MDR was prevented by TET by suppressing Pgp
overexpression in human osteosarcoma cells.
To establish an MDR cell line in vitro, the
cells were treated with stepwise increased concentrations of
paclitaxel in culture medium, which was considered the classic
in vitro serial selection approach (21,23,38). We successfully established the
osteosarcoma MDR cell line from the drug sensitive cell line,
U-2OS, using a similar procedure. The results revealed that the
cells treated with paclitaxel alone acquired MDR with resistance to
paclitaxel and other Pgp substrates, such as doxorubicin, docetaxel
and vincristine. However, the cells treated with the paclitaxel-TET
combination remained sensitive to chemotherapeutic drugs.
Furthermore, the cells treated with paclitaxel alone or with the
paclitaxel-TET combination did not develop drug resistance to the
non-Pgp substrates cisplatin and methotrexate. These results
suggest that TET may inhibit the development of MDR in osteosarcoma
by inhibiting Pgp overexpression.
It has been reported that MDR can be mediated by Pgp
in osteosarcoma (45). We
observed that the long-term treatment of osteosarcoma cells with
paclitaxel induced Pgp overexpression. The overexpression of Pgp
leads to the decreased intracellular accumulation of
chemotherapeutic agents, thus preventing the drugs from exerting
their cytotoxic effects (11,12,46). Our data demonstrated that the
cells treated with paclitaxel alone exhibited reduced drug
intracellular accumulation of the Pgp substrate, Rh123. In
comparison, the cells treated with the paclitaxel-TET combination
displayed no obvious difference with the sensitive control cells,
indicating that TET allowed the retention of chemotherapeutic drugs
during paclitaxel treatment. Consequently, TET enabled the
osteosarcoma cells to maintain sensitivity to chemotherapeutic
drugs and inhibited the introduction of MDR by preventing Pgp
overexpression.
NF-κB is an important transcription factor in
carcinoma. NF-κB is usually located in the cytoplasm of quiescent
cells. In response to stimuli, NF-κB isolates from its inhibitory
partner IκB, then translocates to the nucleus to regulate
downstream genes transcription by binding to κB-binding sites. It
has been reported that TET can inhibit NF-κB activation in various
cells, such as pancreatic cells, peripheral blood T cells and brain
cells (47,48). A previous study revealed that the
decreased NF-κB expression resulted in the downregulation of MDR1
and Pgp, which suggested that NF-κB is involved in MDR regulation
(49). Our results demonstrated
that the cells cultured with paclitaxel alone exhibited
significantly elevated promoter activities of MDR1 and NF-κB, which
were significantly inhibited following paclitaxel-TET combination
treatment, suggesting that TET inhibited the promoter activity of
MDR1, possibly by downregulating NF-κB activity, at least in part.
Subsequently, we demonstrated that the expression levels of p-IκB-α
and nuclear NF-κB were both decreased in the cells cultured with
the paclitaxel-TET combination. Moreover, the NF-κB DNA-binding
activity and the ability of NF-κB to bind to the MDR1 promoter were
attenuated as compared with the cells cultured with paclitaxel
alone, which suggested that TET inhibited NF-κB activation and
subsequently regulated MDR1 gene expression. Collectively, TET
inhibited the overexpression of Pgp by inhibiting NF-κB
signaling.
In conclusion, our findings indicated that TET
prevented the introduction of paclitaxel-induced MDR in
osteosarcoma cells by inhibiting Pgp overexpression through a
mechanism involving the inhibition of NF-κB signaling. Given its
preventive effect on MDR, TET holds promise to extend the long-term
efficacy of chemotherapy in patients with osteosarcoma.
Acknowledgments
The authors would sincerely like to thank the
members of the Department of Orthopaedic Traumatology, Tianjin
Hospital and the Department of Orthopaedics, Jixian People's
Hospital for their valuable input/suggestions concerning the
present manuscript.
References
|
1
|
Lin YT, Huang AC, Kuo CL, Yang JS, Lan YH,
Yu CC, Huang WW and Chung JG: Induction of cell cycle arrest and
apoptosis in human osteosarcoma U-2 OS cells by Solanum lyratum
extracts. Nutr Cancer. 65:469–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang CZ, Zhang X, Li H, Tao YQ, Tao LJ,
Yang ZR, Zhou XP, Shi ZL and Tao HM: Gallic acid induces the
apoptosis of human osteosarcoma cells in vitro and in vivo via the
regulation of mitogen-activated protein kinase pathways. Cancer
Biother Radiopharm. 27:701–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho HJ, Lee TS, Park JB, Park KK, Choe JY,
Sin DI, Park YY, Moon YS, Lee KG, Yeo JH, et al: Disulfiram
suppresses invasive ability of osteosarcoma cells via the
inhibition of MMP-2 and MMP-9 expression. J Biochem Mol Biol.
40:1069–1076. 2007.PubMed/NCBI
|
|
4
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou AJ, Geller DS and Gorlick R: Therapy
for osteosarcoma: where do we go from here? Paediatr Drugs.
10:315–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dieudonné FX, Marion A, Haÿ E, Marie PJ
and Modrowski D: High Wnt signaling represses the proapoptotic
proteoglycan syndecan-2 in osteosarcoma cells. Cancer Res.
70:5399–5408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang ZY, Mei J, Gao YS, Ni M and Yao B:
Primary tumorectomy promotes angiogenesis and pulmonary metastasis
in osteosarcoma-bearing nude mice. Acta Cir Bras. 28:190–194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agarwal R and Kaye SB: Ovarian cancer:
strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Glavinas H, Krajcsi P, Cserepes J and
Sarkadi B: The role of ABC transporters in drug resistance,
metabolism and toxicity. Curr Drug Deliv. 1:27–42. 2004. View Article : Google Scholar
|
|
11
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ozben T: Mechanisms and strategies to
overcome multiple drug resistance in cancer. FEBS Lett.
580:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higgins CF: Multiple molecular mechanisms
for multidrug resistance transporters. Nature. 446:749–757. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bodey B, Taylor CR, Siegel SE and Kaiser
HE: Immunocytochemical observation of multidrug resistance (MDR)
p170 glycoprotein expression in human osteosarcoma cells. The
clinical significance of MDR protein overexpression. Anticancer
Res. 15:2461–2468. 1995.PubMed/NCBI
|
|
15
|
Chano T, Mori K, Scotlandi K, Benini S,
Lapucci C, Manara MC, Serra M, Picci P, Okabe H and Baldini N:
Differentially expressed genes in multidrug resistant variants of
U-2 OS human osteosarcoma cells. Oncol Rep. 11:1257–1263.
2004.PubMed/NCBI
|
|
16
|
Okada T, Tanaka K, Nakatani F, Sakimura R,
Matsunobu T, Li X, Hanada M, Nakamura T, Oda Y, Tsuneyoshi M and
Iwamoto Y: Involvement of P-glycoprotein and MRP1 in resistance to
cyclic tetrapeptide subfamily of histone deacetylase inhibitors in
the drug-resistant osteosarcoma and Ewing's sarcoma cells. Int J
Cancer. 118:90–97. 2006. View Article : Google Scholar
|
|
17
|
Susa M, Iyer AK, Ryu K, Choy E, Hornicek
FJ, Mankin H, Milane L, Amiji MM and Duan Z: Inhibition of ABCB1
(MDR1) expression by an siRNA nanoparticulate delivery system to
overcome drug resistance in osteosarcoma. PLoS One. 5:e107642010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pluchino KM, Hall MD, Goldsborough AS,
Callaghan R and Gottesman MM: Collateral sensitivity as a strategy
against cancer multidrug resistance. Drug Resist Updat. 15:98–105.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shukla S, Ohnuma S and Ambudkar SV:
Improving cancer chemotherapy with modulators of ABC drug
transporters. Curr Drug Targets. 12:621–630. 2011. View Article : Google Scholar
|
|
20
|
Anuchapreeda S, Leechanachai P, Smith MM,
Ambudkar SV and Limtrakul PN: Modulation of P-glycoprotein
expression and function by curcumin in multidrug-resistant human KB
cells. Biochem Pharmacol. 64:573–582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cocker HA, Tiffin N, Pritchard-Jones K,
Pinkerton CR and Kelland LR: In vitro prevention of the emergence
of multidrug resistance in a pediatric rhabdomyosarcoma cell line.
Clin Cancer Res. 7:3193–3198. 2001.PubMed/NCBI
|
|
22
|
Yen WC and Lamph WW: The selective
retinoid X receptor agonist bexarotene (LGD1069, Targretin)
prevents and overcomes multidrug resistance in advanced breast
carcinoma. Mol Cancer Ther. 4:824–834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma AK, Zhang L, Li S, Kelly DL,
Alakhov VY, Batrakova EV and Kabanov AV: Prevention of MDR
development in leukemia cells by micelle-forming polymeric
surfactant. J Control Release. 131:220–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Batrakova EV, Kelly DL, Li S, Li Y, Yang
Z, Xiao L, Alakhova DY, Sherman S, Alakhov VY and Kabanov AV:
Alteration of genomic responses to doxorubicin and prevention of
MDR in breast cancer cells by a polymer excipient: pluronic P85.
Mol Pharm. 3:113–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sarisozen C, Vural I, Levchenko T, Hincal
AA and Torchilin VP: PEG-PE-based micelles co-loaded with
paclitaxel and cyclosporine A or loaded with paclitaxel and
targeted by anticancer antibody overcome drug resistance in cancer
cells. Drug Delivery. 19:169–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kolitz JE, George SL, Marcucci G, Vij R,
Powell BL, Allen SL, DeAngelo DJ, Shea TC, Stock W, Baer MR, et al:
P-glycoprotein inhibition using valspodar (PSC-833) does not
improve outcomes for patients younger than age 60 years with newly
diagnosed acute myeloid leukemia: cancer and leukemia group B study
19808. Blood. 116:1413–1421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gandhi L, Harding MW, Neubauer M, Langer
CJ, Moore M, Ross HJ, Johnson BE and Lynch TJ: A phase II study of
the safety and efficacy of the multidrug resistance inhibitor
VX-710 combined with doxorubicin and vincristine in patients with
recurrent small cell lung cancer. Cancer. 109:924–932. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Brien MM, Lacayo NJ, Lum BL, Kshirsagar
S, Buck S, Ravindranath Y, Bernstein M, Weinstein H, Chang MN,
Arceci RJ, et al: Phase I study of valspodar (PSC-833) with
mitoxantrone and etoposide in refractory and relapsed pediatric
acute leukemia: a report from the Children's Oncology Group.
Pediatr Blood Cancer. 54:694–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelly RJ, Draper D, Chen CC, Robey RW,
Figg WD, Piekarz RL, Chen X, Gardner ER, Balis FM, Venkatesan AM,
et al: A pharmaco-dynamic study of docetaxel in combination with
the P-glycoprotein antagonist tariquidar (XR9576) in patients with
lung, ovarian, and cervical cancer. Clin Cancer Res. 17:569–580.
2011. View Article : Google Scholar
|
|
30
|
Schiff PL Jr: Bisbenzylisoquinoline
alkaloids. J Nat Prod. 50:529–599. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jang BC, Lim KJ, Paik JH, Cho JW, Baek WK,
Suh MH, Park JB, Kwon TK, Park JW, Kim SP, et al:
Tetrandrine-induced apoptosis is mediated by activation of caspases
and PKC-delta in U937 cells. Biochem Pharmacol. 67:1819–1829. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng LH, Zhang H, Hayward L, Takemura H,
Shao RG and Pommier Y: Tetrandrine induces early G1 arrest in human
colon carcinoma cells by down-regulating the activity and inducing
the degradation of G1-S-specific cyclin-dependent kinases and by
inducing p53 and p21Cip1. Cancer Res. 64:9086–9092. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu C, Gong K, Mao X and Li WL:
Tetrandrine induces apoptosis by activating reactive oxygen species
and repressing Akt activity in human hepatocellular carcinoma. Int
J Cancer. 129:1519–1531. 2011. View Article : Google Scholar
|
|
34
|
Wan J1 Liu T, Mei L, Li J, Gong K, Yu C
and Li W: Synergistic antitumour activity of sorafenib in
combination with tetrandrine is mediated by reactive oxygen species
(ROS)/Akt signaling. Br J Cancer. 109:342–350. 2013. View Article : Google Scholar
|
|
35
|
Ao Z and Xia W: Reversal of daunorubicin
resistance by tetrandrine in leukemic cells. Zhonghua Xue Ye Xue Za
Zhi. 16:235–238. 1995.
|
|
36
|
Wei N, Sun H, Wang F and Liu G: H1, a
novel derivative of tetrandrine reverse P-glycoprotein-mediated
multidrug resistance by inhibiting transport function and
expression of P-glycoprotein. Cancer Chemother Pharmacol.
67:1017–1025. 2011. View Article : Google Scholar
|
|
37
|
Shen H, Xu W, Chen Q, Wu Z, Tang H and
Wang F: Tetrandrine prevents acquired drug resistance of K562 cells
through inhibition of mdr1 gene transcription. J Cancer Res Clin
Oncol. 136:659–665. 2010. View Article : Google Scholar
|
|
38
|
Yang X, Yang P, Shen J, Osaka E, Choy E,
Cote G, Harmon D, Zhang Z, Mankin H, Hornicek FJ and Duan Z:
Prevention of multidrug resistance (MDR) in osteosarcoma by
NSC23925. Br J Cancer. 110:2896–2904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang L, Meng Q, Wang C, Liu Q, Peng J, Huo
X, Sun H, Ma X and Liu K: Dioscin restores the activity of the
anticancer agent adriamycin in multidrug-resistant human leukemia
K562/adriamycin cells by down-regulating MDR1 via a mechanism
involving NF-κB signaling inhibition. J Nat Prod. 76:909–914. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chiu LY, Ko JL, Lee YJ, Yang TY, Tee YT
and Sheu GT: L-type calcium channel blockers reverse docetaxel and
vincristine-induced multidrug resistance independent of ABCB1
expression in human lung cancer cell lines. Toxicol Lett.
192:408–418. 2010. View Article : Google Scholar
|
|
41
|
Sun J, Yeung CA, Co NN, Tsang TY, Yau E,
Luo K, Wu P, Wa JC, Fung KP, Kwok TT and Liu F: Clitocine reversal
of P-glycoprotein associated multi-drug resistance through
down-regulation of transcription factor NF-κB in R-HepG2 cell line.
PLoS One. 7:e407202012. View Article : Google Scholar
|
|
42
|
Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe
S, Mills GB and Unate H: Induction of human MDR1 gene expression by
2-acetylaminofluorene is mediated by effectors of the
phosphoinositide 3-kinase pathway that activate NF-kappaB
signaling. Oncogene. 21:1945–1954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leonard GD, Fojo T and Bates SE: The role
of ABC transporters in clinical practice. Oncologist. 8:411–424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Susa M, Choy E, Yang C, Schwab J, Mankin
H, Hornicek F and Duan Z: Multidrug resistance reversal agent,
NSC77037, identified with a cell-based screening assay. J Biomol
Screen. 15:287–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Suto R, Abe Y, Nakamura M, Ohnishi Y,
Yoshimura M, Lee YH, Imanishi T, Yamazaki H, Kijima H, Tokunaga T,
et al: Multidrug resistance mediated by overexpression of
P-glycoprotein in human osteosarcoma in vivo. Int J Oncol.
12:287–291. 1998.PubMed/NCBI
|
|
46
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang H, Li YY and Wu XZ: Effect of
Tetrandrine on LPS-induced NF-kappaB activation in isolated
pancreatic acinar cells of rat. World J Gastroenterol.
12:4232–4236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ho LJ, Juan TY, Chao P, Wu WL, Chang DM,
Chang SY and Lai JH: Plant alkaloid tetrandrine downregulates
IkappaBalpha kinases-IkappaBalpha-NF-kappaB signaling pathway in
human peripheral blood T cell. Br J Pharmacol. 143:919–927. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bentires-Alj M, Barbu V, Fillet M, Chariot
A, Relic B, Jacobs N, Gielen J, Merville MP and Bours V: NF-kappaB
transcription factor induces drug resistance through MDR1
expression in cancer cells. Oncogene. 22:90–97. 2003. View Article : Google Scholar : PubMed/NCBI
|