Introduction
When the intrinsic ability of bone to regenerate is
overpowered by large segmental defects, the patients who suffer
with these defects and have to manage long term operations and
treatments, physicians who have to perform complex surgical
procedures and the health care system which has to bear high costs
for these procedures are faced with major challenges.
While many approaches have been developed to treat
these difficult large bone defects, none of these have proven to be
fully satisfactory. The most commonly used treatments employ
autologous bone, either non-vascularized (1,2) or
vascularized. Several biodegradable bone graft substitutes have
been developed and are commercially available (3–5).
Other treatments include, the Ilizarov technique (6) and vascularized periosteal flaps
(7,8).
Limitations associated with current treatments have
led to the search for alternative treatments using cell-based
tissue engineering protocols (9,10).
While still largely experimental, these methods have generated
great interest in research, industry and clinical settings with
particular emphasis on treatments for bone defects (11,12). Generally these approaches consist
of combining biodegradable scaffolds with different combinations of
bone- and vessel-forming growth factors and cells.
Biodegradable scaffolds or ʻbone graft substitutesʼ
as their name implies, have been developed to replace autologous
bone grafts with their associated limitations and complications
like, limited donor bone availability and donor site morbidity.
These substitutes have been specifically engineered to integrate
with bone tissue while providing structural 3D support (13). While they have been successful in
reducing the need for autologous bone, their ability to duplicate
bone osteogenesis and vessel angiogenesis capacity of autologous
bone grafts has yet to be conclusively demonstrated (14).
We, as well as others have explored the use of
cell-based approaches, which involve seeding scaffolds with
different combinations of bone- and vessel-forming cells and growth
factors (12,15–18). The advantages of this approach are
obvious, as it delivers bone-forming cells and growth factors
directly into the bone defect where they are needed.
Mesenchymal stem cells (MSCs) have been extensively
studied due to their known potential to differentiate into
chondrogenic and/or osteogenic cells, the main cellular mediators
of bone formation. Using this approach, seeding scaffolds with MSCs
into bone defects in animal models, we as well as others have
demonstrated mostly positive results (18–20). Despite these encouraging initial
results, using this approach, the bone-forming capacity observed
when autologous bone grafts are used has not yet been achieved. The
reasons for this may be the limited viability of the transplanted
MSCs, the lack of osteoinductive stimuli and a lack of
angiogenesis. In order to address these issues, investigators have
used genetically modified MSCs (21), using different proteins including,
bone morphogenic protein (BMP) (12,15), vascular endothelial growth factor
(VEGF) (22) and fibroblast
growth factor (FGF) (23). Thus
far, optimal results have been obtained when using a combination of
osteogenic and angiogenic proteins (24,25).
Endothelial progenitor cells (EPCs) are of
hematopoietic origin, and are known to participate in angiogenesis
(26). It has been shown that
EPCs home to ischemic tissue, stimulate blood flow recovery in
ischemic tissues, and are increased in numbers in the blood
following trauma, all qualities that make EPC logical candidates
for treating large bone defects (27,28). Keeping this in mind, we combined
MSCs as a source for osteogenic activity and EPCs due to their
pro-angiogenic activity. In a previous study, we treated large rat
femur bone defects with scaffolds seeded with a combination of MSCs
and EPCs and observed a much higher bone healing response in
animals that received both MSCs and EPCs (18).
Although these tissue-engineering approaches that
combine cells, osteoinductive proteins and osteoconductive
scaffolds have been encouraging, they have not yet achieved
widespread clinical acceptance. This may be due to the logistics of
applying these new techniques in the clinical setting, but also as
they have not conclusively demonstrated clinical superiority over
autologous bone grafts (18,29,30).
Vascularized periosteal flaps, introduced by Doi and
Sakai in the early 1990s, consist of transferring a thin ʻflapʼ of
periosteum with its intact blood supply to cover bone defects
(7). The rich blood supply of
these flaps, together with their thin and pliable structure makes
them very versatile and has enabled surgeons to successfully use
them to reconstruct bone defects in a variety of different
anatomical locations. The periosteum is a rich source of blood
supply and bone-forming cells. Its outer ʻfibrousʼ layer contains a
nerve and blood supply, while its' inner ʻcellularʼ layer contains
different kinds of stem cells (31–33). This inner cellular layer becomes
progressively thinner with age. In adults the periosteum becomes so
thin that it cannot be distinguished from the overlying fibrous
layer (34,35).
We evaluated the effects of a periosteal flap
together with a tissue engineering approach in a former study
(36), and in the present study,
we sought to combine these new cell-based MSC/EPC therapy
approaches with the well-established and clinically proven method
of a vascularized periosteal flap. This combination which has not
been performed in the past by any other research group, at least to
the best of our knowledge, would bring together the recently
demonstrated benefits of tissue engineering approaches with the
well-established clinical success of periosteal flaps. The healing
of critical size bone defects could be facilitated by this method,
since bone grafts with their donor site morbidity would no longer
be necessary and patients would not have to cope with long-term
external fixation, such as that associated with the Ilizarov
technique.
Materials and methods
Animal care
All experiments were performed in accordance with
regulations established and approved (project no. F3/21;
Regierungspräsidium, Darmstadt, Germany) by our Institutional
Animal Care and Oversight Committee according to German law.
Ten-week-old male Sprague-Dawley (SD) rats (Harlan Cytotest Cell
Research GmbH, Rossdorf, Germany) weighing 350–400 g were used.
Animals were caged individually in temperature (21°C), light (12 h
light, 12 h dark), and air flow-controlled rooms. They received
standard rodent chow and water containing tramadol pain killer
ad libitum post-operatively. Rats were monitored daily for
complications or abnormal behavior during the post-operative
period.
Group setup
A total of 80 rats (SD; Harlan Cytotest Cell
Research GmbH) were allocated into 4 groups, consisting of 20
animals/group. Critical size defects were created on their femur
bones and were treated as follows: group 1, vascularized periosteal
flap alone; group 2, vascularized periosteal flap + β-TCP scaffold;
group 3, periosteal flap (with ligated vascular pedicle) + β-TCP
scaffold; group 4, vascularized periosteal flap + β-TCP scaffold +
MSCs/EPCs.
After 8 weeks, the rat femurs were harvested and all
bones were examined using radiological and immunohistochemistry
methods. Eight rats were used for histological analysis and 6 were
used for micro-CT and biomechanical testing. Biomechanical
three-point bending tests were performed in bones, which had been
previously used for radiological examination.
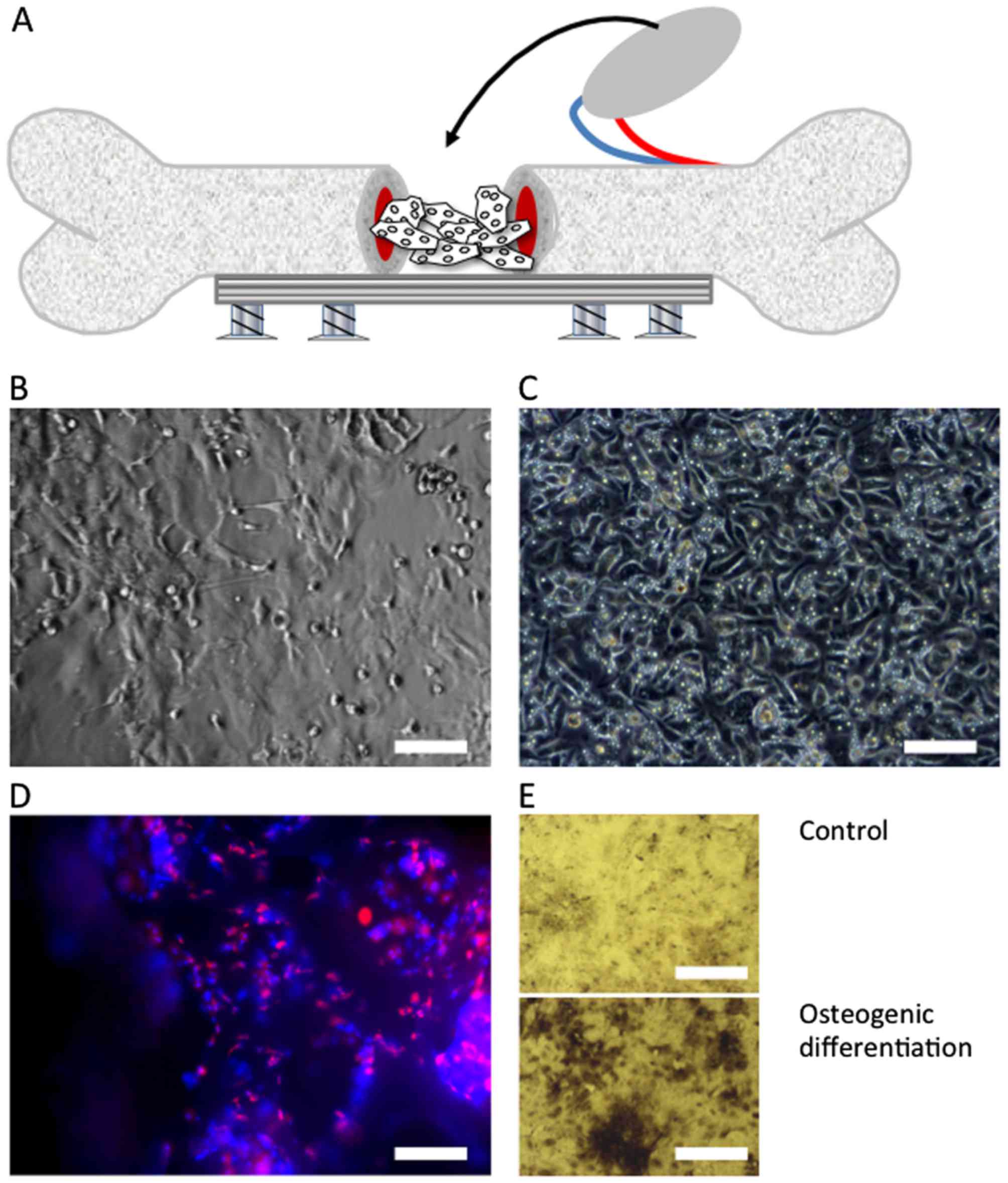
Animal model
Critical size femur bone defect
Under general anesthesia [ketamine chlorhydrate (100
mg/kg) and xylazine hydrochloride (10 mg/kg)] administered
intraperitoneally, the right legs of the rats were shaved, cleaned
and disinfected with antiseptic fluid. A medial longitudinal
incision was made through the skin and fascia over the femur and
the underlying vastus medialis and biceps muscles were separated
bluntly. The ventral aspect of the femur bone was exposed. In order
to provide stability to the bone after creation of the defect 1.5
mm 5-screw stainless-steel plates Compact Hand (Synthes GmbH,
Umkirch, Germany) were secured to the femur bone using 1.5 mm
cortical screws. A 7-mm-long bone defect was then created using a
drill in the mid-shaft of the femur between the second and the
third screw. Special care was taken to ensure that no bone
fragments were left underneath the plate along the 7-mm defect, as
previously described (18).
The periosteum overlaying the medial femoral condyle
and its blood supply (descending genicular artery and vein) were
identified, exposed and the medial condyle periosteal flap was
elevated on its vascular pedicle. The vascular pedicle was
carefully dissected along a trajectory of 15 mm and the flap was
then rotated into the defect and fixed with sutures (5-0 Vicryl;
Ethicon, Norderstedt, Germany) to the medial side of the plate to
bridge the defect. The lateral side of the defect was left free.
After the femur bone defects in the different groups had received
their respective treatments, the fascia was re-approximated with
interrupted 5-0 Vicryl sutures (Ethicon), and the skin sewed up
with intracutaneous sutures (4-0 Prolene; Ethicon).
Scaffold preparation and implantation
into defect
A commercially available bone graft substitute
(chronOS β-TCP, size 0.7–1.4 mm, porosity 60% and pore size 100–500
μm; Synthes GmbH) was incubated for 30 min in a fibronectin
solution (10 μg/ml; Sigma, Deisenhofen, Germany) in
phosphate-buffered saline (PBS) without Mg2+
and Ca2+ (PBS−/−). The supernatant
was then removed after 30 min and replaced by PBS−/−
only. The granules were immediately placed, as a dense single
layer, in a 24-well plate (Nunc, Wiesbaden, Germany) using sterile
forceps. Fibronectin is a commonly used and accepted substrate,
shown to support EPC differentiation and adherence (37). In a previous study we found that
fibronectin coating enhanced EPC adherence to β-TCP (17). This preparation was then used to
fill the femur bone defect prior to implanting the periosteal
flap.
EPC and MSC harvest, isolation and
characterization
Rat early EPCs were isolated from the spleens of
syngeneic male SD rats according to previously described procedures
(38,39). The advantage in doing so is that
the absolute number of harvested cells is much higher if spleen is
used in comparison to bone. The spleen was therefore cut into small
sections (approximately 3 mm) and gently mashed using syringe
plungers. The cell suspension was then filtered through a 100 mm
mesh, washed once with PBS and subjected to Ficoll density gradient
centrifugation (30 min, 900 × g with Ficoll 1.077 g/ml; Biochrom,
Berlin, Germany). Recovered mononuclear cells were washed twice
with cold PBSw/o (10 min, 900 × g), and then each
4×106 cells were cultivated on a fibronectin-coated (10
μg/ml; Sigma) 24-well culture dish in 1 ml of endothelial
basal medium supplemented with endothelial growth medium (both from
Cambrex, Verviers, Belgium) with singlequots at 37°C, 5%
CO2. After 48 h, non-adherent and weakly-adherent cells
were removed, the medium was changed, and the cells were cultivated
for an additional 72 h. Moreover, a parallel preparation was
performed to evaluate the percentage of endothelial-like
differentiated cells. EPCs were identified using a previously
described method (40,41). Briefly, the cells were incubated
with 2.4 μg/ml DiLDL (Cell Systems, St. Katharinen, Germany)
in EBM supplemented with 20% fetal calf serum (FCS) for 1 h. Cells
were then fixed with 2% paraformaldehyde for 10 min and, after
washing with PBS+/+, were incubated with FITC labeled
Ulex europaeus agglutinin-1 (10 μg/ml) (lectin;
Sigma) for 1 h. Cells presenting double-positive fluorescence
(1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled
acetylated low-density lipoprotein, lectin) were considered to be
EPCs. For these experiments, the cells were detached by accutase
treatment (10 min) (PAA Laboratories, Linz, Austria), washed once
with MesenCult + Supplements (Cell Systems), and subsequently
adjusted to a density of 2.5×105 cells in 100
μl.
MSCs were obtained from donor rat femurs. More
precisely, they were isolated from femurs obtained from syngeneic
donor rats by flushing the bone marrow with PBS. The bone marrow
was then re-suspended in PBS and washed once by centrifugation (8
min, 300 × g). The cells obtained from each femur were subsequently
plated into individual 75 cm2 culture flasks using DMEM
supplemented with 10% FCS (Gibco, Darmstadt, Germany) and expanded
over three passages. Subsequently, the cells were detached by
accutase (PAA Laboratories) treatment, washed, and re-suspended in
a medium consisting of 90% FCS and 10% dimethyl sulfoxide (Sigma).
Aliquots were stored in liquid nitrogen until use.
For each experiment, a portion of the cryoconserved
cells was thawed and expanded over two additional passages. MSCs of
the sixth culture passage (P6) were then used for the experiments.
After accutase treatment (10 min), cells were washed (10 min, 300 ×
g) and re-suspended in PBS. Hereafter, the cell suspension was
divided, and one part was adjusted to a density of
2.5×105 cells in 100 μl to be used in the
experiments. The other portion was used to assess MSC osteogenic
differentiation potential by incubating the cells in osteogenic
differentiation medium containing dexamethason (1×10−6
M), ascorbic acid (50 μg/ml), and β-glycerol phosphate
(1×10−1 M) for 3 weeks. The medium was exchanged twice a
week. Osteogenic substances were purchased from Stem Cell
Technologies (Grenoble, France). Extracellular calcium deposition
was evaluated using von Kossa staining (18).
EPC/MSC seeding on scaffolds
Granules were densely placed as bilayer into
individual wells of 48-well plates. Hereafter, the granules were
loaded with either 5×105 cells of a mixture composed of
50% MSCs and 50% EPCs or with 5×105 MSCs or
5×105 EPCs alone (17). The cells were dripped (200
μl) over the bone graft layer and incubated for 10 min at
37°C. The medium containing the non-adhering cells was then
removed, rinsed once again over the granules, and incubated at 37°C
for a further 10 min. This procedure was repeated 3 times.
Subsequently, the granules loaded with cells were subjected to the
animal facility. Granules were constantly kept at 37°C and were
implanted into the bone defects within 2 h after seeding. To
confirm adherence of EPCs and MSCs on biomaterials we used a
parallel setup. DiLDL pre-stained EPCs and MSCs were seeded on the
granules as described. Subsequently, the cells were fixed with 2%
paraformaldehyde in PBS+/+ for 20 min and washed gently
with 2X 200 μl PBS per well and followed by further
incubation with 1 μl DAPI
[2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride; Sigma]
with a final concentration of 1 μg/ml for 10 min at room
temperature. After the staining, the granules were washed three
times with PBS−/− and transferred to a new well in order
to prevent false-positive results caused by the adherent cells at
the bottom of the cultivation well. Finally, the granules were
analyzed by fluorescence microscopy (Axio Observer; Carl Zeiss,
Inc., Göttingen, Germany) and photographed. EPCs appear orange and
MSCs appear blue (Fig. 1).
Euthanasia
The animals were euthanized with an overdose of
pentobarbital (150 mg/kg intraperitoneally) after 8 weeks. The
animal femurs were dissected free and all bones were examined
macro- and microscopically for signs of infection or tumors. Bones
were then frozen and stored at −80°C until preparation for
immunhistological examinations. Thereafter, the bones were fixed in
Zinc-Formal-Fixx (4%; Thermo Electron, Pittsburgh, PA) >20 h and
then subjected to decalcification in a solution containing 0.25 M
Trizma base (Sigma) (17) for 14
days.
Measurements
Various measurements were performed 8 weeks after
surgery as follows:
Bone maturation, vascularization and
inflammation in the defect zone
Samples taken from the bone defect zone were
decalcified, fixed in 4% formaldehyde and embedded in paraffin.
Sections were stained with hematoxylin and eosin or incubated with
antibodies directed against osteocalcin (bone maturation, dilution
1:100, incubation time 1 h, 4°C; Abcam, Cambridge, UK), CD31 (blood
vessels, dilution 1:50, incubation overnight, 4°C; Abcam), Ki-67
(proliferation, M7248, dilution 1:50, incubation time 1 h, 4°C;
Dako, Glostrup, Denmark) and HLA-DR (inflammation, ab23990,
dilution 1:100, incubation time 1 h, 4°C; Abcam). Polyclonal
HRP-coupled secondary antibodies were applied and the sections were
incubated with 3-amino-9-ethyl-carbazole (AEC).
Osteocalcin staining in the defect zone was
evaluated purely descriptive using stitched high resolution images
of the whole defect area using a Keyence Biorevo BZ-9000 microscope
and the software BZ II analyzer (Keyence Deutschland GmbH,
Neu-Isenburg, Germany).
For thedetermination of blood vessel density, the
number of blood vessels (CD31) with a lumen was counted in 5
non-overlapping images of the defect area at a magnification of
×100 in combination with a computer-supported imaging picture
analysis system (Axiovision 4.7; Carl Zeiss, Inc.). Values were
converted to blood vessel/μm and normalized to the
percentage of tissue in the analysed image frame.
Histological Ki-67 and HLA-DR staining was evaluated
in the medial and lateral aspect of the defect zone. The percentage
of Ki-67-positive cells was determined in a standardized area, that
was normalized to the area covered by tissue. The values were
ascertained to 5 categories (0, no Ki-67-positive cells; 1, up to
25%; 2, 25–50%; 3, 50–75%; 4, 75–100% Ki-67-positive cells). The
percentage HLA-DR-positive area was determined using ImageJ
software (https://imagej.nih.gov/ij/) and
normalized to the area covered by tissue in the defect zone. All
histological slides were analyzed in a random order by an
independent observer blinded to the group setup (17,18).
Peripheral quantitative computed
tomography (pQCT) of bone defects
To assess bone density and micro architecture at the
defect site pQCT and micro-CT (SkyScan 1176; Bruker Corp.,
Billerica, MA, USA) were performed on 6 of the 20 femurs harvested
at 8 weeks. For imaging, femurs were oriented along their long axis
orthogonally to the axis of the X-ray beam (90 kV X-ray source,
fully distortion corrected 11-megapixel X-ray camera).
Two-dimensional CT images were scanned and reconstructed using a
standard convolution-back-projection procedure. The isotropic voxel
size was 18 μm. The analyzed volume of interest was placed
at the center of the bone defect with 0.7 mm thickness for all
samples (18). β-TCP signals were
not subtracted from bone mineral density (BMD) values.
Biomechanical testing
In bones (6 in total), that had already been used
for pQCT, biomechanical properties, at the defect site were
measured by a destructive three-point bending procedure using a
material testing machine (zwickiLine Z5.0; Zwick-Roell, Ulm,
Germany). The ʻbending until failureʼ method was performed by
lowering a bar onto the femur, using a constant deflection speed of
0.1 mm/sec, and recording the load and deflection continuously. The
ultimate load was then calculated using testXpert II software
(Zwick-Roell) (18).
Statistical analysis
In this study, differences between the groups were
compared using the non-parametric Kruskal-Wallis test followed by
multiple Conover-Iman comparison with Bonferroni-Holm correction.
The software BIAS 10.11 (Epsilon, Darmstadt, Germany) was used for
group size calculation. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
No deaths occurred during surgery or during the
immediately following post-operative period. Furthermore, the
animals exhibited no abnormal behavior during daily monitoring.
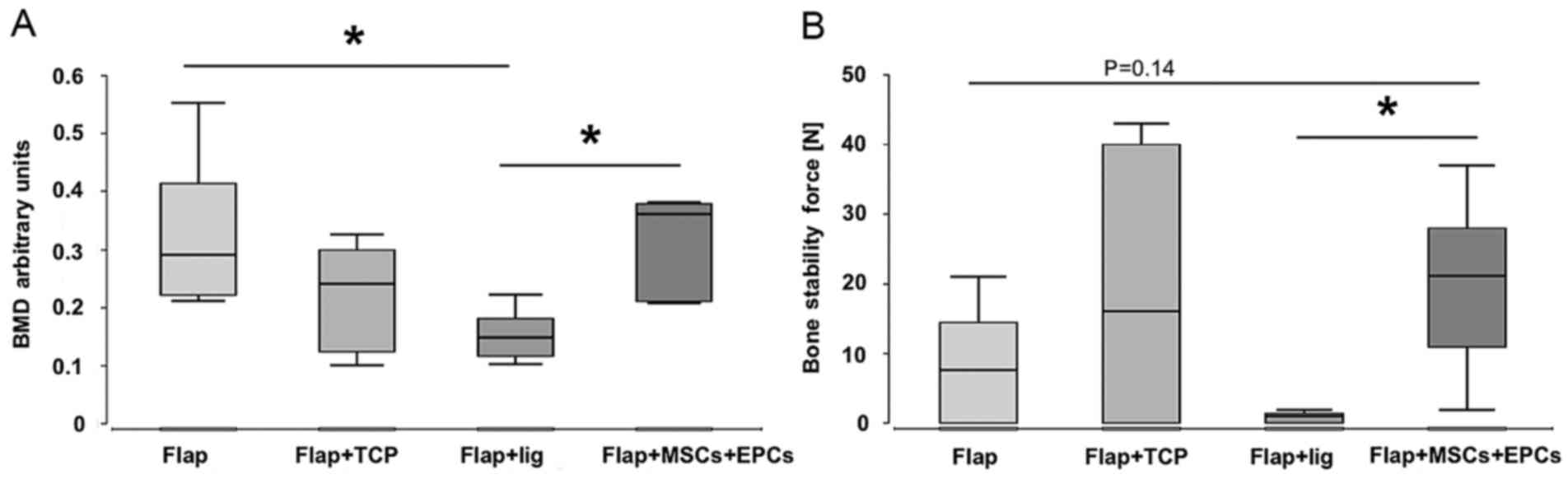
Biomechanical results and BMD
The median BMD values and biomechanical stability at
week 8 were highest in group 4 (flap + β-TCP scaffold + MSCs/EPCs)
compared to all the other groups. However, as regards group 4, this
was only significant compared to group 3 (ligated flap + β-TCP
scaffold). The median BMD values and stability in group 4 (flap +
β-TCP scaffold + MSCs/EPCs) were higher when compared to group 2
(flap + β-TCP scaffold), although not significantly. Stability was
significantly higher in group 4 (flap + β-TCP scaffold + MSCs/EPCs)
in comparison to group 3 (ligated flap + β-TCP scaffold), in which
no stability was observed at all. In addition, group 1 (flap) and
group 2 (flap + β-TCP scaffold) presented higher biomechanical
stability compared to group 3 (ligated flap + β-TCP scaffold). BMD
was found to be significantly lower in group 3 (ligated flap +
β-TCP scaffold) compared to group 1 (flap) and group 4 (flap +
β-TCP scaffold + MSCs/EPCs), and lower (although not significantly)
compared to group 2 (flap + β-TCP scaffold) (Fig. 2).
Gross histological analysis
A thick bony flap was found in group 1 (flap alone)
and bone began to grow into the defect zone originating from the
flap. However, no healing was observed after 8 weeks. A calcified
flap was also observed in group 2 (flap + β-TCP) similar to group
1. Furthermore, signs of bone formation could be found in the
defect zone. However, in group 3 (ligated flap + β-TCP), the flap
appeared completely necrotic and no signs indicating bone healing
were observed. Fibrous, loose tissue formed in the defect instead,
and signs of infection were present in two bones. In group 4 (flap
+ β-TCP + MSCs/EPCs) a thick calcified flap was detected and bone
formation was found throughout the entire defect zone (Fig. 3A–D).
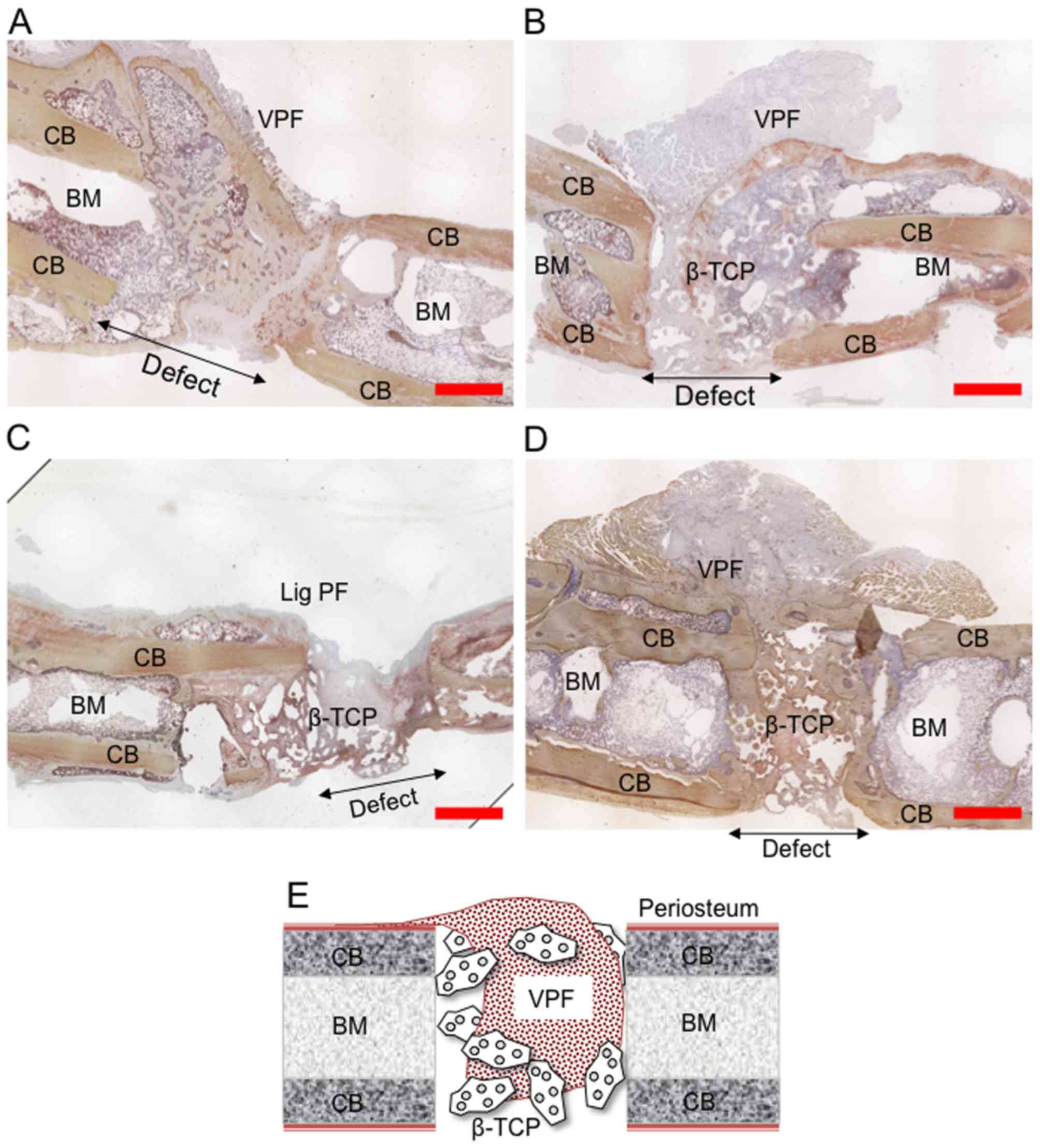 | Figure 3Representative osteocalcin-stained
histological slides of the defect zone 8 weeks after surgery.
Histological slides of the femur defect of (A) group 1 (periosteal
flap), (B) group 2 (periosteal flap + β-TCP), (C) group 3 (ligated
periosteal flap + β-TCP and (D) group 4 (periosteal flap + β-TCP +
MSCs + EPCs) are shown. Brownish color indicates
osteocalcin-positive areas. The vascularized periosteal flap
demonstrates a high degree of osteogenic differentiation.
Pronounced osteogenic differentiation in the defect zone was seen
in (A) group 1 and (B) group 4, whereas (C) minimal bone formation
and resorption of the periosteal flap was seen, if the periosteal
flap was ligated. Defect size is 5 mm. (E) Schematic overview over
the defect zone is shown. Original defect size is 5–6 mm. Red bar
indicates a distance of 2 mm. (F) Representative μCT images
taken from the defect zone 8 weeks after surgery. BM, bone marrow;
CB, cortical bone; VPF, vascularized periosteal flap; LigPF,
ligated periosteal flap; MSCs, mesenchymal stem cells; EPCs,
endothelial progenitor cells. |
Bone formation and healing
Bone formation was observed in close proximity to
the periosteal flap in all cases at 8 weeks, except for defects
treated with flaps with ligated vascular pedicles. In groups 1, 2
and 4, with varying degrees, new bone formation could be seen
protruding from the flap, as well as in the defect zone.
Histological analysis of osteocalcin immunostained slides confirmed
this observation, demonstrating a close correlation between the
periosteal flap and cortical bone formation in all cases at 8
weeks. All defects treated with vascularized flaps revealed thick
bony tissue formation (groups 1, 2, 4). Bone density, thickness and
integration into the defect zone were visibly greater in group 4
(flap + β-TCP scaffold + MSCs/EPCs). By contrast, in animals in
which the vascular pedicle of the periosteal flap was ligated
(group 3), minimal bone formation was observed at the defect, and
the periosteal flap was found to be completely reabsorbed. Finally,
β-TCP resorption appeared to be more advanced in these animals
(Fig. 3A–E).
The results observed in histological analysis were
confirmed by μCT-analysis. In groups 1, 2 and 4, the thick
bony flap and increased healing were observed in the defect, while
in group 3 (ligated vascular flap), only loose non-calcified tissue
was observed (Fig. 3F).
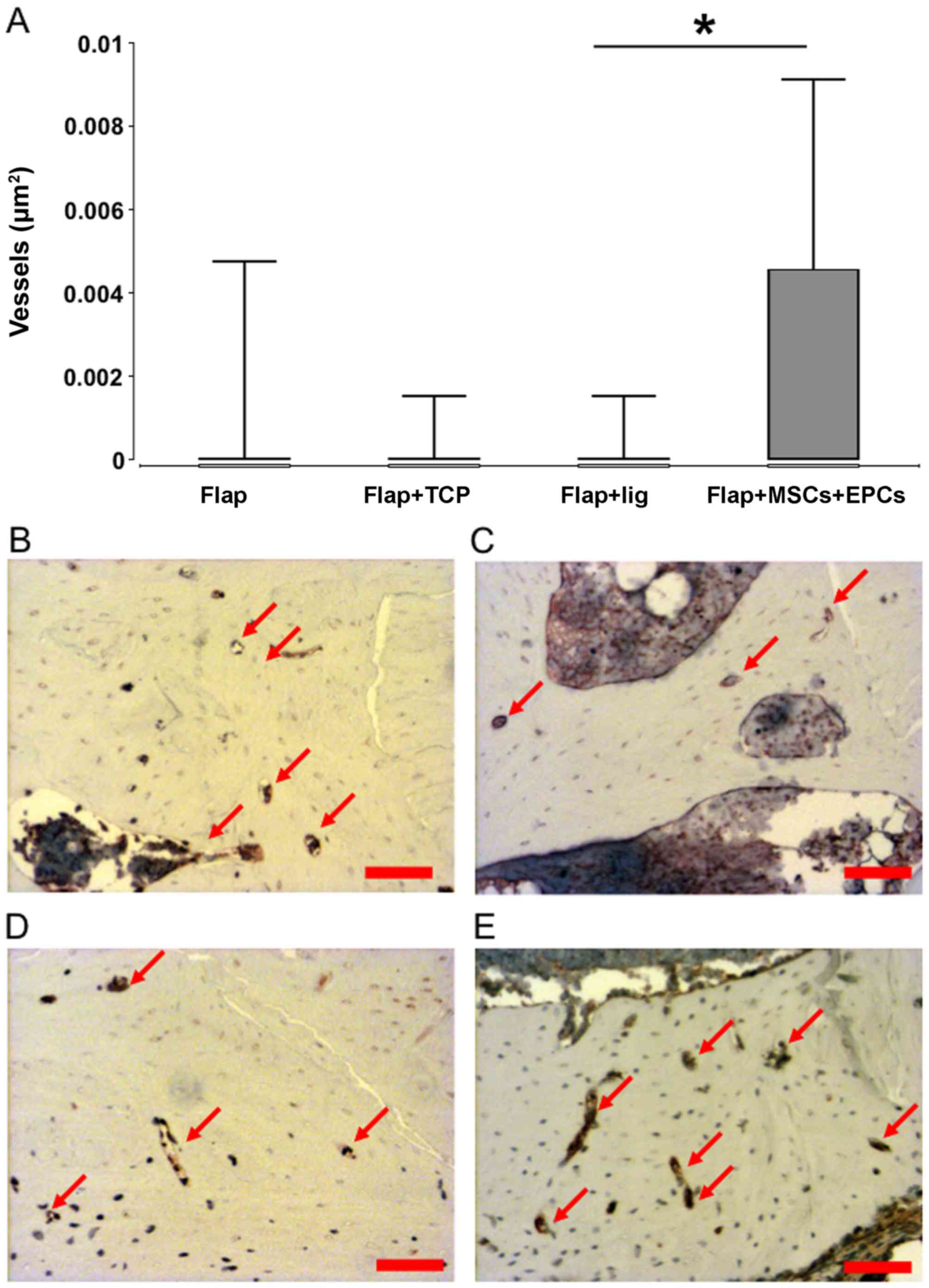
Vascularization
A highest density of blood vessels was observed in
group 4 (flap + β-TCP + MSCs/EPCs) and the values were
significantly increased in comparison to group 3 (ligated flap),
but not to group 1 (flap) and group 2 (flap + β-TCP) (Fig. 4A–E).
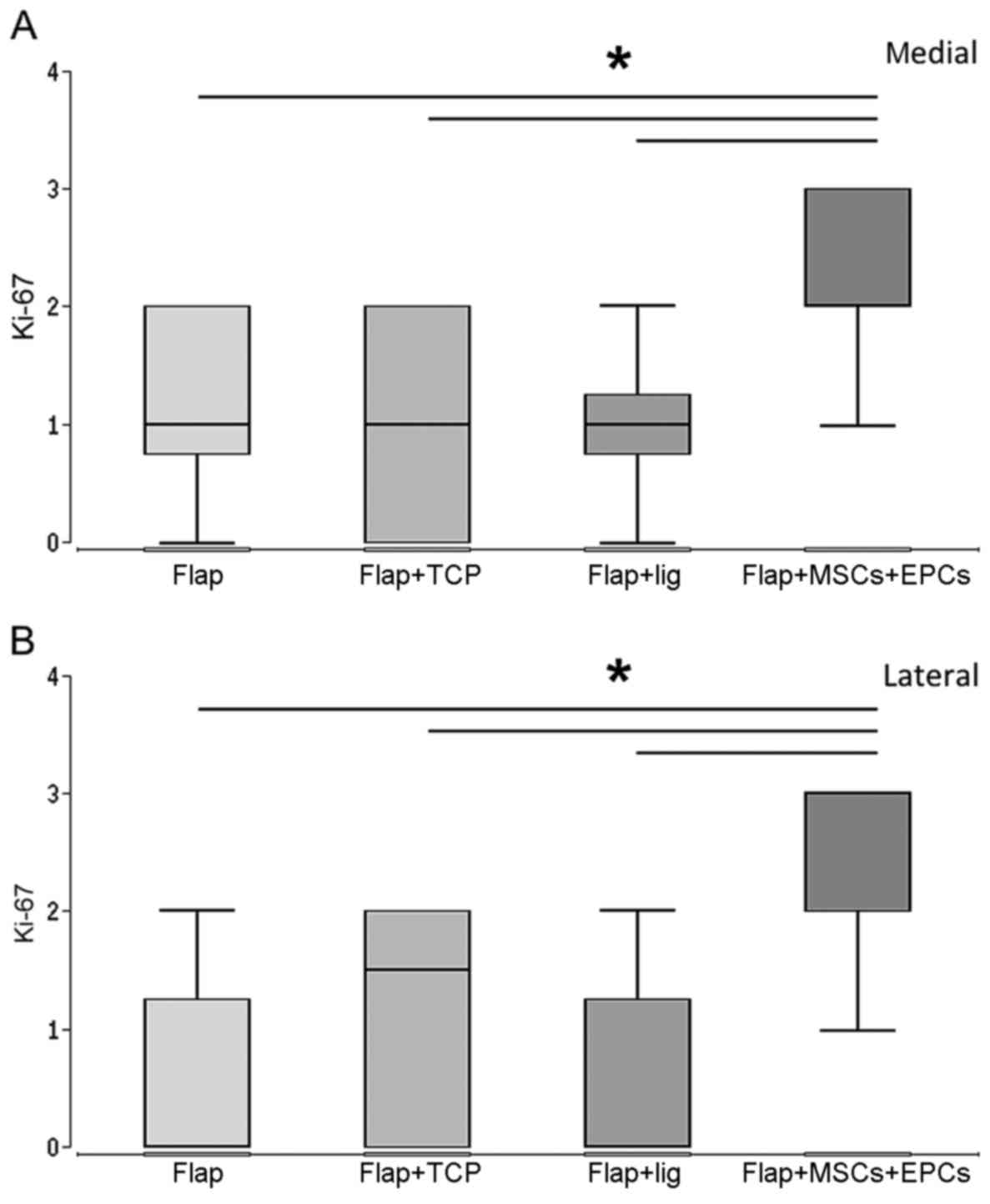
Proliferation and inflammation
Proliferating cells were detected by Ki-67
immunostaining. The greaterst amounts of proliferating cells were
observed in group 4 (flap + β-TCP scaffold + MSCs/EPCs). The
percentage of proliferating cells was significantly higher in group
4 (flap + β-TCP scaffold + MSCs/EPCs) in comparison to all the
other groups after 8 weeks at the medial and the lateral defect
side (Fig. 5A–F).
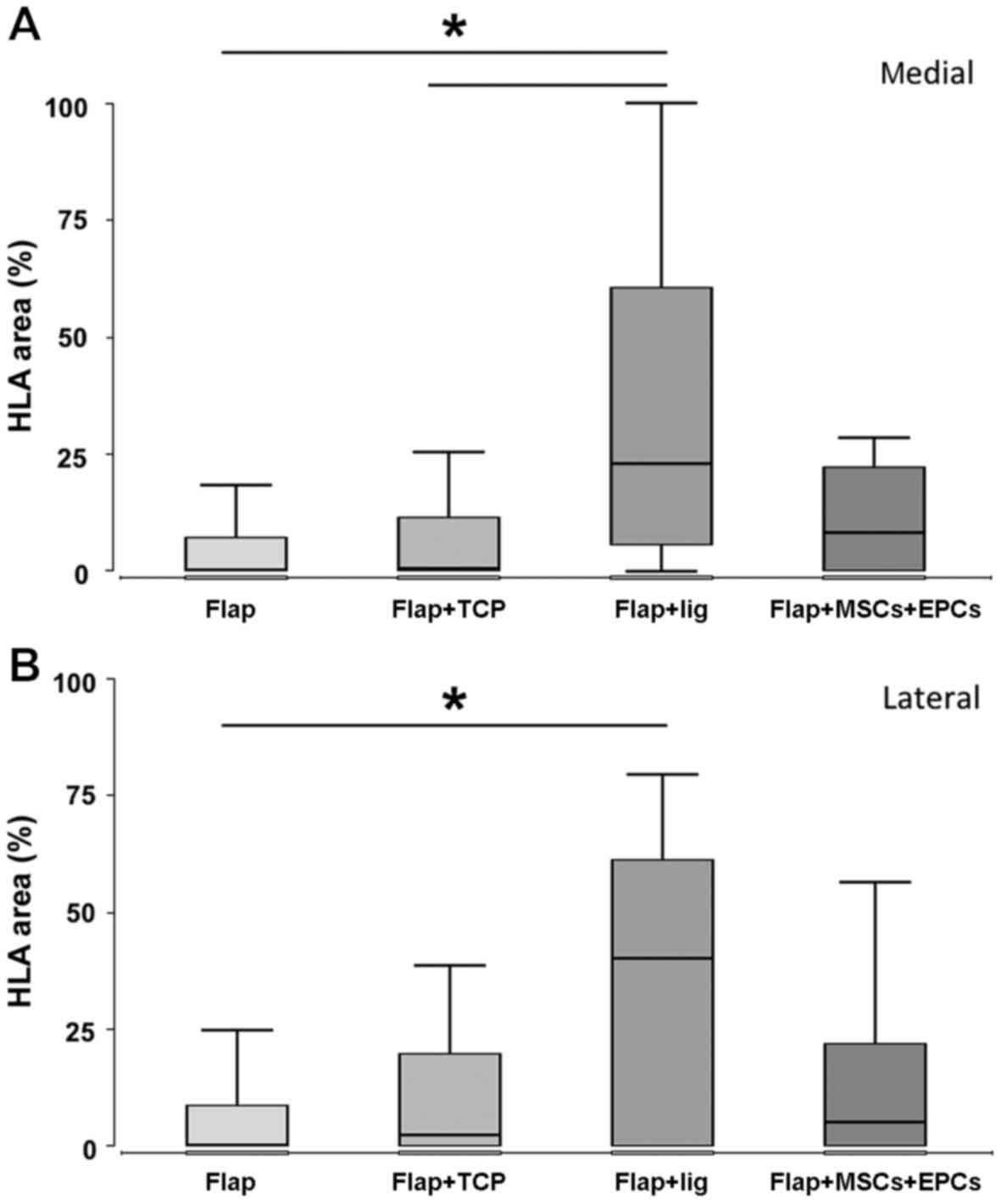
HLA-DR was used to reveal signs of infection. The
amount of inflammatory cells was highest in group 3 (ligated
vascular pedicle) in comparison to all the other groups and
significantly higher compared to group 1 (flap) and in trend
towards group 2 (flap + β-TCP scaffold) (P=0.056) at the lateral
side of the defect. At the medial side where the flap is located,
the numbers of inflammatory cells were significantly increased in
group 3 (ligated vascular pedicle) compared to group 1 (flap) and
group 2 (flap + β-TCP scaffold). A detailed view revealed that
HLA-DR-positive cells were not evenly distributed in the tissue of
the defect zone, but had accumulated around the β-TCP granules
(groups 2 and 4), whereas an extensive distribution of inflammatory
cells was observed in group 3 (ligated periosteal flap). The
formation of multinucleated giant cells in contact to the
β-TCP-scaffold was partly observed in group 2 (flap + β-TCP) and
group 4 (flap + β-TCP scaffold + MSCs/EPCs) (Fig. 6 and Table I).
 | Table IP-value overview. |
Table I
P-value overview.
| Group
comparison | BMD | Force | CD31 | Ki-67 med. | Ki-67 lat. | HLA med. | HLA lat. |
|---|
| Flap vs. flap +
β-TCP | 0.31 | 0.53 | 0.92 | 0.64 | 0.23 | 0.56 | 0.53 |
| Flap vs. flap +
lig | 0.02 | 0.8 | 0.44 | 0.52 | 0.81 | 0.001 | 0.01 |
| Flap vs. flap +
MSCs + EPCs | 0.56 | 0.14 | 0.12 | 0.003 | 0.0003 | 0.16 | 0.27 |
| Flap + β-TCP vs.
flap + lig | 0.22 | 0.38 | 0.62 | 0.89 | 0.37 | 0.008 | 0.056 |
| Flap + β-TCP vs.
flap + MSCs + EPCs | 0.11 | 0.44 | 0.21 | 0.003 | 0.016 | 0.39 | 0.59 |
| Flap + lig vs. flap
+ MSCs + EPCs | 0.005 | 0.007 | 0.036 | 0.001 | 0.0003 | 0.13 | 0.24 |
Discussion
Autologous bone grafts continue to be the ʻgold
standardʼ for the treatment of large bone defects. Their
effectiveness has been attributed to the bone- and vessel-forming
cells and growth factors they contain and furthermore to their 3D
structure which helps bridge the gap left due to missing bone
tissue. Most new treatments developed to substitute autologous bone
grafts have been designed to emulate these qualities with the goal
of matching or exceeding their effectiveness. With this goal in
mind, in the present study, we tested a combination of bone and
vessel forming cells, a 3D scaffold and a vascularized periosteal
tissue flap in a rat femur critical size defect model.
In the present experiments, our vascularized
periosteal flap provided periosteal tissue itself, with its
recognized role in bone growth and repair, a rich blood supply and
its 3D scaffold-like structure that bridged the gap between the
proximal and distal femur bone/periosteum on either side of the
defect. The periosteum plays an important role in bone healing. In
larger bone defects the periosteum of the distal and proximal edges
of the defect grow towards one another in an attempt to bridge the
gap resulting from missing bone. However, when the defect is too
large and the distance between the distal and proximal defect edges
is too great, the ability of the periosteum to bridge the gap is
overpowered and healing is delayed or does not occur at all. In
these cases, by providing a connection between the periosteum at
the distal and proximal edges of the defect, a periosteal flap can
help to span the gap and in doing so promote healing. Periosteum
can be transplanted as a whole tissue on a vascular pedicle, when
placed in a large bone defect, and provides the following
properties: its bone growth/repair capabilities, a rich blood
supply, and its 3D structure that serves as a scaffold upon which
locally generated, systemically derived or transplanted stem cells
and/or growth factors can adhere. Taking advantage of all these
properties, vascularized periosteal flaps have been used
successfully to treat difficult cases of persistent pseudarthrosis,
radiation and avascular necrosis and even large bony defects
created by debridement of osteomyelitis, pseudarthrosis and
infections (12,42,43).
In the present study all animals treated with
vascularized periosteal flaps exhibited a significant increase in
bone formation in the defect, as measured quantitatively by an
increase in BMD and evaluated qualitatively by osteocalcin
immunostaining when compared to group 3 in which the vascular
pedicle was ligated. This effect was greatest in the defects
treated with MSCs, EPCs and β-TCP scaffold, and to a lesser degree
in those that received scaffold solely. As a limitation to mention,
no additional staining was performed to provide more information
regarding tissue composition. This would be an interesting approach
for further studies.
These findings coincide with those of Vögelin et
al, who found in a similar rat femur critical-sized defect
model that the combination of OPLA-HY scaffold, rhBMP-2 and a
vascularized periosteal flap resulted in new callus formation and
bony bridging (12).
In another study, Camilli and Penteado harvested a
periosteal flap from the medial femoral condyle, created a pouch
with it and filled it with cortical bone in one group. One out of
each group was connected to the femoral bone and the other part was
buried in soft tissue. They described that the presence of a
periosteal flap, adjacent to living cortical bone, favours bone
formation in contrast to the ones buried in soft tissue. This
effect was even greater if the pouch was additionally filled with
cortical bone (44).
In the present study, to differentiate between the
role played by the blood supply of the flap vs. the periosteal
tissue itself, in one group, after lifting and transferring the
flap into the bone defect, we ligated its vascular pedicle. Not
surprisingly, we found that instead of newly formed bone, the
defect contained necrotic fibrous tissue. In addition, in this
group of animals, we observed an accumulation of activated
HLA-DR-expressing cells and plate loosening. These observations
highlight the important role which the flap blood supply plays in
bone healing and the prevention of overwhelming inflammation that
is probably caused by the necrotic tissue.
A margin of inflammatory cells was observed around
the β-TCP granules in groups 2 and 4. The accumulation of
inflammatory cells, and the generation of multinucleated giant
cells should be rated as a foreign body response as it is being
triggered by various β-TCP scaffolds as described by Ghanaati et
al (45).
In the present study, we used β-TCP scaffold to fill
the physical gap created by missing bone in our rat femur
critical-sized defect model. While their constitution is not
comparable to the osteoconductive and osteoinductive nature of
autologous bone, bone graft substitutes do eliminate donor-site
morbidity and material limitations (5,46).
We chose this particular scaffold as in previous in vitro
studies, we compared several different commercially available bone
graft substitutes in combination with MSCs + EPCs and found β-TCP
to be superior compared to other synthetic biomaterials in its
osteoconductive and osteoinductive properties (number of invading
cells, cell location, differentiation rate and potential) (3,20,47).
In previous experiments, using the same rat model,
we demonstrated that MSCs promote bone healing. Moreover, by adding
EPCs and MSCs, we demonstrated a further improvement in bone
healing. In mechanically unstable breaks, the lack of vasculature
causes the bulk of the MSCs to develop into bridging cartilage that
eventually spans the defect and then is further stabilized by a
surrounding bony bridge. It is therefore necessary not only to
provide sufficient number of MSCs in the bone defect zone, but also
vascularity and EPCs to promote direct callus formation. We showed
that MSCs alone caused an increase of new bone mass, whereas adding
EPCs resulted in both increased bone mass and improved angiogenesis
probably mediated by the release of VEGF through EPCs (17–19).
Additionally, it has been reported that human MSCs
secrete a distinct set of cytokines constitutively, such as VEGF,
interleukin (IL)-6 or transforming growth factor (TGF)-β, which
stimulate reparative events and inhibit degenerative events
(48–50). Thus, conceivably, MSCs could exert
therapeutic effects by this cytokine secretory activity alone. For
example, it has been described that ʻmesenchymal stem cells support
migration, extracellular matrix invasion, proliferation, and
survival of endothelial cells in vitroʼ by the secretion of
VEGF, basic fibroblast growth factor (bFGF), angiogenin,
procathepsin B, IL-11, and BMP-2 (51).
The combined secretion of various growth factors
through MSCs and EPCs might be the reason for the elevated
proliferative activity as an indicator for an increased
regenerative response observed in group 4 (flap + β-TCP +
MSCs/EPCs) of the present study.
Hence, in comparison to other studies on bone
healing where only BMP was added to a periosteal flap (12), the bone healing response in the
present study benefits additionally from the cytokine secretory
capabilities of MSCs and EPCs.
In this study, we found similar results for bone
healing in the groups with the flap alone and when MSCs/EPCs are
added. We can therefore assume that the vascularized flap already
seems to bring the most important characteristics with it. This
emphasizes the role of the periosteum which includes a wide range
of stem cells as mentioned earlier and the vascularization.
Proliferating cells represented by Ki-67 staining
showed significantly highest amounts in group 4 (flap + β-TCP +
MSCs/EPCs) in comparison to all the other groups. Since BMD and
biomechanical stability were highest in this group we assume a
direct correlation to these proliferating cells. It is not
absolutely clear what type of cells are represented by Ki-67
staining. They are probably the highly active MSCs themselves, but
also osteoblasts since they can also be found in the callus
directly (52).
In conclusion, we combined MSCs, EPCs, β-TCP
scaffold and a vascularized periosteal flap in the present study to
improve osteoconductive and osteoinductive qualities inherently
present in autologous bone grafts.
Acknowledgments
This project was funded in part by a LOEWE Center
for Cell and Gene Therapy Frankfurt grant from the Hessian Ministry
of Higher Education, Research, and the Arts [ref no. III L
4-518/17.004 (2010)] and the Friedrichsheim Foundation in
Frankfurt, Germany.
References
|
1
|
Bauer TW and Muschler GF: Bone graft
materials. An overview of the basic science. Clin Orthop Relat Res.
371:10–27. 2000. View Article : Google Scholar
|
|
2
|
Horner EA, Kirkham J, Wood D, Curran S,
Smith M, Thomson B and Yang XB: Long bone defect models for tissue
engineering applications: Criteria for choice. Tissue Eng Part B
Rev. 16:263–271. 2010. View Article : Google Scholar
|
|
3
|
Giannoudis PV, Dinopoulos H and Tsiridis
E: Bone substitutes: An update. Injury. 36(Suppl 3): S20–S27. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nandi SK, Roy S, Mukherjee P, Kundu B, De
DK and Basu D: Orthopaedic applications of bone graft & graft
substitutes: A review. Indian J Med Res. 132:15–30. 2010.PubMed/NCBI
|
|
5
|
Finkemeier CG: Bone-grafting and
bone-graft substitutes. J Bone Joint Surg Am. 84-A:454–464. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iacobellis C, Berizzi A and Aldegheri R:
Bone transport using the Ilizarov method: A review of complications
in 100 consecutive cases. Strateg Trauma Limb Reconstr. 5:17–22.
2010. View Article : Google Scholar
|
|
7
|
Doi K and Sakai K: Vascularized periosteal
bone graft from the supracondylar region of the femur.
Microsurgery. 15:305–315. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soldado F, Garcia Fontecha C, Haddad S,
Hernandez-Fernandez A, Corona P and Guerra-Farfan E: Treatment of
congenital pseudarthrosis of the tibia with vascularized fibular
periosteal transplant. Microsurgery. 32:397–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsumura G, Hibino N, Ikada Y, Kurosawa H
and Shin'oka T: Successful application of tissue engineered
vascular autografts: Clinical experience. Biomaterials.
24:2303–2308. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doi M, Nagano A and Nakamura Y:
Genome-wide screening by cDNA microarray of genes associated with
matrix mineralization by human mesenchymal stem cells in vitro.
Biochem Biophys Res Commun. 290:381–390. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marcacci M, Kon E, Moukhachev V, Lavroukov
A, Kutepov S, Quarto R, Mastrogiacomo M and Cancedda R: Stem cells
associated with macroporous bioceramics for long bone repair: 6- to
7-year outcome of a pilot clinical study. Tissue Eng. 13:947–955.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vögelin E, Jones NF, Huang JI, Brekke JH
and Lieberman JR: Healing of a critical-sized defect in the rat
femur with use of a vascularized periosteal flap, a biodegradable
matrix, and bone morphogenetic protein. J Bone Joint Surg Am.
87:1323–1331. 2005.PubMed/NCBI
|
|
13
|
Mastrogiacomo M, Scaglione S, Martinetti
R, Dolcini L, Beltrame F, Cancedda R and Quarto R: Role of scaffold
internal structure on in vivo bone formation in macroporous calcium
phosphate bioceramics. Biomaterials. 27:3230–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nauth A, Giannoudis PV, Einhorn TA,
Hankenson KD, Friedlaender GE, Li R and Schemitsch EH: Growth
factors: Beyond bone morphogenetic proteins. J Orthop Trauma.
24:543–546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Y, Tang W, Lin Y, Long J, Wang H, Liu
L and Tian W: Combination of bone tissue engineering and BMP-2 gene
transfection promotes bone healing in osteoporotic rats. Cell Biol
Int. 32:1150–1157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Madeddu P: Therapeutic angiogenesis and
vasculogenesis for tissue regeneration. Exp Physiol. 90:315–326.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henrich D, Seebach C, Kaehling C, Scherzed
A, Wilhelm K, Tewksbury R, Powerski M and Marzi I: Simultaneous
cultivation of human endothelial-like differentiated precursor
cells and human marrow stromal cells on beta-tricalcium phosphate.
Tissue Eng Part C Methods. 15:551–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seebach C, Henrich D, Kähling C, Wilhelm
K, Tami AE, Alini M and Marzi I: Endothelial progenitor cells and
mesenchymal stem cells seeded onto beta-TCP granules enhance early
vascularization and bone healing in a critical-sized bone defect in
rats. Tissue Eng Part A. 16:1961–1970. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seebach C, Henrich D, Wilhelm K, Barker JH
and Marzi I: Endothelial progenitor cells improve directly and
indirectly early vascularization of mesenchymal stem cell-driven
bone regeneration in a critical bone defect in rats. Cell
Transplant. 21:1667–1677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seebach C, Schultheiss J, Wilhelm K, Frank
J and Henrich D: Comparison of six bone-graft substitutes regarding
to cell seeding efficiency, metabolism and growth behaviour of
human mesenchymal stem cells (MSC) in vitro. Injury. 41:731–738.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gamradt SC and Lieberman JR: Genetic
modification of stem cells to enhance bone repair. Ann Biomed Eng.
32:136–147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keramaris NC, Calori GM, Nikolaou VS,
Schemitsch EH and Giannoudis PV: Fracture vascularity and bone
healing: A systematic review of the role of VEGF. Injury. 39(Suppl
2): S45–S57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo X, Zheng Q, Kulbatski I, Yuan Q, Yang
S, Shao Z, Wang H, Xiao B, Pan Z and Tang S: Bone regeneration with
active angiogenesis by basic fibroblast growth factor gene
transfected mesenchymal stem cells seeded on porous beta-TCP
ceramic scaffolds. Biomed Mater. 1:93–99. 2006. View Article : Google Scholar
|
|
24
|
Peng H, Wright V, Usas A, Gearhart B, Shen
HC, Cummins J and Huard J: Synergistic enhancement of bone
formation and healing by stem cell-expressed VEGF and bone
morphogenetic protein-4. J Clin Invest. 110:751–759. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar S, Wan C, Ramaswamy G, Clemens TL
and Ponnazhagan S: Mesenchymal stem cells expressing osteogenic and
angiogenic factors synergistically enhance bone formation in a
mouse model of segmental bone defect. Mol Ther. 18:1026–1034. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Atesok K, Li R, Stewart DJ and Schemitsch
EH: Endothelial progenitor cells promote fracture healing in a
segmental bone defect model. J Orthop Res. 28:1007–1014.
2010.PubMed/NCBI
|
|
28
|
Rozen N, Bick T, Bajayo A, Shamian B,
Schrift-Tzadok M, Gabet Y, Yayon A, Bab I, Soudry M and Lewinson D:
Transplanted blood-derived endothelial progenitor cells (EPC)
enhance bridging of sheep tibia critical size defects. Bone.
45:918–924. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jones AL, Bucholz RW, Bosse MJ, Mirza SK,
Lyon TR, Webb LX, Pollak AN, Golden JD and Valentin-Opran A; BMP-2
Evaluation in Surgery for Tibial Trauma-Allgraft (BESTT-ALL) Study
Group: Recombinant human BMP-2 and allograft compared with
autogenous bone graft for reconstruction of diaphyseal tibial
fractures with cortical defects. A randomized, controlled trial. J
Bone Joint Surg Am. 88:1431–1441. 2006.PubMed/NCBI
|
|
30
|
Daniels T, DiGiovanni C, Lau JT, Wing K
and Younger A: Prospective clinical pilot trial in a single cohort
group of rhPDGF in foot arthrodeses. Foot Ankle Int. 31:473–479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Driscoll SW and Fitzsimmons JS: The role
of periosteum in cartilage repair. Clin Orthop Relat Res. (Suppl):
S190–S207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Driscoll SW, Saris DB, Ito Y and
Fitzimmons JS: The chondrogenic potential of periosteum decreases
with age. J Orthop Res. 19:95–103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jaffe HL: Metabolic, Degenerative and
Inflammatory Diseases of Bone and Joints. 1st edition. Urban and
Schwarzenberg; München-Berlin-Wien: 1972
|
|
34
|
Dwek JR: The periosteum: What is it, where
is it, and what mimics it in its absence? Skeletal Radiol.
39:319–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allen MR, Hock JM and Burr DB: Periosteum:
Biology, regulation, and response to osteoporosis therapies. Bone.
35:1003–1012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nau C, Henrich D, Seebach C, Schröder K,
Fitzsimmons SJ, Hankel S, Barker JH, Marzi I and Frank J: Treatment
of large bone defects with a vascularized periosteal flap in
combination with biodegradable scaffold seeded with bone
marrow-derived mononuclear cells: An experimental study in rats.
Tissue Eng Part A. 22:133–141. 2016. View Article : Google Scholar
|
|
37
|
Arthur A, Zannettino A and Gronthos S: The
therapeutic applications of multipotential mesenchymal/stromal stem
cells in skeletal tissue repair. J Cell Physiol. 218:237–245. 2009.
View Article : Google Scholar
|
|
38
|
Eldesoqi K, Henrich D, El-Kady AM, Arbid
MS, Abd El-Hady BM, Marzi I and Seebach C: Safety evaluation of a
bioglass-polylactic acid composite scaffold seeded with progenitor
cells in a rat skull critical-size bone defect. PLoS One.
9:e876422014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eldesoqi K, Seebach C, Nguyen Ngoc C,
Meier S, Nau C, Schaible A, Marzi I and Henrich D: High calcium
bioglass enhances differentiation and survival of endothelial
progenitor cells, inducing early vascularization in critical size
bone defects. PLoS One. 8:e790582013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Henrich D, Hahn P, Wahl M, Wilhelm K,
Dernbach E, Dimmeler S and Marzi I: Serum derived from multiple
trauma patients promotes the differentiation of endothelial
progenitor cells in vitro: Possible role of transforming growth
factor-beta1 and vascular endothelial growth factor165. Shock.
21:13–16. 2004. View Article : Google Scholar
|
|
41
|
Henrich D, Seebach C, Wilhelm K and Marzi
I: High dosage of simvastatin reduces TNF-alpha-induced apoptosis
of endothelial progenitor cells but fails to prevent apoptosis
induced by IL-1beta in vitro. J Surg Res. 142:13–19. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Del Piñal F, García-Bernal FJ, Regalado J,
Ayala H, Cagigal L and Studer A: Vascularised corticoperiosteal
grafts from the medial femoral condyle for difficult non-unions of
the upper limb. J Hand Surg Eur Vol. 32:135–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fuchs B, Steinmann SP and Bishop AT: Free
vascularized corticoperiosteal bone graft for the treatment of
persistent nonunion of the clavicle. J Shoulder Elbow Surg.
14:264–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Camilli JA and Penteado CV: Bone formation
by vascularized periosteal and osteoperiosteal grafts. An
experimental study in rats. Arch Orthop Trauma Surg. 114:18–24.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghanaati S, Barbeck M, Orth C,
Willershausen I, Thimm BW, Hoffmann C, Rasic A, Sader RA, Unger RE
and Peters F: Influence of β-tricalcium phosphate granule size and
morphology on tissue reaction in vivo. Acta Biomater. 6:4476–4487.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Faour O, Dimitriou R, Cousins CA and
Giannoudis PV: The use of bone graft substitutes in large
cancellous voids: Any specific needs? Injury. 42(Suppl 2): S87–S90.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schultheiss J, Seebach C, Henrich D,
Wilhelm K, Barker JH and Frank J: Mesenchymal stem cell (MSC) and
endothelial progenitor cell (EPC) growth and adhesion in six
different bone graft substitutes. Eur J Trauma Emerg Surg.
37:635–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee DE, Ayoub N and Agrawal DK:
Mesenchymal stem cells and cutaneous wound healing: Novel methods
to increase cell delivery and therapeutic efficacy. Stem Cell Res
Ther. 7:372016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao L, Liu X, Zhang Y, Liang X, Ding Y,
Xu Y, Fang Z and Zhang F: Enhanced cell survival and paracrine
effects of mesenchymal stem cells overexpressing hepatocyte growth
factor promote cardioprotection in myocardial infarction. Exp Cell
Res. 344:30–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Merino-González C, Zuñiga FA, Escudero C,
Ormazabal V, Reyes C, Nova-Lamperti E and Salomón C: Aguayo C.
Mesenchymal stem cell-derived extracellular vesicles promote
angiogenesis: Potencial clinical application. Front Physiol.
7:242016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Potapova IA, Gaudette GR, Brink PR,
Robinson RB, Rosen MR, Cohen IS and Doronin SV: Mesenchymal stem
cells support migration, extracellular matrix invasion,
proliferation, and survival of endothelial cells in vitro. Stem
Cells. 25:1761–1768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hoch AI, Binder BY, Genetos DC and Leach
JK: Differentiation-dependent secretion of proangiogenic factors by
mesenchymal stem cells. PLoS One. 7:e355792012. View Article : Google Scholar : PubMed/NCBI
|