Introduction
Changing life styles in China have contributed to a
rise in atherosclerosis resulting in coronary heart disease (CHD),
causing serious morbidity and mortality (1). Growing evidence has revealed that
long-lasting and low-grade inflammation is a key cause of the
progression of atherosclerosis (2,3).
The abnormal proliferation of vascular smooth muscle cells (VSMCs)
and their expression of various pro-inflammatory cytokines such as
interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) play an
important role in the process of inflammation within
atherosclerotic plaques (4,5).
Angiotensin II (AngII), which is increased significantly in the
serum of patients with acute coronary syndrome (ACS), may promote
the process of atherosclerosis and plaque rupture by recruiting
monocytes, activating macrophages, producing pro-inflammatory
factors and causing oxidative stress (6,7).
More importantly, these pro-atherosclerotic effects of AngII do not
depend on the elevation of systolic blood pressure.
Peroxisome proliferator-activated receptor-γ
(PPAR-γ), a member of the nuclear hormone family, plays a critical
role in regulating glucose homeostasis and adipose metabolism.
After activation, PPAR-γ binds to specific PPAR response elements
(PPREs) and then regulates the transcription of its target genes
(8,9). In recent years growing evidence has
shown that the activity of PPAR-γ may potentially downregulate
inflammatory responses caused by various proinflammatory stimuli
(10–12). Moreover, PPAR-γ also participates
in the production of AngII-induced proinflammatory factors by
VSMCs, and thiazolidinediones (TZDs), synthetic PPAR-γ agonists,
may significantly inhibit this inflammatory effect (13). PPAR-γ also plays an important role
in regulating VSMC proliferation which can significantly affect the
process of cardiac fibrosis (14).
Oxidative stress, characterized by the
overproduction of intercellular reactive oxygen species (iROS), can
trigger multiple pathological responses related to atherosclerosis,
including oxidation of lipids and proteins, proliferation and
migration of VSMCs, and overexpression of proinflammatory cytokines
(15). Common risk factors for
atherosclerosis such as hypertension, hypercholesterolemia, obesity
and smoking may enhance the production of ROS in VSMCs. NADPH
activation is the principle cause of ROS production by VSMCs
(16). Previous studies have
revealed that inhibition of the activation of NADPH reduced
high-fat diet-induced increase in the area of atherosclerostic
plaques in ApoE−/− mice (17,18), compared with the control
group.
Curcumin (Cur) is a natural polyphenol and is the
principal curcuminoid present in Curcuma longa. Cur is
responsible for the yellow color of turmeric and has been used in
herbal remedies to treat inflammation- and infection-related
diseases in China and India since ancient times (19). Cur has many pharmacological
effects including anti-inflammatory, antioxidant, anti-microbial,
anti-proliferative, neuroprotective and cardio-protective
activities (5,14,20,21). Recently, some studies have found
that Cur attenuates the progression of atherosclerosis by
inhibiting pro-inflammatory cytokine expression and ROS production,
in vivo and in vitro (5,22).
Our previous study found that Cur inhibited LPS-induced
inflammation through a TLR4-mediated and ROS-relative signaling
pathway in VSMCs (5).
Furthermore, Cur attenuates cardiac fibrosis by elevating PPAR-γ
activation (14). However, the
molecular mechanisms underlying the anti-inflammatory and
anti-proliferative effects of Cur in AngII-stimulated VSMCs are not
well known.
Therefore, our present study aimed to ascertain
whether Cur can suppress AngII-induced expression of
pro-inflammatory mediators and whether its cardioprotective effect
is partially dependent on PPAR-γ activity and a reduction in
oxidative stress.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were provided by Gibco-BRL (Carlsbad, CA, USA).
Curcumin (purity over 98%), penicillin, streptomycin, Tris, EDTA,
2′,7′-dichlorodihydrofluororescein diacetate (DCFH-DA),
diphenyleneiodonium (DPI),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
GW9662, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased
from Sigma Chemical Co. (St. Louis, MO, USA). The following primary
antibodies were used: PPAR-γ (GW21258-50UG; Sigma Chemical Co.),
p47phox (4312S; Cell Signaling Technology., Inc., Danvers, MA,
USA), inducible NO synthase (iNOS; xy-2977; Xin Yu Biotech Co.,
Shanghai, China) and histone (orb48770; Biorbyt Ltd., Cambridge,
UK). TranZol, TransStrat Green qPCR SuperMix, EasyScript Reverse
Transcriptase and the β-actin antibody (VSC47778; www.biomart.cn) were purchased from TransGen
Biotechnology (Beijing, China). IL-6 and TNF-α enzyme-linked
immunosorbent assay (ELISA) kits were purchased from Thermo Fisher
Scientific (Rockford, IL, USA).
VSMC culture
The present study was approved by the Ethics
Committee of the Laboratory Animal Institute in the School of
Medicine at Zhengzhou University, and carried out in strict
accordance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
publication no. 85–23, revised 1996). Male Sprague-Dawley rats
(weighing 150–180 g) were purchased from the Laboratory Animal
Institute in the School of Medicine at Zhengzhou University. VSMCs
were isolated from the thoracic aorta of rats, according to a
previously described method (23). Cells were cultured in DMEM
containing 20% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C. Cells that were between passage 3 and 10 were used for all
experiments. Cells at 80–90% confluence in culture dishes were
growth-arrested by serum starvation for 16 h.
Cell proliferation assay
Cells were planted at a density of 6,000 cells/well
in 96-well plates. An MTT reduction assay and cell count
experiments were used to determine the amount of cell
proliferation. After the indicated treatments, the medium was
removed and the cells were incubated with MTT (5 mg/ml) for 4 h at
37°C. The dark blue formazan crystals that formed in intact cells
were solubilized with DMSO, and then the absorbance was measured at
490 nm on a microplate reader (Bio-Rad, Hercules, CA, USA). Cell
count experiments were performed as previously described. Cells
were counted by a hemocytometer using light microscopy.
Real-time reverse-transcriptase
polymerase chain reaction
Total RNA was extracted by TransZol reagent
(TransGen Biotechnology). The quality of total mRNA was measured by
denaturing agarose gel electrophoresis containing 1.5%
formaldehyde. Total RNA concentration and purity were determined
using UV-Vis spectroscopy with the Bio-Rad SmartSpec 5000 system
(Bio-Rad). cDNA was synthesized from 1 µg of total RNA in a
20 µl reaction using oligo(dT)18 primers and
TransScript™ reverse transcriptase (TransGen Biotechnology).
Primers for rat PPAR-γ, TNF-α, IL-6, iNOS, p47phox and
glyceraldehyde 3-phosphate dehydroge-nase (GAPDH) were designed
using Beacon designer v6.0 software (Premier Biosoft, Palo Alto,
CA, USA) and are listed in Table
I. GAPDH was used as an endogenous control. mRNA levels of
PPAR-γ, TNF-α, IL-6, iNOS and GAPDH were measured using real-time
PCR on the ABI PRISM 7000 sequence detection PCR system (Applied
Biosystems, Foster City, CA, USA). A melting point dissociation
curve was used to confirm that only a single PCR product was
obtained. Results were expressed as fold difference relative to the
level of GAPDH by the 2−ΔΔCT method.
 | Table IPrimers used for real-time PCR
analysis. |
Table I
Primers used for real-time PCR
analysis.
| Gene | Oligonucleotide
primer sequences (5′-3′) |
|---|
| IL-6 | F:
GAGAAAAGAGTTGTGCAATGGC |
| R:
ACTAGGTTTGCCGAGTAGACC |
| TNF-α | F:
TCCCAACAAGGAGGAGAAGT |
| R:
TGGTATGAAGTGGCAAATCG |
| p47phox | F:
GAGACATACCTGACGGCCAAAGA |
| R:
AGTCAGCGATGGCCCGATAG |
| iNOS | F:
CCACGCTCTTCTGTCTACTGAAC |
| R:
ACGGGCTTGTCACTCGAG |
| GADPH | F:
ATCGGCAATGAGCGGTTCC |
| R:
AGCACTGTGTTGGCATAGAGG |
Western blot analysis
Lysates of ~6×106 VSMCs were prepared
using 200 µl ice-cold lysis buffer (pH 7.4) (50 mmol/l
HEPES, 5 mmol/l EDTA, 100 mmol/l NaCl, 1% Triton X-100, protease
inhibitor cocktail; Roche, Mannheim, Germany) in the presence of
phosphatase inhibitors (50 mmol/l sodium fluoride, 1 mmol/l sodium
orthovanadate, 10 mmol/l sodium pyrophosphate, 1 nmol/l
microcystin). The nuclear PPAR-γ protein was extracted using a
Pierce NE-PER kit (Pierce, Rockford, IL, USA). The protein
concentration was measured using a BCA protein assay kit. Samples
underwent 10 or 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and were transferred onto a
polyvinylidene difluoride membrane in a semi-dry system, which was
blocked with 5% fat-free milk in TBST buffer (20 mmol/l Tris-HCl,
137 mmol/l NaCl and 0.1% Tween-20), and incubated with primary
antibodies recognizing PPAR-γ (1:400), iNOS (1:500), p47phox
(1:400), histone (1:1,000), and β-actin (1:2,000), respectively, in
TBST buffer overnight, washed and incubated with secondary
antibodies for 90 min. The optical density of the bands was scanned
and quantified using a Gel-Pro Analyzer v4.0 (Media Cybernetics LP,
Silver Spring, MD, USA). β-actin was used as an endogenous control.
Data were normalized to β-actin levels.
Enzyme-linked immunoassay for cytokines
and chemokines
VSMCs were cultured into 6-well plates at
5×106 cells/well and incubated with Cur (5, 10 and 20
µmol/l) (14) with or
without AngII (10−7 mol/l) for 24 h. Cells were
pretreated with the PPAR-γ antagonist GW9662 (10 µmol/l) and
the NADPH oxidase inhibitor DPI (25 µmol/l) for 1 h, and
then stimulated with AngII (10−7 mol/l) for another 24
h. The amounts of TNF-α and IL-6 in the supernatants were measured
by ELISA according to the manufacturer's instructions.
Measurement of nitrite
The Griess reaction was used to determine the level
of nitrite, a stable precursor of NO (24). Fifty microliters of the culture
supernatant was mixed with an equal volume of Griess reagent (0.1%
naphthyl-ethylenediamine, 1% sulfanylamide and 2.5% phosphoric
acid). Absorbance was measured on a microplate reader at 540 nm,
using a calibration curve with sodium nitrite standards.
Intracellular ROS assay
The level of intracellular ROS was measured using
the DCFH-DA method, based on the ROS-dependent oxidation of DCFH to
the highly fluorescent DCF. DCFH was dissolved in methanol at 10
mmol/l and then diluted by a factor of 500 in Hank's balanced salt
solution (HBSS) to give a final DCFH concentration of 20
µmol/l. The cells were incubated with DCFH-DA for 1 h and
then treated with HBSS containing Cur (5, 10 or 20 µmol/l)
or AngII (10−7 mol/l) for another 100 min. The
fluorescence was immediately measured using 485 nm for excitation
and 528 nm for emission on the iMark™ microplate absorbance reader
(Bio-Rad).
Stable free radical scavenging
activity
Stable free radical scavenging activity was detected
using the method reported by Jeong et al (25). Briefly, 100 µmol/l of DPPH
radical solution was dissolved in 100% ethanol. The mixture was
shaken vigorously and allowed to stand for 10 min in the dark. The
test materials (100 µl each) were added to 900 µl of
the DPPH radical solution. After incubation at room temperature for
30 min, the absorbance at 517 nm was measured using the SPECTRA
(shell) reader.
Statistical analysis
The significance between groups was analyzed using
ANOVA, and the difference between each of the 2 groups was detected
using the post hoc test using 'Statistic version 8.0' (Statsoft
Inc., Tulsa, OK, USA) software. Data are presented as mean ±
standard deviation (SD). A value of P<0.05 was considered to be
statistically significant.
Results
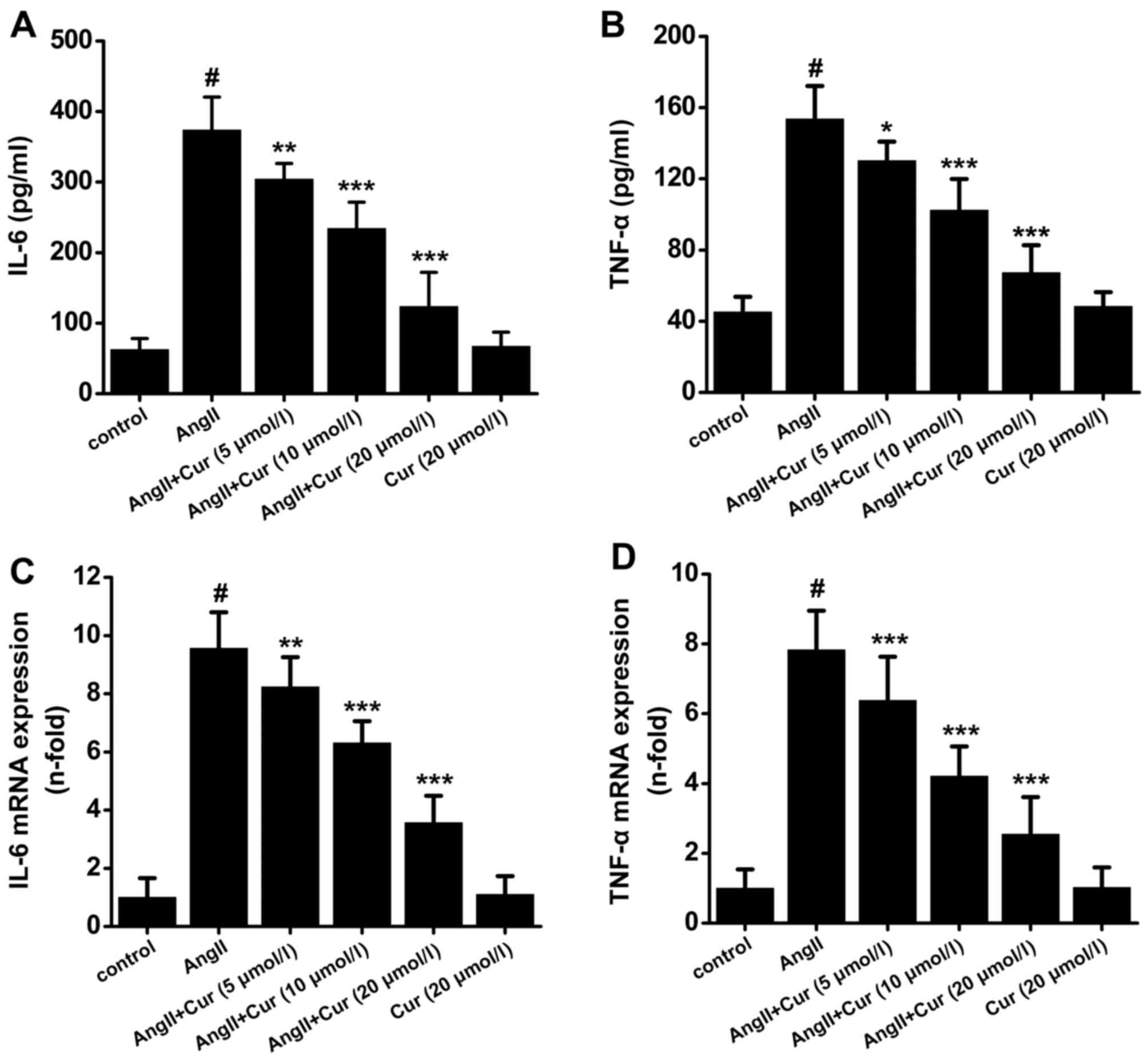
Cur inhibits IL-6 and TNF-α expression in
AngII-stimulated VSCMs
VSMCs express a number of pro-inflammatory cytokines
in response to different stimuli, which can dramatically promote
the progression of atherosclerotic plaques. As shown in Fig. 1, stimulation of VSMCs with AngII
(10−7 mol/l) for 24 h caused a significant increase in
the production of IL-6 and TNF-α. However, pretreatment with Cur
attenuated AngII-induced production of IL-6 and TNF-α in a
concentration-dependent manner (Fig.
1A and B). Moreover, Cur also dose-dependently decreased the
mRNA expression of IL-6 and TNF-α in AngII-stimulated VSMCs
(Fig. 1C and D). Treatment with
Cur (20 µmol/l) did not affect the expression of IL-6 and
TNF-α (Fig. 1).
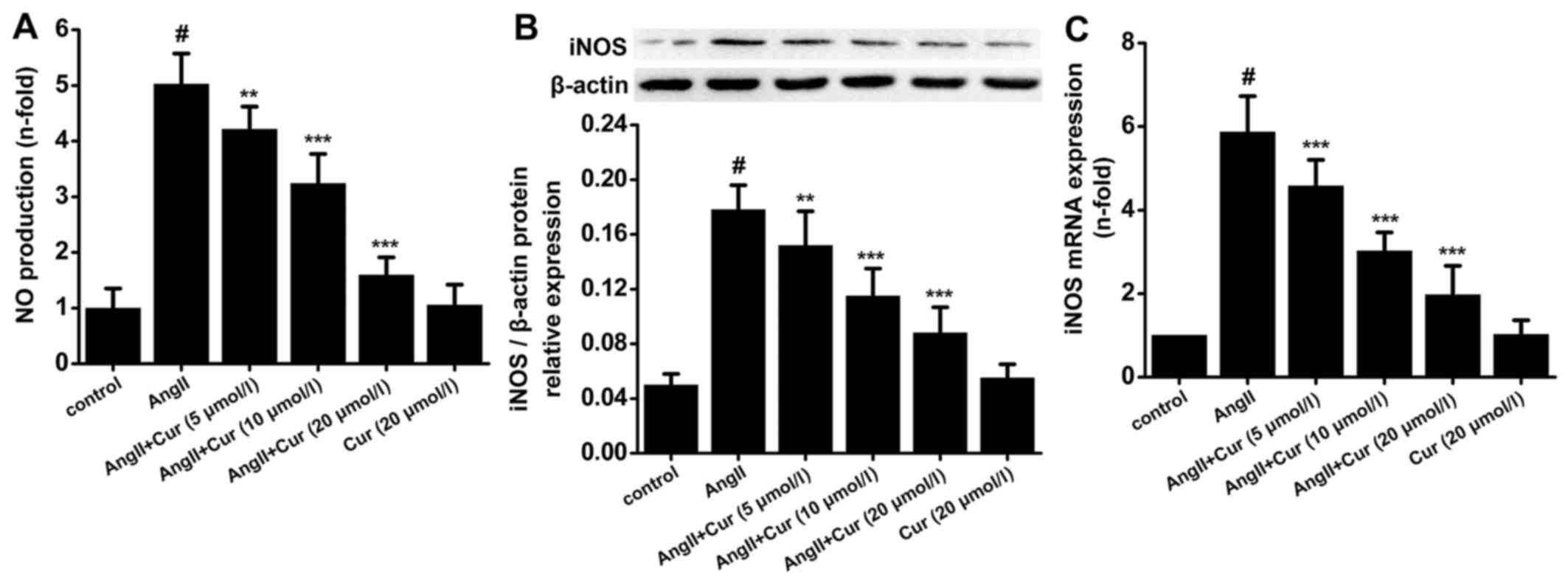
Cur attenuates AngII-induced NO
production and iNOS expression
Cur markedly attenuated AngII-induced NO production
in a concentration-dependent manner, but did not change NO
production in the absence of AngII stimulation (Fig. 2A). Furthermore, AngII
significantly increased the protein and mRNA expression of iNOS in
VSMCs, which was concentration-dependently attenuated by Cur.
Treating VSMCs with only Cur had no affect on the expression of
iNOS mRNA and protein (Fig. 2B and
C). These results indicated that Cur attenuated AngII-induced
NO production in VSMCs, which was partly due to inhibition of iNOS
activity.
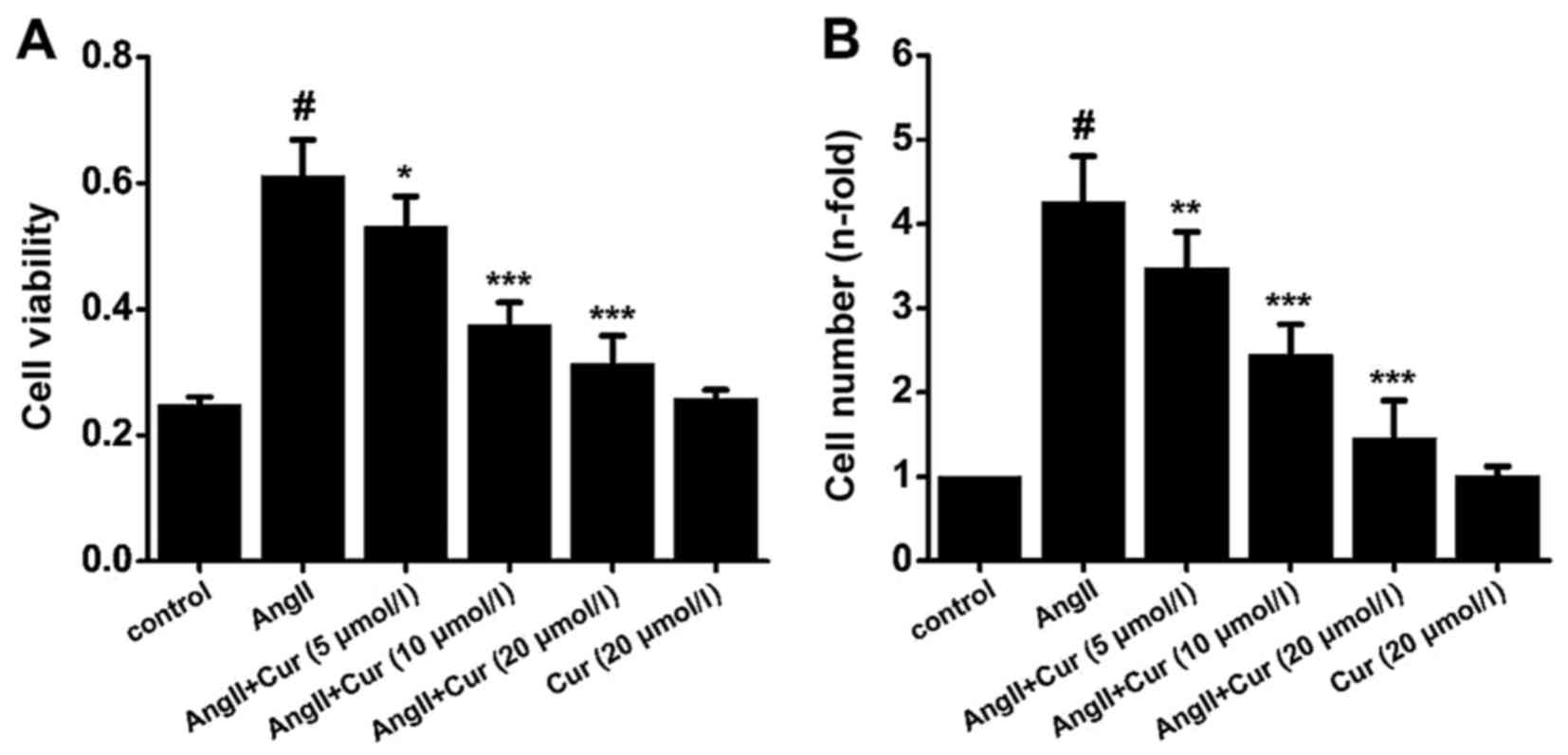
Cur inhibits AngII-induced proliferation
of VSMCs
As shown in Fig.
3, treating VSMCs with AngII (10−7 mol/l) for 24 h
significantly increased their proliferation, while pretreating the
cells with Cur (5, 10 and 20 µmol/l) suppressed this effect.
However, treatment with Cur (20 µmol/l) alone did not affect
the proliferation of VSMCs (Fig.
3).
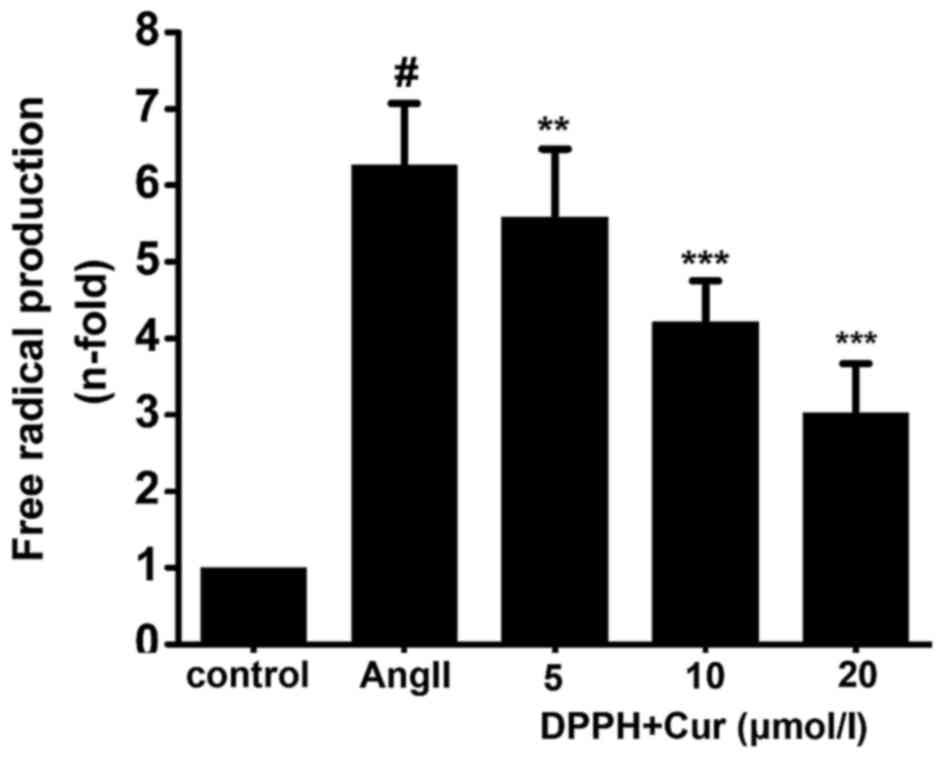
Cur reduces AngII-induced free radical
production in VSMCs
The free radical scavenging activity of Cur in
AngII-stimulated VSMCs was measured using the DPPH reduction assay.
Cur concentration-dependently scavenged the DPPH free radical
caused by ROS in the AngII-stimulated VSMCs (Fig. 4). The results indicated that Cur
exhibited free radical scavenging activity in the AngII-stimulated
VSMCs.
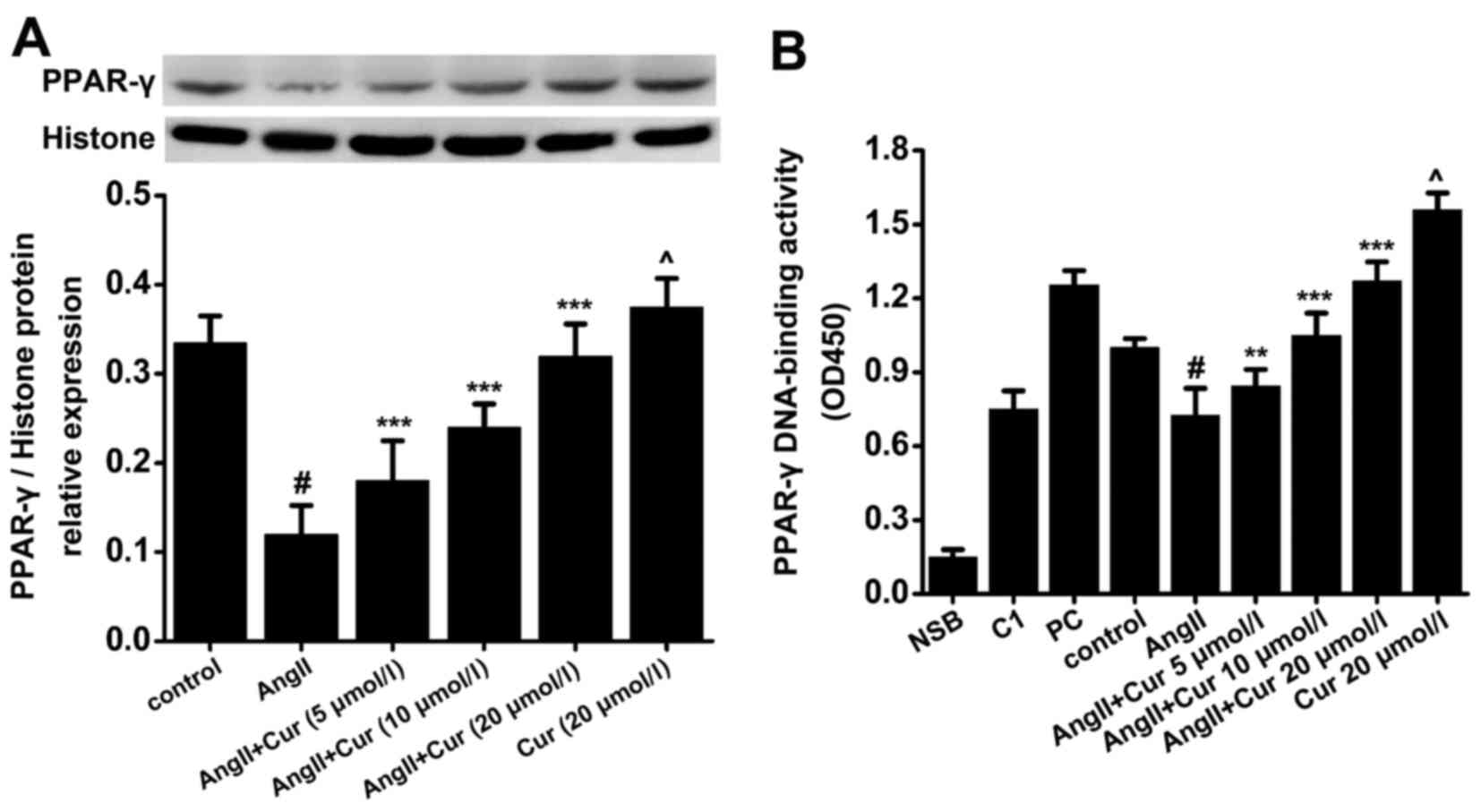
Cur elevates the expression and activity
of PPAR-γ in AngII-stimulated VSMCs
To evaluate the expression and translocation of
PPAR-γ into the nucleus, nuclear protein was extracted and analyzed
by western blot analysis and DNA-binding assay. As shown in
Fig. 4, treating the cells with
AngII (10−7 mol/l) for 24 h markedly decreased the
expression of PPAR-γ in the nucleus and blocked its ability to bind
the DNA response element PPRE. However, pretreating the cells with
Cur significantly increased both PPAR-γ translocation and bound
PPRE in a concentration manner, compared with the AngII-treated
cells (Fig. 5). Moreover,
exposure to Cur (20 µmol/l) alone for 24 h caused an
increase in the nuclear expression and activity of PPAR-γ in the
VSMCs, compared with the control cells (Fig. 5). These results indicated that Cur
may be a potential promoter of PPAR-γ activity in AngII-stimulated
VSMCs.
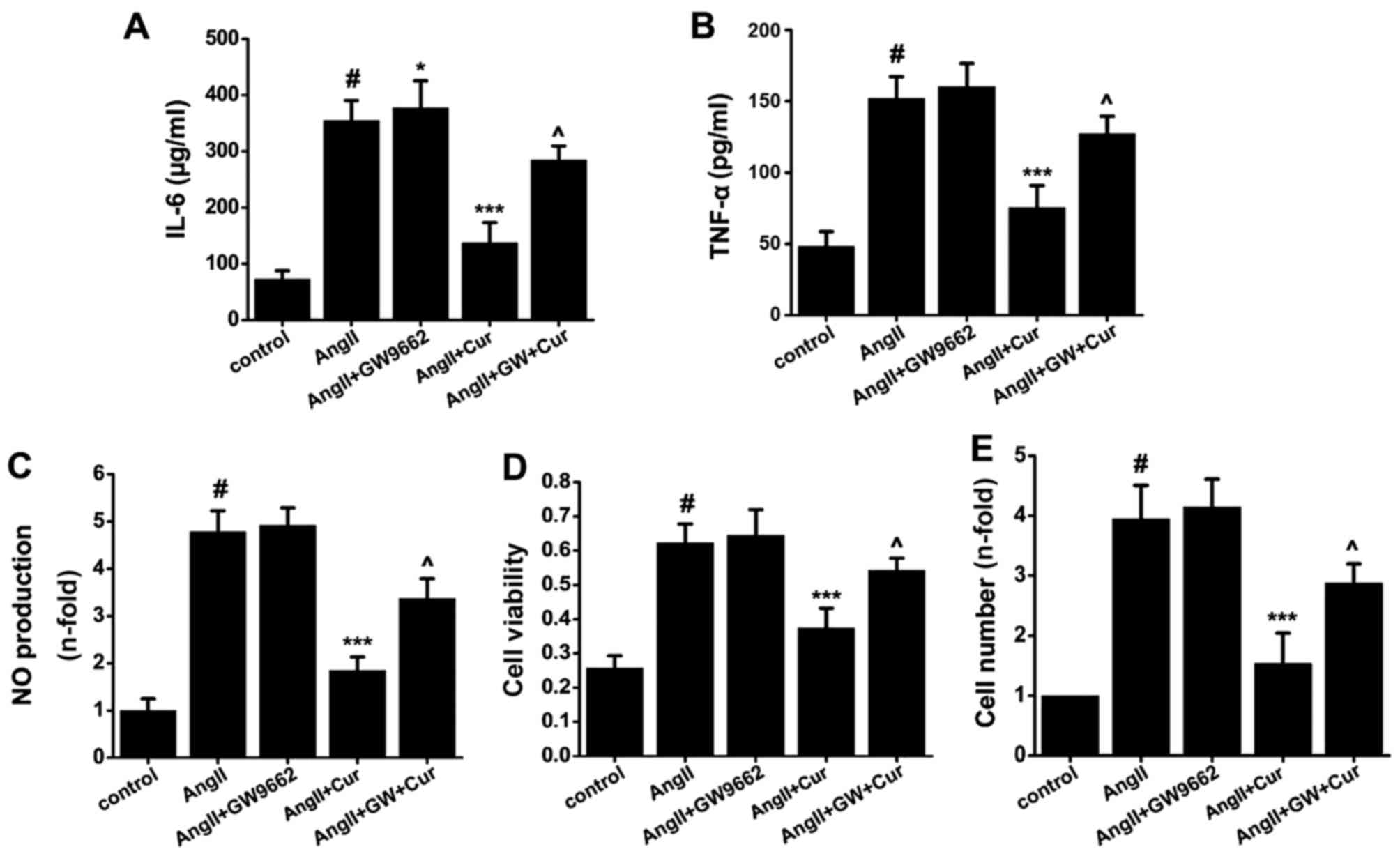
Relationship between PPAR-γ and the
anti-inflammatory and anti-proliferative effect of Cur in
VSMCs
To evaluate whether the anti-inflammatory and
anti-proliferative effects of Cur are dependent upon PPAR-γ, VSMCs
were pretreated with GW9662 (10 µmol/l), an antagonist of
PPAR-γ, for 1 h, treated with Cur (20 µmol/l) for 1 h, and
then exposed to AngII (10−7 mol/l) for another 24 h. As
shown in Fig. 6, compared with
the control cells, AngII significantly increased the production of
IL-6, TNF-α and NO (Fig. 6A–C),
but this was significantly attenuated by Cur treatment. The PPAR-γ
antagonist GW9662 significantly reversed the inhibitory effect of
Cur on the AngII-induced inflammation in VSMCs (Fig. 6A–C). As shown in Fig. 6, Cur significantly inhibited
AngII-induced proliferation of VSMCs, which was reversed by
pretreatment with GW9662 (Fig. 6D and
E). The results suggest that the activation of PPAR-γ plays a
key role in Cur-mediated suppression of inflammatory factor
production. However, compared with AngII and Cur, pretreating the
cells with GW9662 to inhibit the activity of PPAR-γ did not
completely suppress the anti-inflammatory and anti-proliferative
effects of Cur in AngII-stimulated VSMCs, which suggests that other
PPAR-γ-independent molecular mechanisms may partially contribute to
these effects.
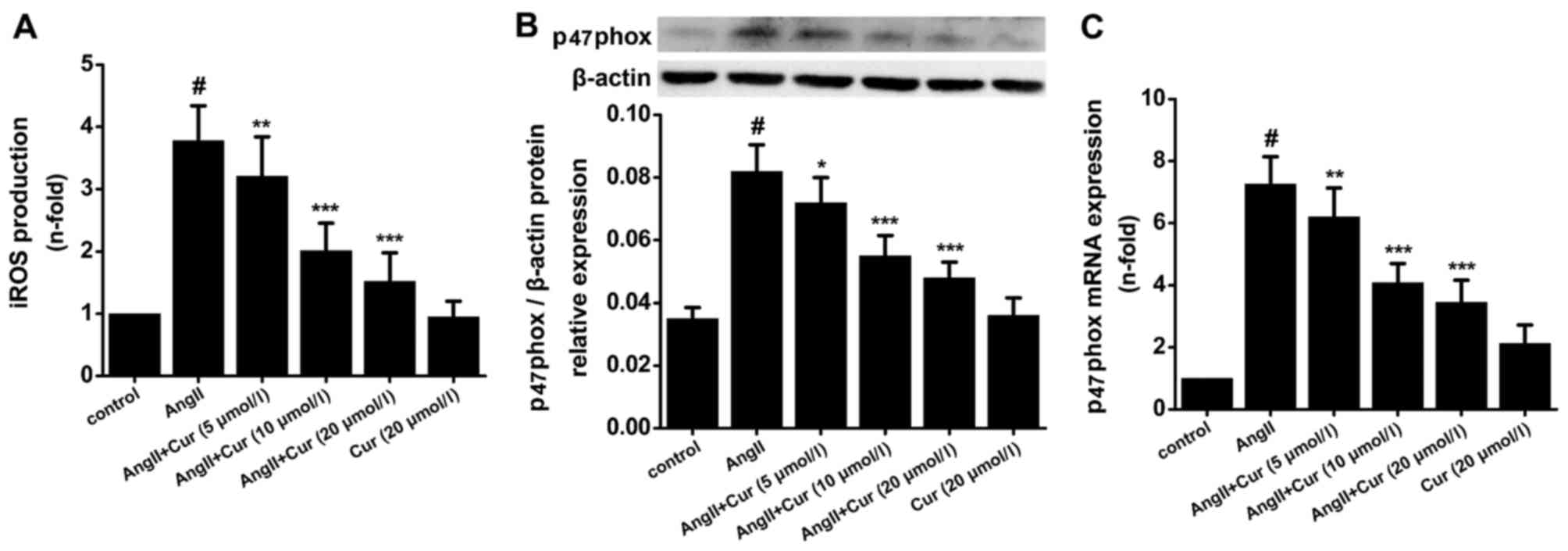
Cur suppresses AngII-induced iROS
production and p47phox expression in VSMCs
The increase in iROS during inflammation causes
various pathological responses such as the expression of
pro-inflammatory cytokines, proliferation of VSMCs, and oxidation
of lipids, which promotes the progression of atherosclerosis.
According to our data, treating VSMCs with AngII (10−7
mol/l) for 24 h significantly increased iROS production, and
pretreatment with Cur concentration-dependently attenuated this
effect (Fig. 7A). p47phox is an
important component of NAPDH oxidase which is the main source of
iROS production in VSMCs. As shown in Fig. 7, Cur concentration-dependently
suppressed AngII-induced expression of p47phox mRNA and protein
(Fig. 7B and C).
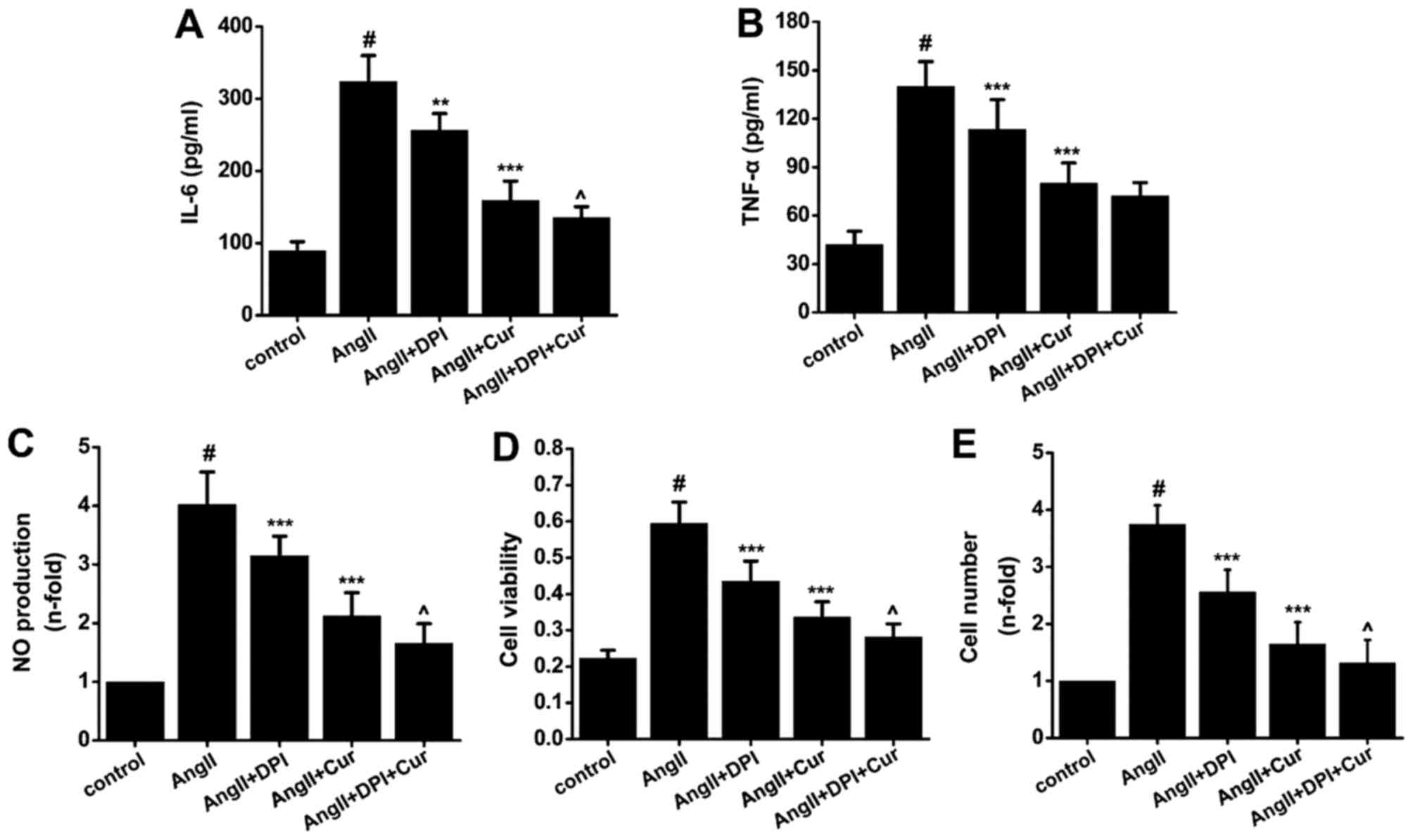
Relationship between oxidative stress and
the anti-inflammatory and anti-proliferative effects of Cur on
VSMCs
To evaluate whether the increase in iROS was related
to the anti-inflammatory and anti-proliferative effect of Cur in
VSMCs, cells were pretreated with or without DPI (25
µmol/l), an antagonist of NADPH oxidase, for 1 h before
treatment with Cur (20 µmol/l) for 1 h, and then incubated
with AngII (10−7 mol/l) for 24 h. Our data indicated
that Cur and DPI both partially inhibited AngII-induced expression
of IL-6 (Fig. 8A), TNF-α
(Fig. 8B) and NO (Fig. 8C) in VSMCs. Pretreatment with a
combination of Cur and DPI synergistically inhibited AngII-induced
inflammation in VSMCs. Proliferation of VSMCs was also decreased
when the cells were pretreated with Cur and DPI (Fig. 8D and E). These results suggest
that Cur inhibits AngII-induced inflammation and proliferation in
VSMCs partly through suppressing NADPH oxidase-mediated iROS.
Discussion
In the present study, we observed that Cur
concentration-dependently suppressed AngII-induced production of
TNF-α, IL-6 and NO and inhibited the proliferation of VSMCs in
vitro. Our results indicated that the inhibitory effect of Cur
on AngII-induced inflammation and proliferation of VSMCs was partly
dependent on enhancing PPAR-γ activity and reducing NADPH-mediated
iROS production. These findings show a novel relationship between
the anti-inflammatory and anti-proliferative effect of Cur and
PPAR-γ activation and oxidative response in AngII-induced VSMCs,
which enables a better understanding of the molecular mechanisms of
the beneficial effect of Cur on atherosclerosis.
Although atherosclerosis is regarded as a complex
pathological disease, the long-lasting and low-grade inflammatory
response within the arterial walls is a critical factor that
enhances the progression of atherosclerosis and plaque instability
(3). IL-6, which is elevated in
the serum of patients with ACS, is a potential pro-inflammatory
factor as it increases the release of fibrinogen and promotes
platelet aggregation (26). TNF-α
enhances the progression of atherosclerosis and this process
depends upon the induction of endothelium dysfunction, inflammatory
cytokine production, and the increased apoptosis of VSMCs (26). AngII promotes inflammatory
responses and oxidative stress of various cell types such as
endothelium, VSMCs, and macrophages, within atheroscle-rotic
plaques (27,28). AngII upregulates the expression of
various pro-inflammatory mediators in VSMCs including TNF-α, MCP-1,
IL-6 and nitric oxide synthase (29). In our previous study, we
demonstrated that Cur significantly suppressed LPS-induced
expression of TNF-α and MCP-1, two mediators that also play an
important role in the progression of inflammatory responses within
atherosclerotic plaques by inducing macrophage chemotaxis and cell
apoptosis in VSMCs (5).
Therefore, we postulated that Cur may inhibit AngII-induced
inflammation in VSMCs. Our data showed that Cur significantly
decreased AngII-induced production of TNF-α and IL-6, and the
anti-inflammatory effect of Cur was concentration-dependent.
Previous studies have shown that Cur also suppressed ox-LDL, TNF-α
and PMA-induced inflammation in different cell types, which
indicates that the anti-inflammatory effect of Cur is multifaceted
and not only dependent upon AngII.
The production of NO in endothelial cells by the
enzyme ecNOS is beneficial for attenuating AngII-induced
dysfunction in endothelial cells in the initial formation of
atherosclerotic plaques (30).
However, high concentrations of NO promote the progression of
atherosclerosis by enhancing intercellular ROS production and
causing significant endothelial cell dysfunction. High amounts of
NO react with superoxide to become peroxynitrite, causing oxidative
stress and then increasing intercellular ROS (31). Moreover, deficiency in iNOS is
accompanied by a decrease in NO production reducing atherosclerosis
in apolipoprotein E-deficient mice (32). These studies indicate that
suppressing the production of NO by iNOS may be a possible way to
delay the progression of atherosclerosis. Additionally, in our
previous study, we found that Cur can inhibit LPS-induced activity
of iNOS and then suppress production of NO in VSMCs (5). In our present experiments, we
observed that Cur suppressed AngII-induced NO production,
accompanied by a decrease in the expression of iNOS (both protein
and mRNA) in VSMCs. These results suggest that Cur can
significantly inhibit AngII-induced NO production by suppressing
the activity of iNOS in VSMCs, providing a new molecular mechanism
for the anti-inflammatory and anti-atherosclerotic effect of
Cur.
Although PPAR-γ was initially thought to regulate
the metabolism of glucose and lipid, it also participates in
controlling various physiological functions, especially
inflammation and proliferation (33). Previous studies have shown that
AngII can inhibit PPAR-γ activity, which accelerates the
progression of atherosclerosis and increases plaque instability by
upregulating the production of numerous types of proinflammatory
factors production (12). After
treatment with AngII, the concentration of MCP-1, VCAM-1 and ICAM-1
were significantly increased in apolipoprotein E-deficient mice
(34). Moreover, some PPAR-γ
agonists such as rosiglitazone and telmis-artan can suppress
AngII-induced inflammation in vivo and in vitro
(13,35). These studies suggest that
increasing PPAR-γ activity inhibits AngII-induced production of
proinflammatory factors, providing an effective method to delay the
progression of atherosclerosis and enhance plaque stability.
Additionally, Cur is a potential PPAR-γ agonist. Siddiqui et
al reported that by upregulating PPAR-γ, Cur inhibited
inflammation in an experimental model of sepsis (36). Cur also suppressed the expression
of TNF-α and ameliorated renal failure by activating PPAR-γ in 5/6
nephrectomized rats (37). In the
liver, Cur increased PPAR-γ activity and attenuated oxidative
stress and suppressed inflammation in CCl4-induced
injury and fibrogenesis (38).
Moreover, our previous study demonstrated that Cur inhibited
hypertension-induced cardiac fibrosis by activating PPAR-γ
(14). In this study, we found
that treating VSMCs with AngII increased the expression of TNF-α
and IL-6 and caused VSMC over-proliferation. This was accompanied
by the suppression of PPAR-γ, which is consistent with previous
studies. Treatment with Cur significantly attenuated AngII-induced
expression of pro-inflammatory cytokines and VSMC proliferation by
increasing PPAR-γ expression and elevating its activation.
Meanwhile, the PPAR-γ antagonist GW9662 partially reversed the
anti-inflammatory and anti-proliferative effects of Cur in
AngII-stimulated VSMCs by inhibiting PPAR-γ activation. These
results suggest that the activation of PPAR-γ, an
inflammation-related nuclear transcription factor, is involved in
the mechanism by which Cur suppresses AngII-induced inflammation
and proliferation in VSMCs.
Growing evidence shows that oxidative stress,
characterized by the overexpression of intercellular ROS, plays a
critical role in the progression of atherosclerosis (15). The common risk factors for
atherosclerosis such as hypercholesterolemia, hypertension, aging,
smoking and diabetes can induce overproduction of intercellular
ROS, not only in VSMCs but also in endothelial cells and
adventitial cells (39). ROS
production affects almost all of the processes of atherosclerosis,
such as lipid overload, abnormal lipid metabolism, calcium-related
signaling pathway inhibition, endoplasmic reticulum stress, VSMC
proliferation and endothelial cell dysfunction (40). ROS cause direct cellular damage,
but it can also act as potential secondary messengers that
participate in the inflammatory response (5). Therefore, we believe that there is a
close relationship between ROS production and inflammation. Some
studies have shown that inhibition of ROS production may be an
effective way to suppress atherosclerosis (41,42). Growing evidence indicates that Cur
may be a potential scavenger of intracellular ROS (5,43,44). In our previous study, we found
that Cur can inhibit NADPH-mediated iROS and free radical
production in LPS-stimulated VSMCs (5). In the present study, we found that
AngII significantly increased intracellular ROS production in
VSMCs, which agrees with previous studies (13,45). Treatment with Cur effectively
attenuated the AngII-induced increase in intracellular ROS and
inhibited free radical production by VSMCs. To detect whether the
anti-inflammatory and anti-proliferative effects of Cur are linked
with inhibition of ROS production, we pretreated the cells with
DPI, a ROS antagonist. Our results indicated that this was
accompanied by a decrease in ROS production, pro-inflammatory
cytokine release and cell proliferation in AngII-stimulated VSMCs.
These responses were further decreased when the cells were treated
with a combination of Cur and DPI. These results indicate that
targeting ROS may be a critical mechanism by which Cur induces its
anti-inflammatory and anti-proliferative effects on
AngII-stimulated VSMCs.
It is generally recognized that the
membrane-associated enzyme NADPH oxidase is the main source of
intracellular ROS production in mammal cells. At present, NADPH
oxidase is expressed in almost all cell types of the cardiovascular
system, including VSMCs, cardiac fibrosis, cardiomyocytes, and
endothelial cells (16). Recent
studies have focused on the relationship between NADPH oxidase
activation and the progression of atherosclerosis (18,46). p47phox is one of the three main
subunits of the NADPH oxidase cytosolic component (16). Stimulation of cells causes an
increase in p47phox expression and an elevation in intracellular
ROS (47). Therefore it is
generally recognized that detection of p47phox expression is an
effective way to determine NADPH oxidase activation. In
vivo, a previous study revealed that impaired p47phox function
significantly reduced atherosclerotic plaque area and attenuated
plaque vulnerability caused by a high-fat diet (compared with the
ApoE−/− mice of normal p47phox function) (17). These previous studies suggest that
inhibition of NADPH oxidase activation may be an effective way to
suppress the progression of atherosclerosis. Moreover, studies have
reported that the inhibitory effect of Cur on NADPH oxidase
activation may play a critical role in its antioxidation by
decreasing intracellular ROS production (5,48,49). As shown by our results, the
expression of p47phox was significantly elevated after stimulation
by AngII, and was accompanied by an increase in ROS production.
Treatment with Cur effectively suppressed AngII-induced elevation
of p47phox expression. These results revealed that the inhibitory
effect of Cur on ROS production may be partially mediated by NADPH
oxidase. It is worth mentioning that other pathways have also been
observed to contribute to ROS production, such as the mitochondrial
electron chain, and it is worthwhile to explore other signaling
pathways that participate in the inhibitory effect of Cur on ROS
production. It is possible that, in addition to NADPH oxidase,
other pathways contribute to this effect in AngII-stimulated
VSMCs.
In conclusion, the present study demonstrated that
Cur attenuated AngII-induced production of TNF-α, IL-6 and NO, and
cell proliferation in VMSCs. The anti-inflammatory and
anti-proliferative effect of Cur may partially depend on an
increase in PPAR-γ activity and suppression of NADPH
oxidase-mediated intracellular ROS production. These results may
provide a novel mechanism to explain the pharmacological effect of
Cur on chronic inflammation-related diseases, and its potentially
beneficial effect on atherosclerosis.
Acknowledgments
This study was supported by the Youth Foundation of
the First Affiliated Hospital of Zhengzhou University (YFFAHZZ
2013020105 to Z.M.).
References
|
1
|
Jiang G, Wang D, Li W, Pan Y, Zheng W,
Zhang H and Sun YV: Coronary heart disease mortality in China: Age,
gender, and urban-rural gaps during epidemiological transition. Rev
Panam Salud Publica. 31:317–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bäck M and Hansson GK: Anti-inflammatory
therapies for atherosclerosis. Nat Rev Cardiol. 12:199–211. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libby P: Inflammation in atherosclerosis.
Arterioscler Thromb Vasc Biol. 32:2045–2051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Vascular smooth muscle cell in atherosclerosis. Acta Physiol
(Oxf). 214:33–50. 2015. View Article : Google Scholar
|
|
5
|
Meng Z, Yan C, Deng Q, Gao DF and Niu XL:
Curcumin inhibits LPS-induced inflammation in rat vascular smooth
muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways.
Acta Pharmacol Sin. 34:901–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pacurari M, Kafoury R, Tchounwou PB and
Ndebele K: The renin-angiotensin-aldosterone system in vascular
inflammation and remodeling. Int J Inflam. 2014:6893602014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Askari AT, Shishehbor MH, Kaminski MA,
Riley MJ, Hsu A and Lincoff AM; GUSTO-V Investigators: The
association between early ventricular arrhythmias,
renin-angiotensin-aldosterone system antagonism, and mortality in
patients with ST-segment-elevation myocardial infarction: insights
from Global Use of Strategies to Open Coronary Arteries (GUSTO) V.
Am Heart J. 158:238–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Derosa G and Maffioli P: Peroxisome
proliferator-activated receptor-γ (PPAR-γ) agonists on glycemic
control, lipid profile and cardiovascular risk. Curr Mol Pharmacol.
5:272–281. 2012. View Article : Google Scholar
|
|
9
|
Usuda D and Kanda T: Peroxisome
proliferator-activated receptors for hypertension. World J Cardiol.
6:744–754. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuentes E, Guzmán-Jofre L, Moore-Carrasco
R and Palomo I: Role of PPARs in inflammatory processes associated
with metabolic syndrome (Review). Mol Med Rep. 8:1611–1616.
2013.PubMed/NCBI
|
|
11
|
Liu WX, Wang T, Zhou F, Wang Y, Xing JW,
Zhang S, Gu SZ, Sang LX, Dai C and Wang HL: Voluntary exercise
prevents colonic inflammation in high-fat diet-induced obese mice
by up-regulating PPAR-gamma activity. Biochem Biophys Res Commun.
459:475–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marchesi C, Rehman A, Rautureau Y, Kasal
DA, Briet M, Leibowitz A, Simeone SM, Ebrahimian T, Neves MF,
Offermanns S, et al: Protective role of vascular smooth muscle cell
PPARγ in angiotensin II-induced vascular disease. Cardiovasc Res.
97:562–570. 2013. View Article : Google Scholar
|
|
13
|
Ji Y, Liu J, Wang Z, Liu N and Gou W:
PPARgamma agonist, rosiglitazone, regulates angiotensin II-induced
vascular inflammation through the TLR4-dependent signaling pathway.
Lab Invest. 89:887–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng Z, Yu XH, Chen J, Li L and Li S:
Curcumin attenuates cardiac fibrosis in spontaneously hypertensive
rats through PPAR-γ activation. Acta Pharmacol Sin. 35:1247–1256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Horke S and Förstermann U: Vascular
oxidative stress, nitric oxide and atherosclerosis.
Atherosclerosis. 237:208–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Madamanchi NR and Runge MS: NADPH oxidases
and atherosclerosis: Unraveling the details. Am J Physiol Heart
Circ Physiol. 298:H1–H2. 2010. View Article : Google Scholar
|
|
17
|
Barry-Lane PA, Patterson C, van der Merwe
M, Hu Z, Holland SM, Yeh ET and Runge MS: p47phox is required for
atherosclerotic lesion progression in ApoE(−/−) mice. J Clin
Invest. 108:1513–1522. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kinkade K, Streeter J and Miller FJ:
Inhibition of NADPH oxidase by apocynin attenuates progression of
atherosclerosis. Int J Mol Sci. 14:17017–17028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as 'Curecumin': From kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008. View Article : Google Scholar
|
|
20
|
Barzegar A and Moosavi-Movahedi AA:
Intracellular ROS protection efficiency and free radical-scavenging
activity of curcumin. PLoS One. 6:e260122011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng HL, Fan H, Dang HZ, Chen XP, Ren Y,
Yang JD and Wang PW: Neuroprotective effect of curcumin to Aβ of
double transgenic mice with Alzheimer's disease. Zhongguo Zhong Yao
Za Zhi. 39:3846–3849. 2014.In Chinese.
|
|
22
|
Sahebkar A: Dual effect of curcumin in
preventing atherosclerosis: The potential role of
pro-oxidant-antioxidant mechanisms. Nat Prod Res. 29:491–492. 2015.
View Article : Google Scholar
|
|
23
|
Griendling KK, Taubman MB, Akers M,
Mendlowitz M and Alexander RW: Characterization of
phosphatidylinositol-specific phospholipase C from cultured
vascular smooth muscle cells. J Biol Chem. 266:15498–15504.
1991.PubMed/NCBI
|
|
24
|
Van Hoogmoed LM, Snyder JR and Harmon F:
In vitro investigation of the effect of prostaglandins and
nonsteroidal anti-inflammatory drugs on contractile activity of the
equine smooth muscle of the dorsal colon, ventral colon, and pelvic
flexure. Am J Vet Res. 61:1259–1266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong JM, Choi CH, Kang SK, Lee IH, Lee JY
and Jung H: Antioxidant and chemosensitizing effects of flavonoids
with hydroxy and/or methoxy groups and structure-activity
relationship. J Pharm Pharm Sci. 10:537–546. 2007. View Article : Google Scholar
|
|
26
|
Mehta JL and Romeo F: Inflammation,
infection and atherosclerosis: Do antibacterials have a role in the
therapy of coronary artery disease? Drugs. 59:159–170. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peters S: Inhibition of atherosclerosis by
angiotensin II type 1 receptor antagonists. Am J Cardiovasc Drugs.
13:221–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Durante A, Peretto G, Laricchia A, Ancona
F, Spartera M, Mangieri A and Cianflone D: Role of the
renin-angiotensin-aldosterone system in the pathogenesis of
atherosclerosis. Curr Pharm Des. 18:981–1004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu Z, Wang M, Gucek M, Zhang J, Wu J,
Jiang L, Monticone RE, Khazan B, Telljohann R and Mattison J: Milk
fat globule protein epidermal growth factor-8: A pivotal relay
element within the angiotensin II and monocyte chemoattractant
protein-1 signaling cascade mediating vascular smooth muscle cells
invasion. Circ Res. 104:1337–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawashima S: The two faces of endothelial
nitric oxide synthase in the pathophysiology of atherosclerosis.
Endothelium. 11:99–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bunderson M, Coffin JD and Beall HD:
Arsenic induces peroxynitrite generation and cyclooxygenase-2
protein expression in aortic endothelial cells: Possible role in
atherosclerosis. Toxicol Appl Pharmacol. 184:11–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Detmers PA, Hernandez M, Mudgett J,
Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock
J, et al: Deficiency in inducible nitric oxide synthase results in
reduced atherosclerosis in apolipoprotein E-deficient mice. J
Immunol. 165:3430–3435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grygiel-Górniak B: Peroxisome
proliferator-activated receptors and their ligands: Nutritional and
clinical implications - a review. Nutr J. 13:172014. View Article : Google Scholar
|
|
34
|
Azhar S: Peroxisome proliferator-activated
receptors, metabolic syndrome and cardiovascular disease. Future
Cardiol. 6:657–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsumura T, Kinoshita H, Ishii N, Fukuda
K, Motoshima H, Senokuchi T, Taketa K, Kawasaki S, Nishimaki-Mogami
T, Kawada T, et al: Telmisartan exerts antiatherosclerotic effects
by activating peroxisome proliferator-activated receptor-γ in
macrophages. Arterioscler Thromb Vasc Biol. 31:1268–1275. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Siddiqui AM, Cui X, Wu R, Dong W, Zhou M,
Hu M, Simms HH and Wang P: The anti-inflammatory effect of curcumin
in an experimental model of sepsis is mediated by up-regulation of
peroxisome proliferator-activated receptor-gamma. Crit Care Med.
34:1874–1882. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghosh SS, Massey HD, Krieg R, Fazelbhoy
ZA, Ghosh S, Sica DA, Fakhry I and Gehr TW: Curcumin ameliorates
renal failure in 5/6 nephrectomized rats: Role of inflammation. Am
J Physiol Renal Physiol. 296:F1146–F1157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu Y, Zheng S, Lin J, Ryerse J and Chen A:
Curcumin protects the rat liver from CCl4-caused injury
and fibrogenesis by attenuating oxidative stress and suppressing
inflammation. Mol Pharmacol. 73:399–409. 2008. View Article : Google Scholar
|
|
39
|
Tousoulis D, Psaltopoulou T, Androulakis
E, Papageorgiou N, Papaioannou S, Oikonomou E, Synetos A and
Stefanadis C: Oxidative stress and early atherosclerosis: Novel
antioxidant treatment. Cardiovasc Drugs Ther. 29:75–88. 2015.
View Article : Google Scholar
|
|
40
|
Li H, Horke S and Förstermann U: Oxidative
stress in vascular disease and its pharmacological prevention.
Trends Pharmacol Sci. 34:313–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun GB, Qin M, Ye JX, Pan RL, Meng XB,
Wang M, Luo Y, Li ZY, Wang HW and Sun XB: Inhibitory effects of
myricitrin on oxidative stress-induced endothelial damage and early
atherosclerosis in ApoE−/− mice. Toxicol Appl Pharmacol.
271:114–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hort MA, Straliotto MR, Netto PM, da Rocha
JB, de Bem AF and Ribeiro-do-Valle RM: Diphenyl diselenide
effectively reduces atherosclerotic lesions in LDLr−/−
mice by attenuation of oxidative stress and inflammation. J
Cardiovasc Pharmacol. 58:91–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rong S, Zhao Y, Bao W, Xiao X, Wang D,
Nussler AK, Yan H, Yao P and Liu L: Curcumin prevents chronic
alcohol-induced liver disease involving decreasing ROS generation
and enhancing antioxidative capacity. Phytomedicine. 19:545–550.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D,
Guo S, Ming Z and Liu C: Curcumin alleviates diabetic
cardiomyopathy in experimental diabetic rats. PLoS One.
7:e520132012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bruder-Nascimento T, Chinnasamy P,
Riascos-Bernal DF, Cau SB, Callera GE, Touyz RM, Tostes RC and
Sibinga NE: Angiotensin II induces Fat1 expression/activation and
vascular smooth muscle cell migration via Nox1-dependent reactive
oxygen species generation. J Mol Cell Cardiol. 66:18–26. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gray SP, Di Marco E, Okabe J,
Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C,
El-Osta A, Andrews KL, et al: NADPH oxidase 1 plays a key role in
diabetes mellitus-accelerated atherosclerosis. Circulation.
127:1888–1902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ushio-Fukai M: Localizing NADPH
oxidase-derived ROS. Sci STKE. 2006:re82006.PubMed/NCBI
|
|
48
|
Zhao WC, Zhang B, Liao MJ, Zhang WX, He
WY, Wang HB and Yang CX: Curcumin ameliorated diabetic neuropathy
partially by inhibition of NADPH oxidase mediating oxidative stress
in the spinal cord. Neurosci Lett. 560:81–85. 2014. View Article : Google Scholar
|
|
49
|
Derochette S, Franck T, Mouithys-Mickalad
A, Ceusters J, Deby-Dupont G, Lejeune JP, Neven P and Serteyn D:
Curcumin and resveratrol act by different ways on NADPH oxidase
activity and reactive oxygen species produced by equine
neutrophils. Chem Biol Interact. 206:186–193. 2013. View Article : Google Scholar : PubMed/NCBI
|