Introduction
Tumor progression relies on a series of sequential
events, including tumor initiation, growth, angiogenesis and
metastasis, which occur in a complex and dynamic microenvironment
and are regulated by a number of mediators and signaling
transduction pathways (1–3). Growing evidence indicates the
crucial role of angiogenesis in tumor growth and metastasis
(4,5). Tumor cells can develop their
microenvironment by the secretion of vascular endothelial growth
factor (VEGF) or cytokines to promote abnormal tumor neovasculature
formation, which can provide nutrients for tumor growth and a route
for metastasis (6). Owing to the
key role of angiogenesis, inhibition of tumor angiogenesis has been
considered as an effective strategy for suppressing tumor
progression (7,8). It has also been demonstrated that
agents with anti-angiogenic properties can effectively inhibit
tumor cell growth and metastasis (9). Thus, searching for novel agents with
anti-angiogenic activity represents an effective strategy for the
overall control of human cancers.
Salinomycin (SAL), a polyether antibiotic, isolated
from bacterium Streptomyces albus, has been used extensively
to improve the nutrient absorption and feeding efficiency of
poultry (10). SAL was identified
as a potent anticancer agent among 16,000 compounds against human
breast cancer stem cells (CSCs) for the first time in 2009
(11). It was also demonstrated
that SAL displays antitumor activities in other types of human
CSCs, including colorectal, lung, gastric, pancreatic and
osteosarcoma CSCs (12–16). Recently, several studies have
shown that SAL displays broad-spectrum anticancer properties,
including inhibition of proliferation, migration and invasion, and
induction of autophagy and apoptosis in cancer cells (17–21). Additionally, accumulating evidence
indicates that SAL can enhance the cytotoxic effects of several
chemotherapeutic drugs, such as doxorubicin and etoposide (22,23). An increasing number of studies
have reported that induction of DNA damage (23), mitochondrial dysfunction (24), reactive oxygen species (ROS)
accumulation (20), signal
regulation of FOXO3a, Wnt/β-catenin, STAT3/Skp2 and Akt/NF-κB/mTOR
(25–28), all contribute to SAL-mediated
anticancer mechanisms. However, little information concerning the
anti-angiogenic potential of SAL is available. In this study,
SAL-mediated angiogenic activity was examined, and the underlying
molecular mechanisms in human glioma were also evaluated.
Materials and methods
Materials
SAL and conventional chemicals were all obtained
from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified
Eagle's medium (DMEM)-F12 and fetal bovine serum (FBS) were
purchased from Beyotime (Beijing, China). All of the antibodies and
inhibitors (LY294002 and PF-562271) used in this study were
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
All solvents used were of high-performance liquid chromatography
(HPLC) grade. Water used in this study was obtained from a Milli-Q
from Millipore (Billerica, MA, USA).
Cell culture
The U251 human glioma cell line and human umbilical
vein endothelial cells (HUVECs) were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). HUVECs were
cultured in DMEM-F12 supplemented with FBS (10%) at 37°C in a
humidified incubator with 5% CO2 atmosphere.
Measurement of cell viability (MTT
assay)
The effect of SAL on HUVEC growth was determined by
MTT assay. Briefly, HUVECs (6,000 cells/well) were seeded in
96-well culture plates for 24 h and incubated with different
concentrations of SAL. In the preliminary experiments, SAL
treatment for 12, 24, 48 and 72 h showed time-dependent effects on
cell growth inhibition. However, treatment for 48 h was the optimal
time and was selected for further mechanism evaluation. After SAL
treatment for 48 h, 20 µl/well of MTT solution (5 mg/ml) was
added and incubated for 5 h. The medium was aspirated and replaced
with 200 µl/well of DMSO to dissolve the formazan salt
formed. The color intensity of the formazan solution was measured
at 570 nm by a microplate spectrophotometer. The cell viability was
expressed as % of the control (as 100%).
Scratch motility (wound-healing)
assay
HUVECs were incubated in 6-well plates and allowed
to grow to full confluence. After serum starvation for 4 h, the
cells were scratched using pipette tips, washed with
phosphate-buffered saline (PBS) and photographed by using a
phase-contrast microscope. Fresh medium supplemented with 1% FBS
and 4 µM SAL was added into the well. VEGF (200 ng/ml) and 5
µM cisplatin were employed as the positive and negative
control, respectively. After 48 h of treatment, cells were
photographed again at three random areas. The migrated cells were
quantified by manual counting and the migration ratio was
calculated.
Transwell invasion assay
HUVECs were placed on a Transwell Boyden chamber
(8-µm pore; Corning Inc., Lowell, MA, USA) pre-coated with
Matrigel for 4 h at 37°C. One hundred micro-liters of cell
suspension (2×105 cells/ml) in FBS-free medium was added
into the upper compartment of the chamber. The bottom chambers were
supplemented with 500 µl complete medium (10% FBS)
containing the indicated concentrations of SAL with or without 200
ng/ml VEGF. After 24 h of treatment, the non-invaded cells from the
upper face were scraped using a cotton swab. The invaded cells on
the lower face were fixed with methanol, stained with Giemsa and
photographed by a phase-contrast microscope. The invaded cells were
quantified by manual counting and the invasion ratio was expressed
as % of the control.
Tube formation assay
HUVECs (1×104 cells/well) were seeded in
a Matrigel pre-coated 48-well plate. VEGF (200 ng/ml) and 5
µM cisplatin were employed as the positive and negative
control, respectively. After 24 h of treatment, the tube formation
was visualized with an inverted microscope and the tube number was
quantified by manual counting.
Western blotting
After treatment, the cells were harvested and
incubated with cell lysis buffer overnight at −20°C. The protein
concentrations were detected using a BCA protein assay kit. After
electrophoresis, the separated proteins were transferred onto
nitrocellulose membrane for 75 min at 110 V and blocked with 5%
non-fat milk in TBS buffer for 1 h. Subsequently, the membranes
were washed with TBST buffer and incubated with primary antibodies
[p-FAK (#8556), VEGF (#2463), p-VEGFR2 (#2478s), VEGFR2 (#9698),
p-AKT (#4058), AKT (#4691), Ki67 (#9027) and CD34 (#3569); Cell
Signaling Technology, Inc.] overnight at 4°C and then secondary
antibody [IgG (#3452), Cell Signaling Technology, Inc.] for 2 h at
room temperature. The target proteins were detected on X-ray film
using a chemiluminescence reagent. β-actin was used to confirm the
equal loading and transfer of proteins.
In vivo study
Human glioma U251 cells (1×107) suspended
in 100 µl PBS were injected into the right lower hind flank
of each 6-week-old male nude mouse. The mice were then randomly
assigned into three groups of 10 mice in each group. After one
week, SAL (5 and 10 mg/kg) was administered into the caudal vein
every other day for 16 days. Control mice received an equal volume
of vehicle (saline) only. Body weight and tumor volume were
monitored every two days. At the end of the experiments, tumors
were excised, photographed, and weighed. Tumors from each group
were used for western blotting and immunohistochemical (IHC) assay.
All animal experiments were approved by the Animal Experimentation
Ethics Committee of Taishan Medical University (no. 2015026).
Statistical analysis
Experiments were carried out at least in triplicate
and the results are expressed as mean ± SD. Statistical analysis
was performed using SPSS version 13 (SPSS, Inc., Chicago, IL, USA).
A difference between two groups was analyzed by two-tailed
Student's t-test. Difference with p<0.05 or p<0.01 was
considered statistically significant.
Results
SAL inhibits the proliferation of human
HUVECs
Due to the essential role of HUVECs in angiogenesis,
in the present study, HUVECs were chosen to evaluate the
anti-angiogenic potential of SAL. Firstly, the inhibitory effect of
SAL on the growth of HUVECs was determined by MTT assay. As shown
in Fig. 1A, treatment of HUVECs
with the indicated concentrations (0.1–8 µM) of SAL resulted
in a dose-dependent inhibition of cell growth, accounting for 32.1
and 59.2% inhibition at 4 and 8 µM, respectively. In the
phase contrast observation (Fig.
1B), HUVECs exposed to 2, 4 and 8 µM of SAL for 48 h
showed a dose-dependent reduction in cell number and a change in
cell morphology, such as cell shrinkage and cell rounding. These
results demonstrated that SAL could inhibit HUVEC growth in
vitro.
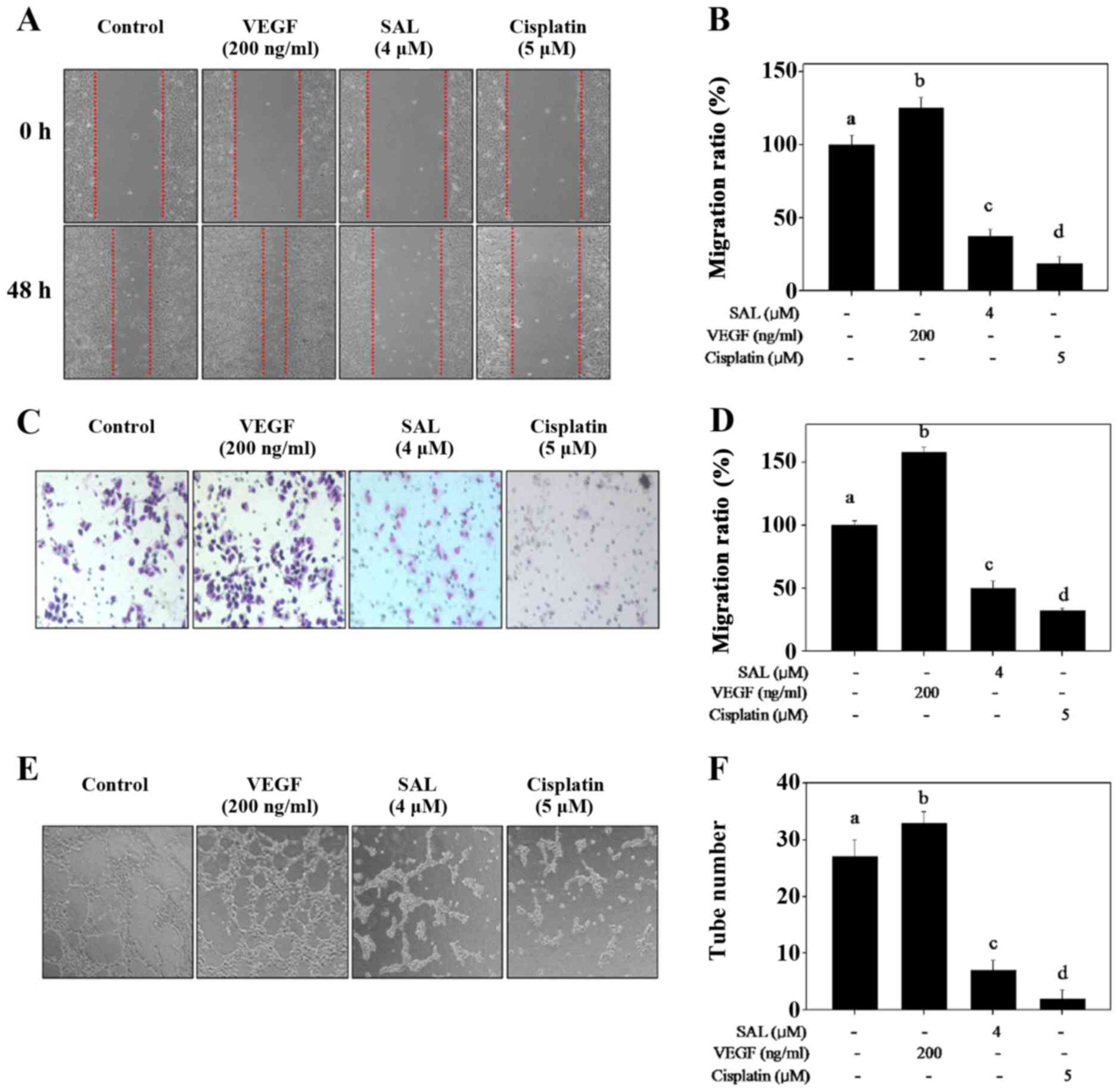
SAL blocks HUVEC migration, invasion and
capillary-like tube formation
Migration, invasion and tube formation of HUVECs are
crucial components in the process of tumor-induced
neovascularization. Thus, we evaluated the effects of SAL on the
metastatic potential of HUVECs in vitro. Scratch motility
and Transwell invasive assays were used to detect the migration and
invasion of HUVECs after SAL treatment, respectively. As shown in
Fig. 2A–D, VEGF treatment
obviously promoted HUVEC migration and invasion. However, SAL
treatment effectively inhibited HUVEC migration and invasion at the
concentration of 4 µM after 48 h of treatment (Fig. 2A–D). A similar effect was also
observed in the HUVECs exposed to cisplatin (negative control).
To further confirm the effect of SAL on
angiogenesis, a tube formation assay was carried out to investigate
whether SAL can inhibit the capillary-like tube formation of
HUVECs. After pretreatment with VEGF, elongated and robust
tube-like structures were observed, while a significant disruption
in capillary-like tube formation was observed in the HUVECs exposed
to 4 µM of SAL for 24 h (Fig.
2E and F). These results indicated that SAL inhibited
angiogenesis of HUVECs in vitro.
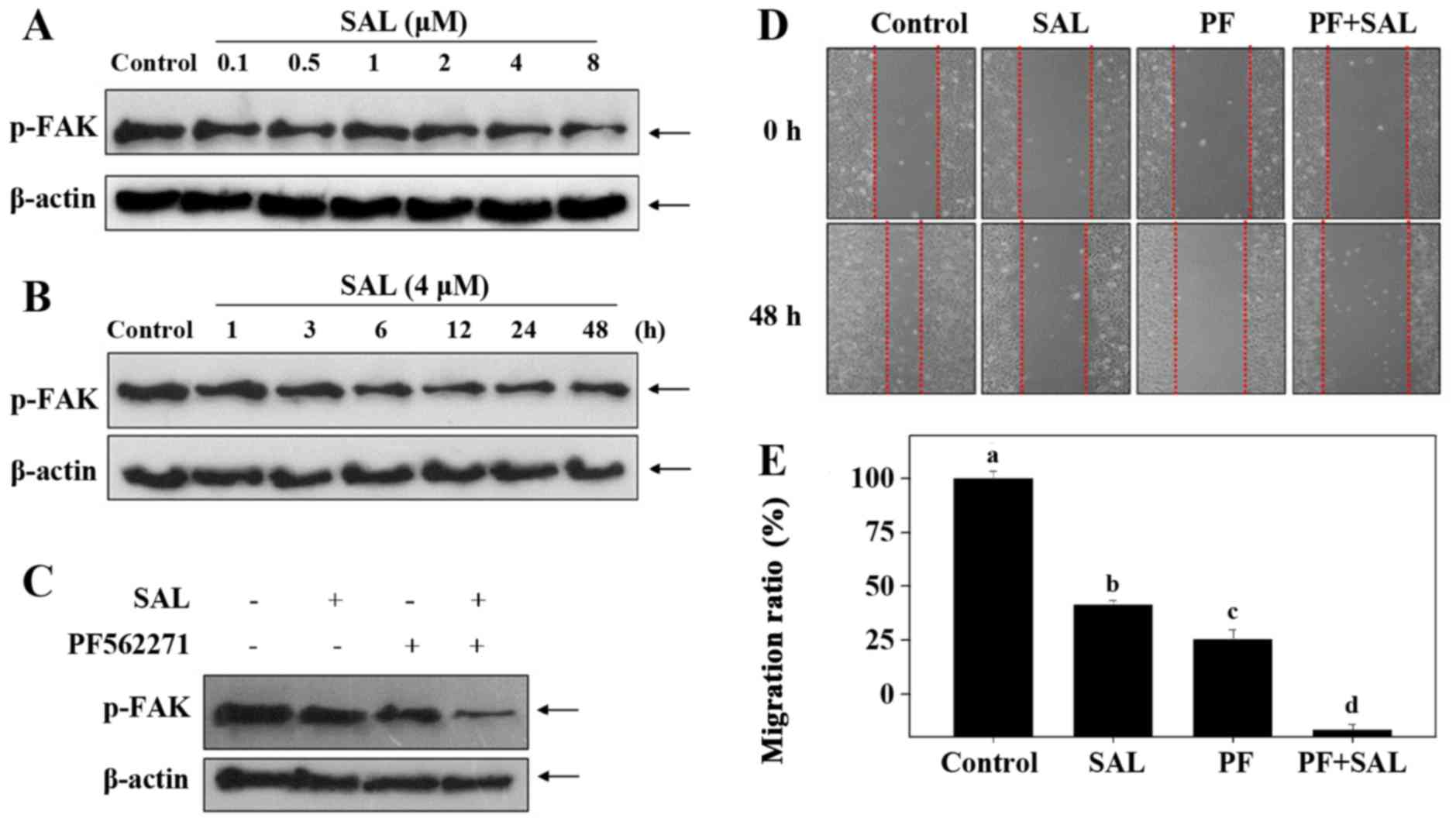
SAL suppresses FAK phosphorylation in
HUVECs
Further investigation of the underlying mechanisms
was evaluated. FAK, a cytoplasmic protein tyrosine kinase, plays a
vital role in cell proliferation, survival and metastasis. To
illustrate whether the inactivation of FAK is involved, we examined
the levels of phosphorylated level of FAK in HUVECs after SAL
treatment. As shown in Fig. 3A and
B, exposure of HUVECs to SAL significantly suppressed the
expression levels of phosphorylated (p)-FAK in a time- and
dose-dependent manner. In addition, PF562271 (FAK inhibitor) was
used to confirm the role of FAK inactivation in SAL-mediated
inhibition of cell migration. As shown in Fig. 3C–E, treatment with 10 nM PF562271
or 4 µM SAL alone for 24 h both displayed notable inhibitory
effects on FAK phosphorylation and HUVEC migration. Notably,
pretreatment with PF562271 markedly enhanced SAL-induced inhibition
against FAK phosphorylation and HUVEC migration. Taken together,
these results indicated that FAK dephosphorylation contributed to
SAK-mediated inhibition against HUVEC migration.
SAL disturbs the VEGF-VEGFR2-AKT
signaling axis
As a crucial mediator of angiogenesis in the tumor
microenvironment, VEGF promotes tumor angiogenesis via interacting
with VEGFR to regulate downstream signaling transduction. In the
present study, the expression of VEGF, VEGFR2, AKT and their
phosphorylated levels in HUVECs were detected by western blotting.
As shown in Fig. 4A, little
change was observed in the expression levels of total VEGFR2 and
AKT after SAL treatment. However, HUVECs exposed to SAL showed a
significant decrease in VEGF expression, leading to the reduction
of phosphorylated VERGR2 and AKT. To further study the role of AKT
in SAL-mediated anti-angiogenesis in vitro, we examined the
effects of AKT-upstream inhibitor (LY294002) on HUVEC growth by MTT
assay. As shown in Fig. 4B,
pretreatment with LY294002 resulted in a marked decrease in cell
viability, which supports the key role of AKT inactivation in
SAL-induced cell growth inhibition. Taken together, our results
indicated that SAL inhibited HUVEC angiogenesis by disturbing the
VEGF-VEGFR2-AKT signaling axis.
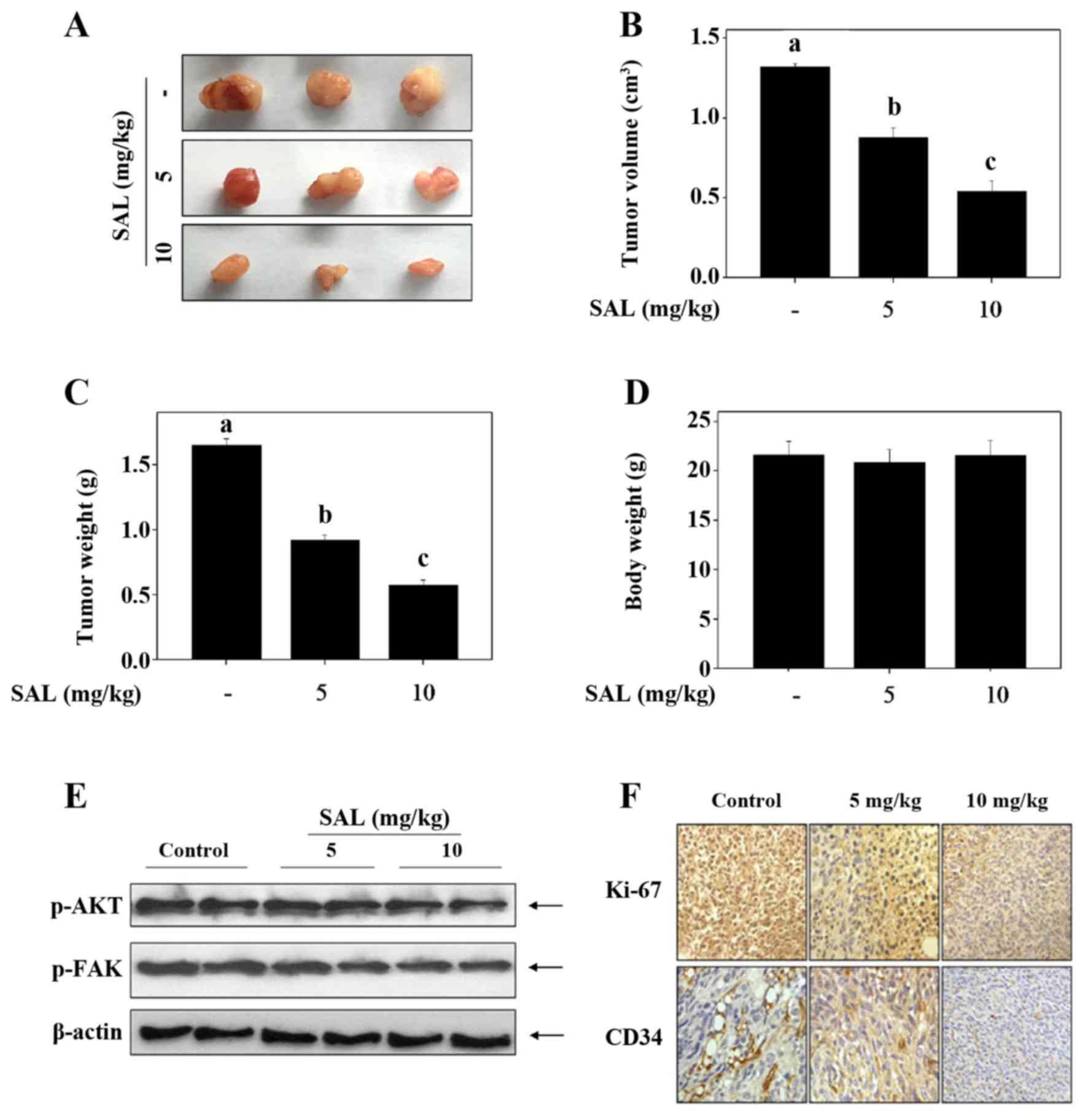
SAL inhibits glioma growth in vivo by
inhibiting angiogenesis
To evaluate the anticancer and anti-angiogenic
potential of SAL in vivo, we treated tumor-bearing nude mice
with 5 and 10 mg/kg of SAL. As shown in Fig. 5A–C, the average tumor volume and
tumor weight were significantly suppressed by SAL treatment. The
body weight of the mice showed no significant change (Fig. 5D). Moreover, consistent with the
in vitro model, we also found that SAL moderately decreased
FAK and AKT phosphorylation in the tumor model (Fig. 5E). IHC staining showed that the
expression of Ki-67, a biomarker of proliferation, was
significantly inhibited by SAL treatment (Fig. 5F). CD34, an important marker of
hematopoietic progenitor cells and the small vessel endothelium was
highly expressed in the control tumor tissue (Fig. 5F). However, SAL treatment
significantly inhibited the in vivo angiogenesis (Fig. 5F). Taken together, these findings
revealed that SAL hindered the U251 human glioma cell growth in
vivo via inhibition of angiogenesis with involvement of AKT and
FAK dephosphorylation.
Discussion
Glioma is one of the most common malignant brain
cancers worldwide and has become a great threat to human health
(29). Glioma is highly
aggressive and is associated with a very poor prognosis, and the
median survival rate is ~12–15 months (30,31). Even though the current clinical
treatments for glioma have achieved satisfactory results, adjuvant
chemotherapy after surgical resection and radiotherapy are still
irreplaceable (32–34). This forms a rationale for the
development of novel chemotherapeutic agents for the treatment of
glioma. Preclinical data indicate that angiogenesis is essential
for the proliferation and survival of glioma cells (35,36). Thus, searching for novel
anti-angiogenic agents to treat human glioma is urgently needed.
Herein, in the present study, we investigated the anti-angiogenic
potential of SAL in the treatment of human glioma and clarify the
underlying mechanisms.
Tumor cells can release growth factors and cytokines
into the microenvironment, which activate the sprouting and
proliferation of formerly quiescent endothelial cells on nearby
blood vessels (3). VEGF, as one
of the most crucial mediators in angiogenesis, can effectively
promote the metastasis of endothelial cells. HUVEC migration,
invasion and tube formation were significantly enhanced by the
addition of VEGF. However, this tendency was significantly
suppressed by SAL, indicating the anti-angiogenic potential of SAL
in vitro. Growing evidence suggests that the interaction of
VEGF and receptors (VEGFR1/2/3) promote the activation of
downstream signaling transduction cascades (37,38). Among these VEGF receptors, VEGFR2
plays a major role in the transduction of angiogenic signaling. The
activation of VEGFR2 by VEGF stimulation subsequently results in
activation of several downstream pathways, including Ras/MEK/ERK
and PI3K/Akt, which can positively regulate pro-survival and
pro-angiogenic signals (3,37).
Recent research has shown that SAL decreases VEGF-induced
phosphorylation of VEGFR2 in HUVECs (17). Consistent with the previous data,
we found that the expression levels of VEGF and phosphorylated
VEGFR2 were both downregulated by the treatment of SAL, leading to
the inactivation of the pro-survival PI3K/Akt signaling pathway
(Fig. 4). In addition,
pretreatment with the PI3K inhibitor (LY294002) markedly enhanced
the growth inhibitory effects of SAL, indicating that the
VEGF-VEGFR2-AKT signaling axis plays an essential role in
SAL-induced HUVEC growth inhibition in vitro.
FAK, a cytoplasmic protein tyrosine kinase, plays a
vital role in cell proliferation, survival and migration, and is
associated with integrin-mediated signal transduction (39). Integrin clustering-mediated
activation of FAK results in the phosphorylation of Tyr397, which
is a binding site for PI3K (40).
Previous studies have shown that the activation of FAK by integrin
engagement or growth factor stimulation both promote the activation
of downstream PI3K/Akt signaling pathways, which leads to the
activation or overexpression of pro-metastatic proteins, such as
matrix metalloproteinases, urokinase-type plasminogen activator and
VEGF (39,41). Inactivation of FAK was involved in
the SAL-induced suppression of metastatic potential in HUVECs
(Fig 3). Moreover, the
introduction of FAK inhibitor (PF562271) further confirmed the
above result.
Accumulative studies have demonstrated that SAL has
the ability to inhibit cancer cell growth in nude mouse xeno-graft
models, including human gastric cancer (17), human nasopharyngeal carcinoma
(18) and human hepatocellular
carcinoma (19) through induction
of apoptotic cell death and anti-angiogenesis. To make the in
vitro data more convincible, we detected the inhibitory effect
of SAL on U251 tumor xenografts. The results revealed that SAL
effectively inhibited human glioma growth by inhibiting FAK and AKT
phosphorylation and angiogenesis, which validated that the
anti-angiogenic potential of SAL contributes to SAL-induced growth
inhibition of U251 human glioma cells in vivo.
SAL is a potential anticancer agent, and its action
mechanism has been extentively expolored. For example, Qin et
al found that SAL inhibited human glioma cell growth by
ROS/p53/cyclophilin-D signaling-mediated necrosis (42). Zhang et al found that SAL
can inhibit the growth of colorectal carcinoma by targeting tumor
stem cells (43). Kim et
al reported that SAL can induce apoptosis and autophagy, and
also acts as a sensitizer to enhance the effects of doxorubicin on
human cancer cells (20,23). Researchers also found that
ROS-mediated oxidative damage (20,23), mitochondrial dysfunction (24), and several signaling pathway, such
as FOXO3a, Wnt/β-catenin, STAT3/Skp2 and Akt/NF-κB/mTOR (25–28), all contribute to the anticancer
mechanisms of SAL. However, we provide new evidence that SAL can
act as a potent anti-angiogenic agent in vitro and in
vivo to inhibit human glioma growth via suppression of the
VEGF-VEGFR2-AKT/FAK signaling axis. Our findings validate the
potential of SAL as a promising anti-angiogenic candidate for the
treatment of human glioma in clinical trails.
Acknowledgments
This study was supported by the Science and
Technology Research Projects of Shandong, China (no. 2012GSF2180 to
Shi-Liang Jiang).
References
|
1
|
Sautès-Fridman C, Cherfils-Vicini J,
Damotte D, Fisson S, Fridman WH, Cremer I and Dieu-Nosjean MC:
Tumor microenvironment is multifaceted. Cancer Metastasis Rev.
30:13–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valastyan S and Weinberg RA: Tumor
metastasis: molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weis SM and Cheresh DA: Tumor
angiogenesis: molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: The role of the tumor-microenvironment in lung
cancer-metastasis and its relationship to potential therapeutic
targets. Cancer Treat Rev. 40:558–566. 2014. View Article : Google Scholar
|
|
6
|
Fokas E, McKenna WG and Muschel RJ: The
impact of tumor microenvironment on cancer treatment and its
modulation by direct and indirect antivascular strategies. Cancer
Metastasis Rev. 31:823–842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Albini A and Sporn MB: The tumour
microenvironment as a target for chemoprevention. Nat Rev Cancer.
7:139–147. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang H and Declerck YA: Targeting the
tumor microenvironment: from understanding pathways to effective
clinical trials. Cancer Res. 73:4965–4977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen L, Fifis T and Christophi C:
Vascular disruptive agent OXi4503 and anti-angiogenic agent
Sunitinib combination treatment prolong survival of mice with CRC
liver metastasis. BMC Cancer. 16:5332016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyazaki Y, Shibuya M, Sugawara H,
Kawaguchi O and Hirsoe C: Salinomycin, a new polyether antibiotic.
J Antibiot (Tokyo). 27:814–821. 1974. View Article : Google Scholar
|
|
11
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong TT, Zhou HM, Wang LL, Feng B, Lv B
and Zheng MH: Salinomycin selectively targets 'CD133+'
cell subpopulations and decreases malignant traits in colorectal
cancer lines. Ann Surg Oncol. 18:1797–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y: Effects of salinomycin on cancer
stem cell in human lung adenocarcinoma A549 cells. Med Chem.
7:106–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhi QM, Chen XH, Ji J, Zhang JN, Li JF,
Cai Q, Liu BY, Gu QL, Zhu ZG and Yu YY: Salinomycin can effectively
kill ALDH(high) stem-like cells on gastric cancer. Biomed
Pharmacother. 65:509–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang GN, Liang Y, Zhou LJ, Chen SP, Chen
G, Zhang TP, Kang T and Zhao YP: Combination of salinomycin and
gemcitabine eliminates pancreatic cancer cells. Cancer Lett.
313:137–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ,
Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, et al: Salinomycin
inhibits osteosarcoma by targeting its tumor stem cells. Cancer
Lett. 311:113–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li T, Liu X, Shen Q, Yang W, Huo Z, Liu Q,
Jiao H and Chen J: Salinomycin exerts anti-angiogenic and
anti-tumorigenic activities by inhibiting vascular endothelial
growth factor receptor 2-mediated angiogenesis. Oncotarget.
7:26580–26592. 2016.PubMed/NCBI
|
|
18
|
Wu D, Zhang Y, Huang J, Fan Z, Shi F and
Wang S: Salinomycin inhibits proliferation and induces apoptosis of
human nasopharyngeal carcinoma cell in vitro and suppresses tumor
growth in vivo. Biochem Biophys Res Commun. 443:712–717. 2014.
View Article : Google Scholar
|
|
19
|
Wang F, He L, Dai WQ, Xu YP, Wu D, Lin CL,
Wu SM, Cheng P, Zhang Y, Shen M, et al: Salinomycin inhibits
proliferation and induces apoptosis of human hepatocellular
carcinoma cells in vitro and in vivo. PLoS One. 7:e506382012.
View Article : Google Scholar
|
|
20
|
Kim SH, Choi YJ, Kim KY, Yu SN, Seo YK,
Chun SS, Noh KT, Suh JT and Ahn SC: Salinomycin simultaneously
induces apoptosis and autophagy through generation of reactive
oxygen species in osteosarcoma U2OS cells. Biochem Biophys Res
Commun. 473:607–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He L, Wang F, Dai WQ, Wu D, Lin CL, Wu SM,
Cheng P, Zhang Y, Shen M, Wang CF, et al: Mechanism of action of
salinomycin on growth and migration in pancreatic cancer cell
lines. Pancreatology. 13:72–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim KY, Kim SH, Yu SN, Park SK, Choi HD,
Yu HS, Ji JH, Seo YK and Ahn SC: Salinomycin enhances
doxorubicin-induced cytotoxicity in multidrug resistant MCF-7/MDR
human breast cancer cells via decreased efflux of doxorubicin. Mol
Med Rep. 12:1898–1904. 2015.PubMed/NCBI
|
|
23
|
Kim JH, Chae M, Kim WK, Kim YJ, Kang HS,
Kim HS and Yoon S: Salinomycin sensitizes cancer cells to the
effects of doxorubicin and etoposide treatment by increasing DNA
damage and reducing p21 protein. Br J Pharmacol. 162:773–784. 2011.
View Article : Google Scholar :
|
|
24
|
Managò A, Leanza L, Carraretto L, Sassi N,
Grancara S, Quintana-Cabrera R, Trimarco V, Toninello A, Scorrano
L, Trentin L, et al: Early effects of the antineoplastic agent
salinomycin on mitochondrial function. Cell Death Dis. 6:e19302015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Liang C, Xue F, Chen W, Zhi X,
Feng X, Bai X and Liang T: Salinomycin decreases doxorubicin
resistance in hepatocellular carcinoma cells by inhibiting the
β-catenin/TCF complex association via FOXO3a activation.
Oncotarget. 6:10350–10365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koo KH, Kim H, Bae YK, Kim K, Park BK, Lee
CH and Kim YN: Salinomycin induces cell death via inactivation of
Stat3 and downregulation of Skp2. Cell Death Dis. 4:e6932013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parajuli B, Lee HG, Kwon SH, Cha SD, Shin
SJ, Lee GH, Bae I and Cho CH: Salinomycin inhibits Akt/NF-κB and
induces apoptosis in cisplatin resistant ovarian cancer cells.
Cancer Epidemiol. 37:512–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huse JT and Holland EC: Targeting brain
cancer: advances in the molecular pathology of malignant glioma and
medulloblastoma. Nat Rev Cancer. 10:319–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho AL, Koch MJ, Tanaka S, Eichler AF,
Batchelor TT, Tanboon J, Louis DN, Cahill DP, Chi AS and Curry WT
Jr: Impact of histopathological transformation and overall survival
in patients with progressive anaplastic glioma. J Clin Neurosci.
31:99–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dewan MC, White-Dzuro GA, Brinson PR,
Thompson RC and Chambless LB: Perioperative seizure in patients
with glioma is associated with longer hospitalization, higher
readmission, and decreased overall survival. J Neurosurg.
125:1033–1041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaff LR and Lassman AB: Indications for
treatment: is observation or chemotherapy alone a reasonable
approach in the management of low-grade gliomas? Semin Radiat
Oncol. 25:203–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blondin NA and Becker KP: Anaplastic
gliomas: radiation, chemotherapy, or both? Hematol Oncol Clin North
Am. 26:811–823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Viaccoz A, Lekoubou A and Ducray F:
Chemotherapy in low-grade gliomas. Curr Opin Oncol. 24:694–701.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Onishi M, Ichikawa T, Kurozumi K and Date
I: Angiogenesis and invasion in glioma. Brain Tumor Pathol.
28:13–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tate MC and Aghi MK: Biology of
angiogenesis and invasion in glioma. Neurotherapeutics. 6:447–457.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang X, Xu F, Li X, Ma C, Zhang Y and Xu
W: VEGF signal system: the application of antiangiogenesis. Curr
Med Chem. 21:894–910. 2014. View Article : Google Scholar
|
|
39
|
Zhang J and Hochwald SN: The role of FAK
in tumor metabolism and therapy. Pharmacol Ther. 142:154–163. 2014.
View Article : Google Scholar
|
|
40
|
Chen HC, Appeddu PA, Isoda H and Guan JL:
Phosphorylation of tyrosine 397 in focal adhesion kinase is
required for binding phosphatidylinositol 3-kinase. J Biol Chem.
271:26329–26334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Smith HW and Marshall CJ: Regulation of
cell signalling by uPAR. Nat Rev Mol Cell Biol. 11:23–36. 2010.
View Article : Google Scholar
|
|
42
|
Qin LS, Jia PF, Zhang ZQ and Zhang SM:
ROS-53-cyclophilin-D signaling mediates salinomycin-induced glioma
cell necrosis. J Exp Clin Cancer Res. 34:572015. View Article : Google Scholar
|
|
43
|
Zhang C, Tian Y, Song F, Fu C, Han B and
Wang Y: Salinomycin inhibits the growth of colorectal carcinoma by
targeting tumor stem cells. Oncol Rep. 34:2469–2476.
2015.PubMed/NCBI
|