Introduction
Myocardial infarction (MI) is one of the main causes
of death and disability in both developed and developing countries.
According to the statistics of the American Heart Association
(AHA), approximately every 43 sec, one American suffers MI
(1). In 2011, ~160,000
individuals died of MI (1). In
China, cardiovascular disease is the leading cause of death and
acute MI has become the primary cause of cardiac-related deaths in
2013 (2). MI not only affects
human health, but also results in a heavy financial burden to the
patients' family and society. Thus, exploring effective therapies
for MI is of great importance for improving the health status of
populations in China and worldwide.
MI is characterized by the loss of function of
cardiomyocytes caused by reduced blood supply from coronary
arteries to the heart (3). In
patients with MI, necrotic myocardium is gradually replaced by scar
tissue, leading to loss of cardiac contraction and possibly to
heart failure. A variety of therapeutic methods for treating MI
have been applied in the clinic such as coronary intervention
(4), thrombolytic therapy
(5) and drug therapy
[anticoagulants (6), β-blockers
(7), angiotensin-receptor
blockers (8) and antiarrhythmic
drugs (9)]. The main goal of the
current clinical treatment is revascularization after MI.
Successful revascularization can increase the blood supply to the
infarcted area and effectively reduce the myocardial necrosis of
the remaining healthy myocardial cells. Reperfusion is an effective
method for tissue survival, yet it may lead to tissue damage, such
as cardiomyocyte loss and microcirculatory disturbances during the
pathological process of ischemia-reperfusion injury in prolonged
myocardial ischemia (10). As
mentioned previously, cardiomyocytes regenerate at ~1%/year
throughout human life, thus the capacity for regeneration is
limited (11). Notably,
cardiomyocytes are incapable of sufficient regeneration to correct
cell losses after MI (11).
Therefore, therapeutic strategies stimulating cardiomyocyte renewal
may be an effective method for treating cardiac pathologies, and
cell transplantation for cardiomyocyte renewal has attracted
increased attention for cardiac dysfunction after MI.
Endothelial progenitor cells (EPCs) are derived from
peripheral mononuclear cells that can differentiate into vascular
endothelial cells (12). EPCs
play important roles in angiogenesis after birth and repair of
injured endothelium (12). When
required for vascular repair or angiogenesis, circulating EPCs
migrate to the areas of endothelial damage, differentiate into
vascular endothelial cells and aggregate to form new vessels
(13). Previous studies have
demonstrated the promoting effects on angiogenesis following
transplantation of EPCs in the infarcted area (14,15). However, most EPCs die after
transplantation due to inflammation and presence of apoptotic
factors, leading to insufficient capacity of EPC transplantation
for treating cardiac disease (16,17). The key for treating MI is
promoting the survival and function of EPCs after
transplantation.
Thymosin β4 (Tβ4) is a pleiotropic polypeptide and
is abundantly expressed in EPCs (18). Recent studies indicate that Tβ4 is
involved in angiogenesis, and may promote endothelial cell
differentiation, migration and angiogenesis in vitro
(18,19). Thus, in the present study, we
incorporated both in vitro and in vivo animal
experiments to explore the cardioprotective roles of Tβ4 on
EPC-based transplantation in rats and to further investigate the
mechanisms underlying the cardioprotective effects of EPCs.
Materials and methods
Animals
Adult Sprague-Dawley rats, weighing 200±20 g were
obtained from the Experimental Animal Center, Shanghai Medical
College of Fudan University. Rats were bred and kept in
special-pathogen-free (SPF) condition, with free access of food and
water. All animal experiments were approved by the Ethics Committee
of Shanghai Medical College of Fudan University and performed in
accordance with institutional guidelines for animal
experiments.
Isolation, cultivation and
characterization of EPCs
The male rats were sacrificed by cervical
dislocation and immersed in 75% alcohol for 8 min for
sterilization. Subsequently, the skin and fascia were cut with eye
scissors and the muscles were stripped. Then the femurs and tibias
were isolated and placed in pre-cooled Dulbecco's modified Eagle's
medium (DMEM; Gibco, Carlsbad, CA, USA). Afterwards, the bone
marrow was flushed from femurs and tibias of the rats using a
syringe with DMEM, collected in a 15-ml centrifuge tube and
dissociated into a single-cell suspension by pipetting. Then the
single-cell suspension was slowly transferred to a new centrifuge
tube containing 10 ml lymphocyte separation medium and centrifuged
for 20 min at 2,000 rpm. The liquid in the middle layer was
separated and resuspended with DMEM. After centrifugation at 1,000
rpm for 10 min, EPCs were obtained and suspended with Endothelial
Basal Medium-2 (EBM-2; Lonza, Basel, Switzerland), and inoculated
in a 6-cm culture dish pre-coated with 0.1% fibronectin at
5×105 cells/ml. Cells were incubated in a 37°C and 5%
CO2 incubator. When cells were grown to 70–80%
confluence, they were used for further analysis.
The cultured EPCs (passage 0) were digested with
0.25% trypsin-EDTA solution for 2 min at room temperature after
washing with phosphate-buffered saline (PBS). Then the cells were
collected, counted and resuspended with PBS containing 0.5% bovine
serum albumin (BSA) at 1×108 cells/ml. Afterwards, the
cells were incubated with FITC-conjugated antibodies against CD34,
PE-conjugated antibodies against CD133 and VEGFR-2 at 4°C for 10
min. After centrifugation, the harvested cells were smeared onto
glass slides and observed under confocal microscopy.
In vitro asses′′sments
The identified EPCs (passage 0) were seeded into
24-well plates at 1×105 cells/ml and treated with DMEM
(control) and Tβ4 at different concentrations (0.05, 0.1 and 0.2
µM), respectively.
Cell viability
Twenty-four hours after Tβ4 treatment, 0.5% (w/v)
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
in PBS was added to each well and the mixture was incubated at 37°C
for 4 h. Afterwards, dimethyl-sulfoxide (DMSO) was added to the
mixture and the mixture was incubated with gentle shaking for 10
min. Cell viability was determined by the absorbance at 570 nm
using a micro-plate reader. Three replicate trials were conducted
for each concentration of Tβ4.
Hypoxia and serum deprivation
treatment
Twenty-four hours before the experiment, DMEM was
placed in a hypoxic chamber (1% O2, 94% N2
and 5% CO2) for pretreatment. After cells were treated
with Tβ4 at different concentrations, they were incubated in
pretreated DMEM without fetal bovine serum (FBS) in the hypoxic
chamber (1% O2, 94% N2 and 5% CO2)
for 12 h. Subsequently, a portion of the cells was added together
with 0.5% w/v MTT solution for detection of cell viability. The
other portion of cells was stained with ethidium bromide and
acridine orange (EB/AO) and observed under a fluorescence
microscope. Five random fields in each well were analyzed for
calculating surviving and apoptotic cells.
Cell migration
The changes in EPC migration after Tβ4 treatment
were measured in Transwell cell culture chambers with 6.5-mm
diameter polycarbonate membrane filters (8-µm pore). The
Transwell chambers were placed in 24-well plates, and the membrane
filters were coated with 40 ml of Matrigel solution [diluted with
FBS-free DMEM solution]. After drying, EPCs were seeded into the
chamber at a density of 5×105/chamber and then treated
with Tβ4 at different concentrations. FBS-free EBM-2 was added into
the upper chamber of the devise, whereas the lower chamber was
filled with EBM-2 containing 5% FBS. After 12 h of incubation at
37°C, non-migrated cells were removed from the upper surface of the
membrane using a cotton swab. Then the filters were fixed in 90%
alcohol for 30 min and stained with crystal violet for 10 min. The
migrated cells were observed under a microscope and determined by
the absorbance at 600 nm using a microplate reader after addition
of 10% acetic acid.
Angiogenesis assay
Firstly, the 96-well plates were filled with
pre-cooled Matrigel solution (50 µl in each well) and placed
in a cell culture chamber with 5% CO2 at 37°C for 30
min. Then EPCs were inoculated into the 96-well plates at a density
of 1×105/well and treated with Tβ4 at different
concentrations. In addition, 5% FBS was added to each well and EPCs
were incubated in 5% CO2 at 37°C for 6 h. Three
different images from each well were captured, and the total tubule
number was calculated by two independent investigators.
Western blot analysis
EPCs after treatment of Tβ4 at different
concentrations were lysed with RIPA lysis buffer containing 10 mM
phenylmethylsulfonyl fluoride (PMSF). Then the lysates were
isolated by centrifugation and total protein was quantified using a
standard bicinchoninic acid (BCA) assay (Pierce, Rockford, IL,
USA). After quantification, the proteins were separated using 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), and transferred onto a polyvinylidene difluoride
membrane. The membranes were incubated in 5% milk in 0.1%
Tris-buffered saline (TBS) at room temperature for 1 h. Afterwards,
the membranes were incubated with anti-Akt and anti-p-AKT
antibodies (1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). Subsequently, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies
(Abcam, Cambridge, MA, USA) at a dilution of 1:5,000. Finally, the
membranes were washed and visualized using an enhanced
chemiluminescence system (GE Healthcare, Piscataway, NJ, USA).
MI model and treatment
MI was induced by surgical ligation of the left
anterior descending coronary artery in 24 female rats. Firstly, the
animals were anesthetized with ketamine (80 mg/kg) and placed in a
supine position. Then they were intubated and mechanically
ventilated with a rodent ventilator with a respiratory rate of 80
beats/min, and tidal volume of 7 ml. A left-side thoracotomy was
performed to expose the heart. After removing the pericardium, the
left arterial descending coronary artery was permanently ligated.
After establishment of the MI model, rats were randomized into
three groups, with 8 rats in each group: control group, EPC group
and Tβ4-EPC group. Rats in the control group received an
intramyocardial injection of 100 µl PBS; rats in the EPC
group received an intramyocardial injection of 100 µl PBS
containing 2×106 EPCs; and rats in the Tβ4-EPC group
were administered 2×106 EPCs pretreated with Tβ4 (2
µM). Another 8 mice underwent a sham operation including
every step except coronary ligation and were assigned into the sham
group. Surgery was finally completed by chest closure and muscle
and skin suture. Rats in each group were treated with penicillin
after surgery to prevent infection.
Measurement of cardiac function
Four weeks after EPC injection, the cardiac function
of all rats was monitored using an ultrasound system with a 15 MHz
probe, sample volume of 0.6 mm and scanning speed of 100 mm/sec.
M-mode images were obtained by drawing an anatomical M-mode line
perpendicular to the interventricular septum and left ventricular
posterior wall. The measurements for left ventricular end-diastolic
diameter (LVEDD), left ventricular end-systolic diameter (LVESD),
left ventricular end-diastolic volume (LVEDV) and left ventricular
end-systolic volume (LVESV) were carried out according to the
recommendations of the American Society of Echocardiography. The
ejection fraction of the left ventricle (EF) and fractional
shortening (FS) were calculated using the Teichholz formula:
EF(%)=[(LVEDV−LVESV)/LVEDV]×100%FS(%)=[(LVEDD−LVESD)/LVEDD]×100%.
The value of each parameter was obtained from three
different cardiac cycles.
Histologic examination
After echocardiography, the rats were anesthetized
with ketamine and the heart was removed from the open chest of the
rats. Then the hearts were washed with normal saline, fixed in 4%
paraformaldehyde for 24 h, immersed in 15 and 30% sucrose in PBS
for dehydration and embedded in OCT compound. Then the tissue
samples were cut into 5-µm sections and stained with
Masson's trichrome. Myocardial tissue and scar tissue in the
infarcted area were observed under light microscopy and the
epicardial perimeters of the infarcted area and the whole
ventriculus sinister were assessed using Image-Pro Plus 6.0
software. The infarcted size was determined by the following
formula:
Infarcted size=epicardial perimeters of the infarcted area/epicardial perimeters of the whole ventriculus sinister×100%.
Immunohistochemistry
For immunohistochemistry, tissue sections were
blocked in goat serum. Then the sections were incubated with mouse
specific primary antibodies [anti-CD31 and smooth muscle actin-α
(α-SMA)] at a dilution of 1:100 at 4°C overnight, followed by
incubated with DyLight 594-conjugated and FITC-conjugated goat
anti-mouse secondary antibodies at room temperature for 1 h. Cell
nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Vascular structures positive for the expression of both CD31 and
α-SMA were observed under fluorescence microscopy at ×200
magnification. Five random fields from each slide were used to
calculate the microvessel density and three slides selected from
the upper, middle and lower region of the infarcted area of the
heart were used for analysis.
Fluorescence in situ hybridization (FISH)
for the Y-chromosome
Tissue sections were first immersed in PBS
containing 0.2% Triton X-100. After washing with PBS, the sections
were treated with 0.2 N HCl solution for 20 min at room temperature
and digested with 0.1 mg/ml proteinase K solution in a humidified
atmosphere. Then probe buffer was added onto the slides at 37°C for
30 min to block non-specific binding. Afterwards, the sections were
incubated with cDNA probe buffer containing 1 ng/µl
Y-chromosome probe at 95°C for 10 min and at 37°C overnight. On the
next day, the slides were washed with pre-warmed 2× SSC solution
[17.53 g of sodium chloride (0.29 M), 8.82 g of sodium citrate and
distilled water in a final volume of 1,000 ml, pH 7.0], 2× SSC
solution and 0.1× SSC solution, respectively. After blocking with
5% goat serum for 5 min, the slides were treated with FITC-labeled
streptavidin and then incubated for 1 h at room temperature.
Finally, the sections were immunostained with the anti-CD31
antibody at a dilution of 1:100. DAPI was used to stain cell
nuclei. The distribution and endothelial differentiation of cells
positive for Y-chromosome were observed under a microscope.
Statistical analysis
All statistical analysis was conducted with SPSS
19.0 software (SPSS Inc., Chicago, IL, USA). Data were expressed as
mean ± standard deviation (SD) and independent t-test was utilized
to perform comparison between two groups. A value of P<0.05 was
considered to indicate a statistically significantly
difference.
Results
Identification of EPCs
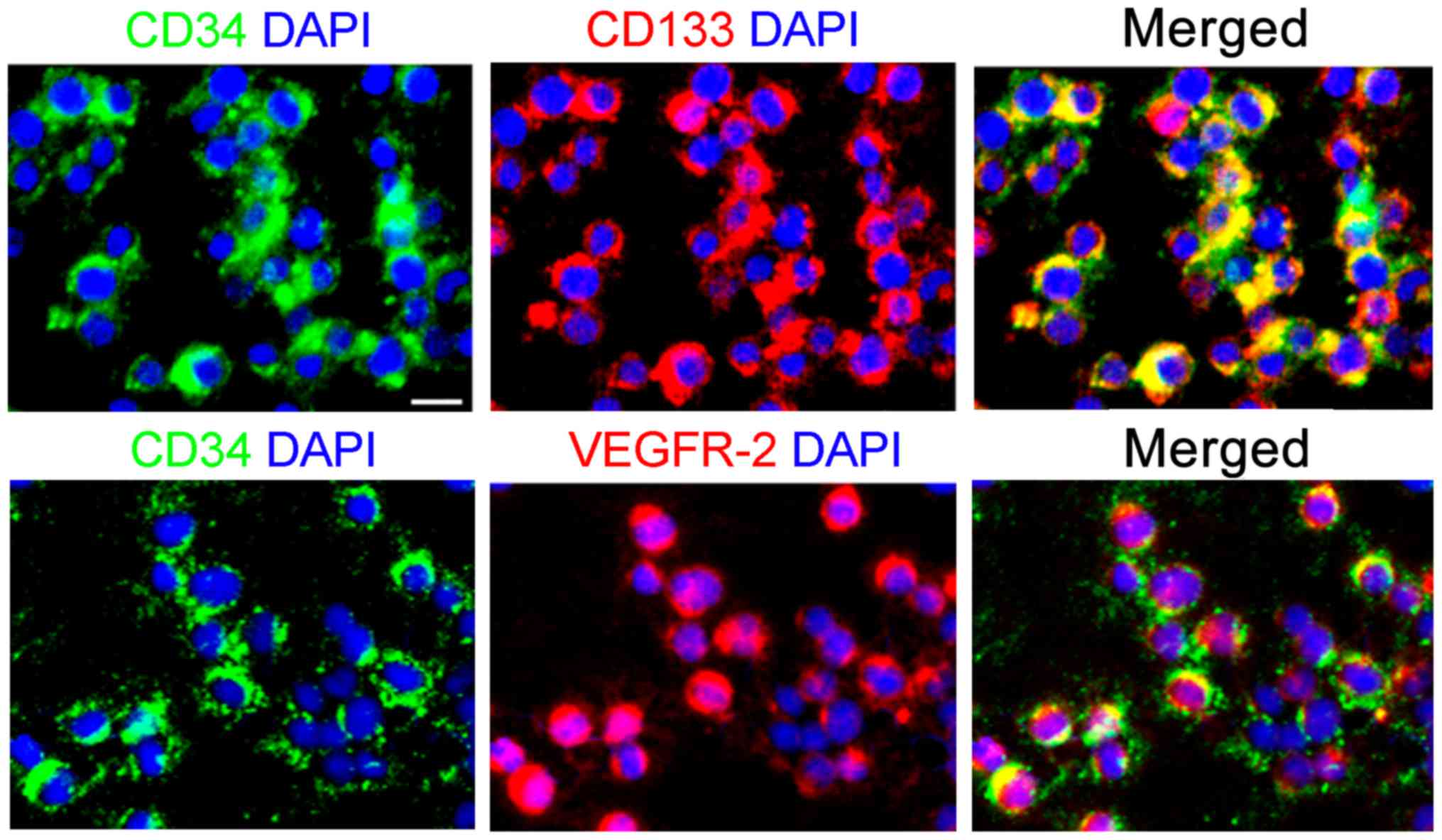
EPCs were isolated from bone marrow and cultured in
a 37°C and 5% CO2 incubator. Three days after
inoculation, colony growth occurred on adherent cells. After
additional culture of 2 days, the cells were grown to 80%
confluence. The cells were spindle-shaped and covered with
projections. The results from the immunofluorescent staining
revealed that >90% of the cells expressed CD34, CD133 and
VEGFR-2 (Fig. 1). The results
indicate that the EPCs were successfully prepared.
Tβ4 promotes the cell function of EPCs
Tβ4 enhances EPC viability
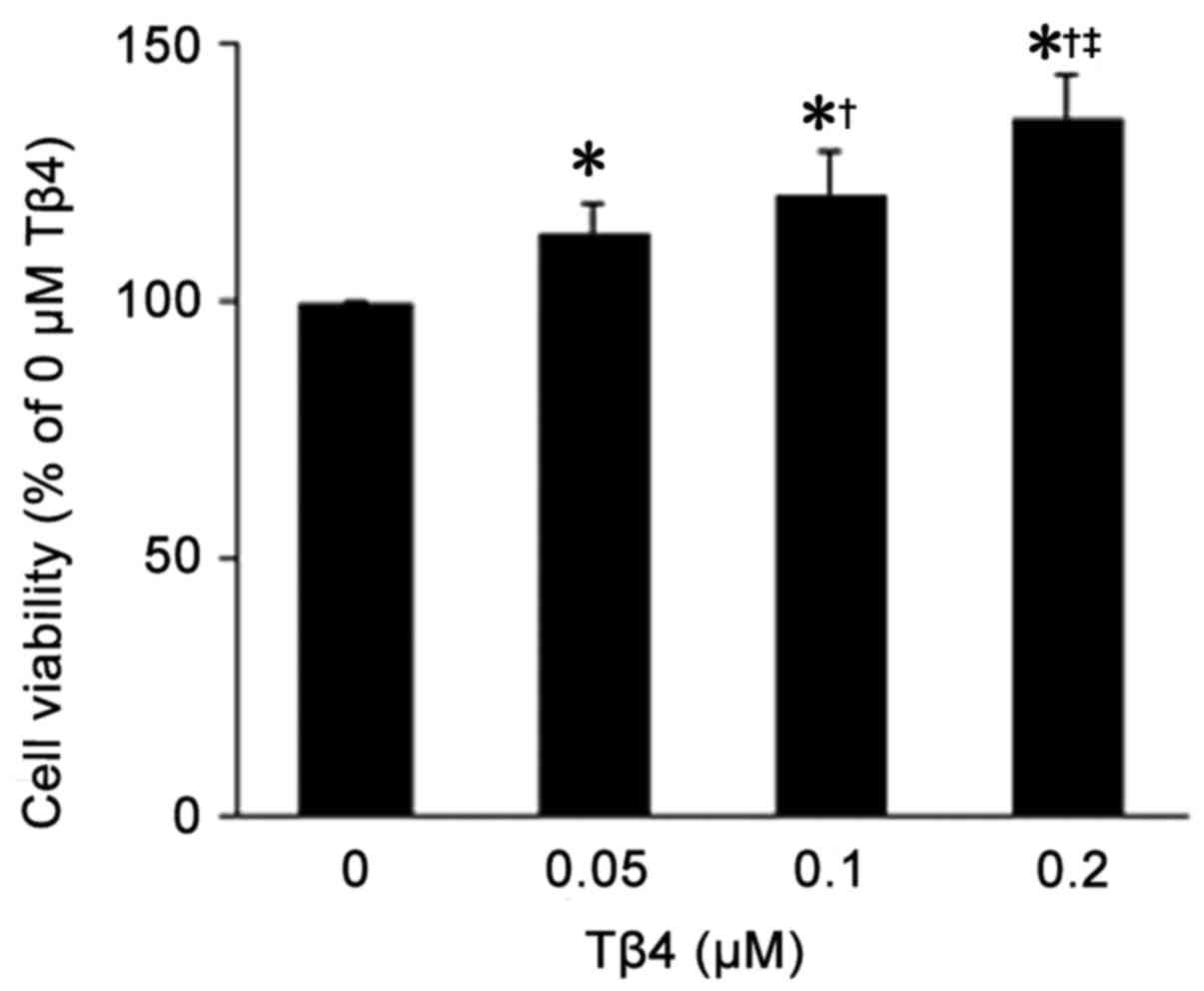
As shown in Fig.
2, there was a significant increase in cell viability in the
EPCs following treatment with Tβ4 compared to the viability of EPCs
without Tβ4 treatment (P<0.05). The effects of Tβ4 in promoting
EPC viability were in a dose-dependent manner (0.05 vs. 0.1
µM, 0.05 vs. 0.2 µM and 0.1 vs. 0.2 µM:
113.2±6.23 vs. 120.9±8.73%, 113.2±6.23 vs. 135.7±8.71% and
120.9±8.73 vs. 135.7±8.71%, P<0.05).
Tβ4 protects EPCs against hypoxia and
serum deprivation
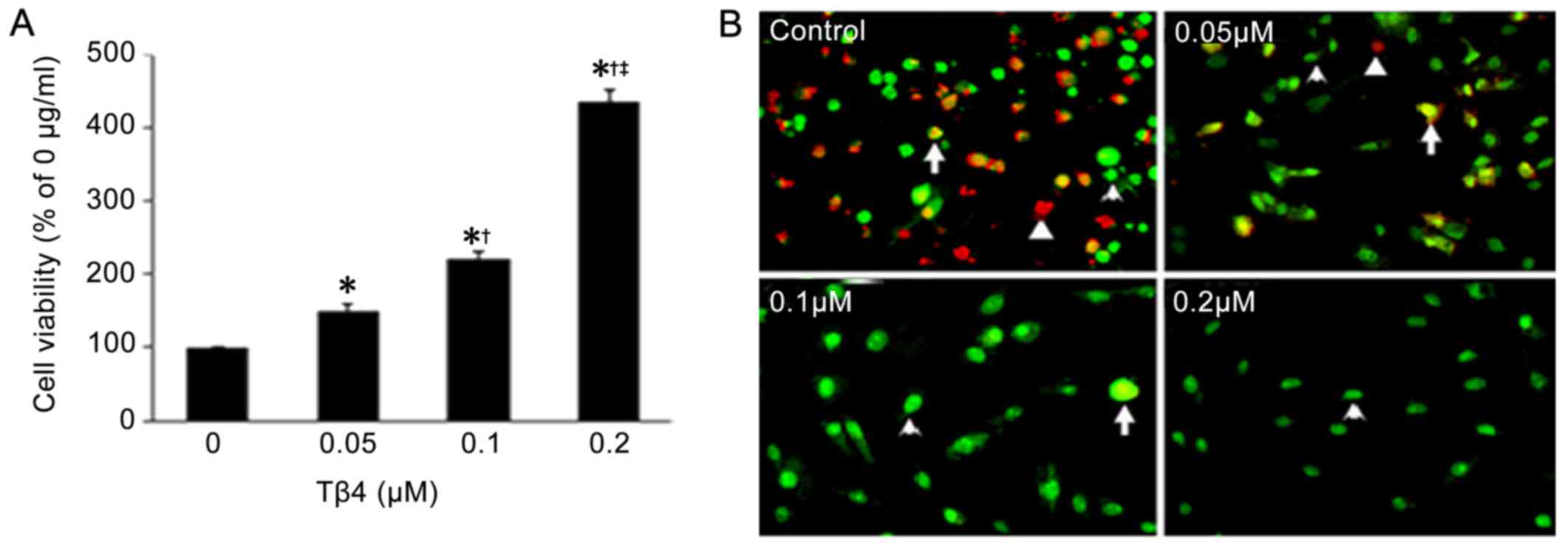
Based on the results from the MTT assay (Fig. 3A), compared to the EPCs without
Tβ4 treatment, there was an obvious increase in cell viability in
the EPCs after Tβ4 treatment at different concentrations
(P<0.05) under hypoxia and serum deprivation and the
growth-promoting effects were also in a dose-dependent manner. In
addition, after staining with EB/AO, the apoptotic cells in the Tβ4
treatment groups were less than those without Tβ4 treatment
(Fig. 3B).
Tβ4 promotes EPC migration
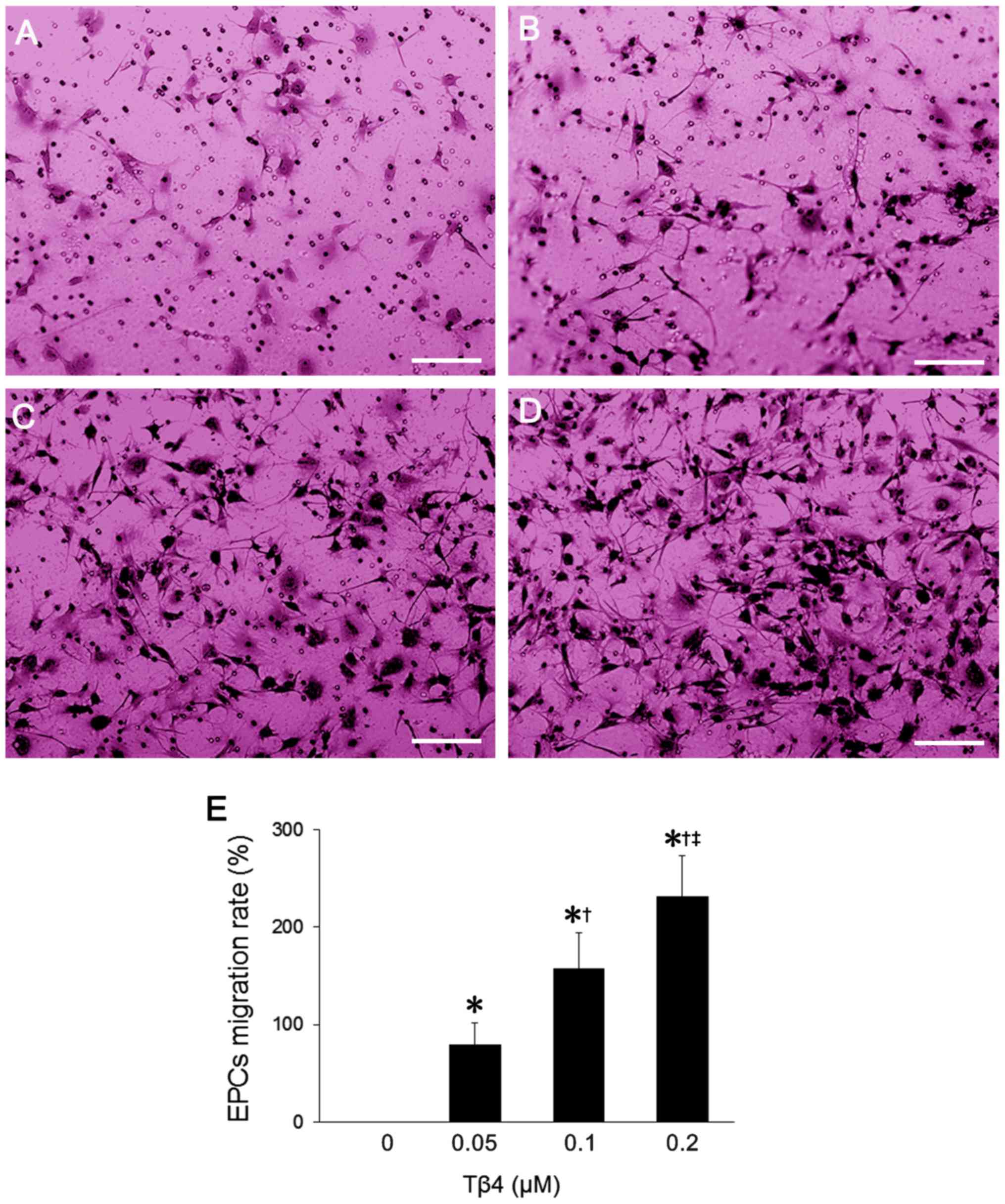
As shown in Fig.
4A–D, the number of migrated cells in the Tβ4 treatment group
was increased compared to that in the control group. After staining
with crystal violet, the numbers of migrated cells from each group
were quantified. A significant increase in the number of migrated
cells was observed in the Tβ4 treatment group (P<0.05) and the
migration-promoting effects of Tβ4 was in a dose-dependent manner
(Fig. 4E).
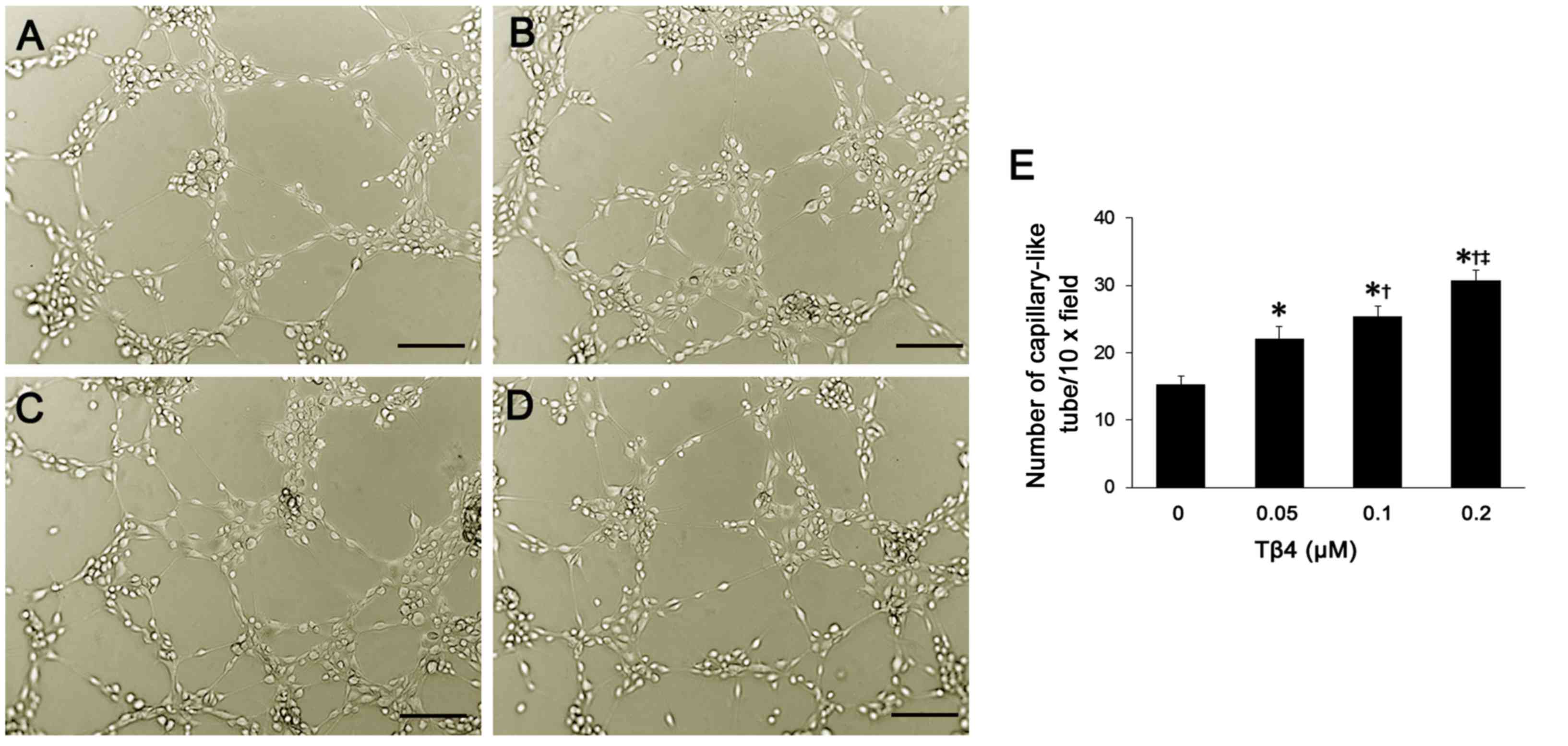
Tβ4 promotes EPC-based
neovascularization
In each group, 6 h after inoculation of EPCs into
Matrigel, most of the cells were covered with projections. These
projections connected with each other, leading to the formation of
tubular structure. Moreover, Tβ4 promoted the formation of
EPC-based vascular structure. As the concentration of Tβ4 was
increased, more tubular structures were observed (Fig. 5A–D). After quantification
analysis, compared to the number of tubular structures in the
control group, there was a significant increase in EPCs treated
with Tβ4 at different concentrations (P<0.05) (Fig. 5E). In addition, the
promoting-effect of Tβ4 on EPC-based neovascularization was in a
dose-dependent manner.
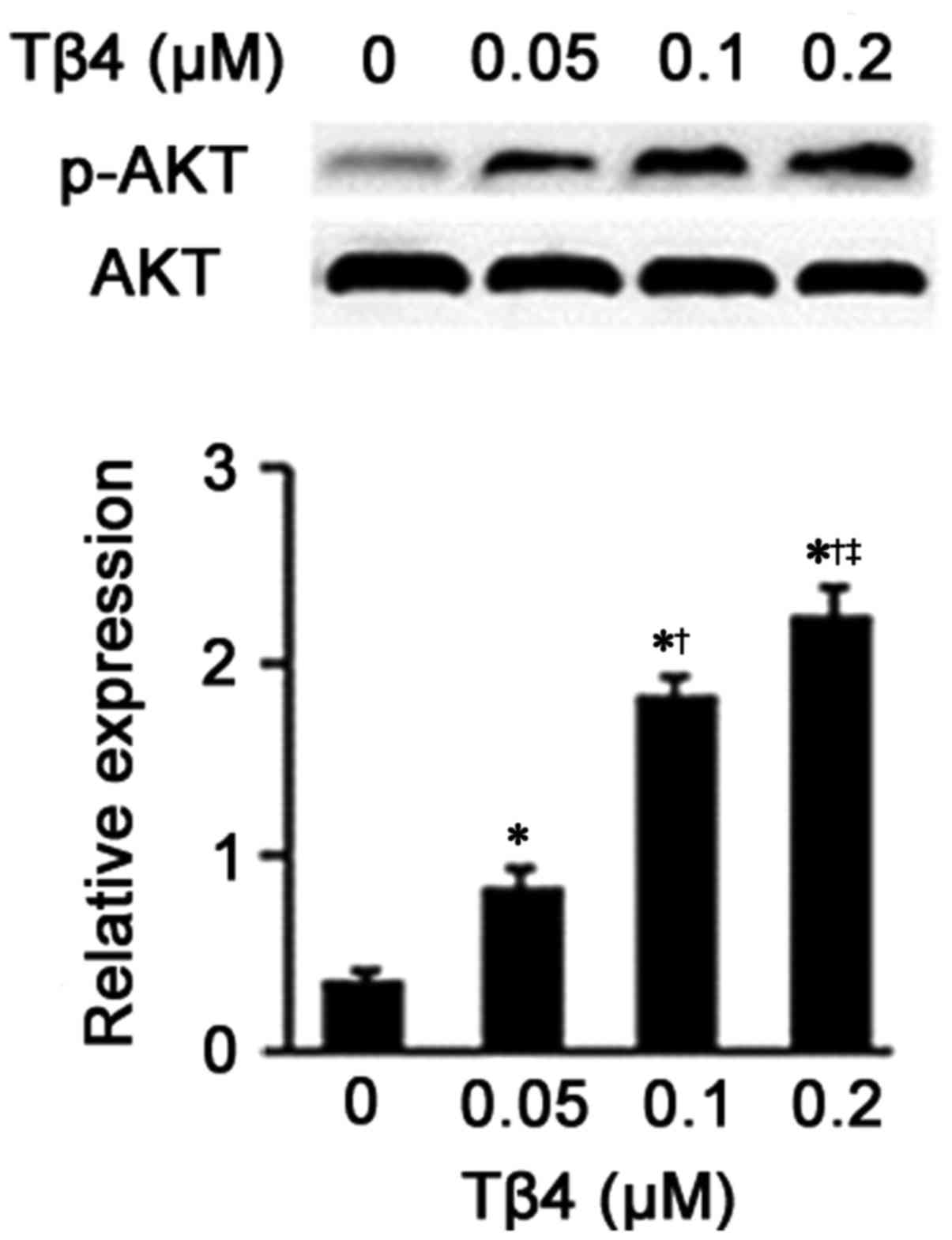
Tβ4 upregulates the expression of
p-Akt
Following the results from the western blot analysis
(Fig. 6), an obviously
upregulation of the expression of phosphorylated Akt was noted in
the EPCs treated with Tβ4 at different concentrations compared to
the EPCs treated with DMEM (P<0.05). Additionally, as the dose
of Tβ4 increased, the promoting effects of Tβ4 on p-Akt expression
were also elevated (0.05 vs. 0.1 µM, 0.05 vs. 0.2 µM
and 0.1 vs. 0.2 µM, P<0.05).
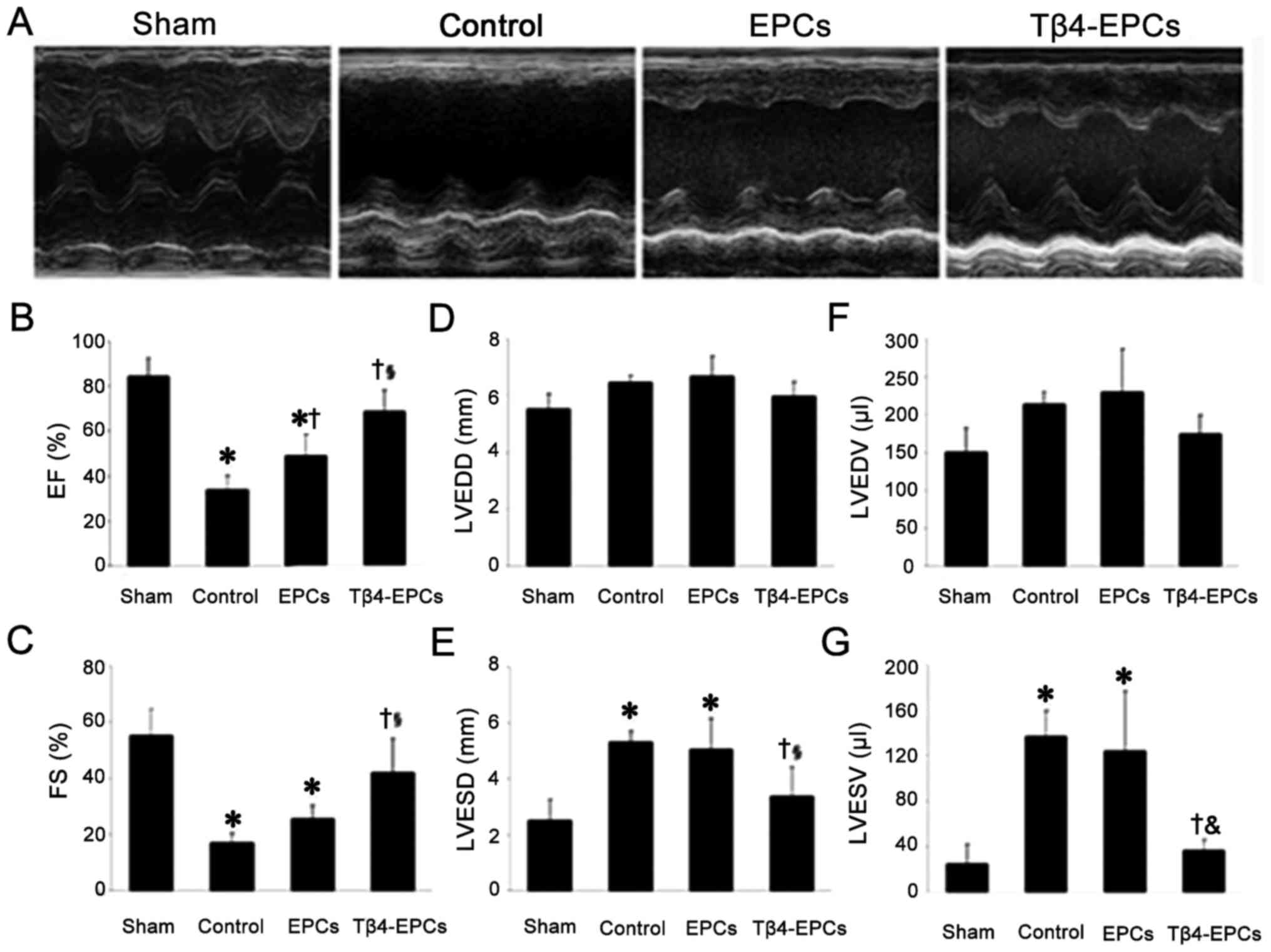
Transplantation of Tβ4-treated EPCs
improves cardiac function in rats with MI
Based on the results of echocardiography,
transplantation of the EPCs or Tβ4-EPCs both reduced left
ventricular chamber and increased the left ventricular wall
thickness in rats with MI (Fig.
7A). After statistical analysis (Fig. 7B–G), compared to the rats in the
control group, both EF and FS were significantly increased in the
rats transplanted with EPCs and Tβ4-EPCs (P<0.05). Moreover,
both parameters were also significantly higher in the rats
transplanted with Tβ4-EPCs than those with EPCs (P<0.05).
Furthermore, both LVESD and LVESV were significantly decreased in
the rats transplanted with Tβ4-EPCs compared to the rats in the
control group (P<0.05).
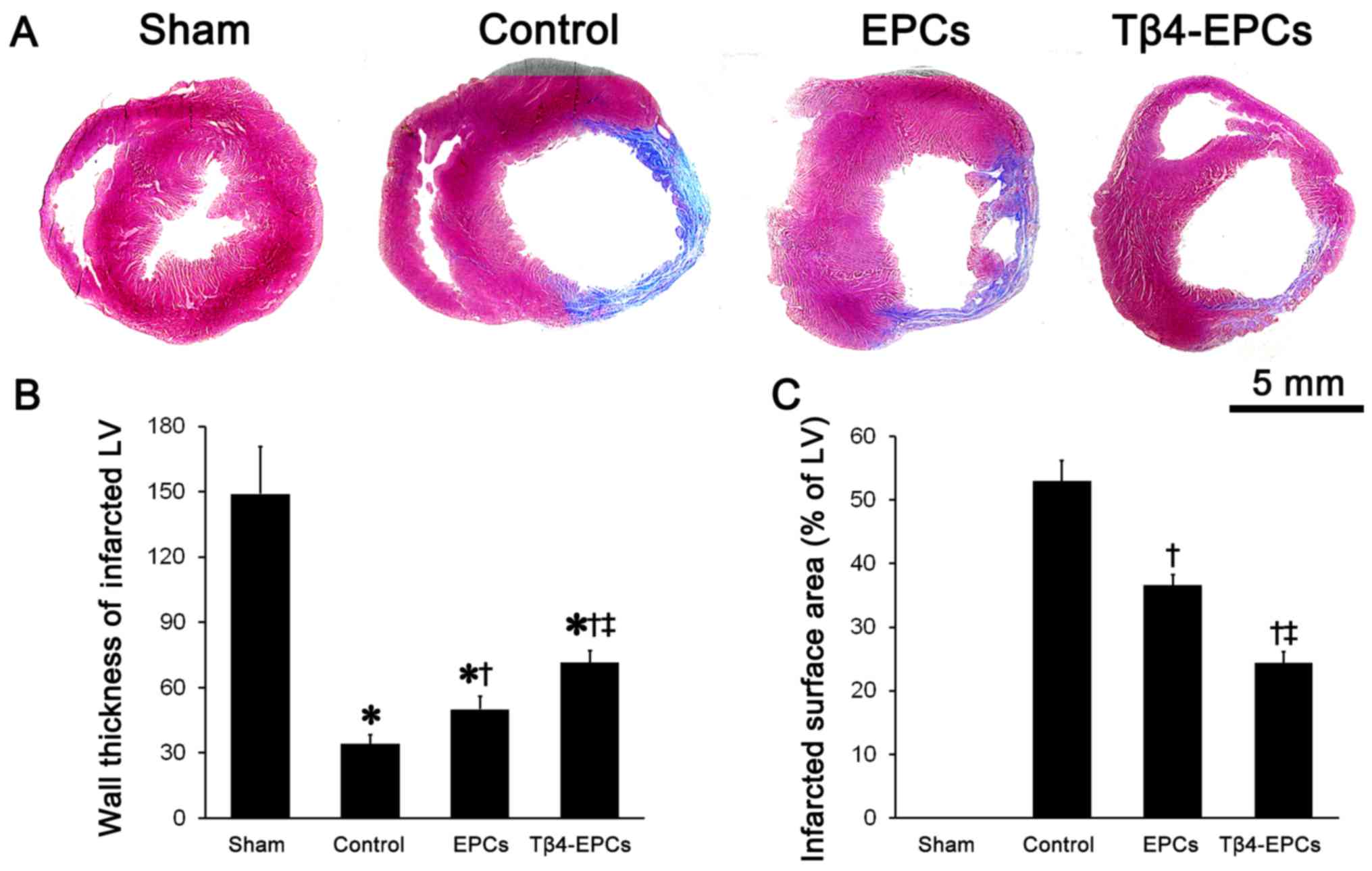
Tβ4 promotes EPC-based cardiac repair in
rats with MI
Masson's trichrome staining showed an obvious
expansion of the left ventricular chamber and thinning of the left
ventricular wall in rats that underwent surgical ligation of the
left anterior descending coronary artery. Moreover, most of the
myocardium in the infarcted area was replaced by scar tissue.
Whereas, in the MI-induced rats transplanted with EPCs or Tβ4-EPCs,
the left ventricular chamber was smaller, the left ventricular wall
was thicker and there was much less scar tissue and more myocardial
cells compared to the rats in the control group (Fig. 8A). Statistical results indicated
that in rats transplanted with Tβ4-EPCs the left ventricular wall
thickness was significantly larger and the infarcted size was
significantly smaller than these parameters in the control and EPC
groups (P<0.05) (Fig. 8B and
C).
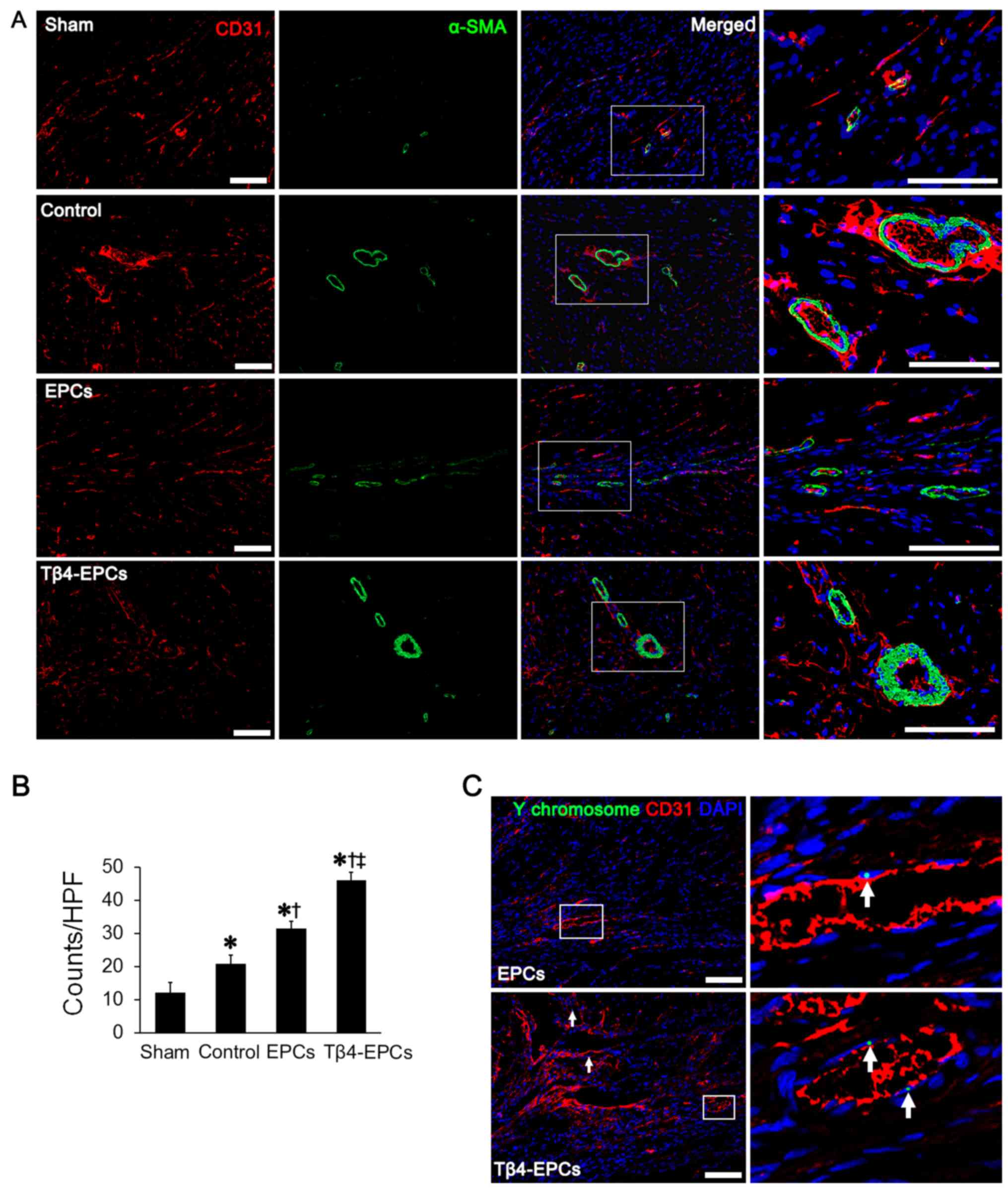
Tβ4 promotes EPC-based angiogensis in
rats with MI
Angiogenesis was observed in the marginal zone of
the infarcted area as confirmed by immunofluorescence.
Transplantation with EPCs promoted angiogenesis as confirmed by the
increase in CD31- and αSMA-positive cells in the infarcted area
(Fig. 9A). In addition,
transplantation with Tβ4-EPCs showed more promoting effects on
angiogenesis. Based on the statistical results, the microvessel
density was significantly higher in rats transplanted with Tβ4-EPCs
than that in rats in the EPC and control groups (P<0.05)
(Fig. 9B).
Furthermore, based on the results from FISH,
Y-chromosome-positive cells were observed in the marginal zone of
the infarcted area, which constitutes the endothelial tissue. There
were a higher number of Y-chromosome-positive cells in rats
transplanted with Tβ4-EPCs than the number in rats transplanted
with EPCs (P<0.05) (Fig.
9C).
Discussion
The aim of the present study was to explore the
cardioprotective effects of the transplantation of Tβ4-treated EPCs
in rats with MI. Both in vivo and in vitro
experiments were conducted. Tβ4 is a pleiotropic factor involved in
a wide variety of physiological and pathological processes. As
described previously, Tβ4 promotes cell migration through binding
actin and regulating the balance between polymerization and
depolymerization of actin (20).
In addition, Tβ4 also modulates the synthesis and secretion of
various cytokines including laminin-5, intercellular adhesion
molecule (ICAM)-1 and matrix metalloproteinase-2 (MMP-2) to promote
cell adhesion and migration (21). Recently, Tβ4 has been reported to
exert a profound cardioprotective effect (22). Tβ4 has been shown to be involved
in the protection and regeneration of injured or damaged tissues.
It also promotes angiogenesis by stimulating endothelial cell
adhesion and migration, tubule formation, as well as EPC
differentiation into endothelial cells (23). For the in vitro experiments
in our study, Tβ4 promoted EPC survival, migration and EPC-based
tubule formation, all of which were consistent with previous
studies. In addition, EPC survival in the hypoxia and ischemic
microenvironment of injured or damaged tissues is the key to EPC
transplantation for treating cardiac diseases. In our study, Tβ4
also protected EPCs from hypoxia and serum deprivation, which may
be attributed to the protective effects of Tβ4 on mitochondria by
reducing cytochrome enzyme c release and upregulating the
expression of Bcl-2 (24).
The Akt signaling pathway plays an important role in
Tβ4-mediated biological processes. After binding to adaptor protein
LIMS1 and integrin-linked kinase (ILK), Tβ4 activates the Akt
signaling pathways, thus promoting cell migration (22). Additionally, Tβ4 decreases the
expression of caspase-3 and caspase-9 by activating the ILK-Akt
signaling pathway under serum deprivation, then suppresses cell
apoptosis (24). Consistent with
a study by Qiu et al (25), Tβ4 increased the expression of
p-Akt, indicating that the Akt signaling pathway was activated by
Tβ4, which may be the mechanism underlying the promoting effects of
Tβ4 on EPC viability, migration and angiogenesis. In addition, this
mechanism was also confirmed in nude mice, and the same conclusion
was obtained. All of this evidence indicates that the pathway of
Tβ4-induced EPC migration in vitro was consistent with that
in vivo.
We also established an MI model by surgical ligation
of the left anterior descending coronary artery in rats. A previous
study indicated that EPC transplantation after MI can improve
cardiac function and promote angiogenesis in the damaged myocardium
(26). In the present study,
compared to the control group, rats with EPC transplantation had
improved cardiac function, smaller myocardial infarcted size and
higher microvessel density. However, the low survival rate of EPCs
limits the expanded application of EPC transplantation for treating
MI (17). EPCs pretreated with
Tβ4 were also transplanted into rats with MI. Based on the results
from our study, Tβ4-treated EPCs significantly improved cardiac
function in rats at 4 weeks post-infarction confirmed by the
elevated levels of EF and FS and increase in the left ventricular
wall thickness. Moreover, Tβ4-treated EPCs obviously decreased the
infarcted size and promoted cardiac repair in the MI model. Our
findings were consistent with a previous study (27). Furthermore, Tβ4 also promoted
EPC-based neovascularization as confirmed by the significant
increase in CD31- and αSMA-positive cells and Y-chromosome-positive
cells. Due to the massive false-positive results provided by the
α-SMA antibody, the flow cytometry was restricted and the
double-positive immunostaining method was selected in this study.
In spite of this, the immunostaining also provided us with
significant information for this comparison. All of these results
indicated that Tβ4 promoted cardioprotective effects of EPC
transplantation in MI.
Collectively, Tβ4 is able to promote EPC survival,
migration, tubule formation and also protect EPCs from hypoxia and
serum deprivation, which may be attributed to the activation of the
Akt signaling pathway. Additionally, the transplantation and
survival of EPCs in MI can be increased by pretreatment with Tβ4.
Treatment with both EPCs and Tβ4 can obviously improve cardiac
function, promote cardiac repair and stimulate neovascularization
in rats with MI.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81270200 and grant no.
81470385) and the Scientific Research Foundation of State Education
Commission (grant no. 20130071110080).
References
|
1
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP,
Fullerton HJ, et al: Heart Disease and Stroke Statistics-2016
Update: A report from the American Heart Association. Circulation.
133:e38–e360. 2016. View Article : Google Scholar
|
|
2
|
Diseases NCFC Report on cardiovascular
disease in China 2014. Encyclopedia of China Publishing House;
2015
|
|
3
|
White HD and Chew DP: Acute myocardial
infarction. Lancet. 372:570–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hochman JS, Lamas GA, Buller CE, Dzavik V,
Reynolds HR, Abramsky SJ, Forman S, Ruzyllo W, Maggioni AP, White
H, et al Occluded Artery Trial Investigators: Coronary intervention
for persistent occlusion after myocardial infarction. N Engl J Med.
355:2395–2407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keeley EC, Boura JA and Grines CL: Primary
angioplasty versus intravenous thrombolytic therapy for acute
myocardial infarction: A quantitative review of 23 randomised
trials. Lancet. 361:13–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White H; Hirulog and Early Reperfusion or
Occlusion (HERO)-2 Trial Investigators: Thrombin-specific
anticoagulation with bivalirudin versus heparin in patients
receiving fibrinolytic therapy for acute myocardial infarction: The
HERO-2 randomised trial. Lancet. 358:1855–1863. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freemantle N, Cleland J, Young P, Mason J
and Harrison J: beta Blockade after myocardial infarction:
systematic review and meta regression analysis. BMJ. 318:1730–1737.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verma S and Strauss M: Angiotensin
receptor blockers and myocardial infarction. BMJ. 329:1248–1249.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McMurray J, Køber L, Robertson M, Dargie
H, Colucci W, Lopez-Sendon J, Remme W, Sharpe DN and Ford I:
Anti-arrhythmic effect of carvedilol after acute myocardial
infarction: Results of the Carvedilol Post-Infarct Survival Control
in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll
Cardiol. 45:525–530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X, Cohen MV and Downey JM: Mechanism
of cardioprotection by early ischemic preconditioning. Cardiovasc
Drugs Ther. 24:225–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bergmann O, Bhardwaj RD, Bernard S, Zdunek
S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA,
Druid H, et al: Evidence for cardiomyocyte renewal in humans.
Science. 324:98–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Werner N, Kosiol S, Schiegl T, Ahlers P,
Walenta K, Link A, Böhm M and Nickenig G: Circulating endothelial
progenitor cells and cardiovascular outcomes. N Engl J Med.
353:999–1007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao EH, Fukuda N, Matsumoto T, Katakawa M,
Yamamoto C, Han Y, Ueno T, Kobayashi N and Matsumoto K: Effects of
the antioxidative β-blocker celiprolol on endothelial progenitor
cells in hypertensive rats. Am J Hypertens. 21:1062–1068. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sen S, Merchan J, Dean J, Ii M, Gavin M,
Silver M, Tkebuchava T, Yoon YS, Rasko JE and Aikawa R: Autologous
transplantation of endothelial progenitor cells genetically
modified by adeno-associated viral vector delivering insulin-like
growth factor-1 gene after myocardial infarction. Hum Gene Ther.
21:1327–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dubois C, Liu X, Claus P, Marsboom G,
Pokreisz P, Vandenwijngaert S, Dépelteau H, Streb W, Chaothawee L,
Maes F, et al: Differential effects of progenitor cell populations
on left ventricular remodeling and myocardial neovascularization
after myocardial infarction. J Am Coll Cardiol. 55:2232–2243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higuchi T, Anton M, Dumler K, Seidl S,
Pelisek J, Saraste A, Welling A, Hofmann F, Oostendorp RA,
Gansbacher B, et al: Combined reporter gene PET and iron oxide MRI
for monitoring survival and localization of transplanted cells in
the rat heart. J Nucl Med. 50:1088–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao Y, Li Y, Ma G, Liu N, Ju S, Jin J,
Chen Z, Shen C and Teng G: In vivo magnetic resonance imaging of
injected endothelial progenitor cells after myocardial infarction
in rats. Mol Imaging Biol. 13:303–313. 2011. View Article : Google Scholar
|
|
18
|
Kupatt C, Horstkotte J, Vlastos GA,
Pfosser A, Lebherz C, Semisch M, Thalgott M, Büttner K, Browarzyk
C, Mages J, et al: Embryonic endothelial progenitor cells
expressing a broad range of proangiogenic and remodeling factors
enhance vascularization and tissue recovery in acute and chronic
ischemia. FASEB J. 19:1576–1578. 2005.PubMed/NCBI
|
|
19
|
Smart N, Risebro CA, Clark JE, Ehler E,
Miquerol L, Rossdeutsch A, Marber MS and Riley PR: Thymosin β4
facilitates epicardial neovascularization of the injured adult
heart. Ann NY Acad Sci. 1194:97–104. 2010. View Article : Google Scholar
|
|
20
|
Sosne G, Xu L, Prach L, Mrock LK, Kleinman
HK, Letterio JJ, Hazlett LD and Kurpakus-Wheater M: Thymosin beta 4
stimulates laminin-5 production independent of TGF-beta. Exp Cell
Res. 293:175–183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan L, Guan W, Hu Y, Xiaofan Y, Songshan
X, Liya N and Yongzhe C: Recombinant thymosin β4 accelerates skin
wound healing by regulating ICAM-1, MMP-2 and LN-5. J Tissue
Engineering and Reconstructive Surgery. 163:142–145. 2008.
|
|
22
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin β4 activates integrin-linked
kinase and promotes cardiac cell migration, survival and cardiac
repair. Nature. 432:466–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smart N, Risebro CA, Melville AA, Moses K,
Schwartz RJ, Chien KR and Riley PR: Thymosin β4 induces adult
epicardial progenitor mobilization and neovascularization. Nature.
445:177–182. 2007. View Article : Google Scholar
|
|
24
|
Sosne G, Siddiqi A and Kurpakus-Wheater M:
Thymosin-β4 inhibits corneal epithelial cell apoptosis after
ethanol exposure in vitro. Invest Ophthalmol Vis Sci. 45:1095–1100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu FY, Song XX, Zheng H, Zhao YB and Fu
GS: Thymosin β4 induces endothelial progenitor cell migration via
I3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol.
53:209–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burlacu A, Grigorescu G, Rosca AM, Preda
MB and Simionescu M: Factors secreted by mesenchymal stem cells and
endothelial progenitor cells have complementary effects on
angiogenesis in vitro. Stem Cells Dev. 22:643–653. 2013. View Article : Google Scholar :
|
|
27
|
Chang ZT, Hong L, Wang H, Lai HL, Li LF
and Yin QL: Application of peripheral-blood-derived endothelial
progenitor cell for treating ischemia-reperfusion injury and
infarction: A preclinical study in rat models. J Cardiothorac Surg.
8:332013. View Article : Google Scholar : PubMed/NCBI
|