Introduction
Atherosclerosis is a chronic inflammatory disease
which develops in response to injury in the vessel wall (1,2). A
number of chronic diseases are known to be involved in the
pathogenesis of inflammation, including atherosclerosis,
Alzheimer's disease and Parkinson's disease (3). Endothelial cells (ECs) recruit
circulating monocytes via a multi-step process mediated by a
combination of cell surface adhesion molecules, such as
intercellular cell adhesion molecule-1 (ICAM-1) during inflammation
(2). Pro-inflammatory cytokines,
such as tumor necrosis factor α (TNFα) commonly found in
inflammation, can activate nuclear factor-κB (NF-κB) which plays a
key role in the expression of cell adhesion molecules (1,4).
Following stimulation with TNFα, a kinase cascade is activated that
induces subsequent IκB phosphorylation. Phosphorylated IκB is
rapidly degraded, resulting in the release of NF-κB, which enters
the nucleus and regulates gene transcription (5).
Lycopene is a natural carotenoid that is present in
tomatoes and tomato-based products. Other dietary sources of
lycopene also include pink grapefruit, papaya, watermelon and dried
apricotscarotenoid (6). It has
been reported that the dietary intake of tomatoes containing
lycopene reduces the risk of chronic diseases and various types of
cancer (7). Previous
epidemiologic studies have found that the dietary intake of
lycopene was significantly associated with plasma lycopene
concentrations (8). The mean
plasma level of lycopene has been found to be 0.59 µM,
ranging from 0.07 to 1.79 µM and it contributes to
approximately 21–43% of the total carotenoids (8,9).
Lycopene exerts several biological functions, such as acting as an
antioxidant and reducing low density lipoprotein cholesterol levels
(10); it also reduces
pro-inflammatory cytokine and chemokine expression in macrophages
(11,12). Indeed, lycopene has been found to
decrease the expression of several genes by modulating the NF-κB
signaling pathway in ECs (13).
These results provide a possible mechanism for the
anti-inflammatory effects of lycopene related to its antioxidant
activity. However, the detailed mechanisms responsible for the
anti-inflammatory effects of lycopene remain to be elucidated.
The transcription factor, nuclear factor-erythroid 2
related factor 2 (Nrf2), which binds to the antioxidant response
element (ARE), is essential for the induction of phase II
detoxification and antioxidant enzymes (14–16). A previous study further
demonstrated that the activation of Nrf2 abolishes inflammatory
gene expression in ECs (17).
Heme oxygenase (HO) is a cytoprotective and a rate-limiting enzyme,
and it degrades heme to bilirubin, carbon monoxide (CO) and iron
(18). HO-1 is an Nrf2-mediated
phase II enzyme upregulated in conditions of oxidative stress,
cellular injury and disease (18). In our previous study, we reported
that the Nrf2-induced expression of HO-1 and the related signaling
pathways exerted anti-inflammatory effects in ECs (19). Recently, we also demonstrated that
HO-1 exerts anti-inflammatory effects by its derivative CO
production (20,21). However, the detailed mechanisms
responsible for the anti-inflammatory effects of lycopene in ECs
have not yet been fully elucidated. Thus, in the present study, we
investigated the molecular mechanisms underlying the
anti-inflammatory properties of lycopene in ECs. We aimed to
investigate whether HO-1 expression contributes to
lycopene-regulated anti-inflammatory responses. We found that the
lycopene-induced accumulation of Nrf2 in the nuclei was closely
associated with the upregulation of HO-1, which led to
anti-inflammatory effects in ECs.
Materials and methods
The p3xARE/Luc and NF-κB/Luc vectors were
constructed by introducing the Nrf2 binding site or NF-κB binding
site into the pGL3 promoter plasmid (Promega, Madison, WI, USA),
respectively as described in a previous study (22). Luciferase assay kits were
purchased from Promega. Antibodies against HO-1 (SPA-896) and p65
(KAS-TF110) were purchased from Stressgen Biotechnologies (SB, San
Diego, CA, USA). ICAM-1 (sc-7891), IκBα (sc-847), lamin B1
(sc-56143), α-tubulin (sc-53646) and Nrf2 (sc-722) antibodies were
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Bacterially-derived TNFα was purchased from Calbiochem (San Diego,
CA, USA). All other reagents, including lycopene and tricarbonyl
dichlororuthenium (II) dimer (TCDC) were purchased from Sigma (St.
Louis, MO, USA). The CO donor, TCDC, was activated by adding the
compound to culture medium to liberate CO (20).
Culture of ECs
The human umbilical vein cell line, EA.hy926 (ATCC
CRL-2922) was cultured in Dulbecco's modified Eagle's medium (DMEM;
Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal
bovine serum at 37°C under 5% CO2 in air. When the ECs
were grown to confluence, the culture medium was then replaced with
serum-free DMEM and the cells were incubated for 12 h prior to the
experimental treatments.
Cell adhesion assay
Adhesion assay for monocytes and ECs was performed
as previously described (23).
Briefly, ECs grown to confluency in a 96-well plate were
pre-treated with lycopene for 1–12 h and/or 100 U/ml TNFα for 4 h
to allow for the expression of ICAM-1. The cells were washed with
control DMEM without supplements and were co-cultured with
5×105 cells of calcein-labeled THP-1 cells
(ATCC® TIB202™) in the control medium for 30 min. After
washing twice with RPMI medium, the adherent cells were examined
using a Fluoroscan enzyme-linked immunosorbent assay (ELISA) plate
reader (FLx800; Bio-Tek Instruments, Inc., Winooski, VT, USA) at
485 nm excitation and 538 nm emission wavelengths.
Fluorescence-labeled adherent cells were photographed using an
Axiovert S100 microscope (Zeiss, Jena, Germany).
Western blot analysis
The cells (106) were lysed on ice in
lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and a
protease inhibitor mixture) and whole-cell extracts were boiled for
5 min prior to separation on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins
were then transferred onto nitrocellulose membranes (Millipore,
Bedford, MA, USA) in Tris-glycine buffer at 10 volts for 1.5 h. The
membranes were then blocked with PBS containing 5% non-fat milk and
incubated with primary antibodies for 2 h at 4°C with gentle
shaking. After washing with PBS, the membranes were incubated with
the secondary antibodies, horseradish peroxidase-conjugated goat
anti-rabbit (34083) or anti-mouse (34081) antibody (Thermo Fisher
Scientific, Waltham, MA, USA). The antibodies against α-tubulin and
lamin were used as internal controls of the total or nuclear
protein lysates, respectively. The results were visualized by
chemiluminescence using ECL in according with the manufacturer's
instructions.
RNA isolation and RT-PCR
Total RNA was isolated using TRIzol reagent and was
reverse transcribed using SuperScript II reverse transcriptase
(both from Invitrogen, Carlsbad, CA, USA) using an oligo(dT) primer
according to the manufacturer's instructions. cDNA was subjected to
PCR amplification using the following forward and reverse primer
sets: ICAM-1, 5′-AGCAATGTGCAAGAAGATAGCCAA-3′ and 5′-GGT
CCCCTGCGTGTTCCACC-3′; glyceraldehyde 3-phosphate dehydrogenase
(GAPDH), 5′-TATCGTGGAAGGACTCA TGACC-3′ and
5′-TACATGGCAACTGTGAGGGG-3′; glutamate-cysteine ligase modifier
subunit (GCLM), 5′-CAG CGA GGA GCT TCA TGA TTG-3′ and 5′-TGA TCA
CAG AAT CCA GCT GTG C-3′; glutamate-cysteine ligase catalytic
subunit (GCLC), 5′-GTT CTT GAA ACT CTG CAA GAG AAG-3′ and 5′-ATG
GAG ATG GTG TAT TCT TGT CC-3′ (24). PCR products were separated on 1.5%
agarose gels and visualized by ethidium bromide staining.
Preparation of subcellular fractionation
for immunoblotting
The ECs were collected by scraping and lysed with
cell lysis buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl,
0.5 mM DTT, 0.5 mM PMSF and 0.3% nonidet P-40). The cell lysate was
separated into cytoplasmic nuclear fractions by centrifugation at
500 × g for 5 min at 4°C, and the supernatant was collected and
designated as the cytosolic fraction. The nuclei were washed with
nuclei washing buffer and the nuclear protein was extracted using a
buffer containing 25% glycerol, 20 mM HEPES, 0.6 M KCl, 1.5 mM
MgCl2 and 0.2 mM EDTA for 15 min at 4°C.
Luciferase reporter assays
The ECs were transfected with NF-κB/Luc or
p3xARE/Luc using Lipofectamine 2000 (Invitrogen), as previously
described (22). For the
luciferase assays, the cells were lysed and luciferase activity was
determined by use luciferase substrate solution (Promega) and the
resulting luciferase activity was measured using a luminometer. For
each experiment, luciferase activity was determined in triplicate
and normalized to β-galactosidase activity.
Cytotoxicity
To examine cytotoxicity, the resazurin reduction
test, which is an index of the metabolic activity of living cells,
was carried out using the Alamar blue® assay kit
according to the instructions provided by the manufacturer
(Serotec, Oxford, UK). The ECs were plated into 96-well microtiter
plates (Falcon, Pittsburgh, PA, USA) at 20,000 cells/well and
incubated in Alamar blue® reagent for 2 h at 37°C.
Fluorescence was then measured using a Fluorescence Reader (FLx800,
Bio-Tek, Winooski, VT, USA) at excitation/emission wavelengths of
570/600 nm.
Determination of GSH levels
The GSH levels were determined using the method
originally described by Kamencic et al (25). Briefly, the cells cultured in
6-well plates were first washed with PBS, and 40 µM of the
fluorescent probe, monochlorobimane (MCB), with PBS was added
followed by incubation for 20 min at 37°C, and then washed again
with PBS. Fluorescence was monitored at excitation and emission
wavelengths of 405 and 510 nm, respectively, using a
spectrofluorophotometer (Shimadzu, Rf-5301PC; Shimadzu, Kyoto,
Japan).
RNA interference using siRNA against
HO-1
The siRNA nucleotide sequence for human HO-1 was as
follows: 5′-CUGUGUCCCUCUCUCUGGA-3′ and was obtained from Sigma
(NM_002133). A non-targeting siRNA, 5-GCAAGCUGACCCUGAAGUUCAU-3, was
purchased from Ambion (Austin, TX, USA). The EA.hy926 cells were
transfected with siRNA against HO-1 (HO-1 siRNA) or non-targeting
siRNA using Lipofectamine 2000 reagent according to the
manufacturer's instructions. After 24 h of transfection, the ECs
were then cultured in medium without serum for another 12 h prior
to the treatments.
Statistical analysis
The values are expressed as the means ± standard
error of the mean (SEM) of at least 3 independent experiments.
Statistical significance was assessed by one-way analysis of
variance (ANOVA) followed by Tukey's test. A confidence limit of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Lycopene inhibits both monocyte adhesion
and ICAM-1 expression in ECs
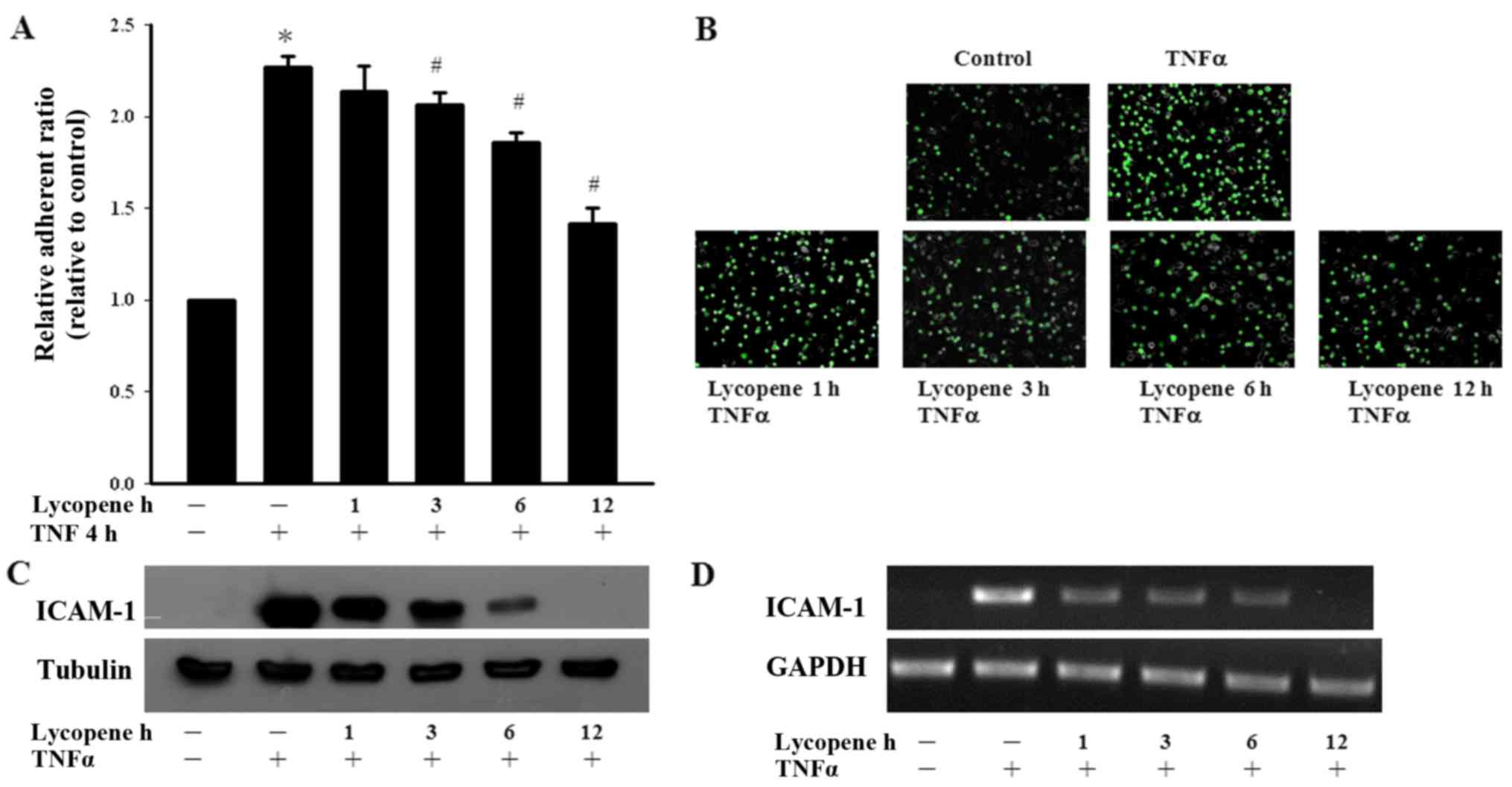
We first examined the effects of lycopene on
monocyte adhesion to ECs. Monocyte adhesion was quantified by
measuring the fluorescence intensity using fluorescent-labeled
monocytes adhering to lycopene and/or TNFα pre-treated ECs. We
found that TNFα significantly increased monocyte adhesion to the
ECs and this adhesion process was abolished by treatment with
lycopene (Fig. 1A and B). We then
investigated the inhibitory effects of lycopene upon the
TNFα-induced expression of adhesion molecules in ECs. We found that
pre-treatment of the ECs with 2 µM lycopene after 12 h
significantly inhibited the TNFα-induced ICAM-1 expression at the
protein and mRNA level (Fig. 1C and
D). In the present study, we examined the cytotoxicity of
lycopene in ECs using an Alamar blue assay, which indicated no
adverse effects (data not shown).
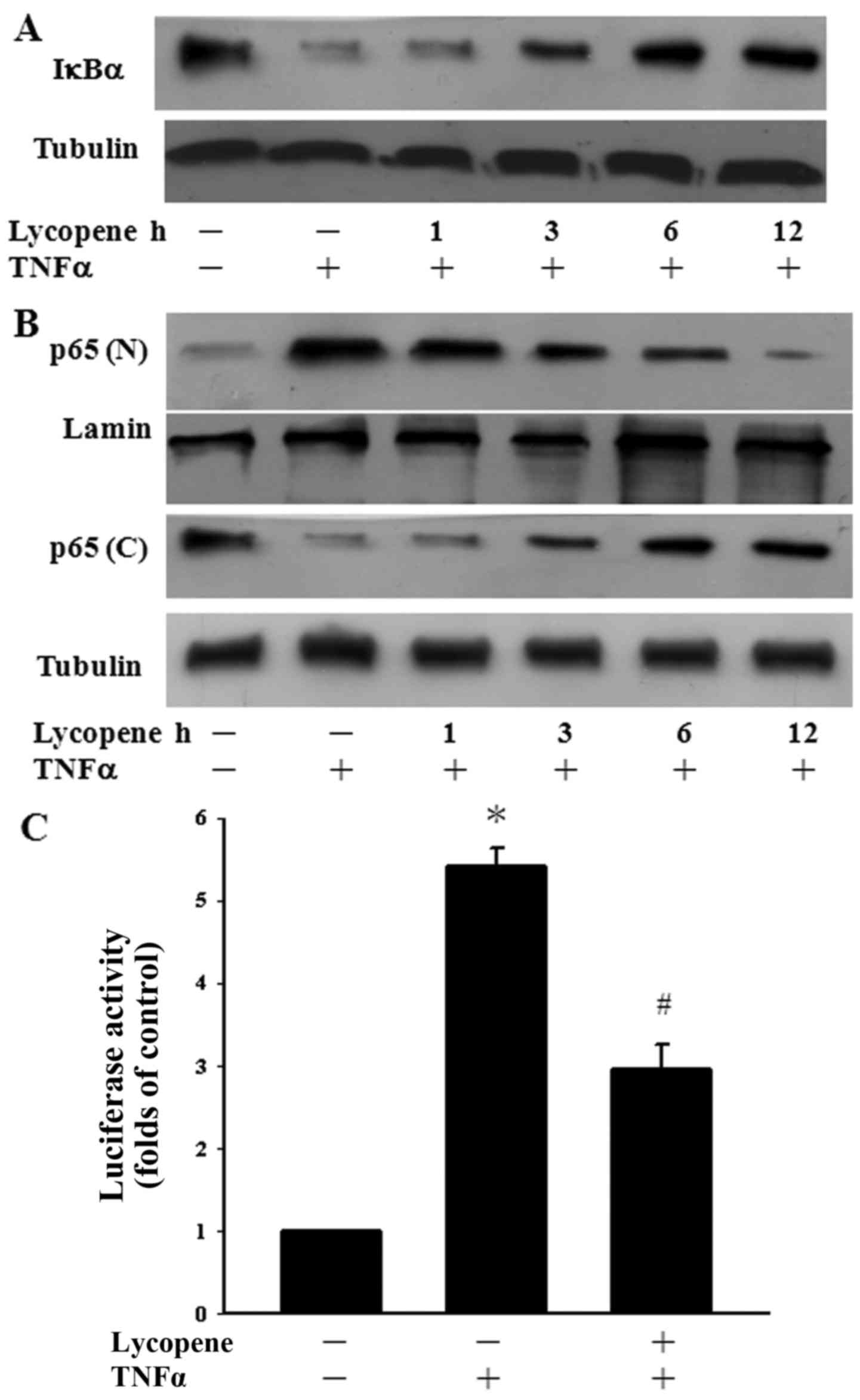
Influence of lycopene on TNFα-induced
IκBα degradation and NF-κB nuclear translocation
Since the NF-κB pathway is a well-known inflammatory
pathway (2), we examined whether
lycopene regulates TNFα-induced NF-κB activation. To clarify the
inhibitory mechanisms of lycopene in TNFα-induced NF-κB activation,
the degradation of IκBα was determined over a time course manner.
TNFα alone induced the significant degradation of IκBα after 1 h of
exposure. However, pre-treatment with lycopene for 6 and 12 h
inhibited the TNFα-induced degradation of IκBα in ECs (Fig. 2A). The cells were also pre-treated
with lycopene over a 12-h time period to examine whether this agent
regulates p65 translocation to the nucleus in TNFα-stimulated ECs.
We found the TNFα-induced p65 nuclear translocation decreased
following pre-treatment with lycopene for 6 to 12 h (Fig. 2B). In addition, we examined the
inhibition of TNFα-induced p65 activation by lycopene at the
transcriptional level. Following pre-treatment with lycopene for 12
h, we found that the TNFα-induced NF-κB activation was indeed
inhibited using a luciferase assay (Fig. 2C). Our results thus indicate that
lycopene inhibits NF-κB nuclear translocation and transactivation
following pre-treatment with lycopene for 12 h.
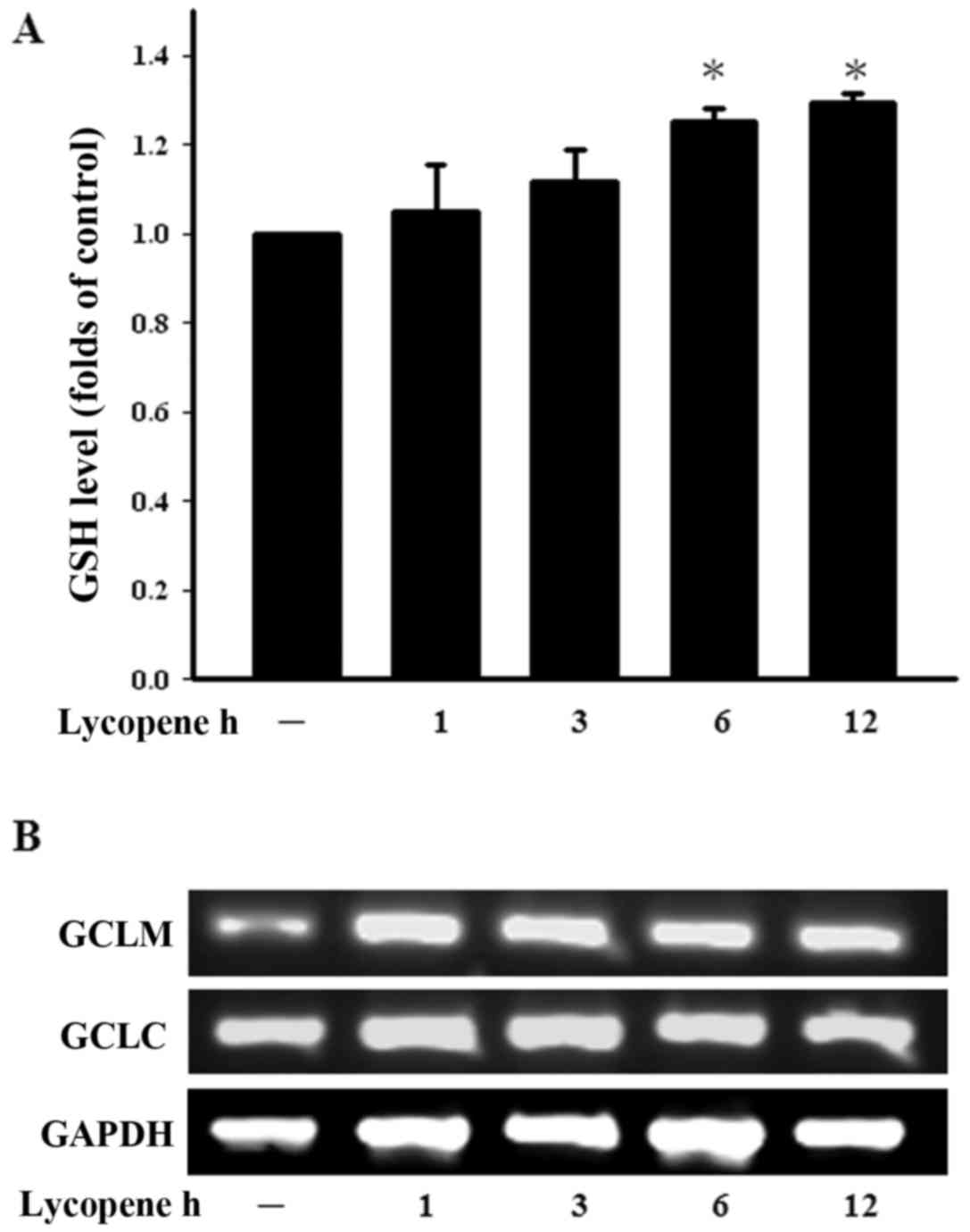
Lycopene increases the GSH level in
ECs
GSH, a well-studied tri-peptide, plays numerous
protective roles in cells, protecting them against oxidative stress
and maintaining the cellular thiol redox status. We therefore
examined the GSH levels in ECs treated with lycopene in a time
course manner. The intracellular GSH levels were found to be
increased following treatment with lycopene for 6 h and this
increased persisted until 12 h of treatment (Fig. 3A). The enzyme in the de
novo synthesis of GSH is GCL and consists of GCLC and GCLM.
Treatment with lycopene increased the GCLM expression levels over
the indicated incubation period (Fig.
3B). These findings demonstrate that lycopene increases the GSH
levels and modulates the cellular redox status.
Lycopene induces Nrf2 activation
Lycopene increases the intracellular GSH levels
which are synthesised by Nrf2-related glutamylcysteine synthetase
(26). It has previously been
reported that Nrf2 encoding phase II detoxifying and antioxidant
enzymes provide cytoprotective effects in ECs (27). In this study, the ECs treated with
lycopene exhibited a continuous increase in Nrf2 nuclear
accumulation for up to 3 h (Fig.
4A). Nrf2 regulates the ARE that drives the expression of
specific genes (27). We found
lycopene that indeed led to an increase in Nrf2 transcriptional
activity by transfecting ECs with ARE-luciferase reporter construct
(Fig. 4B).
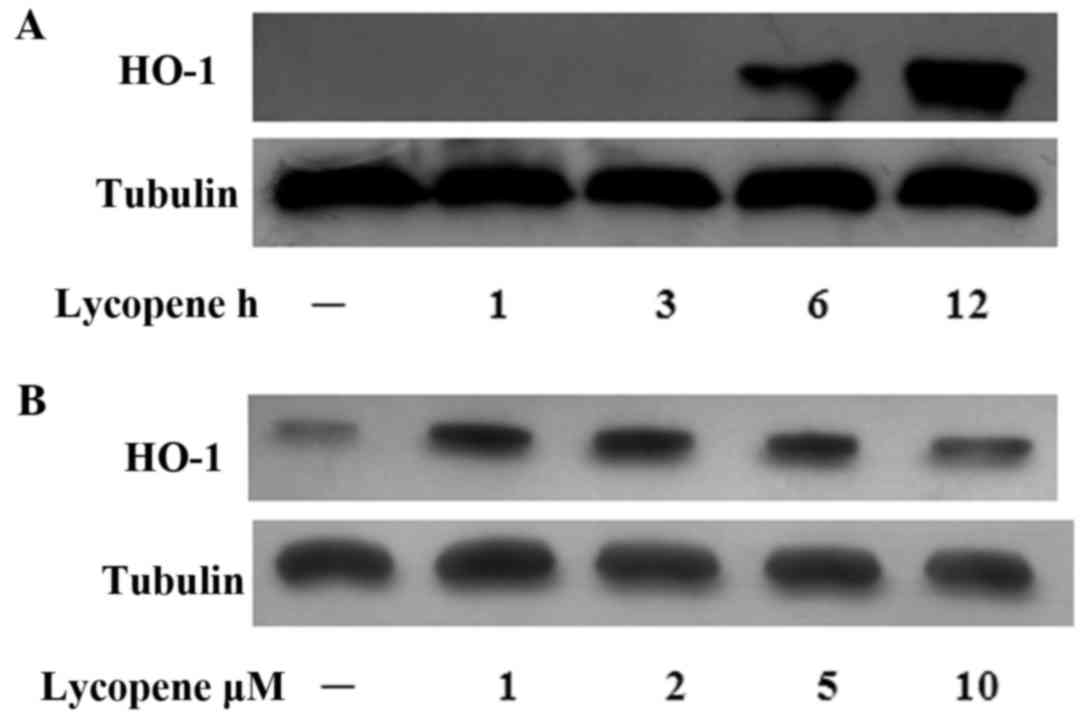
Lycopene induces HO-1 expression
In our previous study, we reported that the
Nrf2-induced expression of HO-1 exerted anti-inflammatory effects
in ECs (19). Hence, in this
study, we examined the HO-1 levels over the time course of lycopene
pre-treatment and found that the HO-1 protein levels increased
after 6 h of lycopene pre-treatment and this increased persisted
for up to 12 h (Fig. 5A). In the
ECs treated with various concentrations of lycopene, HO-1 protein
expression was increased (at the concentrations of 1–5 µM)
(Fig. 5B).
The inhibitory effects of lycopene are
dependent on the induction of HO-1 expression
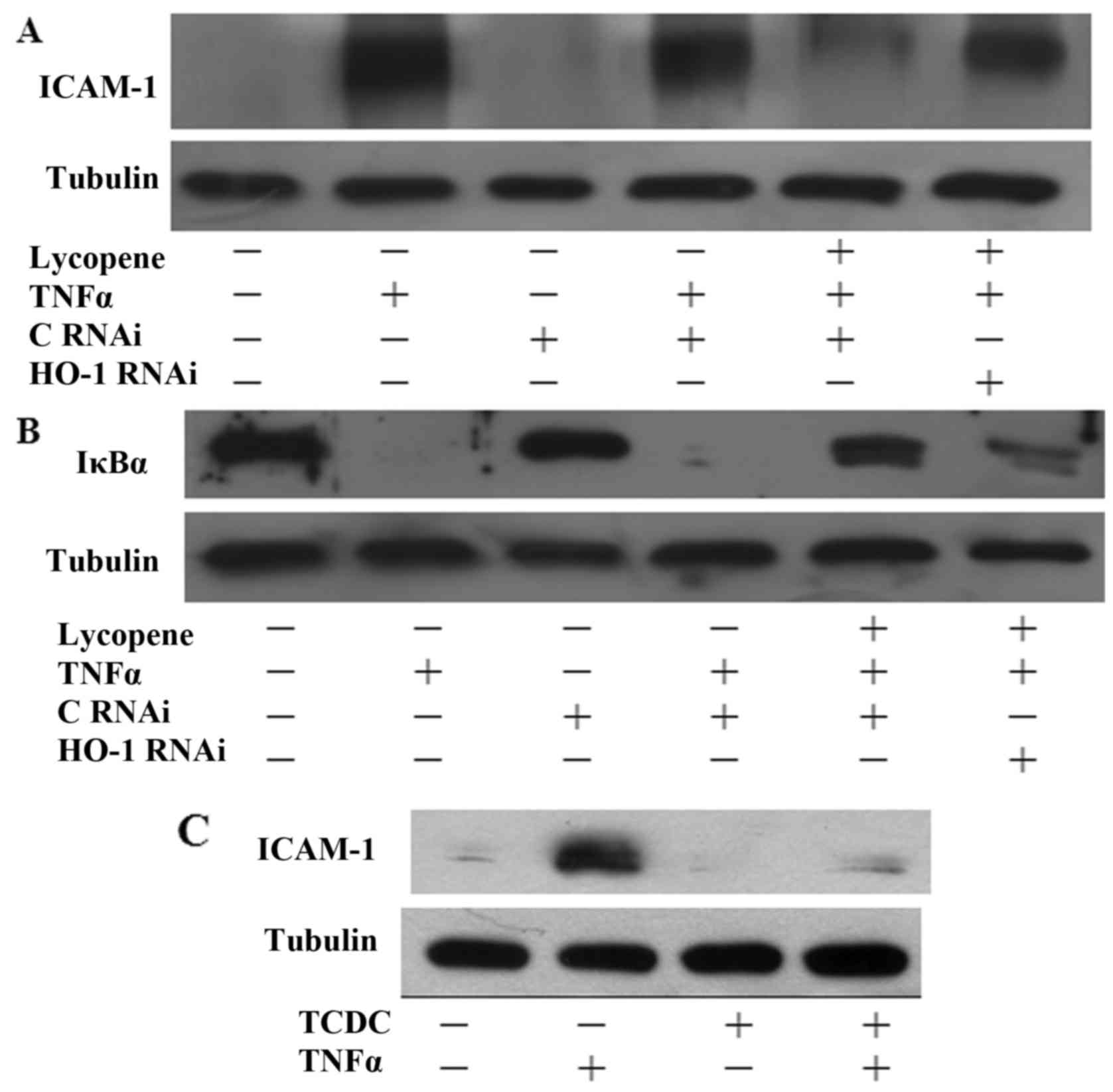
To investigate whether HO-1 induction contributes to
the protective effects of lycopene, HO-1 siRNA experiments were
performed. Indeed, transfection with HO-1 siRNA suppressed the
inhibitory effects of lycopene on ICAM-1 expression (Fig. 6A). Consistently, transfection of
the ECs with HO-1 siRNA also reversed the inhibitory effects of
lycopene on IκB degradation following treatment with lycopene for
12 h (Fig. 6B). Our previous data
found that CO seems to be the major mediator of the
anti-inflammatory effects of HO-1 (20,21). In this study, we found that
pre-treatment of the ECs with 25 µM CO donor
[tricarbonyldichlororuthenium (II) dimer] (TCDC) for 3 h inhibited
TNFα-induced ICAM-1 expression (Fig.
6C). Therefore, our data indicate that the lycopene-induced
expression of HO-1 is involved in the anti-inflammatory effects in
ECs.
Discussion
Lycopene is a natural carotenoid that is present in
tomatoes and tomato-based products and our present findings
indicated that lycopene suppressed TNFα-induced monocyte adhesion
to ECs by downregulating the expression of ICAM-1. Our data also
indicated that the inhibitory effects of lycopene upon the
activation of inflammatory transcriptional factor, NF-κB, were
mediated through the blocking of the degradation of IκBα. However,
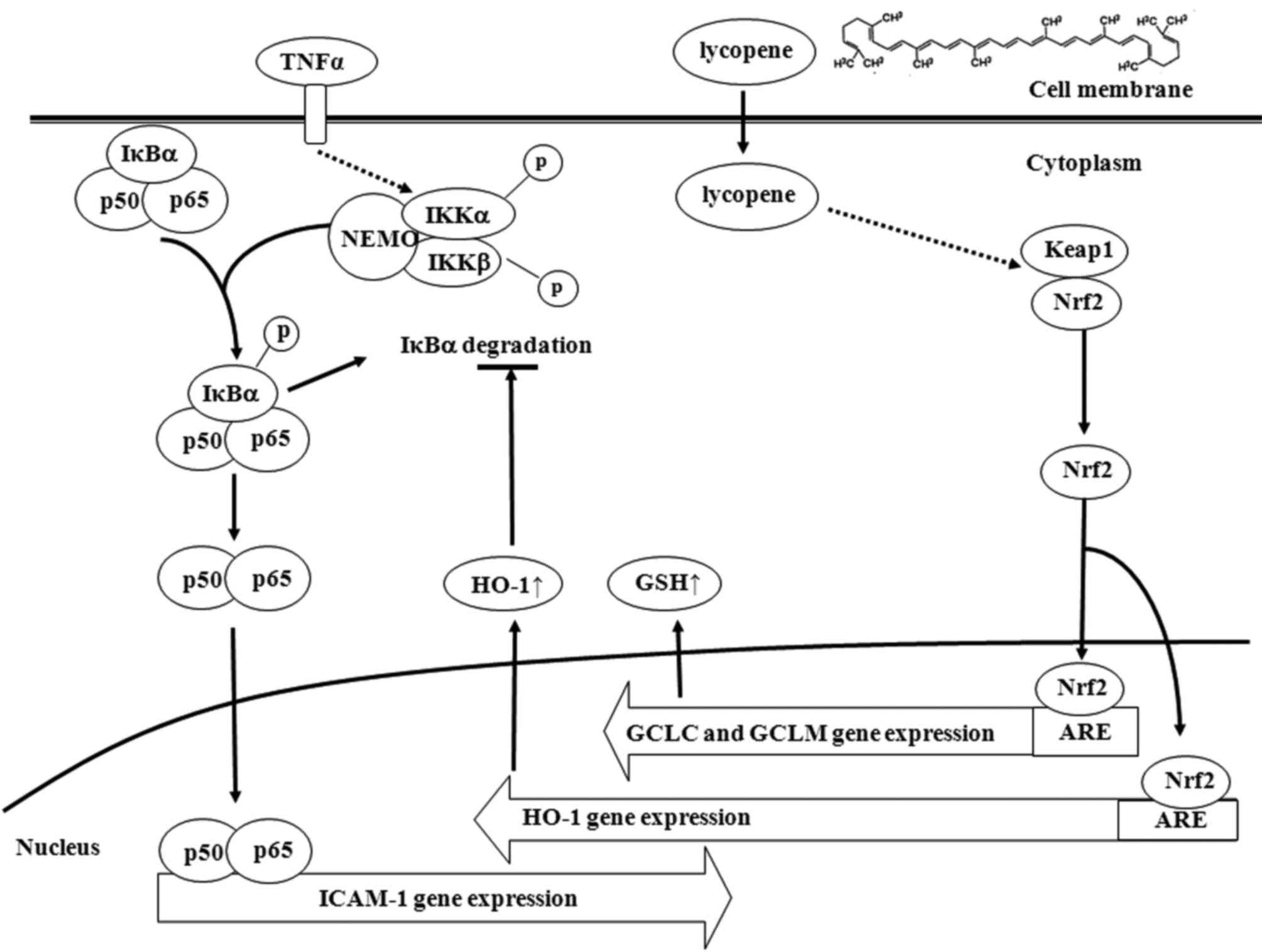
with this chemical, this effect appears to be exerted through the
induction of Nrf2-related genes, particularly HO-1. HO-1 was
suggested as the major effector of lycopene for its
anti-inflammatory effects and the increase in the levels of GSH and
HO-1 expression were mediated by promoting Nrf2 nuclear
translocation (Fig. 7).
It has been reported that the regulation of ICAM
expression involves the NF-κB pathway. The activation of the
transcription factor, NF-κB, by TNFα is required for the
transcriptional activation of EC adhesion molecules, and NF-κB is
believed to be a pivotal transcription factor in chronic
inflammatory diseases (5).
Overall, this suggests that lycopene inhibits adhesion molecule
expression, possibly by blocking NF-κB activation. Our data
revealed that lycopene inhibited the TNFα-induced degradation of
IκBα, following pre-treatment with lycopene for more than 3 h
(Fig. 2A). Hence, the inhibition
of NF-κB activity by lycopene was mediated through the modulation
of upstream targets in the NF-κB pathway. However, lycopene exerted
an inflammatory effect via the NF-κB pathway and it modulated EC
activation according to different incubation times. A short
incubation (3 h) with lycopene only had a partial inhibitory effect
on the nuclear appearance of NF-κB in ECs (Fig. 2B). Otherwise, the significant
inhibitory effects of lycopene on p65 translocation were observed
at 12 h. These findings further indicate that the cytoprotective
effects of lycopene are more promiment with prolonged
treatment.
GSH is a well-studied tri-peptide and has numerous
roles in the protection of cells from oxidants and maintaining the
cellular thiol redox status. Our previous studies have demonstrated
that the intracellular GSH levels are a major modulator of the
effects of EGCG and piceatannol (19,28). In this study, we therefore
investigated the intracellular level of GSH following lycopene
treatment. We observed that the GSH levels increased after 6 h of
lycopene treatment. Additional experiments demonstrated that
lycopene increased the gene expression of GCL, the key
rate-limiting enzyme in GSH synthesis (Fig. 3B). The reversible redox reactions
of GSH regulate diverse biological processes, including enzyme
catalysis, gene expression, cell proliferation and atherosclerosis
(29). In a previous study of
ours, we found that GSH played a critical role in protein
glutathionylation during mile oxidative stress (30). Thus, the regulation of GSH by Nrf2
plays an important role in the glutathionylation of NF-κB and this
may be one of the potential mechanisms underlying the
anti-inflammatory effects. Thus, we cannot exclude the mechanisms
involved in the elevated intracellular GSH levels. It has been
reported that GCLC and GCLM expression are mediated by Nrf2
activation (26). Subsequently,
additional experiments are required to evaluate the role of
lycopene in regulating Nrf2 activity, since GCLC was found to be
regulated by Nrf2 activation.
Nrf2 has previously been reported to be a key factor
inducing phase II detoxifying and antioxidant enzymes (27). A recent study also demonstrated
that lycopene enhanced the activation of the phosphoinositide
3-kinase/Akt pathway, followed by the induction of Nrf2 nuclear
translocation in ECs (31). Our
present findings indicated that lycopene induced phase II enzyme
GCL and HO-1 expression and further demonstrate that Nrf2
translocates into the nucleus and binds to ARE to activate
transcription.
It has been previously determined that HO-1
functions as part of a cytoprotective mechanism derived from its
antioxidant and anti-inflammatory properties, and thus has
potential as a therapeutic target for cardiovascular diseases
(20). In atherosclerotic
plaques, the increased expression of HO-1 attenuates
atherosclerosis and this further establishes the protective role of
HO-1 against this disease (32).
The anti-inflammatory effects of lycopene-induced HO-1 have been
investigated in previous studies (33,34). In this study, we found that the
anti-inflammatory effects of lycopene were mediated through the
inhibition of IκBα degradation and ICAM-1 expression was reduced by
transfection with HO-1 siRNA (Fig. 6A
and B). Previous studies have shown that CO donors exert their
effects via HO-1 induction in various systems (20,21). In the present study, as shown in
Fig. 6C, CO donor mediated the
inhibition of ICAM-1 expression.
In conclusion, the findings of the present study
indicated that there is a pivotal role of Nrf2-regulated pathways
in the mechanisms underlying the inhibition of NF-κB in
lycopene-treated ECs. The cytoprotective effects of lycopene
require the upregulation of HO-1 expression in ECs. A clearer
understanding of the working mechanisms of lycopene in the future
may contribute to the development of a therapeutic application
which may be used in inflammation-associated diseases.
Acknowledgments
This study was supported by grants from the National
Science Council, Taiwan (no. 102-2320-B-415-002-MY3) and from the
Chiayi Christian Hospital, Taiwan.
References
|
1
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glass CK and Witztum JL: Atherosclerosis.
the road ahead. Cell. 104:503–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernandes A, Miller-Fleming L and Pais TF:
Microglia and inflammation: Conspiracy, controversy or control?
Cell Mol Life Sci. 71:3969–3985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ledebur HC and Parks TP: Transcriptional
regulation of the inter-cellular adhesion molecule-1 gene by
inflammatory cytokines in human endothelial cells. Essential roles
of a variant NF-kappaB site and p65 homodimers. J Biol Chem.
270:933–943. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Story EN, Kopec RE, Schwartz SJ and Harris
GK: An update on the health effects of tomato lycopene. Annu Rev
Food Sci Technol. 1:189–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang XD: Lycopene metabolism and its
biological significance. Am J Clin Nutr. 96:1214S–1222S. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mayne ST, Cartmel B, Silva F, Kim CS,
Fallon BG, Briskin K, Zheng T, Baum M, Shor-Posner G and Goodwin WJ
Jr: Plasma lycopene concentrations in humans are determined by
lycopene intake, plasma cholesterol concentrations and selected
demographic factors. J Nutr. 129:849–854. 1999.PubMed/NCBI
|
|
9
|
Schierle J, Bretzel W, Bühler I, Faccin N,
Hess D, Steiner K and Schüep W: Content and isomeric ratio of
lycopene in food and human blood plasma. Food Chem. 59:459–465.
1997. View Article : Google Scholar
|
|
10
|
Karppi J, Nurmi T, Kurl S, Rissanen TH and
Nyyssönen K: Lycopene, lutein and beta-carotene as determinants of
LDL conjugated dienes in serum. Atherosclerosis. 209:565–572. 2010.
View Article : Google Scholar
|
|
11
|
Simone RE, Russo M, Catalano A, Monego G,
Froehlich K, Boehm V and Palozza P: Lycopene inhibits
NF-κB-mediated IL-8 expression and changes redox and PPARγ
signalling in cigarette smoke-stimulated macrophages. PLoS One.
6:e196522011. View Article : Google Scholar
|
|
12
|
Marcotorchino J, Romier B, Gouranton E,
Riollet C, Gleize B, Malezet-Desmoulins C and Landrier JF: Lycopene
attenuates LPS-induced TNF-α secretion in macrophages and
inflammatory markers in adipocytes exposed to
macrophage-conditioned media. Mol Nutr Food Res. 56:725–732. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Armoza A, Haim Y, Bashiri A, Wolak T and
Paran E: Tomato extract and the carotenoids lycopene and lutein
improve endothelial function and attenuate inflammatory NF-κB
signaling in endothelial cells. J Hypertens. 31:521–529. 2013.
View Article : Google Scholar
|
|
14
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alam J, Stewart D, Touchard C, Boinapally
S, Choi AM and Cook JL: Nrf2, a Cap'n'Collar transcription factor,
regulates induction of the heme oxygenase-1 gene. J Biol Chem.
274:26071–26078. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McMahon M, Itoh K, Yamamoto M, Chanas SA,
Henderson CJ, McLellan LI, Wolf CR, Cavin C and Hayes JD: The
Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2
p45-related factor 2) controls both constitutive and inducible
expression of intestinal detoxification and glutathione
biosynthetic enzymes. Cancer Res. 61:3299–3307. 2001.PubMed/NCBI
|
|
17
|
Chen XL, Dodd G, Thomas S, Zhang X,
Wasserman MA, Rovin BH and Kunsch C: Activation of Nrf2/ARE pathway
protects endothelial cells from oxidant injury and inhibits
inflammatory gene expression. Am J Physiol Heart Circ Physiol.
290:H1862–H1870. 2006. View Article : Google Scholar
|
|
18
|
Calay D and Mason JC: The multifunctional
role and therapeutic potential of HO-1 in the vascular endothelium.
Antioxid Redox Signal. 20:1789–1809. 2014. View Article : Google Scholar
|
|
19
|
Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH
and Wung BS: Upregulation of heme oxygenase-1 by
Epigallocatechin-3-gallate via the phosphatidylinositol
3-kinase/Akt and ERK pathways. Life Sci. 78:2889–2897. 2006.
View Article : Google Scholar
|
|
20
|
Yeh PY, Li CY, Hsieh CW, Yang YC, Yang PM
and Wung BS: CO-releasing molecules and increased heme oxygenase-1
induce protein S-glutathionylation to modulate NF-κB activity in
endothelial cells. Free Radic Biol Med. 70:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang YC, Huang YT, Hsieh CW, Yang PM and
Wung BS: Carbon monoxide induces heme oxygenase-1 to modulate STAT3
activation in endothelial cells via S-glutathionylation. PLoS One.
9:e1006772014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun
YW and Wung BS: Cinnamaldehyde inhibits the tumor necrosis
factor-alpha-induced expression of cell adhesion molecules in
endothelial cells by suppressing NF-kappaB activation: Effects upon
IkappaB and Nrf2. Toxicol Appl Pharmacol. 229:161–171. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Braut-Boucher F, Pichon J, Rat P, Adolphe
M, Aubery M and Font J: A non-isotopic, highly sensitive,
fluorimetric, cell-cell adhesion microplate assay using calcein
AM-labeled lymphocytes. J Immunol Methods. 178:41–51. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neurohr C, Lenz AG, Ding I, Leuchte H,
Kolbe T and Behr J: Glutamate-cysteine ligase modulatory subunit in
BAL alveolar macrophages of healthy smokers. Eur Respir J.
22:82–87. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kamencic H, Lyon A, Paterson PG and
Juurlink BH: Monochlorobimane fluorometric method to measure tissue
glutathione. Anal Biochem. 286:35–37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mulcahy RT, Wartman MA, Bailey HH and Gipp
JJ: Constitutive and beta-naphthoflavone-induced expression of the
human gamma-glutamylcysteine synthetase heavy subunit gene is
regulated by a distal antioxidant response element/TRE sequence. J
Biol Chem. 272:7445–7454. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen B, Lu Y, Chen Y and Cheng J: The role
of Nrf2 in oxidative stress-induced endothelial injuries. J
Endocrinol. 225:R83–R99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wung BS, Hsu MC, Wu CC and Hsieh CW:
Piceatannol upregulates endothelial heme oxygenase-1 expression via
novel protein kinase C and tyrosine kinase pathways. Pharmacol Res.
53:113–122. 2006. View Article : Google Scholar
|
|
29
|
Pimentel D, Haeussler DJ, Matsui R,
Burgoyne JR, Cohen RA and Bachschmid MM: Regulation of cell
physiology and pathology by protein S-glutathionylation: Lessons
learned from the cardiovascular system. Antioxid Redox Signal.
16:524–542. 2012. View Article : Google Scholar :
|
|
30
|
Liao BC, Hsieh CW, Lin YC and Wung BS: The
glutaredoxin/glutathione system modulates NF-kappaB activity by
glutathionylation of p65 in cinnamaldehyde-treated endothelial
cells. Toxicol Sci. 116:151–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sung LC, Chao HH, Chen CH, Tsai JC, Liu
JC, Hong HJ, Cheng TH and Chen JJ: Lycopene inhibits cyclic
strain-induced endothelin-1 expression through the suppression of
reactive oxygen species generation and induction of heme
oxygenase-1 in human umbilical vein endothelial cells. Clin Exp
Pharmacol Physiol. 42:632–639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morita T: Heme oxygenase and
atherosclerosis. Arterioscler Thromb Vasc Biol. 25:1786–1795. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sahin K, Tuzcu M, Sahin N, Ali S and Kucuk
O: Nrf2/HO-1 signaling pathway may be the prime target for
chemoprevention of cisplatin-induced nephrotoxicity by lycopene.
Food Chem Toxicol. 48:2670–2674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sahin K, Orhan C, Tuzcu M, Sahin N, Ali S,
Bahcecioglu IH, Guler O, Ozercan I, Ilhan N and Kucuk O: Orally
administered lycopene attenuates diethylnitrosamine-induced
hepatocarcinogenesis in rats by modulating Nrf-2/HO-1 and Akt/mTOR
pathways. Nutr Cancer. 66:590–598. 2014. View Article : Google Scholar : PubMed/NCBI
|