Introduction
Approximately 300,000 women die of cervical cancer
every year worldwide. Cervical cancer is one of the most common
gynecological malignancies and the third-leading cause of
cancer-related deaths among females in middle-income countries. In
China, its incidence ranks second after breast cancer among
gynecological malignancies (1,2).
In recent years, the average age of patients has gradually become
younger. Research data have shown that cervical cancer occurs most
frequently among females aged 50 years or older. The disease
develops from cervical intraepithelial neoplasia to cervical cancer
via an evolutionary process that takes ~10–20 years. This
pre-malignant period is an important stage for the prevention of
cervical cancer as, in theory, the development of a malignant tumor
could be blocked using targeted therapies. It is therefore
necessary to perform the screening, diagnosis and treatment of
cervical cancer patients at the earliest stage possible.
At present, the methods mainly used for the
screening of cervical cancer include colposcopy examination,
cytology and human papilloma virus detection (3,4).
Nevertheless, these methods have certain drawbacks, such as the
possibility of false-positives and false-negatives, as well as high
cost. Although cervical biopsy is a significant method for the
diagnosis of cervical cancer, it causes traumatic injury.
Therefore, the development of a fluorescence probe with high
sensitivity and specificity is highly desirable and significant for
the early diagnosis and prognosis of cancer (5–8).
Extracellular matrix-degrading enzymes mainly fit
into 3 categories: serine proteases, cysteine proteinases, and
matrix metalloproteinases (MMPs). Among these enzymes, MMPs account
for >70% of the total activity of extracellular matrixdegrading
enzymes (9). MMPs are specific
endopeptidases that can degrade a variety of extracellular matrix
proteins and remodel damaged tissue. In addition, MMPs play a key
role in mediating tumor invasion and metastasis by compromising the
integrity of barriers composed of extracellular matrix at the
interfaces between different tissues. The activation of MMPs is
considered to be the rate-limiting step in the degradation of the
extracellular matrix (10,11).
The ene encoding MMP-2 is located on human chromosome 16q21 and
consists of 13 exons and 12 introns, and the molecular weight of
the MMP-2 protein is 72 kDa (12,13). MMP-2 is also known as type IV
collagenase or gelatinase and functions as a zinc-dependent
protease. The primary substrate of MMP-2 is type IV collagen, but
it also has activity towards collagen types V, VII, IX and X, as
well as fibronectin and elastin. In various tumors, MMP-2 is often
overexpressed in an inactive zymogen form that can be converted
into active MMP-2 through lysis by membrane type matrix
metalloproteinase-1 (MT1-MMP). MMP-2 and MT1-MMP are considered to
be closely involved in tumor invasion, metastasis and angiogenesis
(14). Tissue invasion and
metastasis are the leading causes of death among cancer patients
(15). Therefore, building a
molecular imaging probe for the evaluation of MMP-2 and MT1-MMP
proteolytic activity would help to predict tumor malignancy.
MMP-2 expression is low or absent in normal cervical
epithelium, but generally high in cervical intraepithelial
neoplasia and cervical cancer (16). Among cervical cancers, those with
a higher expression of MMP-2 tend to have a poorer prognosis
(16–19). MMP-2 expression can also be used
as a marker for the differential diagnosis of benign cervical
lesions and cervical cancer. Wang et al showed that MMP-2
expression is significantly upregulated in cervical cancer,
compared with benign cervical lesions, and that MMP-2 expression is
related to patient survival (17). Schröpfer et al analyzed the
expression of MMPs in various gynecologic cancer cell lines and
found that MMP-2 expression was moderate to strong in HeLa, CaSki
and SiHa cervical cancer cell lines (18). Xie et al conducted a study
involving 230 patients with cervical cancer and 230 healthy
controls to investigate the relationship between the susceptibility
and clinical outcomes of cervical cancer and genetic polymorphisms
in the MMP-2, -3, -7 and -9 genes in a Chinese Han population. The
results showed that certain genotypes of the MMP-2 and MMP-7 genes
were associated with the progression of cervical cancer to the
advanced stages and a poor survival in cervical cancer patients
(19).
The results of the studies described above provide
an important foundation for the exploration of a cervical cancer
detection method based on targeting MMP-2. To date, the methods
used to detect MMP-2 have mainly included western blotting,
immunohistochemistry, zymography and enzyme- linked immunosorbent
assay. However, none of these methods can be used for the direct or
real-time analysis of living cells or animals (20–25). Li et al prepared CdTe
quantum dots as fluorophores for the detection of MMP-2 in tumors.
However, the application of the probe was limited due to its high
level of toxicity (26).
Non-invasive imaging technologies are important for
understanding the operation and control of living systems,
especially at the cellular level. Fluorescence imaging technologies
have the advantages of detailed visualization, high sensitivity,
and the potential for low toxicity. Such technologies can be used
for the qualitative or quantitative detection of specific
biomolecules in cells, tissues and whole animals in vivo,
and can enable the precise localization of target analytes.
Real-time monitoring of living processes is also possible using
fluorescence imaging techniques (27).
In this study, a coumarin-based fluorescence
resonance energy transfer (FRET) probe was prepared by coupling the
fluorophore 7-[diethylamino]-2-oxo-2H-chromene-3- succinimidyl
ester (Coumarin-Osu) with the quencher 4-
(4-[dimethylamino]phenylazo)benzoic acid N-succinimidyl ester
(Dabcyl-Osu) via a polypeptide chain (GPLGVRGKGG) that can be
cleaved by MMP-2 (28–33). Through research using cervical
cancer cell lines, it was demonstrated that this probe can detect
MMP-2 in living cells and has promise for use in the screening,
diagnosis and prognosis of cervical cancer.
Materials and methods
Reagents
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
hydrochloride and N-hydroxysuccinimide were purchased from
Sahn Chemical Technology (Shanghai, China). Dulbecco's modified
Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased
from Thermo Fisher Scientific (Waltham, MA, USA). TRIzol® reagent
and a 100-bp DNA marker were purchased from Invitrogen (Carlsbad,
CA, USA). Reverse transcription-polymerase chain reaction (RT-PCR)
kit, Taq DNA polymerase and dNTPs were obtained from Takara
Bio (Otsu, Japan). Primers were obtained from Invitrogen Trading
(Shanghai, China). Goat anti-MMP-2 polyclonal antibody (sc-6838)
was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). HRP-conjugated AffiniPure rabbit anti-goat IgG (H+L)
(SA-00001-4), mouse anti-β-actin monoclonal antibody (66009-1-Ig),
and HRP-conjugated AffiniPure goat anti-mouse IgG (H+L)
(SA-00001-1) were purchased from Proteintech (Rosemont, IL, USA).
Polyvinylidene fluoride membrane,
N,N′-methylenebis(acrylamide), sodium dodecyl sulfate,
ammonium sulfate, N,N,N′N′-
tetramethylethylenediamine, glycine, Whatman filter paper, non-fat
milk powder, phenylmethanesulfonyl fluoride, trypsin,
trishydroxymethyl-aminomethane (Tris), and Coomassie Brilliant Blue
G-250 were purchased from Merck (Darmstadt, Germany). Recombinant
MMP-2 protein was purchased from SinoBiological Inc. (Beijing,
China). Human MMP-1 protein was purchased from PeproTech (Rocky
Hill, NJ, USA). 4-Aminophenyl mercuric acetate (APMA) was obtained
from InnoChem Technology (Beijing, China). All other chemicals were
of analytic grade and used as received. Stock solutions
(2.0×10−2 M) of Fe3+, K+,
Na+, Mg2+, Ca2+, Cu2+,
Fe2+, Zn2+, HCO3−,
NO3−, ClO4−,
F−, Br−, and amino acids such as
L-tryptophan, L-serine, and L-aspartic acid were prepared in
aqueous solutions. Stock solutions of 7-(diethylamino)-2-oxo-
2H-chromene-3-succinimidyl ester and MMP-2 probe (20 μM) for
spectral measurements were prepared in dimethyl sulfoxide
(DMSO):H2O (1:1,000, v/v) solution. A stock solution of
the MMP-2 probe for fluorescence imaging in cells was prepared in
DMSO. TCNB buffer was comprised of 50 mM Tris, 10 mM
CaCl2, 150 mM NaCl and 0.05% (v/v) Brij® 35 (with pH
7.5).
Instruments
1H nuclear magnetic resonance (NMR) and
13C NMR spectra were measured using an Ascend™ 400
spectrometer (Bruker, Billerica, MA, USA) and chemical shifts were
reported as ppm, with tetramethylsilane as the internal standard.
Mass spectrometric data were obtained using a Microtof-QIII™ mass
spectrometer (Bruker). UV-vis absorption spectra were recorded
using a UV2550 spectrophotometer (Shimadzu, Kyoto, Japan).
Fluorescence spectra were measured using a FS5 fluorescence
spectrometer (Edinburgh Instruments, Livingston, UK). Other
instruments used were as follows: FV10SW confocal laser scanning
microscope (Olympus, Tokyo, Japan), JY92-II ultrasonic cell crusher
(Ningbo Xinzhi Biological Polytron Technologies, Ningbo, China),
CO2 incubator (model no. 5215-2; Shellab, Cornelius, OR,
USA), StepOne™ real-time fluorescence quantitative PCR (qPCR)
instrument (Applied Biosystems, Foster City, CA, USA), TE-70
semi-dry transfer film (GE Healthcare Life Sciences, Little
Chalfont, UK), electrophoresis apparatus (model no. DYCP-31C; Liuyi
Biotechnology, Beijing, China), and BioPhotometer® D30 nucleic acid
protein analyzer (Eppendorf, Hamburg, Germany).
Cell lines
CaSki, SiHa, C33A and HeLa cervical cancer cell
lines were obtained from the China Infrastruture of Cell Line
Resources (catalog nos. 3142C0001000000829, 3142C0001000000089,
3142C0001000000132 and 3142C0001000000009, respectively). The cells
were cultured in DMEM high glucose medium supplemented with 10%
(v/v) FBS, 100 U/ml penicillin, and 100 U/ml streptomycin. Cells
were cultured at 37°C in a humidified incubator with 5%
CO2.
Activation and measurement of MMP-2
Initially, 75 μl of MMP-2 (160 mM) and 75
μl of APMA (5 mM) were mixed and reacted in an oscillator at
37°C for 2 h to activate MMP-2. Then, 150 μl of activated
MMP-2 (80 mM) was added to 150 μl of the MMP-2 probe (20
μM) and mixed in the oscillator at 37°C for 180 min. The
test solutions were placed back into the oscillator at 37°C
immediately after each measurement.
qPCR method
The total volume of each PCR reaction was 20
μl, which included 10 μl 2X SYBR® Premix Ex Taq™ II,
0.8 μl forward primer (10 μM), 0.8 μl reverse
primer (10 μM), 0.4 μl ROX reference dye, 2 μl
cDNA, and 6 μl ddH2O. The reaction conditions
were as follows: 95°C for 30 sec, then 40 cycles of 95°C for 5 sec
and 60°C for 30 sec.
RT-PCR method
Total RNA was isolated from cells using TRIzol®
purification. After denaturing RNA at 94°C for 5 min, 500 ng of RNA
was transcribed into cDNA. Next, cDNA was amplified using the
primers and target fragments. The amplification was performed using
a thermocycler for 32 cycles according to the following program: 30
sec at 94°C, 30 sec at 58°C, and 30 sec at 72°C. Final extension
was performed at 72°C for 10 min.
Western blot analysis
Firstly, 40 μg of total proteins were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on a 10–12% (v/v) gradient gel. Subsequently,
proteins were transferred to polyvinylidene fluoride membranes and
incubated with blocking buffer [phosphate- buffered saline (PBS)
containing 5% (w/v) non-fat skim milk] for 2 h at room temperature.
Then, the membranes were incubated with primary antibodies (goat
anti-MMP-2) with gentle shaking overnight at 4°C, washed twice with
PBS for 5 min, and further incubated with secondary antibodies for
2 h at room temperature. Finally, MMP-2 was detected using a
chemiluminescence reaction. Band staining intensity was measured
using BandScan 5.0 software. Protein expression levels were
normalized to β-actin protein.
Fluorescence imaging method
The MMP-2 fluorescence probe (2 μM) was
dissolved in PBS. CaSki, SiHa, C33A and HeLa cells were inoculated
into confocal culture dishes. After 24 h, the living cells, which
were in the exponential phase of growth, were incubated with MMP-2
fluorescence probe for 120 min at 37°C. Then, the cells were washed
3 times with PBS and observed using confocal laser scanning
microscopy.
Statistical analysis
The collected data were analyzed using SPSS 19.0
software (IBM, Armonk, NY, USA) by one-way analysis of variance.
Multiple comparisons among groups were performed using Fisher's
least significant difference test. A P-value of <0.05 was
considered to indicate a statistically significant result.
Results and Discussion
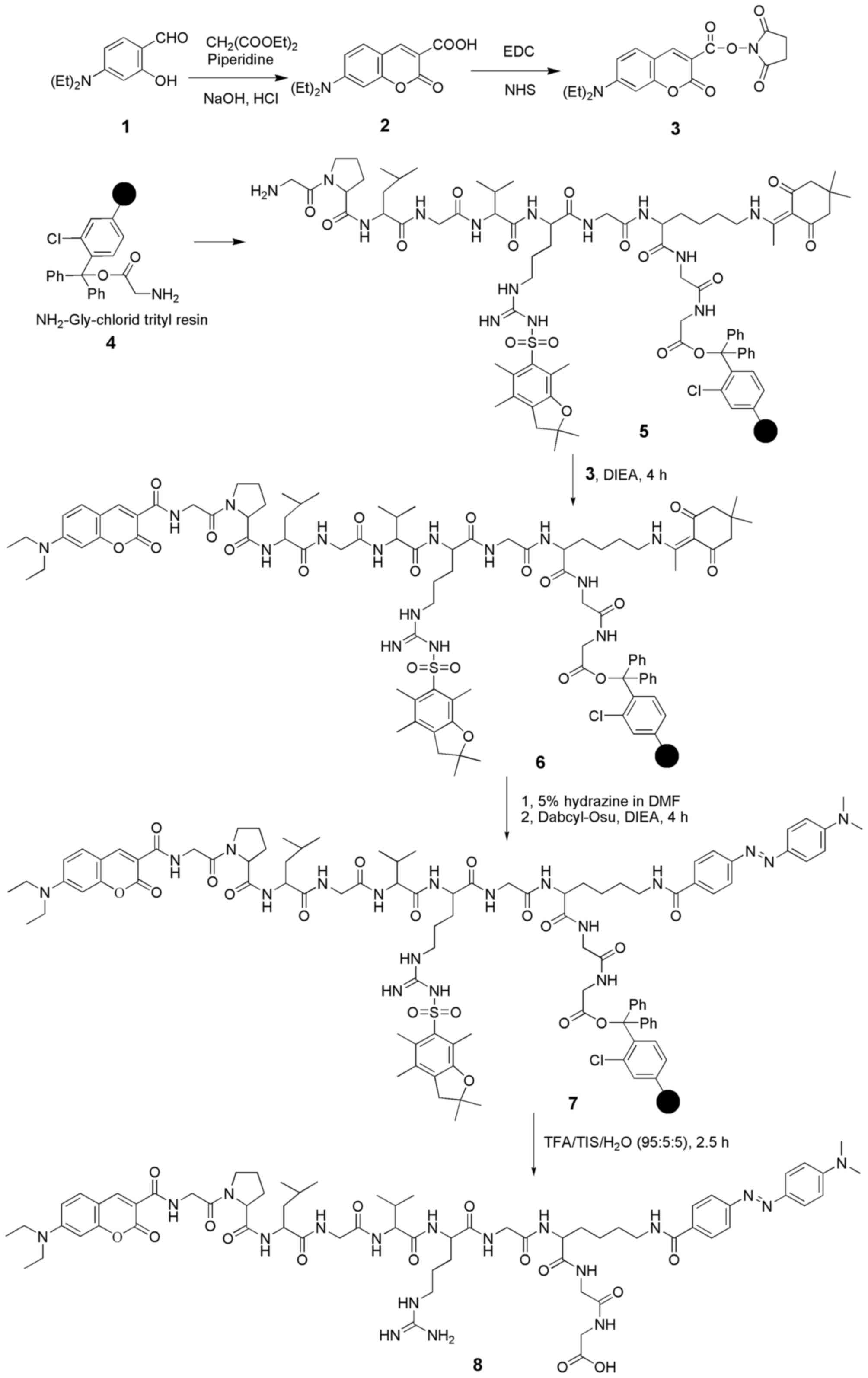
Preparation of the MMP-2 fluorescence
probe
The procedures for the synthesis of the MMP-2 probe
are shown in Fig. 1. Coumarin-Osu
was used as a fluorescent chromophore and Dabcyl-Osu was used as a
quencher for the preparation of the fluorescent MMP-2 probe.
Coumarin-Osu was prepared conveniently according to previous
literature (34–36). The UV-vis absorption spectrum of
Coumarin-Osu (10 μM) in DMSO:H2O (1:1,000, v/v)
solution exhibited a broad coumarin-based n-n* transition band
around 445 nm. The fluorescence spectrum of the same solution
showed a broad emission peak around 480 nm with strong green
fluorescence. In contrast, Dabcyl-Osu, which shows maximum
absorption at 480 nm, is greatly effective in restraining the blue
to green emission spectra of various fluorescent dyes and has often
been used as a quencher in other studies. The emission spectrum of
Coumarin-Osu and the absorption spectrum of Dabcyl-Osu overlapped
enough to provide a basis for the synthesis of a probe. Thus, a
coumarin-based fluorescence FRET probe was prepared by coupling the
fluorophore (Coumarin-Osu) with the quencher (Dabcyl-Osu) through a
polypeptide chain (GPLGVRGKGG) that can be cleaved by MMP-2. The
constructed probe was characterized by matrix-assisted laser
desorption/ionization-time of flight mass spectrometry (MALDI-TOF)
and high-pressure liquid chromatography (HPLC). MALDI-TOF:
calculated for [M+H]+: 1391.72, found:
1392.09. Purity (HPLC): 98.0533%. In the normal biological
environment, FRET between the fluorophore and the quencher caused
effective fluorescence quenching. However, upon cleavage of the
polypeptide chain (GPLGVRGKGG) between G and V of the core MMP-2
substrate sequence PLGVR, the fluorophore and quencher were
separated (Fig. 2) (37,38). Thus, the fluorescence of coumarin
was recovered.
Fluorescence responses of the probe to
MMP-2, MMP-1 and biologically relevant chemical species
To explore the practicality of the designed probe
for the measurement of MMP-2 in living systems, the dynamic
fluorescence spectra of the probe (10 μM, DMSO:TCNB buffer =
1:1,000, v/v) which was incubated with MMP-2 were measured over
time. As shown in Fig. 3, the
fluorescence intensity of the probe itself was very weak in the
beginning. However, it increased obviously with emission at 480 nm
as time went on and then leveled off after ~2 h. The fluorescence
intensity of the probe increased by ~10-fold (39). Selective experiments of the probe
for the target molecule were carried out at lower concentrations
for practical application. When other biologically relevant species
such as MMP-1 (10 nM), K+, Mg2+,
Na+, Fe3+, Cu2+, Zn2+,
Ca2+, L-tryptophan, L-serine, L-aspartic acid,
HCO3−, NO3−,
ClO4−, F− and Br− (all
10 mM) were incubated with the probe (20 nM) at 37°C for 2 h, no
obvious changes in the fluorescence spectra were observed (Fig. 4). For practical application, it
was necessary to investigate the limit of detection (LOD) and the
detection range of the probe targeting MMP-2 by means of
calibrating the fluorescence assay (40,41). The fluorescence titration of the
probe (10 μM) upon the addition of activated MMP-2 (0.0,
0.5, 1.0, 2.0, 3.0, 4.5, 5.5, 7.0, 8.5 and 10 ng/ml) in TCNB buffer
solution was performed (Fig. 5).
Each test was conducted after the probe was incubated with MMP-2
for 2 h. The linear regression of the fluorescence intensity
(F/F0, at 480 nm) vs. the concentration of MMP-2 is
shown in Fig. 5 inset. The probe
featured an LOD for MMP-2 up to 0.05 ng/ml with the emission
intensity increasing ~23%. Moreover, the fluorescence intensity was
linearly correlated with the concentration of MMP-2 in the range of
0.05–10 ng/ml. The lower limit of LOD hinted a higher sensitivity
to detect MMP-2, indicating the possibility of potent application
for the early diagnosis of cervical carcinoma through collection of
exfoliated cells from the vagina, and following regular cell
culture technique to obtain massive cervical carcinoma cells. The
cells can be used for MMP-2 fluorescence analysis via a fluorescent
reader. Definitely a reliable control is required based on
pathological criteria, which will be the focus of subsequent
research by us.
Detection of MMP-2 expression using
qPCR
qPCR was used to detect the expression of MMP-2 mRNA
in the 4 cervical cancer cell lines for preliminary screening.
Total RNA was isolated from cells using TRIzol® purification and
first-strand cDNA was synthesized using a qPCR kit. Specific
primers were as follows: MMP-2 sense, 5′-TGGGGCCTCTCCTGACATTGA-3′
and antisense, 5′-CACAGTCCGCCAAATGAACCG-3′; glyceraldehyde
3-phosphate dehydrogenase (GAPDH) sense, 5′-AGAAGGCTGGGGCTCATTTG-3′
and antisense, 5′-AGGGGCCATCCACAGTCTTC-3′. The target fragments
were 157 and 258 bp, respectively. The expression of MMP-2 was
detected by fluorescence qPCR using cDNA as a template. The
amplification plot and melting curve showed a single amplification
product without non-specific amplification and without
miscellaneous melting curve peaks. The results indicated that MMP-2
expression in cervical cancer cell lines was ordered as CaSki >
SiHa > C33A > HeLa (Fig.
6). Each reaction was performed in triplicate and analyzed
individually. The results were calculated using the
2−ΔΔCt relative quantification method and normalized
using HeLa cells as a reference control and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal
standard (Table I).
 | Table IMMP-2 mRNA expression levels in 4
cervical cell lines, as determined by qPCR. |
Table I
MMP-2 mRNA expression levels in 4
cervical cell lines, as determined by qPCR.
| Cell lines | 2−ΔΔCt
(mean ± SD) | F-value | P-value |
|---|
| CaSki |
11.028+0.074a | 3467.610 | 0.000 |
| SiHa | 9.700+0.023a | | |
| C33-A | 3.151+0.148a | | |
| HeLa | 0.000±0.260a | | |
Detection of MMP-2 expression using
RT-PCR
RT-PCR was used to examine the expression of MMP-2
mRNA in the 4 cervical cancer cell lines. Specific primers were as
follows: MMP-2 sense, 5′-TGGGGCCTCTCCTGACATTGA-3′ and antisense,
5′-CACAGT CCGCCAAAT GAACCG-3′, 157 bp; GAPDH sense,
5′-AATCCCATCACCATCTTCCA-3′ and antisense,
5′-CCTGCTTCACCACCTTCTTG-3′, 580 bp. PCR fragments were separated by
electrophoresis on a 1.5% (w/v) agarose gel with GAPDH as an
internal control. The results indicated that the relative MMP-2
gene expression levels in the 4 cervical cancer cell lines were
ordered as follows: CaSki > SiHa > C33A > HeLa (Fig. 7). The PCR products were analyzed
and data calculations were performed using GeneTools gel analysis
software (Syngene, Cambridge, UK), and GAPDH was used as an
internal control for data normalization (Table II).
 | Table IIExpression of MMP-2 mRNA in 4
cervical cancer cell lines, as determined by RT-PCR. |
Table II
Expression of MMP-2 mRNA in 4
cervical cancer cell lines, as determined by RT-PCR.
| Cell lines | Grayscale ratio
(mean ± SD) |
|---|
| CaSki | 0.880±0.016a |
| SiHa | 0.695±0.018a |
| C33-A | 0.221±0.010a |
| HeLa |
0.0951+0.008a |
| F-value | 2245.500 |
| P-value | 0.000 |
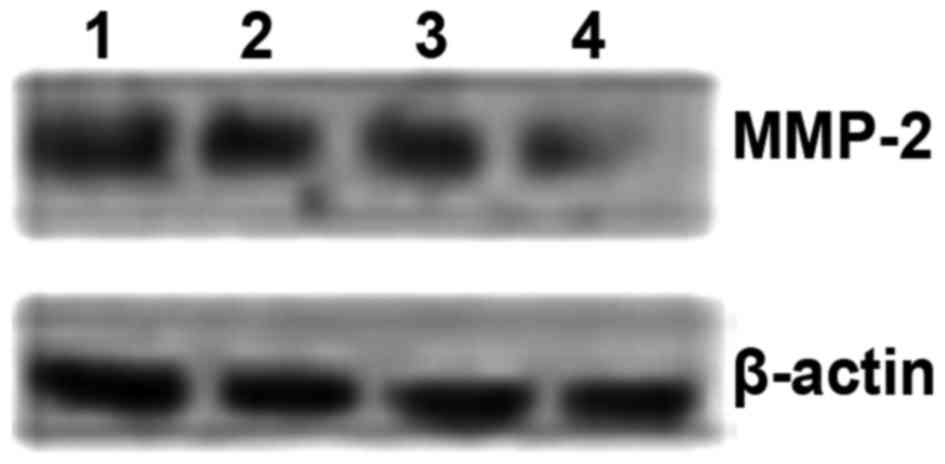
Detection of MMP-2 expression by western
blotting
Western blotting was used to investigate the
expression of MMP-2 protein in the 4 cervical cancer cell lines.
Western blot results revealed that the expression of MMP-2 protein
in the 4 cervical cancer cell lines was ordered as follows: CaSki
> SiHa > C33A > HeLa (Fig.
8 and Table III).
 | Table IIIExpression of MMP-2 protein in 4
cervical cancer cell lines, as determined by western blotting. |
Table III
Expression of MMP-2 protein in 4
cervical cancer cell lines, as determined by western blotting.
| Cell lines | Grayscale ratio
(MMP2/p-actin) | F-value | P-value |
|---|
| CaSki | 1.044+0.069a | 163.726 | 0.000 |
| SiHa | 0.870+0.038b | | |
| C33-A | 0.766+0.014 | | |
| HeLa | 0.316±0.023c | | |
Fluorescence imaging
The mRNA and protein expression of the MMP-2 gene in
4 cervical cancer cell lines was examined by qPCR, RT-PCR and
western blotting. The results illustrated that the expression of
the MMP-2 gene differed among the 4 tested cell lines, which
provided a basis for the cell imaging experiments. After the 4
cervical cancer cell lines were incubated individually with the
fluorescence probe for 120 min at 37°C, fluorescence images were
acquired using confocal laser scanning microscopy. The fluorescence
intensities were in the following order: CaSki > SiHa > C33A
> HeLa, which were consistent with the experimental results of
qPCR, RT-PCR and western blotting (Fig. 9).
In conclusion, a coumarin-based FRET probe targeting
MMP-2 was successfully designed, synthesized and used to measure
MMP-2 expression in 4 cervical cancer cell lines. The cell lines
showed green fluorescence with intensities in the order of CaSki
> SiHa > C33A > HeLa. These results were consistent with
those of qPCR, RT-PCR and western blotting. Taken together, the
findings indicate that the fluorescence probe accurately measures
MMP-2 expression in living cervical cancer cells. In vivo
fluorescence imaging experiments and clinical trials are
underway.
Acknowledgments
The present study was financially supported by the
National Natural Science Foundation (nos. 21371148 and 21571153),
the Key Scientific and Technological Project of Henan Province (no.
122102310196), and the Education Project of Henan Province (no.
12A150019).
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar
|
|
2
|
Wang T, Wu MH, Wu YM and Zhang WY: A
population-based study of invasive cervical cancer patients in
Beijing: 1993–2008. Chin Med J (Engl). 128:3298–3304. 2015.
View Article : Google Scholar
|
|
3
|
Sankaranarayanan R, Qiao YL and Keita N:
The next steps in cervical screening. Wom Health Lond. 11:201–212.
2015. View Article : Google Scholar
|
|
4
|
Catarino R, Petignat P, Dongui G and
Vassilakos P: Cervical cancer screening in developing countries at
a crossroad: Emerging technologies and policy choices. World J Clin
Oncol. 6:281–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi DK, Sun IC, Ryu JH, Koo H, Park CW,
Youn IC, Choi K, Kwon IC, Kim K and Ahn CH: Matrix
metalloproteinase sensitive gold nanorod for simultaneous
bioimaging and photothermal therapy of cancer. Bioconjug Chem.
21:2173–2177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Shen P, Li C, Wang Y and Liu Z:
Upconversion fluorescence resonance energy transfer based biosensor
for ultrasensitive detection of matrix metalloproteinase-2 in
blood. Anal Chem. 84:1466–1473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Udukala DN, Samarakoon TN, Basel
MT, Kalita M, Abayaweera G, Manawadu H, Malalasekera A, Robinson C,
Villanueva D, et al: Nanoplatforms for highly sensitive
fluorescence detection of cancer-related proteases. Photochem
Photobiol Sci. 13:231–240. 2014. View Article : Google Scholar
|
|
8
|
Zhu L, Xie J, Swierczewska M, Zhang F,
Quan Q, Ma Y, Fang X, Kim K, Lee S and Chen X: Real-time video
imaging of protease expression in vivo. Theranostics. 1:18–27.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madsen DH and Bugge TH: The source of
matrix-degrading enzymes in human cancer: Problems of research
reproducibility and possible solutions. J Cell Biol. 209:195–198.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Theocharis AD, Skandalis SS, Gialeli C and
Karamanos NK: Extracellular matrix structure. Adv Drug Deliv Rev.
97:4–27. 2016. View Article : Google Scholar
|
|
11
|
Lombard C, Saulnier J and Wallach J:
Assays of matrix metalloproteinases (MMPs) activities: A review.
Biochimie. 87:265–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tveitaras MK, Skogstrand T, Leh S, Helle
F, Iversen BM, Chatziantoniou C, Reed RK and Hultstrom M: Matrix
metalloproteinase-2 knockout and heterozygote mice are protected
from hydronephrosis and kidney fibrosis after unilateral ureteral
obstruction. PLoS One. 10:e01433902015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu C, Wang C, Cai QF, Zhang Q, Weng L, Liu
GM, Su WJ and Cao MJ: Matrix metalloproteinase 2 (MMP-2) plays a
critical role in the softening of common carp muscle during chilled
storage by degradation of type I and V collagens. J Agric Food
Chem. 63:10948–10956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yi GZ, Feng WY, Zhou Q, Liu YW and Qi ST:
The impact of MMP-2 and its specific inhibitor TIMP-2 expression on
the WHO grade and prognosis of gliomas in Chinese population: A
Meta-Analysis. Mol Neurobiol. 54:22–30. 2017. View Article : Google Scholar :
|
|
15
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghosh A, Moirangthem A, Dalui R, Ghosh T,
Bandyopadhyay A, Dasgupta A, Banerjee U, Jana N and Basu A:
Expression of matrix metalloproteinase-2 and 9 in cervical
intraepithelial neoplasia and cervical carcinoma among different
age groups of premenopausal and postmenopausal women. J Cancer Res
Clin Oncol. 140:1585–1593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Wang Q, Li HL and Han LY:
Expression of MiR200a, miR93, metastasis-related gene RECK and
MMP2/MMP9 in human cervical carcinoma - relationship with
prognosis. Asian Pac J Cancer Prev. 14:2113–2118. 2013. View Article : Google Scholar
|
|
18
|
Schropfer A, Kammerer U, Kapp M, Dietl J,
Feix S and Anacker J: Expression pattern of matrix
metalloproteinases in human gynecological cancer cell lines. BMC
Cancer. 10:5532010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie B, Zhang Z, Wang H, Chen Z, Wang Y,
Liang H, Yang G, Yang X and Zhang H: Genetic polymorphisms in MMP2,
3, 7, and 9 genes and the susceptibility and clinical outcome of
cervical cancer in a Chinese Han population. Tumour Biol.
37:4883–4888. 2016. View Article : Google Scholar
|
|
20
|
Dutra KL, Cordeiro MM, Vieira DS and
Rivero ER: Immunohistochemical expression of matrix
metalloproteinases in ameloblastomas and pericoronal follicles. J
Oral Pathol Med. 45:586–590. 2016. View Article : Google Scholar
|
|
21
|
Gunawardena I, Arendse M, Jameson MB,
Plank LD and Gregor RT: Prognostic molecular markers in head and
neck squamous cell carcinoma in a New Zealand population: Matrix
metalloproteinase-2 and sialyl Lewis x antigen. ANZ J Surg.
85:843–848. 2015. View Article : Google Scholar
|
|
22
|
Lee S, Ryu JH, Park K, Lee A, Lee SY, Youn
IC, Ahn CH, Yoon SM, Myung SJ, Moon DH, et al: Polymeric
nanoparticle- based activatable near-infrared nanosensor for
protease determination in vivo. Nano Lett. 9:4412–4416. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang GX, Pan HC, Li Y, Jiang LP, Zhang JR
and Zhu JJ: Near infrared sensing based on fluorescence resonance
energy transfer between Mn:CdTe quantum dots and Au nanorods.
Biosens Bioelectron. 24:3693–3697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bremer C, Bredow S, Mahmood U, Weissleder
R and Tung CH: Optical imaging of matrix metalloproteinase-2
activity in tumors: Feasibility study in a mouse model. Radiology.
221:523–529. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zajac A, Song D, Qian W and Zhukov T:
Protein microarrays and quantum dot probes for early cancer
detection. Colloids Surf B Biointerfaces. 58:309–314. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Deng D, Xue J, Qu L, Achilefu S and
Gu Y: Quantum dots based molecular beacons for in vitro and in vivo
detection of MMP-2 on tumor. Biosens Bioelectron. 61:512–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schaferling M: The art of fluorescence
imaging with chemical sensors. Angew Chem Int Ed Engl.
51:3532–3554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Knight CG, Willenbrock F and Murphy G: A
novel coumarin- labelled peptide for sensitive continuous assays of
the matrix metalloproteinases. FEBS Lett. 296:263–266. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aggarwal M, Sharma R, Kumar P, Parida M
and Tomar S: Kinetic characterization of trans-proteolytic activity
of Chikungunya virus capsid protease and development of a
FRET-based HTS assay. Sci Rep. 5:147532015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang M, Yin BC, Wang XF and Ye BC:
Interaction of peptides with graphene oxide and its application for
real-time monitoring of protease activity. Chem Commun (Camb).
47:2399–2401. 2011. View Article : Google Scholar
|
|
31
|
Myochin T, Hanaoka K, Komatsu T, Terai T
and Nagano T: Design strategy for a near-infrared fluorescence
probe for matrix metalloproteinase utilizing highly cell permeable
boron dipyr- romethene. J Am Chem Soc. 134:13730–13737. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim J, Cote LJ, Kim F and Huang J:
Visualizing graphene based sheets by fluorescence quenching
microscopy. J Am Chem Soc. 132:260–267. 2010. View Article : Google Scholar
|
|
33
|
Feng D, Zhang Y, Feng T, Shi W, Li X and
Ma H: A graphene oxide-peptide fluorescence sensor tailor-made for
simple and sensitive detection of matrix metalloproteinase 2. Chem
Commun (Camb). 47:10680–10682. 2011. View Article : Google Scholar
|
|
34
|
Ma Y, Luo W, Quinn PJ, Liu Z and Hider RC:
Design, synthesis, physicochemical properties, and evaluation of
novel iron chelators with fluorescent sensors. J Med Chem.
47:6349–6362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He G, Guo D, He C, Zhang X, Zhao X and
Duan C: A color- tunable europium complex emitting three primary
colors and white light. Angew Chem Int Ed Engl. 48:6132–6135. 2009.
View Article : Google Scholar
|
|
36
|
Albers AE, Okreglak VS and Changng CJ: A
FRET-based approach to ratiometric fluorescence detection of
hydrogen peroxide. J Am Chem Soc. 128:9640–9641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu L, Zhang F, Ma Y, Liu G, Kim K, Fang
X, Lee S and Chen X: In vivo optical imaging of membrane-type
matrix metalloproteinase (MT-MMP) activity. Mol Pharm. 8:2331–2338.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fields GB: Using fluorogenic peptide
substrates to assay matrix metalloproteinases. Methods Mol Biol.
622:393–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Netzel-Arnett S, Mallya SK, Nagase H,
Birkedal-Hansen H and Van Wart HE: Continuously recording
fluorescent assays optimized for five human matrix
metalloproteinases. Anal Biochem. 195:86–92. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song E, Cheng D, Song Y, Jiang M, Yu J and
Wang Y: A graphene oxide-based FRET sensor for rapid and sensitive
detection of matrix metalloproteinase 2 in human serum sample.
Biosens Bioelectron. 47:445–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Udukala DN, Wang H, Wendel SO,
Malalasekera AP, Samarakoon TN, Yapa AS, Abayaweera G, Basel MT,
Maynez P, Ortega R, et al: Early breast cancer screening using
iron/iron oxide-based nanoplatforms with sub-femtomolar limits of
detection. Beilstein J Nanotechnol. 7:364–373. 2016. View Article : Google Scholar : PubMed/NCBI
|