Introduction
Despite improvement in the scheduling and
administration of chemotherapy against human epithelial ovarian
cancer (EOC), EOC remains the most lethal gynecologic malignancy,
and the 5-year overall survival has achieved only a marginal
improvement during the last decade (1,2).
EOC is the fifth leading cause of cancer-related death in women, at
least partially due to late diagnosis and frequent chemotherapy
resistance. The majority of EOC patients are diagnosed with locally
advanced or metastatic disease (FIGO stages III and IV) (1–3).
Although initial therapy with surgery and chemotherapy may be
effective, the majority of patients suffer relapse, often resulting
in drug resistance. Thus, identifying diagnostic markers and
developing novel therapeutic strategies are important challenges to
combat EOC.
In our previous study, we successfully inhibited the
proliferation of EOC cells in vitro and in a xenograft
model, using a novel triazole nucleoside analog, which led to
suppressed expression of heat shock factor 1 (HSF1) (4). HSF1 is the crucial transcription
factor of molecular chaperones and heat shock proteins, which is a
highly conserved mechanism to protect organisms against various
stresses. The cytoprotective and anti-apoptotic activities of HSF1
have been well documented, and research concerning its
physiological function has mainly focused on its regulatory roles
in the expression of chaperone genes (5,6).
Nevertheless, in the past few years, the capacity of HSF1 to
orchestrate a genome-wide transcriptional program has attracted
growing interest, and HSF1 has revealed an important role in
multiple physiological processes (7–9).
In both human cancer cell lines and tumor tissues of
various origins, including breast, colon, lung, cervix, pancreatic,
prostate and mesenchymal tumors, highly amplified HSF1 was
detected, while low expression of the protein was observed in
normal cells (9–15). There is a growing understanding of
the carcinogenesis-promoting role of HSF1, and its overexpression
has been found to be associated with reduced survival (7,8,11,16–19). More importantly, HSF1 orchestrates
a genomic transcriptional program to support malignant
transformation. Cancer cells show a large dependency on HSF1 to
maintain proliferation and survival compared with normal cells
(7,9,19).
Additionally, HSF1 was found to regulate more genes in highly
malignant cancer cells than in less aggressive or non-transformed
cells, and many of these genes are cancer-specific (7). Therefore, HSF1 is regarded as an
attractive target for cancer therapy (16,20–23). Despite the well-uncovered
physiological roles of HSF1 in diverse tumor types, its expression
pattern and carcinogenesis function in EOC remain elusive.
Recently, we reported a nucleoside analog that
attenuated the proliferative activity and tumorigenesis ability of
EOC cells by inhibition of HSF1 expression (4). The finding motivated us to define
the role of HSF1 in ovarian carcinogenesis and to address the
correlation between inhibition of the HSF1 expression and the
antitumor effect in EOC. The objective of present study included
the investigation of the HSF1 expression pattern in EOC tissues and
the antitumor effect of HSF1 in EOC cells.
Materials and methods
Tissue collection and immunohistochemical
assay
Healthy and EOC ovarian tissue blots were purchased
from Alen abio (Xi'an, China) and Shanghai Outdo Biotech Co., Ltd.
(Shanghai, China). The collected tissues included 42 benign (30
serous cystadenoma, 12 mucinous cystadenoma), 9 borderline (7
serous adenocarcinoma, 2 mucinous adenocarcinoma), 126 malignant
(88 serous adenocarcinoma, 11 mucinous adenocarcinoma, 17
endometrioid adenocarcinoma, 10 clear cell carcinoma) and 62
healthy ovarian tissues. The stages and histological grades were
established according to the FIGO criteria.
A rabbit polyclonal antibody (4356; Cell Signaling
Technology, Inc., Danvers, MA, USA) using a Histostain-Plus IHC kit
(MRBiotech, Shanghai, China) was used to detect HSF1 protein.
Following 3,3′-diaminobenzidine (DAB) staining and hematoxylin
counterstaining, the immunostained sections were scrutinized using
light microscopy. Staining extent was calculated by a
semi-quantitative scoring system, and the percentage of stained
cells was scored as follows: 1 (<25%), 2 (25–49%), 3 (50–75%)
and 4 (>75%); and the staining intensity was subjectively
estimated as: 1 (+), 2 (++) and 3 (+++). The final scores are
presented as the 'percentage of stained cells' × 'staining
intensity'. Depending on the expression score, the specimens were
grouped as negative (0), low expression (1–5)
and high expression (6–12).
The Mann-Whitney U test and t-test were used for
statistical analysis; p<0.05 was considered statistically
significant. Statistical analysis was performed using SPSS
statistical software (SPSS, Inc., Chicago, IL, USA).
Cell culture and infection
Human EOC cell line SKOV3 was purchased from the
Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). SKOV3 and its derivatives were maintained in McCoy's 5A
medium (Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine
serum (FBS; Gibco, Carlsbad, CA, USA) at 37°C with 5%
CO2.
Short hairpin RNAs (shRNAs) against HSF1 and the
corresponding expression vectors were designed and constructed by
GenePharma (Shanghai, China). Lentiviral particles, expressing
specific shRNAs against HSF1, were also prepared by GenePharma.
SKOV3 cells were infected with lentiviral particles expressing the
scramble shRNA or shRNA against HSF1 in the presence of 5
µg/ml polybrene, generating SKOV3-NC and SKOV3-shHSF1 cells,
respectively. SKOV3-NC and SKOV3-shHSF1 clones were cultured and
screened with puromycin (1 µg/ml; Merck Millipore,
Billerica, MA, USA) after infection. The HSF1 inhibitory effect was
examined at both the mRNA and protein levels.
Cell proliferation assessment
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Sigma-Aldrich) assay was employed to assess the cell
viability and to determine cell proliferative activity. The assay
was performed according to a previously published procedure
(4). Briefly, ~1×104
cells were seeded per well in a 96-well plate, and incubated in 100
µl medium. Fresh medium was added every 48 h. For cell
viability assessment, 10 µl MTT (5 mg/ml) was added,
followed by a 4-h incubation at 37°C. The medium was replaced by
150 µl dimethyl sulfoxide (DMSO; Sigma-Aldrich), and
absorbance at 490 nm was determined in a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Each assay contained at
least six wells and all experiments were independently repeated at
least 3 times.
Flow cytometric analysis
To determine the cell cycle distribution, cells in
the logarithmic phase were dissociated by 0.25%
trypsin-ethylenediaminetetraacetic acid (Life Technologies,
Carlsbad, CA, USA), seeded in a 6-well plate and then allowed to
attach and proliferate for 24 h. Cells were harvested by trypsin
digestion, rinsed with phosphate-buffered saline (PBS), and fixed
with 70% cold-ethanol for at least 12 h at −20°C. The resultant
cells were centrifuged and resuspended, and were treated with RNase
A for 30 min at 37°C. Then propidium iodide (PI) was added and
incubated at 4°C for 30 min.
Annexin V-PE/7-AAD Apoptosis Detection kit (KeyGen,
Nanjing, China) was used to evaluate cell apoptosis. Cells in a
logarithmic phase were dissociated and seeded in 6-cm dish. After a
48-h culture, the resulting cells were harvested by trypsin
digestion, and analyzed by following the manufacturer's
instructions.
The flow cytometric detection and data analysis were
performed with a flow cytometer (BD Accuri™ C6; BD Biosciences,
Franklin Lakes, NJ, USA).
RNA extraction and quantitative PCR
RNA preparation and quantitative PCR were performed
as described in the literature (4,24).
Cells were harvested and rinsed, and RNA was extracted with TRIzol
reagent (Life Technologies) according to the manufacturer's
instructions. Genomic DNA contamination was removed by treatment
with DNase I (Fermentas, Hanover, MD, USA). Reverse-transcription
was performed with M-MLV Reverse Transcriptase (Promega, Madison,
WI, USA). Quantitative PCR was performed with the SYBR Real-Time
PCR kit (GenePharma) on a Mastercycler ep realplex (Eppendorf,
Hamburg, Germany).
DNA oligo pairs used in this study were as follows:
GAPDH forward, GAGTCAACGGATTTGGTCGT and reverse, TTG
ATTTTGGAGGGATCTCG; HSF1 forward, CATGAAGCAT GAGAATGAGGCT and
reverse, ACTGCACCAGTGAGATC AGGA. Relative RNA concentration was
calculated from cycle thresholds and GAPDH was used as the internal
standard control. Three independent experiments were performed.
Immunoblot assay
Routine western blot analysis was carried out
according to a previously a published protocol (4). Anti-human HSF1 rabbit polyclonal
antibody (4356; Cell Signaling Technology, Inc.) and anti-human
β-actin mouse monoclonal antibody (A5441; Sigma-Aldrich) were used
as primary antibodies. Horseradish peroxidase (HRP)-conjugated
antibodies (goat anti-rabbit IgG-HRP, 111-035-003; goat anti-mouse
IgG-HRP, 115-035-003; Jackson ImmunoResearch Inc., West Grove, PA,
USA) were applied as secondary antibodies. Immunoblot signals were
visualized with SuperSignal West Pico chemiluminescent substrate
(Thermo Fisher Scientific, Inc., Rockford, IL, USA).
Xenograft tumor construction
Animal maintenance and experiments were performed in
compliance with the guidelines of the Institutional Animal Care and
Use Committee of Shanghai Jiaotong University (Shanghai, China).
SKOV3-NC and SKOV3-shHSF1 cells in a logarithmic phase were
harvested by trypsin digestion and washed twice with PBS. Cells
(1×106) were injected subcutaneously into 4-week-old
female BALB/c nude mice. After 2 weeks, the tumor growth of
SKOV3-NC cells was well-established. The mouse weight and tumor
size were monitored every 3 or 4 days. At the end of the
experiment, the mice were sacrificed by cervical dislocation, and
tumors were excised.
Results
HSF1 expression is elevated in malignant
EOC tissues
Hyperexpression of HSF1 is considered to be an
aggressiveness marker in certain malignant carcinomas. However, to
the best of our knowledge the clinical and prognostic significance
of HSF1 expression in ovarian neoplasms has not been well studied.
In order to investigate whether HSF1 is elevated in EOC patients,
the HSF1 expression pattern was determined in normal ovarian
tissues and EOC tissue samples, which were derived from
patients.
We investigated the relationship of HSF1 in EOC to
clinical parameters in a series of primary tumors. Forty-two benign
(30 serous cystadenoma, 12 mucinous cystadenoma), 9 borderline (7
serous adenocarcinoma, 2 mucinous adenocarcinoma), 126 malignant
(88 serous adenocarcinoma, 11 mucinous adenocarcinoma, 17
endometrioid adenocarcinoma, 10 clear cell carcinoma) and 62
healthy ovarian tissues were included.
The expression of HSF1 was examined by
immunochemical staining. As shown in Fig. 1A, HSF1 was barely detected in the
normal ovarian tissues. The expression of HSF1 was low and largely
restricted in the infiltrating stroma and tumor areas bordering
necrosis in the benign and borderline tumors (Fig. 1B and C), while dense and massive
HSF1 staining was observed in the malignant EOC tissues (Fig. 1D).
A semi-quantification scoring system was used to
quantify the HSF1 expression pattern. The expression patterns of
HSF1 in EOC tissues with different clinicopathological features are
listed in Table I. Correlation of
the HSF1 status with clinical parameters was further delineated by
Mann-Whitney U test analysis. Compared with the normal ovarian
tissues, malignant EOC tissues, including serous, mucinous,
endometrioid and clear cell, showed significantly elevated HSF1
expression. High HSF1 expression was detected in malignant tissues
only, including serous, mucinous, endometrioid and clear cell
carcinoma. This suggests an association between high HSF1
expression and a more malignant phenotype.
 | Table IThe HSF1 expression patterns in EOC
tissues. |
Table I
The HSF1 expression patterns in EOC
tissues.
| Clinicopathological
characteristics | No HSF1 n (%) | Low HSF1 n (%) | High HSF1 n (%) |
|---|
| Normal ovarian
tissue | 60 (96.77) | 2 (3.23) | 0 (0.00) |
| Serous |
| Benign serous
cystadenomaa | 9 (30.00) | 21 (70.00) | 0 (0.00) |
| Borderline serous
adenocarcinomaa | 3 (42.86) | 4 (57.14) | 0 (0.00) |
| Malignant serous
adenocarcinomaa | 29 (32.95) | 42 (47.73) | 17 (19.32) |
| Mucinous |
| Benign mucinous
cystadenoma | 11 (91.67) | 1 (8.33) | 0 (0.00) |
| Borderline mucinous
adenocarcinoma | 1 (50.00) | 1 (50.00) | 0 (0.00) |
| Malignant mucinous
adenocarcinomaa,c | 4 (36.36) | 6 (54.55) | 1 (9.09) |
| Endometrioid |
| Malignant
endometrioid adenocarcinomab | 12 (70.59) | 3 (17.65) | 2 (11.76) |
| Clear cell |
| Malignant clear cell
carcinomaa | 1 (10.00) | 4 (40.00) | 5 (50.00) |
In Fig. 2A–D the
HSF1 staining scores are shown in serous, mucinous, endometrioid
and clear cell EOC tissues, respectively. The HSF1 expression was
significantly higher in benign serous tissues and serous EOC
tissues, including borderline and malignant, than the normal
ovarian tissues (Fig. 2A).
Compared with the benign serous cystadenoma, malignant serous
adenocarcinoma showed higher HSF1 staining scores. However, there
was no significant difference between the benign and borderline
tissues, or the borderline and malignant tissues. In malignant
mucinous adenocarcinoma, the HSF1 expression was significantly
elevated (Fig. 2B), and the HSF1
staining score was higher in malignant carcinoma than that in the
corresponding benign tumors. Endometrioid (Fig. 2C) and clear cell (Fig. 2D) carcinoma also had higher
expression of HSF1 than that in the normal ovarian tissues.
The results showed that HSF1 is activated in the
malignant state, suggesting HSF1 to be a candidate biomarker for
EOC, and implying its biological significance in ovarian
malignancy. The elevated expression of HSF1 also suggests it as a
therapeutic target against EOC, and motivated us to examine whether
targeting HSF1 leads to an anticancer effect in EOC cells.
HSF1 knockdown leads to reduced growth
and induced apoptosis in SKOV3 cells
As discussed above, HSF1 represents an attractive
therapeutic target for several human cancers, and our data
confirmed its elevated expression in EOC. Thus, we aimed to
ascertain whether targeting HSF1 leads to antitumor activities in
EOC. To demonstrate the potential effect of targeting HSF1 in EOC
treatment, we firstly investigated the biological function of HSF1
knockdown in SKOV3 cells, which is an EOC-derived immortalized cell
line.
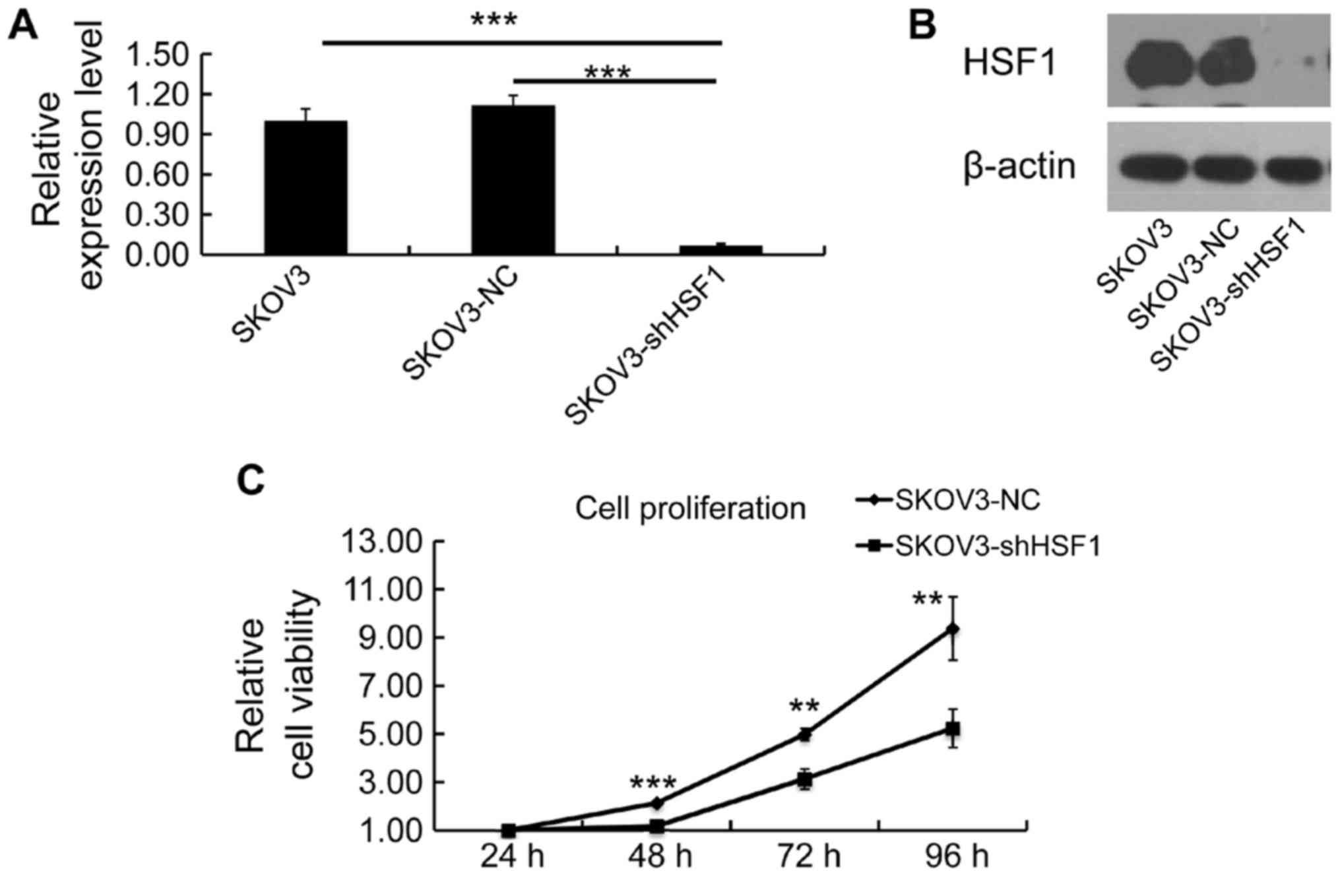
In the attempt to obtain stable HSF1-knockdown SKOV3
cells, scramble and HSF1-specific shRNA lentiviral particles were
generated and transfected into SKOV3 cells, generating SKOV3-NC and
SKOV3-shHSF1 cell lines respectively. Both mRNA and protein
expression levels of HSF1 were examined to confirm the silencing
efficiency. As expected, the expression of HSF1 was silenced in the
SKOV3-shHSF1 cells, while its expression remained the same in the
SKOV3-NC cells (Fig. 3A and
B).
To assess the significance of targeting HSF1 in cell
proliferation regulation, cell viability was determined by MTT
assay, and a growth curve was evaluated for the SKOV3-NC and
SKOV3-shHSF1 cells. Notably, a reduction in proliferation was
observed in the SKOV3-shHSF1 cells (Fig. 3C), indicating that downregulation
of HSF1 led to inhibition of cell growth.
Cell cycle distribution and cell apoptosis were
investigated in the SKOV3-shHSF1 and SKOV3-NC cells. The percentage
of early apoptotic cells was markedly increased in the SKOV3-shHSF1
cells (11.87%), relative to that in the SKOV3-NC cells (6.47%)
(Fig. 4A and B). Additionally,
the percentage of late apoptotic cells was slightly increased in
the SKOV3-shHSF1 group (Fig. 4A).
This implies that downregulation of HSF1 in the SKOV3 cells led to
induction of cell apoptosis.
Then, cell cycle distribution was investigated in
both SKOV3-shHSF1 and SKOV3-NC cells. As illustrated in Fig. 4C, the percentage of cells at the
G1 phase was increased in the SKOV3-shHSF1 cells, while the
percentages of S and G2/M phase cells were decreased in the
SKOV3-shHSF1 cells. The observed differences in cell cycle
distribution confirmed that the suppression of proliferative
activity resulted from HSF1 knockdown. These results demonstrated
the antitumor effect of targe ting HSF1 against EOC in
vitro.
Depletion of HSF1 in SKOV3 cells results
in reduced tendency of carcinogenesis
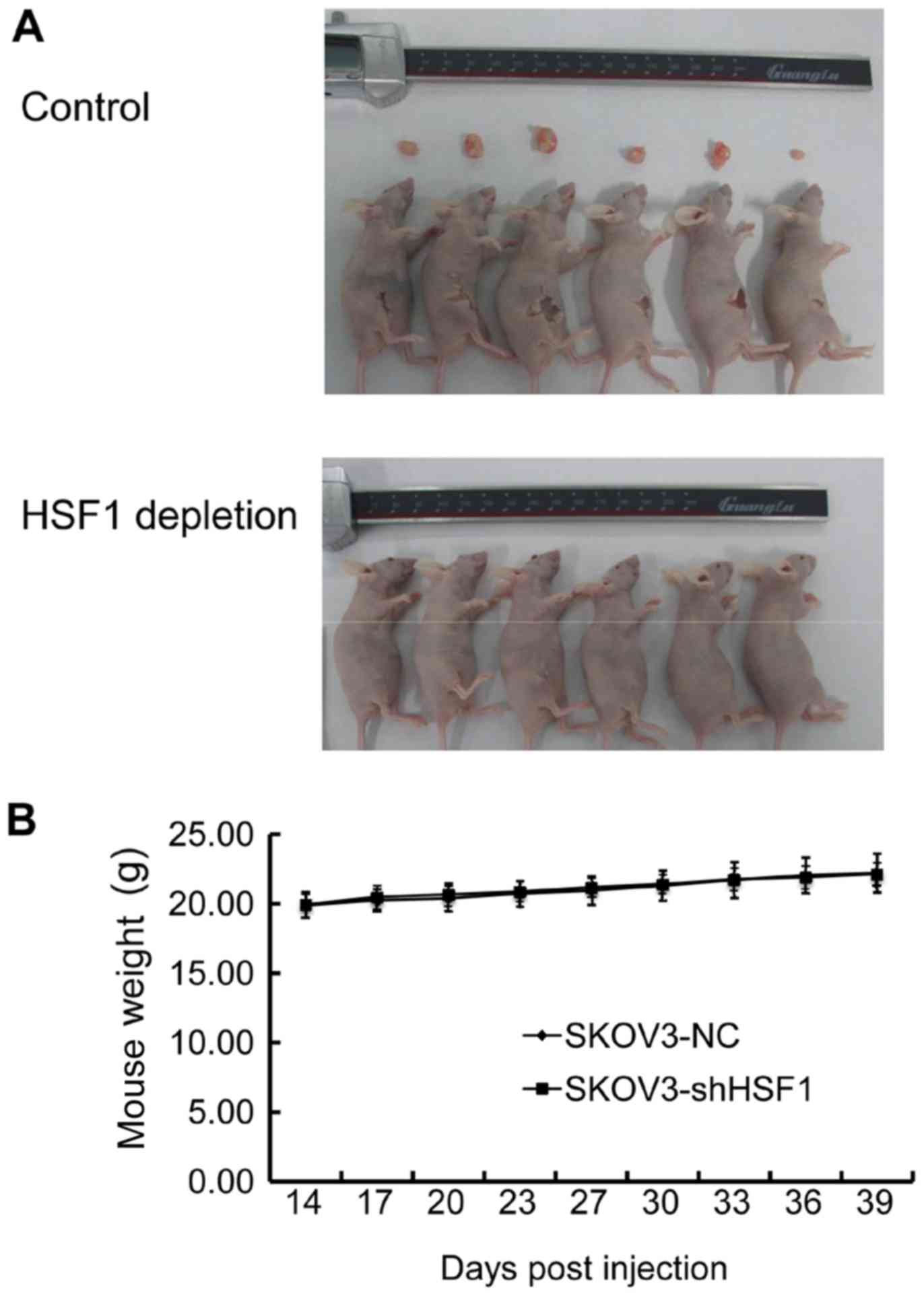
An animal study was performed to confirm the
antitumor activity arising from the downregulation of HSF1 in
vivo. After injection into immunodeficiency nude female mice
for 14 days, the control cells formed noticeable tumors. However,
the HSF1-depleted cells (SKOV3-shHSF1 cells) exhibited no tumor
genesis until 39 days post-injection (Fig. 5A). In addition, the body weights
of the mice were also recorded, and no discernible weight
alteration was observed between the two groups (Fig. 5B). These data revealed that
targeting HSF1 in SKOV3 cells also led to an antitumor effect in
vivo, suggesting that HSF1 may be used as a potential
therapeutic target against EOC.
Discussion
The biological functions of HSF1 in promoting cell
protection and survival against stress have been well understood
for decades. In addition, it is believed that the regulatory
function of HSF1 is performed by coordinating chaperone protein
expression. However, in the past few years, the biological function
of HSF1 has been found to be more extensive than previously
assumed, especially in regards to tumor cells (7,9,11).
Increasing evidence indicates this ancient transcriptional
regulator to be a genome-wide regulator of carcinogenesis and tumor
progression. The critical cellular roles of HSF1 include
maintaining or promoting tumorigenesis, facilitating tumorigenesis
and malignant transformation, maintaining protein homostasis and
hindering cell apoptosis. More importantly, several lines of
evidence suggest that downregulation of HSF1 is a promising
antitumor strategy for several types of malignancies. Therefore,
HSF1 has been regarded as a promising candidate target for cancer
therapy (20–23), and strategies that are capable of
modulating HSF1 expression to elicit anticancer activity have
recently raised considerable interest (25). However, data concerning the
expression pattern of HSF1 in human ovarian cancer, and the
antitumor effect of HSF1 modulation against human ovarian cancer
have not been investigated.
Our previous study demonstrated that suppression of
HSF1 expression, probed by a nucleoside analog, efficiently
inhibited the proliferation activity and tumorigenesis in
EOC-derived cells (4), suggesting
the potential to combat EOC by down regulation of HSF1 expression.
In the present study, we evaluated the HSF1 expression pattern in
human EOC tissues, and investigated the antitumor function of the
attenuated expression of HSF1 in EOC-derived cell lines (SKOV3). We
observed that HSF1 was highly expressed in malignant serous,
mucinous, endometrioid, and clear cell ovarian carcinoma and low
HSF1 expression was detected in benign serous and mucinous
cystadenoma. In contrast, HSF1 was barely detected in healthy
ovarian tissues.
The observed overexpression of HSF1 in human EOC
tissues led us to suggest HSF1 as a molecular target against
ovarian cancer. Additionally, the present study showed that HSF1
knockdown induced early apoptosis and inhibited proliferative
activity in SKOV3 cells. Moreover, downregulation of HSF1 was found
to result in suppression of EOC carcinogenesis.
Taken together, our results demonstrated that HSF1
expression plays an important role in the malignant potential of
human EOC and HSF1 may be a promising diagnotic biomarker for
malignant epithelial ovarian tumors. Furthermore, our data showed
the potential value of manipulating HSF1 as a therapeutic strategy
against EOC. In conclusion, our findings, although still
preliminary, provided critical information concerning the
understanding of the cellular function of HSF1 in ovarian cancer.
This study may facilitate the early diagnosis and efficacious
treatment of human EOC.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81401216 and 81603189),
Shanghai Municipal Health and Family Planning Commission (no.
20134Y128), Shanghai Jiaotong University School of Medicine (no.
13XJ10067), and Shanghai Committee of Science and Technology (no.
14YF1408200), as well as the Innovation Team of Natural Science
Foundation of the Department of Education of Guizhou Province (no.
QJHRCTDZ[2015]57).
References
|
1
|
Coward JI, Middleton K and Murphy F: New
perspectives on targeted therapy in ovarian cancer. Int J Womens
Health. 7:189–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Syrios J, Banerjee S and Kaye SB: Advanced
epithelial ovarian cancer: from standard chemotherapy to promising
molecular pathway targets - where are we now? Anticancer Res.
34:2069–2077. 2014.PubMed/NCBI
|
|
3
|
Landriscina M, Amoroso MR, Piscazzi A and
Esposito F: Heat shock proteins, cell survival and drug resistance:
the mitochondrial chaperone TRAP1, a potential novel target for
ovarian cancer therapy. Gynecol Oncol. 117:177–182. 2010.
View Article : Google Scholar
|
|
4
|
Chen YF, Dong Z, Xia Y, Tang J, Peng L,
Wang S and Lai D: Nucleoside analog inhibits microRNA-214 through
targeting heat-shock factor 1 in human epithelial ovarian cancer.
Cancer Sci. 104:1683–1689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anckar J and Sistonen L: Regulation of
HSF1 function in the heat stress response: implications in aging
and disease. Annu Rev Biochem. 80:1089–1115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vihervaara A and Sistonen L: HSF1 at a
glance. J Cell Sci. 127:261–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mendillo ML, Santagata S, Koeva M, Bell
GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L and Lindquist
S: HSF1 drives a transcriptional program distinct from heat shock
to support highly malignant human cancers. Cell. 150:549–562. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Home T, Jensen RA and Rao R: Heat shock
factor 1 in protein homeostasis and oncogenic signal integration.
Cancer Res. 75:907–912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai C, Whitesell L, Rogers AB and
Lindquist S: Heat shock factor 1 is a powerful multifaceted
modifier of carcinogenesis. Cell. 130:1005–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calderwood SK: HSF1, a versatile factor in
tumorogenesis. Curr Mol Med. 12:1102–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ciocca DR, Arrigo AP and Calderwood SK:
Heat shock proteins and heat shock factor 1 in carcinogenesis and
tumor development: an update. Arch Toxicol. 87:19–48. 2013.
View Article : Google Scholar
|
|
12
|
Meng L, Gabai VL and Sherman MY:
Heat-shock transcription factor HSF1 has a critical role in human
epidermal growth factor receptor-2-induced cellular transformation
and tumorigenesis. Oncogene. 29:5204–5213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santagata S, Hu R, Lin NU, Mendillo ML,
Collins LC, Hankinson SE, Schnitt SJ, Whitesell L, Tamimi RM,
Lindquist S, et al: High levels of nuclear heat-shock factor 1
(HSF1) are associated with poor prognosis in breast cancer. Proc
Natl Acad Sci USA. 108:18378–18383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin X, Moskophidis D and Mivechi NF: Heat
shock transcription factor 1 is a key determinant of HCC
development by regulating hepatic steatosis and metabolic syndrome.
Cell Metab. 14:91–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang F, Chang R and Yang L: Heat shock
factor 1 promotes invasion and metastasis of hepatocellular
carcinoma in vitro and in vivo. Cancer. 118:1782–1794. 2012.
View Article : Google Scholar
|
|
16
|
Li Y, Xu D, Bao C, Zhang Y, Chen D, Zhao
F, Ding J, Liang L, Wang Q, Liu L, et al: MicroRNA-135b, a HSF1
target, promotes tumor invasion and metastasis by regulating RECK
and EVI5 in hepatocellular carcinoma. Oncotarget. 6:2421–2433.
2015. View Article : Google Scholar :
|
|
17
|
Santagata S, Mendillo ML, Tang YC,
Subramanian A, Perley CC, Roche SP, Wong B, Narayan R, Kwon H,
Koeva M, et al: Tight coordination of protein translation and HSF1
activation supports the anabolic malignant state. Science.
341:12383032013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scherz-Shouval R, Santagata S, Mendillo
ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M,
Stemmer SM, Whitesell L, et al: The reprogramming of tumor stroma
by HSF1 is a potent enabler of malignancy. Cell. 158:564–578. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai C and Sampson SB: HSF1: guardian of
proteostasis in cancer. Trends Cell Biol. 26:17–28. 2016.
View Article : Google Scholar :
|
|
20
|
Whitesell L and Lindquist S: Inhibiting
the transcription factor HSF1 as an anticancer strategy. Expert
Opin Ther Targets. 13:469–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar S, Tomar MS and Acharya A:
HSF1-mediated regulation of tumor cell apoptosis: a novel target
for cancer therapeutics. Future Oncol. 9:1573–1586. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Chen J, Loo A, Jaeger S,
Bagdasarian L, Yu J, Chung F, Korn J, Ruddy D, Guo R, et al:
Targeting HSF1 sensitizes cancer cells to HSP90 inhibition.
Oncotarget. 4:816–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossi A, Ciafrè S, Balsamo M, Pierimarchi
P and Santoro MG: Targeting the heat shock factor 1 by RNA
interference: a potent tool to enhance hyperthermochemotherapy
efficacy in cervical cancer. Cancer Res. 66:7678–7685. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YF, Dong Z, Jiang L, Lai D and Guo L:
Mouse primed embryonic stem cells could be maintained and
reprogrammed on human amnion epithelial cells. Stem Cells Dev.
22:320–329. 2013. View Article : Google Scholar
|
|
25
|
Agarwal T, Annamalai N, Khursheed A, Maiti
TK, Arsad HB and Siddiqui MH: Molecular docking and dynamic
simulation evaluation of Rohinitib - Cantharidin based novel HSF1
inhibitors for cancer therapy. J Mol Graph Model. 61:141–149. 2015.
View Article : Google Scholar : PubMed/NCBI
|