Airway bronchoconstriction (BC) and
hyperresponsiveness are hallmarks of asthma, a chronic,
irreversible inflammatory disease (1). Asthma-related BC can be induced by
exercise, infection or allergen exposure according to the
phenotypic characteristics of each patient (2) and is frequently relieved by inhaled
corticosteroids and β2 adrenergic agonists (3). Basically, it can be described as the
consequence of the airway smooth muscle (ASM) contraction developed
by increases in intracellular Ca2+ concentration
([Ca2+]i) in response to agonists or membrane
depolarization (4). On the other
hand, asthma patients also show airway hyperresponsiveness commonly
evaluated by inhalation of methacholine or histamine and this
exacerbated ASM response also involves cellular Ca2+
handling mechanisms (5–8). In addition to Ca2+,
Na+ is paramount in ASM contraction. The literature
available, although not yet conclusive, indicates a possible link
between altered ASM cell Na+ handling mechanisms and BC
and this evidence is discussed in the present review.
It is well know that excitable cells possess a
characteristic resting membrane potential, a state of equilibrium
between inner and outer ionic concentrations. In airway myocytes,
resting membrane potential fluctuates between −40 and −50 mV
(9) and reflects many membrane
mechanisms that keep the balance between internal and external
ionic concentrations. In this tissue, stimuli that trigger
contraction can be characterized as chemical (neurotransmitters,
cytokines and terpenoids) and physical (inspired air volume, air
pressure and temperature). Although different in nature, both types
of stimuli act on membrane proteins that facilitate the passage of
ions in and out, causing a current and a voltage change.
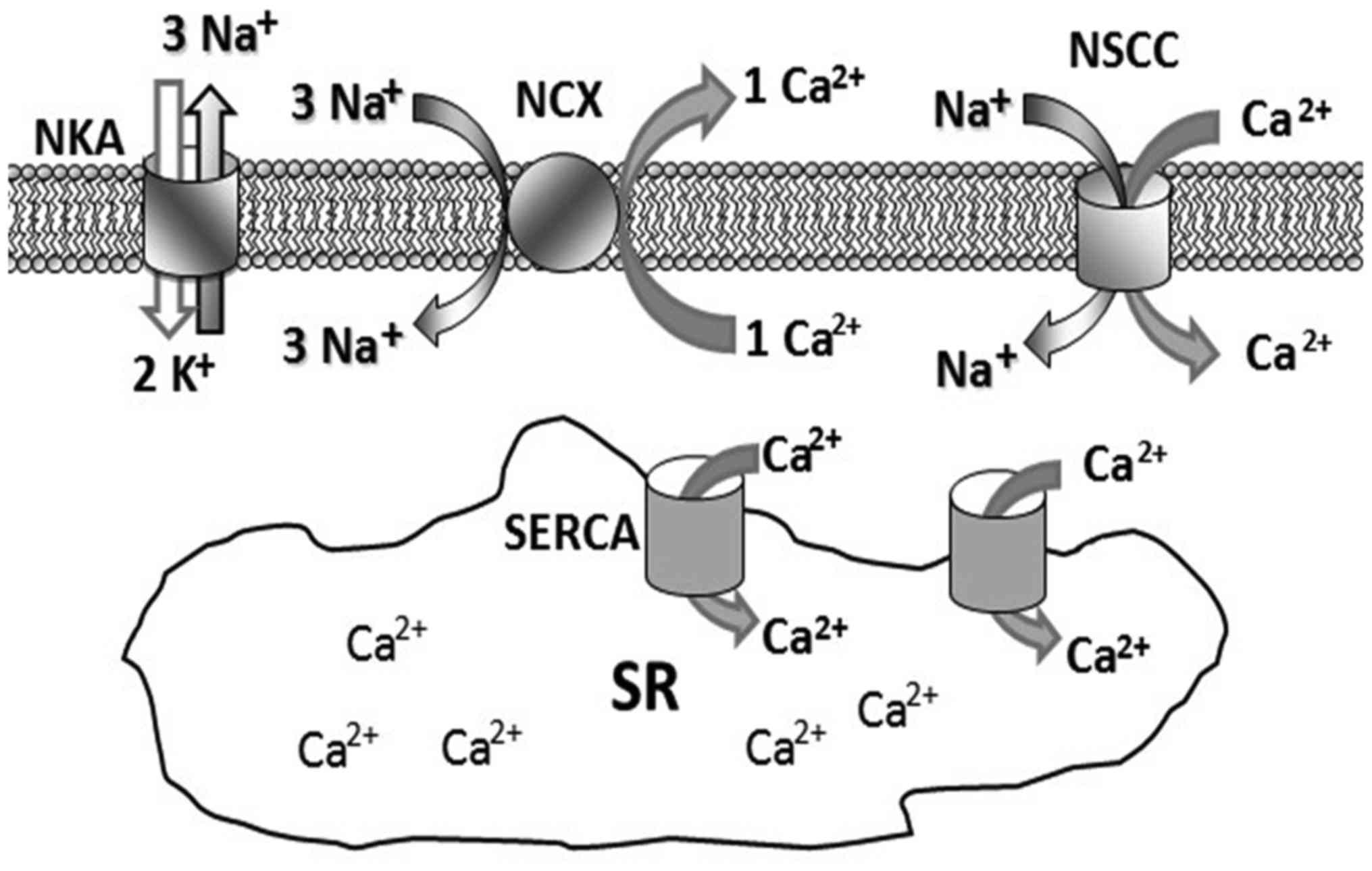
Na+ transit through the cell membrane has been known to
play the leading role in preserving membrane potential at rest. It
is extracted from the cells by the Na+/K+
ATPase (NKA) pump or introduced into the cytoplasm by the
Na+/Ca2+ exchanger (NCX) (Fig. 1). At rest, ASM has a basal tension
(tone) which results from the continuous adjustment of
Ca2+ and Na+ intracytoplasmic
concentrations.
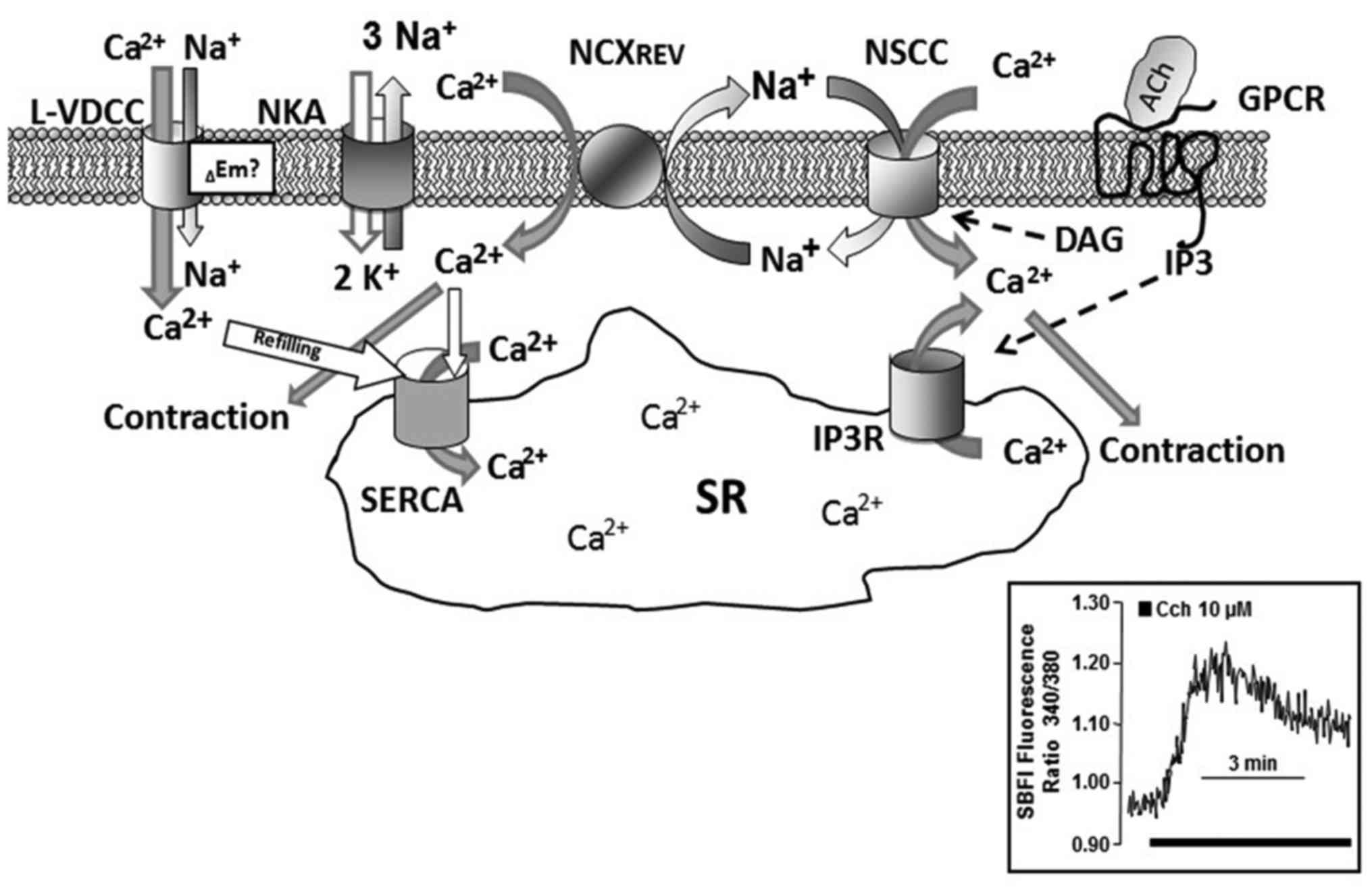
This group is constituted by ion channels of varied
nature that permeate mainly ions such as Ca2+ and
Na+ and are designated as NSCC. They can be clasified in
two groups based on the cellular mechanisms that activate them:
store operated (SOC) channels permate cations in response to a
decrease in the SR Ca2+ content and receptor operated
(ROC) channels which, once the agonist (e.g., acetylcholine)
ocupies its receptor activating a signaling pathway, open in
response to secondary messengers (e.g., diacylglycerol) (50). The components of NSCCs have not
been fully defined, but the TRPC channels are considered as an
essential part of most of them. In general, all TRPs have six
transmembranal regions (TM1-6) and oligomerize to form
homotetramers or heterotetramers (51). The TRPC family consists of seven
members (1–7) and TRPCs are present in excitable and
non-excitable cells allowing nonspecific cation entry through the
membrane. The current hence generated depolarizes the membrane,
triggering the opening of voltage-dependent channels involved in
diverse cellular functions. Thus, the TRPCs may cause action
potentials in excitable cells (51). These channels also contribute to
an increase in intracellular Na+ concentration to induce
NCXREV (Fig. 2). TRPCs
were initially characterized in Drosophila; in this species,
certain mutation prevents the passage of Ca2+ through
the phospholipase C coupled channels. Normally, these
photoreceptors remain depolarized when exposed to a continuous
light source but those cells from specimens with the mutation show
a transient response, therefore 'TRPC'. In mammals, the equivalent
of the TRPC studied in Drosophila is classified into four
subfamilies: TRPC1, TRPC2, TRPC3/6/7 and TRPC4/5 (sometimes TRPC1
is included in the subfamily TRPC4/5) (52–54). TRPC1 is considered paradigmatic
for the study of SOCs as it has been shown to participate in SR
Ca2+ refilling (capacitative entry) and in the sustained
contraction. TRPC1 forms part of many types of NSCCs, from
relatively selective to strictly non-selective channels (regarding
Ca2+ permeability) and this diversity may be related to
its heteromeric association with other TRPCs. Some studies even
suggest that the presence of TRPC1 depends entirely on its
interaction with another TRPC (TRPC4) (55). It has also been reported that the
association of TRPC1 with two other proteins (STIM1 on the SR
membrane and Orai on the plasma membrane) is highly selective for
Ca2+ and that they only permeate a capacitative
Ca2+ current known as calcium release-activated calcium
current (ICRAC) (50,56–58).
With regard to those channels operated by the
receptor, it has been found that TRPC3 (and most likely TRPC6 and
TRPC7) are activated by inositol triphosphate (IP3) and/or the IP3
receptor (IP3R) (59–61). Therefore, TRPC3 is used as a model
channel to study what is considered a conformational coupling
between the channel and the IP3R; the latter requires at least its
N-terminal portion to activate TRPC3 (62). Nevertheless, IP3 presence is not
necessary to induce Ca2+ entry through these channels,
as pharmacological methods other than agonist stimulation
(thapsigargin or ionomycin) induce the same response. It has also
been reported that the presence of TRPC3 is crucial to maintain the
resting membrane potential in cells of healthy ASM, while in
myocytes from asthmatic airways, TRPC1 seems to play an important
role (63). Many of the functions
of the different TRPC isoforms remain unclear; it has even been
proposed that TRPCs are not indispensable for SOCs, but rather to
ROCs due to their sensitivity to diacylglycerol (64).
Multiple studies of patients with atopic asthma
and/or allergic rhinitis report the presence of plasmatic,
endogenous ouabain, indicating a probable intracellular
Na+ accumulation (21–26). In fact, the study by Tribe et
al (25) shows an increased
intracellular Na+ concentration in mononuclear cells
from healthy individuals incubated with plasma of asthmatic blood
donors. Apparently, endogenous ouabain acts not only on ASM NKAs,
but also on inflammatory cells perhaps contributing to consolidate
airway hyperresponsiveness. Knox et al (69) postulate that the difference lies
on the tissue, since ASM contracts in vitro in response to
exogenous ouabain but does not predispose tissue to
hyperresponsivenes. Moreover, Knudsen et al (70) demonstrated that ouabain addition
to rat peritoneal mast cells induced histamine release while
simultaneously blocking intracellular Rb+ increase (used
as surrogate to evaluate K+ cell entry through
Na+/K+ ATPase activity).
The origin of endogenous ouabain found in atopic
asthmatic plasma has not been clarified completely, but documentary
evidence indicates that mammals produce an endogenous ouabain
analog. It is a hormone found in blood plasma, synthesized mainly
by the adrenal, hypothalamus and pituitary glands (27–33). In vitro, the release of
ouabain in primary cultures of bovine adrenocortical cells is
favored by the addition of ACTH, angiotensin II, vasopressin and
phenylephrine (34,35).
On the other hand, ouabain has been claimed to have
anti-inflammatory capabilities by suppressing TNFα production
(71). In this regard, the high
ouabain generation seen in atopic asthma may be associated with a
compensatory process intended to regulate circulating TNF
concentrations (72).
Excercise-induced bronchoconstriction (EIB) is
currently defined as a syndrome where exercise or an increase in
ventilation triggers airflow obstruction that may last 30–90 min if
not attended. Although it occurs most frequently in asthmatic
patients, cross sectional studies show that only a portion of
patients with asthma have EIB when tested with a specific challenge
test (73). The indirect
challenges commonly used to define EIB are exercise itself,
eucapnic voluntary hyperpnea, hypertonic (4.5%) saline, mannitol
and adenosine (74). These
'specific challenge tests' differ from conventional methacholine or
histamine challenges. Theoretically, exercise increases the amount
of inhaled air but augments airway lining fluid osmolarity by
dehydration, favoring lung mast cell activation and
pro-inflammatory mediator release from the epithelium involving a
soluble phospholipase A (75,76). Most of these pro-inflammatory
mediators can induce ASM contraction directly or favor a primed
response to other agonists (hyperractivity). It is conceivable that
airway lining fluid Na+ content could augment osmolarity
in the airways during exercise. In this regard, Schmitt et
al (77) established that, in
airway epithelia from asthmatic patients with EIB, transepithelial
nasal potential carried out by Cl− and Na+ is
not modified during exercise, indicating that a Na+
handling mechanism is at stake. Also associated with impaired
cellular Na+ homeostasis, is the inhibition of the NKA
in atopy and EIB. Mast cells express NKAs and NCXs (78); the [Ca2+]i
increase induced by NCXREV in these cells favors the
release of inflammatory mediators, pointing out this exchanger's
role in inflammation. In fact, the use of an NCXREV
inhibitor (KB-R7943) significantly reduced the amount of histamine
released by stimulated peritoneal mast cells which correlated with
a decrease in [Ca2+]i (79).
In conclusion, dietary sodium intake seems not to
play a pivotal role in asthma nor in EIB, rather it is more
plausible that Na+ handling mechanisms are partially
responsible for EIB and augmented inflammatory mediator release in
both ailments. This argument warrants further and deeper research
in ASM sodium handling mechanisms.
Authors are grateful to National Institute of
Respiratory Diseases for the financial support in the publication
of this manuscript.
|
1
|
Woloski JR, Heston S and Escobedo Calderon
SP: Respiratory Allergic Disorders. Prim Care. 43:401–415. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bostantzoglou C, Delimpoura V, Samitas K,
Zervas E, Kanniess F and Gaga M: Clinical asthma phenotypes in the
real world: Opportunities and challenges. Breathe Sheff.
11:186–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lommatzsch M and Stoll P: Novel strategies
for the treatment of asthma. Allergo J Int. 25:11–17. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koopmans T, Anaparti V, Castro-Piedras I,
Yarova P, Irechukwu N, Nelson C, Perez-Zoghbi J, Tan X, Ward JP and
Wright DB: Ca2+ handling and sensitivity in airway
smooth muscle: Emerging concepts for mechanistic understanding and
therapeutic targeting. Pulm Pharmacol Ther. 29:108–120. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carbajal V, Vargas MH, Flores-Soto E,
Martínez-Cordero E, Bazán-Perkins B and Montaño LM: LTD4 induces
hyperrespon-siveness to histamine in bovine airway smooth muscle:
Role of SR-ATPase Ca2+ pump and tyrosine kinase. Am J
Physiol Lung Cell Mol Physiol. 288:L84–L92. 2005. View Article : Google Scholar
|
|
6
|
Liu C, Tazzeo T and Janssen LJ:
Isoprostane-induced airway hyperresponsiveness is dependent on
internal Ca2+ handling and Rho/ROCK signaling. Am J
Physiol Lung Cell Mol Physiol. 291:L1177–L1184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morin C and Rousseau E: Enhanced
Ca2+ sensitivity in hyper-responsive cultured bronchi is
mediated by TNFalpha and NF-kappaB. Can J Physiol Pharmacol.
84:1029–1041. 2006. View
Article : Google Scholar
|
|
8
|
Sweeney D, Hollins F, Gomez E, Saunders R,
Challiss RA and Brightling CE: [Ca2+]i
oscillations in ASM: Relationship with persistent airflow
obstruction in asthma. Respirology. 19:763–766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fleischmann BK, Washabau RJ and Kotlikoff
MI: Control of resting membrane potential by delayed rectifier
potassium currents in ferret airway smooth muscle cells. J Physiol.
469:625–638. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blaustein MP: Sodium ions, calcium ions,
blood pressure regulation, and hypertension: A reassessment and a
hypothesis. Am J Physiol. 232:C165–C173. 1977.PubMed/NCBI
|
|
11
|
Orrenius S, Zhivotovsky B and Nicotera P:
Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol
Cell Biol. 4:552–565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eder P, Poteser M, Romanin C and Groschner
K: Na(+) entry and modulation of Na(+)/Ca(2+) exchange as a key
mechanism of TRPC signaling. Pflugers Arch. 451:99–104. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blaustein MP and Lederer WJ:
Sodium/calcium exchange: Its physiological implications. Physiol
Rev. 79:763–854. 1999.PubMed/NCBI
|
|
14
|
Blaustein MP and Wier WG: Local sodium,
global reach: Filling the gap between salt and hypertension. Circ
Res. 101:959–961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poburko D, Fameli N, Kuo KH and van
Breemen C: Ca2+ signaling in smooth muscle: TRPC6, NCX
and LNats in nanodomains. Channels (Austin). 2:10–12. 2008.
View Article : Google Scholar
|
|
16
|
Dai JM, Kuo KH, Leo JM, Paré PD, van
Breemen C and Lee CH: Acetylcholine-induced asynchronous calcium
waves in intact human bronchial muscle bundle. Am J Respir Cell Mol
Biol. 36:600–608. 2007. View Article : Google Scholar
|
|
17
|
Flores-Soto E, Reyes-García J, Sommer B
and Montaño LM: Sarcoplasmic reticulum Ca(2+) refilling is
determined by L-type Ca(2+) and store operated Ca(2+) channels in
guinea pig airway smooth muscle. Eur J Pharmacol. 721:21–28. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perusquía M, Flores-Soto E, Sommer B,
Campuzano-González E, Martínez-Villa I, Martínez-Banderas AI and
Montaño LM: Testosterone-induced relaxation involves L-type and
store-operated Ca2+ channels blockade, and
PGE2 in guinea pig airway smooth muscle. Pflugers Arch.
467:767–777. 2015. View Article : Google Scholar
|
|
19
|
Sommer B, Flores-Soto E, Reyes-García J,
Díaz-Hernández V, Carbajal V and Montaño LM: Na(+) permeates
through L-type Ca(2+) channel in bovine airway smooth muscle. Eur J
Pharmacol. 782:77–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lingrel JB: The physiological significance
of the cardiotonic steroid/ouabain-binding site of the Na,
K-ATPase. Annu Rev Physiol. 72:395–412. 2010. View Article : Google Scholar
|
|
21
|
Agrawal A, Agrawal KP, Ram A, Sondhi A,
Chhabra SK, Gangal SV and Mehta D: Basis of rise in intracellular
sodium in airway hyperresponsiveness and asthma. Lung. 183:375–387.
2005. View Article : Google Scholar
|
|
22
|
Chhabra SK, Khanduja A and Jain D:
Increased intracellular calcium and decreased activities of
leucocyte Na+, K+-ATPase and
Ca2+-ATPase in asthma. Clin Sci (Lond). 97:595–601.
1999. View Article : Google Scholar
|
|
23
|
Gentile DA and Skoner DP: The relationship
between airway hyperreactivity (AHR) and sodium, potassium
adenosine triphosphatase (Na+, K+ ATPase)
enzyme inhibition. J Allergy Clin Immunol. 99:367–373. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skoner DP, Gentile D and Evans RW: A
circulating inhibitor of the platelet Na+, K+
adenosine triphosphatase (ATPase) enzyme in allergy. J Allergy Clin
Immunol. 87:476–482. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tribe RM, Barton JR, Poston L and Burney
PG: Dietary sodium intake, airway responsiveness, and cellular
sodium transport. Am J Respir Crit Care Med. 149:1426–1433. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Deusen MA, Gentile DA and Skoner DP:
Inhibition of the sodium, potassium adenosine triphosphatase enzyme
in peripheral blood mononuclear cells of subjects with allergic
rhinitis. Ann Allergy Asthma Immunol. 78:259–264. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamlyn JM, Blaustein MP, Bova S, DuCharme
DW, Harris DW, Mandel F, Mathews WR and Ludens JH: Identification
and characterization of a ouabain-like compound from human plasma.
Proc Natl Acad Sci USA. 88:6259–6263. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferrandi M, Manunta P, Balzan S, Hamlyn
JM, Bianchi G and Ferrari P: Ouabain-like factor quantification in
mammalian tissues and plasma: Comparison of two independent assays.
Hypertension. 30:886–896. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laredo J, Hamilton BP and Hamlyn JM:
Ouabain is secreted by bovine adrenocortical cells. Endocrinology.
135:794–797. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schoner W: Ouabain, a new steroid hormone
of adrenal gland and hypothalamus. Exp Clin Endocrinol Diabetes.
108:449–454. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Komiyama Y, Nishimura N, Munakata M, Mori
T, Okuda K, Nishino N, Hirose S, Kosaka C, Masuda M and Takahashi
H: Identification of endogenous ouabain in culture supernatant of
PC12 cells. J Hypertens. 19:229–236. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
el-Masri MA, Clark BJ, Qazzaz HM and
Valdes R Jr: Human adrenal cells in culture produce both
ouabain-like and dihydroouabain-like factors. Clin Chem.
48:1720–1730. 2002.PubMed/NCBI
|
|
33
|
Murrell JR, Randall JD, Rosoff J, Zhao JL,
Jensen RV, Gullans SR and Haupert GT Jr: Endogenous ouabain:
Upregulation of steroidogenic genes in hypertensive hypothalamus
but not adrenal. Circulation. 112:1301–1308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Laredo J, Shah JR, Lu ZR, Hamilton BP and
Hamlyn JM: Angiotensin II stimulates secretion of endogenous
ouabain from bovine adrenocortical cells via angiotensin type 2
receptors. Hypertension. 29:401–407. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shah JR, Laredo J, Hamilton BP and Hamlyn
JM: Effects of angiotensin II on sodium potassium pumps, endogenous
ouabain, and aldosterone in bovine zona glomerulosa cells.
Hypertension. 33:373–377. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saklani P and Skanes A: Novel
anti-arrhythmic medications in the treatment of atrial
fibrillation. Curr Cardiol Rev. 8:302–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cortijo J, Sarria B, Mata M, Naline E,
Advenier C and Morcillo EJ: Effects of ouabain on human bronchial
muscle in vitro. Naunyn Schmiedebergs Arch Pharmacol. 368:393–403.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blaustein MP and Hamlyn JM: Signaling
mechanisms that link salt retention to hypertension: Endogenous
ouabain, the Na(+) pump, the Na(+)/Ca(2+) exchanger and TRPC
proteins. Biochim Biophys Acta. 1802:1219–1229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katz A, Lifshitz Y, Bab-Dinitz E,
Kapri-Pardes E, Goldshleger R, Tal DM and Karlish SJ: Selectivity
of digitalis glycosides for isoforms of human Na, K-ATPase. J Biol
Chem. 285:19582–19592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Floyd R and Wray S: Calcium transporters
and signalling in smooth muscles. Cell Calcium. 42:467–476. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
DiPolo R and Beaugé L: Sodium/calcium
exchanger: Influence of metabolic regulation on ion carrier
interactions. Physiol Rev. 86:155–203. 2006. View Article : Google Scholar
|
|
42
|
Kofuji P, Lederer WJ and Schulze DH:
Mutually exclusive and cassette exons underlie alternatively
spliced isoforms of the Na/Ca exchanger. J Biol Chem.
269:5145–5149. 1994.PubMed/NCBI
|
|
43
|
Quednau BD, Nicoll DA and Philipson KD:
Tissue specificity and alternative splicing of the
Na+/Ca2+ exchanger isoforms NCX1, NCX2, and
NCX3 in rat. Am J Physiol. 272:C1250–C1261. 1997.PubMed/NCBI
|
|
44
|
Mejía-Elizondo R, Espinosa-Tanguma R and
Saavedra-Alanis VM: Molecular identification of the NCX isoform
expressed in tracheal smooth muscle of guinea pig. Ann NY Acad Sci.
976:73–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Philipson KD, Nicoll DA, Ottolia M,
Quednau BD, Reuter H, John S and Qiu Z: The
Na+/Ca2+ exchange molecule: An overview. Ann
NY Acad Sci. 976:1–10. 2002. View Article : Google Scholar
|
|
46
|
Pitt A and Knox AJ: Molecular
characterization of the human airway smooth muscle
Na+/Ca2+ exchanger. Am J Respir Cell Mol
Biol. 15:726–730. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Janssen LJ, Walters DK and Wattie J:
Regulation of [Ca2+] in canine airway smooth muscle by
Ca(2+)-ATPase and Na+/Cai2+ exchange
mechanisms. Am J Physiol. 273:L322–L330. 1997.PubMed/NCBI
|
|
48
|
Flores-Soto E, Carbajal V, Reyes-García J,
García-Hernández LM, Figueroa A, Checa M, Barajas-López C and
Montaño LM: In airways ATP refills sarcoplasmic reticulum via P2X
smooth muscle receptors and induces contraction through P2Y
epithelial receptors. Pflugers Arch. 461:261–275. 2011. View Article : Google Scholar
|
|
49
|
Liu B, Peel SE, Fox J and Hall IP: Reverse
mode Na+/Ca2+ exchange mediated by STIM1
contributes to Ca2+ influx in airway smooth muscle
following agonist stimulation. Respir Res. 11:1682010. View Article : Google Scholar
|
|
50
|
Putney JW and Tomita T: Phospholipase C
signaling and calcium influx. Adv Biol Regul. 52:152–164. 2012.
View Article : Google Scholar
|
|
51
|
Pedersen SF, Owsianik G and Nilius B: TRP
channels: An overview. Cell Calcium. 38:233–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Trebak M, Lemonnier L, Smyth JT, Vazquez G
and Putney JW Jr: Phospholipase C-coupled receptors and activation
of TRPC channels. Handb Exp Pharmacol. 179:593–614. 2007.
View Article : Google Scholar
|
|
53
|
Trebak M, Vazquez G, Bird GS and Putney JW
Jr: The TRPC3/6/7 subfamily of cation channels. Cell Calcium.
33:451–461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vazquez G, Wedel BJ, Aziz O, Trebak M and
Putney JW Jr: The mammalian TRPC cation channels. Biochim Biophys
Acta. 1742:21–36. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ong HL and Ambudkar IS: The dynamic
complexity of the TRPC1 channelosome. Channels (Austin). 5:424–431.
2011. View Article : Google Scholar
|
|
56
|
Yuan JP, Lee KP, Hong JH and Muallem S:
The closing and opening of TRPC channels by Homer1 and STIM1. Acta
Physiol (Oxf). 204:238–247. 2012. View Article : Google Scholar
|
|
57
|
Cheng KT, Liu X, Ong HL, Swaim W and
Ambudkar IS: Local Ca2+ entry via Orai1
regulates plasma membrane recruitment of TRPC1 and controls
cytosolic Ca2+ signals required for specific
cell functions. PLoS Biol. 9:e10010252011. View Article : Google Scholar
|
|
58
|
Soboloff J, Madesh M and Gill DL: Sensing
cellular stress through STIM proteins. Nat Chem Biol. 7:488–492.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhu X, Jiang M, Peyton M, Boulay G, Hurst
R, Stefani E and Birnbaumer L: Trp, a novel mammalian gene family
essential for agonist-activated capacitative Ca2+ entry.
Cell. 85:661–671. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kiselyov K, Xu X, Mozhayeva G, Kuo T,
Pessah I, Mignery G, Zhu X, Birnbaumer L and Muallem S: Functional
interaction between InsP3 receptors and store-operated Htrp3
channels. Nature. 396:478–482. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
61
|
McKay RR, Szymeczek-Seay CL, Lievremont
JP, Bird GS, Zitt C, Jüngling E, Lückhoff A and Putney JW Jr:
Cloning and expression of the human transient receptor potential 4
(TRP4) gene: Localization and functional expression of human TRP4
and TRP3. Biochem J. 351:735–746. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kiselyov K, Mignery GA, Zhu MX and Muallem
S: The N-terminal domain of the IP3 receptor gates store-operated
hTrp3 channels. Mol Cell. 4:423–429. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xiao JH, Zheng YM, Liao B and Wang YX:
Functional role of canonical transient receptor potential 1 and
canonical transient receptor potential 3 in normal and asthmatic
airway smooth muscle cells. Am J Respir Cell Mol Biol. 43:17–25.
2010. View Article : Google Scholar :
|
|
64
|
Putney JW: The physiological function of
store-operated calcium entry. Neurochem Res. 36:1157–1165. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bradley E, Webb TI, Hollywood MA, Sergeant
GP, McHale NG and Thornbury KD: The cardiac sodium current Na(v)1.5
is functionally expressed in rabbit bronchial smooth muscle cells.
Am J Physiol Cell Physiol. 305:C427–C435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Snetkov VA, Hirst SJ and Ward JP: Ion
channels in freshly isolated and cultured human bronchial smooth
muscle cells. Exp Physiol. 81:791–804. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jo T, Nagata T, Iida H, Imuta H, Iwasawa
K, Ma J, Hara K, Omata M, Nagai R, Takizawa H, et al: Voltage-gated
sodium channel expressed in cultured human smooth muscle cells:
Involvement of SCN9A. FEBS Lett. 567:339–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nakajima T, Jo T, Meguro K, Oonuma H, Ma
J, Kubota N, Imuta H, Takano H, Iida H, Nagase T, et al: Effect of
dexamethasone on voltage-gated Na+ channel in cultured
human bronchial smooth muscle cells. Life Sci. 82:1210–1215. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Knox AJ, Ajao P, Britton JR and
Tattersfield AE: Effect of sodium-transport inhibitors on airway
smooth muscle contractility in vitro. Clin Sci (Lond). 79:315–323.
1990. View Article : Google Scholar
|
|
70
|
Knudsen T, Bertelsen H and Johansen T:
Ouabain enhancement of compound 48/80 induced histamine secretion
from rat peritoneal mast cells: Dependence on extracellular sodium.
Pharmacol Toxicol. 70:412–418. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
de Vasconcelos DI, Leite JA, Carneiro LT,
Piuvezam MR, de Lima MR, de Morais LC, Rumjanek VM and
Rodrigues-Mascarenhas S: Anti-inflammatory and antinociceptive
activity of ouabain in mice. Mediators Inflamm. 2011:9129252011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Choi JP, Kim YS, Kim OY, Kim YM, Jeon SG,
Roh TY, Park JS, Gho YS and Kim YK: TNF-alpha is a key mediator in
the development of Th2 cell response to inhaled allergens induced
by a viral PAMP double-stranded RNA. Allergy. 67:1138–1148. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hallstrand TS: New insights into
pathogenesis of exercise-induced bronchoconstriction. Curr Opin
Allergy Clin Immunol. 12:42–48. 2012. View Article : Google Scholar :
|
|
74
|
Anderson SD: Indirect challenge tests:
Airway hyperresponsiveness in asthma: its measurement and clinical
significance. Chest. 138(Suppl): 25S–30S. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hallstrand TS, Moody MW, Wurfel MM,
Schwartz LB, Henderson WR Jr and Aitken ML: Inflammatory basis of
exercise-induced bronchoconstriction. Am J Respir Crit Care Med.
172:679–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hallstrand TS, Lai Y, Ni Z, Oslund RC,
Henderson WR Jr, Gelb MH and Wenzel SE: Relationship between levels
of secreted phospholipase A2 groups IIA and X in the
airways and asthma severity. Clin Exp Allergy. 41:801–810. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Schmitt L, Wiebel M, Frese F, Dehnert C,
Zugck C, Bärtsch P and Mairbäurl H: Exercise reduces airway sodium
ion reabsorption in cystic fibrosis but not in exercise asthma. Eur
Respir J. 37:342–348. 2011. View Article : Google Scholar
|
|
78
|
Aneiros E, Philipp S, Lis A, Freichel M
and Cavalié A: Modulation of Ca2+ signaling by
Na+/Ca2+ exchangers in mast cells. J Immunol.
174:119–130. 2005. View Article : Google Scholar
|
|
79
|
Praetorius HA, Friis UG, Praetorius J and
Johansen T: Evidence for a Na+/Ca2+ exchange
mechanism in rat peritoneal mast cells. Pflugers Arch. 437:86–93.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Rundell KW and Jenkinson DM:
Exercise-induced bronchospasm in the elite athlete. Sports Med.
32:583–600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gotshall RW, Mickleborough TD and Cordain
L: Dietary salt restriction improves pulmonary function in
exercise-induced asthma. Med Sci Sports Exerc. 32:1815–1819. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
McKeever TM, Lewis SA, Smit HA, Burney P,
Cassano PA and Britton J: A multivariate analysis of serum nutrient
levels and lung function. Respir Res. 9:67–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mickleborough TD, Lindley MR and Ray S:
Dietary salt, airway inflammation, and diffusion capacity in
exercise-induced asthma. Med Sci Sports Exerc. 37:904–914.
2005.PubMed/NCBI
|
|
84
|
Pogson Z and McKeever T: Dietary sodium
manipulation and asthma. Cochrane Database Syst Rev.
3:CD0004362011.
|
|
85
|
Ardern KD and Ardern KD: Dietary salt
reduction or exclusion for allergic asthma. Cochrane Database Syst
Rev. 2:CD0004362004.
|