Introduction
Atherosclerosis is a chronic inflammatory disease
which is characterized by the formation of atherosclerotic plaques
in the arterial wall of large and medium-sized arteries (1). Various pathological events,
including proliferation and migration of vascular smooth muscle
cells (VSMCs) and endothelial cells (ECs), formation of foam cells,
deposition of extracellular lipid, persistent inflammation and
oxidative stress, contribute to the progression of atherosclerosis
(1–3). Atherosclerosis has been reported to
be the primary cause of myocardial infarction, heart failure,
myocardial ischemia as well as stroke and accounts for high
mortality and morbidity worldwide (4,5).
Although many therapeutic medicines for atherosclerosis have been
widely applied in the clinic, there are subgroups of patients who
remain at high risk of the aforementioned cardiovascular diseases.
Therefore, exploring new therapeutic targets or/and developing more
effective treatments for atherosclerosis is a top priority.
LOX-1, lectin-like oxidized low-density lipoprotein
(oxLDL) receptor (also named as OLR1) expressed in VSMCs, vascular
ECs and macrophages, plays a critical role in the pathogenesis of
atherosclerosis and has attracted considerable research attention
(6,7). Accumulating evidence indicates that
oxLDL-induced endothelial dysfunction, VSMC proliferation, and foam
cell formation are associated with LOX-1 overexpression (8,9).
In addition, it has been shown that LOX-1 is strongly upregulated
in atherosclerotic plaques from experimental animals and human
atherosclerosis (10,11). Moreover, deletion of LOX-1 was
found to lead to a marked reduction in atherosclerotic lesions in
LOX-1-knockout mice fed a high cholesterol diet (12). Accordingly, LOX-1 has been
suggested as a potential therapeutic target for treating
atherosclerosis (5).
MicroRNAs (miRNAs or miRs) are endogenous small
non-coding RNAs that can bind to the 3′ untranslated region (3′UTR)
of target mRNAs to negatively regulate their expression (13). Increasing evidence implicates
specific miRNAs as essential modulators for vascular functions and
diseases including atherosclerosis (14,15). Several studies support the notion
that miR-145-targeted therapy reduces atherosclerosis in
vitro and in vivo and have highlighted the potential
application of miRNA-based gene therapy in atherosclerosis
(16–18). Therefore, discovering new
miRNA-based therapeutic strategies to combat atherosclerosis is
warranted.
miR-let-7g, a key member of the let-7 family which
is highly conserved across animal species, has recently attracted
considerable attention due to its various biological functions.
Several studies indicate that let-7g participates in oxLDL-induced
proliferation and apoptosis of ECs and VSMCs (19–21). More recently, Liao et al
demonstrated that let-7g contributes to maintaining endothelial
function and vascular homeostasis by targeting transforming growth
factor-β (TGF-β) and SIRT-1 (22). All of these findings motivated us
to speculate that the targeting of miR-let-7g may be a potential
miRNA-based treatment for atherosclerosis. In the present study, to
test this hypothesis, the effects of miR-let-7g on atherosclerosis
and its underlying mechanisms were investigated in vitro and
in vivo. We demonstrated that the overexpression of
miR-let-7g significantly inhibited cell proliferation and migration
in LOX-1-overexpressed VSMCs by targeting LOX-1. Moreover,
intravenous delivery of miR-let-7g mimics obviously attenuated
atherosclerotic lesions and neointima formation as well as
downregulated the expression of LOX-1 in a hyperlipidemic
apolipoprotein E knockout (ApoE−/−) mouse model. Our
results suggest that miR-let-7g is a valuable therapeutic tool for
atherosclerosis, and provides novel insight into miRNA-based
therapy for this disease.
Materials and methods
Cell culture and transfection
Human aortic smooth muscle cells (ASMCs) were
purchased from CHI Scientific, Inc. (Jiangsu, China). ASMCs were
maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco,
Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS;
Hyclone, Logan, UT, USA) and 1% penicillin-streptomycin at 37°C in
a humidified atmosphere with 5% CO2.
The LOX-1 overexpression vector (pCMV3-LOX-1),
pcDNA3.1-let-7g vector, and pcDNA3.1-let-7g-mutant vector were
constructed by Wanleibio Co., Ltd. (Shenyang, China). For cell
transfection, ASMCs were seeded into cell culture plates or dishes
and cultured for 24 h. Following incubation with serum-free medium
for 1 h, the cells were transfected with the pCMV3-LOX-1 or
pcDNA3.1-let-7g vector, or co-transfected with both vectors using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer's instructions. The
pcDNA3.1-let-7g-mutant vector served as the negative control for
pcDNA3.1-let-7g, while pCMV3 and pcDNA3.1 vectors were used as
internal controls. After 5 h of transfection, the medium was
replaced with DMEM containing 10% FBS, and the cells were used for
the subsequent experiments.
Cell proliferation assay
Cell proliferation was detected using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, ASMCs were seeded in quintuplicate wells of 96-well
plates at 1×103 cells/well for 24 h, and then
transfected with plasmids as described above. At 5 h of
post-transfection, the cell medium was replaced with DMEM
containing 10% FBS and the cells continued to incubate for up to 0,
24, 48, 72 or 96 h. After that, 20 μl MTT solution was added
to each well and incubated for 4 h at 37°C. Subsequently, the
supernatant was discarded and 200 μl dimethyl sulfoxide
(DMSO) was added to dissolve the formazan crystals. Finally,
absorbance was determined at 490 nm with a microplate reader
(ELX-800; BioTek Instruments, Inc., Winooski, VT, USA).
Wound healing assay
ASMCs were seeded in triplicate wells of 6-well
plates for 24 h and then transfected as described above. When the
transfected cells reached 90% confluence, the cells were deprived
of medium and wounded with sterile 200-μl pipette tips.
After washing with serum-free medium to remove cellular debris,
photographic images of the wound area were captured under a
phase-contrast microscope (AE31; Motic, Xiamen, China).
Subsequently, the cells were incubated with serum-free medium for
another 24 h, and the images were captured again. The wound closure
was assessed by measuring the horizontal distance of the migrating
cells from the initial wound.
Transwell migration assay
After transfection with the indicated plasmids as
described above, the cells were detached by 0.25% trypsin-EDTA and
resuspended in serum-free DMEM at a density of 5×104
cells/ml. Then, 0.8 ml medium containing 20% FBS was added to each
bottom well, and 200 μl cell suspension was placed in the
upper chamber of the Transwell chamber (Corning Inc., Lowell, MA,
USA). Following incubation for 24 h at 37°C, the cells in the upper
chamber were removed by wiping the top surface of the membrane with
cotton swabs. Subsequently, the migrated cells that had attached to
the under surface were fixed with 4% paraformaldehyde for 20 min
and stained with 0.5% crystal violet solution for 5 min. After
washing with distilled water, the cells in 5 fields of view from
each membrane were counted under a light microscope.
Luciferase reporter assay
ASMCs were seeded in triplicate in 6-well plates at
2×105 cells/well and cultured for 24 h. Following
incubation with serum-free medium for 1 h, the cells were
transiently co-transfected with pmirGLO or pmirGLO-LOX-1-3′UTR
reporter plasmid and pcDNA3.1, pcDNA3.1-let-7g or
pcDNA3.1-let-7g-mutant vector using Lipofectamine 2000 according to
the manufacturer's instructions. At 24 h post-transfection, the
cells were harvested, and luciferase activity was detected using
the Dual Luciferase Reporter Assay kit (Promega, Madison, WI, USA)
according to the manufacturer's instructions. The data of
luciferase activity was normalized to the Renilla luciferase
activity.
Animal model and treatment
All animal care and experimental protocols were
approved by the Animal Care Ethics and Use Committee of Capital
Medical University and performed in accordance with the guidelines
of this committee. Eight-week-old male ApoE−/− mice were
purchased from Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China). After acclimatization for 1 week, the mice were
randomly divided into four groups: the control, the model, the
scramble miRNA and miR-let-7g mimic groups. The mice in the control
group were fed with a standard rodent diet for 12 weeks. To
accelerate the development of spontaneous atherosclerosis in
ApoE−/− mice, the animals in the other three groups
received a high-fat diet (0.15% cholesterol plus 21% fat) for 12
weeks. Starting from the 13th week, the mice in the miR-let-7g
mimics group were intravenously administered 20 mg/kg miR-let-7g
mimics for 3 weeks (twice a week). Meanwhile, each mouse in the
scramble microRNA group was given an equal volume of scramble
microRNA for 3 weeks. At the end of the treatment, the mice were
sacrificed by cervical dislocation under anesthesia, and complete
aortas were removed. Some samples were flash-frozen in liquid
nitrogen and kept at −80°C, whereas the others were fixed in
paraformaldehyde.
Hematoxylin and eosin (H&E) staining
assay
After 24 h of fixation in 4% paraformaldehyde, the
mouse aortas were dehydrated and then embedded in paraffin.
Subsequently, paraffin-embedded aortic tissues were cross-sectioned
into 5-μm sections, dewaxed and rehydrated. Serial sections
were stained with H&E solution following the manufacturer's
instructions. Images were captured using an optical microscope
(DP73; Olympus, Tokyo, Japan) to estimate vascular morphology and
neointimal formation.
Quantitative (real-time) polymerase chain
reaction (qPCR)
Total RNAs were extracted from ASMCs and mouse
aortas using the RNApure Rapid Extraction Total kit (BioTeke
Corporation, Beijing, China) according to the manufacturer's
suggested protocols. cDNAs were produced from equal samples of
total RNA by reverse transcription in a reaction system containing
random primers and M-MLV reverse transcriptase. Quantitative
(real-time) PCR analysis was performed in Exicycler 96 Real-Time
Quantitative Thermal Block (Bioneer, Daejeon, Korea). The reaction
was initiated by heating at 95°C for 10 min, and then 40 cycles of
95°C for 10 sec, 60°C for 20 sec and 72°C for 30 sec, followed by a
final incubation at 4°C for 5 min. The primers used are shown in
Table I. The relative expression
of mRNA was normalized to the internal control β-actin and
calculated using the 2−ΔΔCT threshold method.
 | Table ISequences of the primers used for
real-time PCR. |
Table I
Sequences of the primers used for
real-time PCR.
| Gene names | Primer sequences
(5′ to 3′) | Product size
(bp) |
|---|
| Homo LOX-1 | F:
GGTCCTTTGCCTGGGATTAG
R: TTCCGAGCAAGGGTTTCTATC | 207 |
| Homo β-actin | F:
CTTAGTTGCGTTACACCCTTTCTTG
R: CTGTCACCTTCACCGTTCCAGTTT | 156 |
| Mus LOX-1 | F:
TTCCCTGCTGCTATGACTCT
R: GTAAGGTTCGCTTGGTATTG | 119 |
| Mus β-actin | F:
CTGTGCCCATCTACGAGGGCTAT
R: TTTGATGTCACGCACGATTTCC | 155 |
Western blot analysis
Total protein from ASMCs and mouse aortas was
extracted using RIPA buffer supplemented with PMSF, and protein
concentrations were quantified using the BCA protein assay kit (all
from Beyotime Institute of Biotechnology, Jiangsu, China) according
to the manufacturer's instructions. Equal amounts of proteins were
separated by 11% SDS-PAGE and subsequently transferred to
polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica,
MA, USA). The membranes were then blocked with 5% non-fat milk in
Tris-buffered saline with Tween-20 (TBST) for 1 h, followed by
incubation with the primary antibodies against LOX-1 (1:500;
D160550; Sangon Biotech Co., Ltd., Shanghai, China) and β-actin
(1:1,000; sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) overnight at 4°C. After washing with TBST, the blots were
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibody (1:5,000; A0208 or A0216; Beyotime Institute of
Biotechnology) at 37°C for 45 min. Thereafter, the proteins of
interest were visualized using enhanced chemiluminescence (ECL;
7Sea Biotech Co., Ltd., Shanghai, China) and densitometric analysis
was performed using Gel-Pro Analyzer system (Liuyi Instrument
Factory, Beijing, China). The relative expression of protein was
calculated by normalization to the internal control β-actin.
Immunofluorescence assay
The paraffin-embedded aortic tissue sections were
dewaxed with xylene, hydrated with gradient ethanol, and microwaved
for antigen retrieval for 10 min. After washing with
phosphate-buffered saline (PBS), the sections were blocked with
goat serum (Solarbio, Beijing, China) at room temperature for 30
min, followed by incubation with the primary antibody against LOX-1
(1:100; D160550; Sangon Biotech Co., Ltd.) in a humidified chamber
at 4°C overnight. After rinsing with PBS, the slides were incubated
with Cy3-labeled goat anti-rabbit IgG (1:200; A0516; Beyotime
Institute of Biotechnology) at room temperature for 60 min. Then,
nuclear staining was carried out with DAPI (BioSharp, Hefei,
China). Thereafter, the slides were mounted with neutral glycerine
and observed under a fluorescence microscope (BX53; Olympus).
Statistical analysis
Data are presented as mean ± standard deviation (SD)
from at least three independent experiments. Statistical analyses
were performed using one-way analysis of variance (ANOVA) followed
by Newman-Keul's test. At P-values <0.05, the results were
considered statistically significant.
Results
LOX-1 overexpression induces ASMC
proliferation and migration
Previous studies have shown that ox-LDL induces SMC
proliferation via LOX-1 (20). In
order to examine whether LOX-1 overexpression stimulates SMC
proliferation, the pCMV3-LOX-1 vector was transfected into ASMCs,
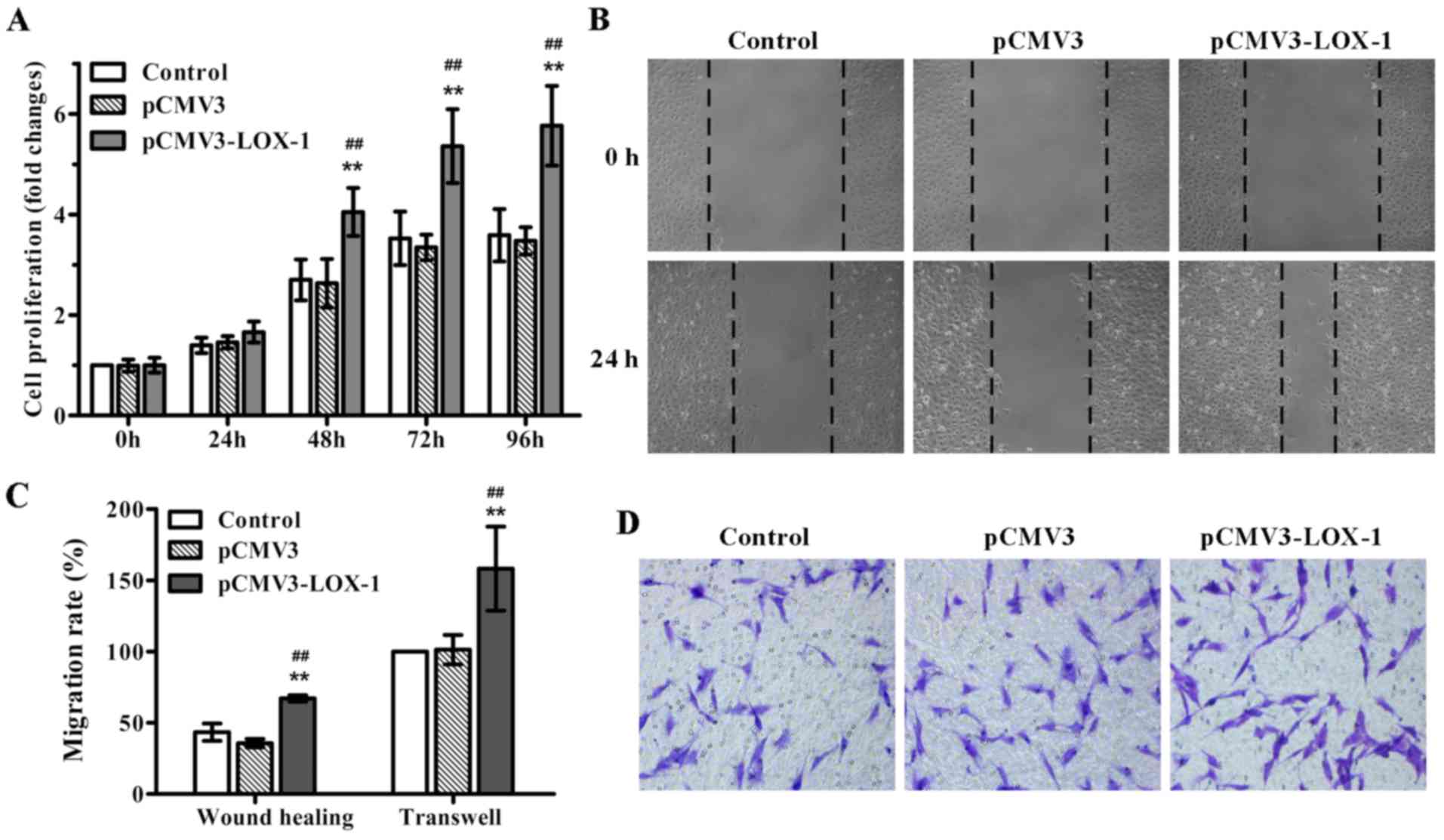
and MTT assay was performed. As expected, LOX-1 transfection
significantly induced ASMC proliferation in a time-dependent
manner, in particular, after 48 h of transfection (Fig. 1A). Additionally, we also evaluated
the impact of LOX-1 overexpression on ASMC migration, an important
event contributing to the development of atherosclerosis. The
results of the wound healing and Transwell migration assays showed
that LOX-1 overexpression markedly enhanced ASMC migration compared
with the non-transfected and pCMV3-transfected ASMCs (Fig. 1B–D). Together, these data
indicated that LOX-1 overexpression induced not only ASMC
proliferation, but also cell migration.
miR-let-7g attenuates LOX-1-induced ASMC
proliferation and migration
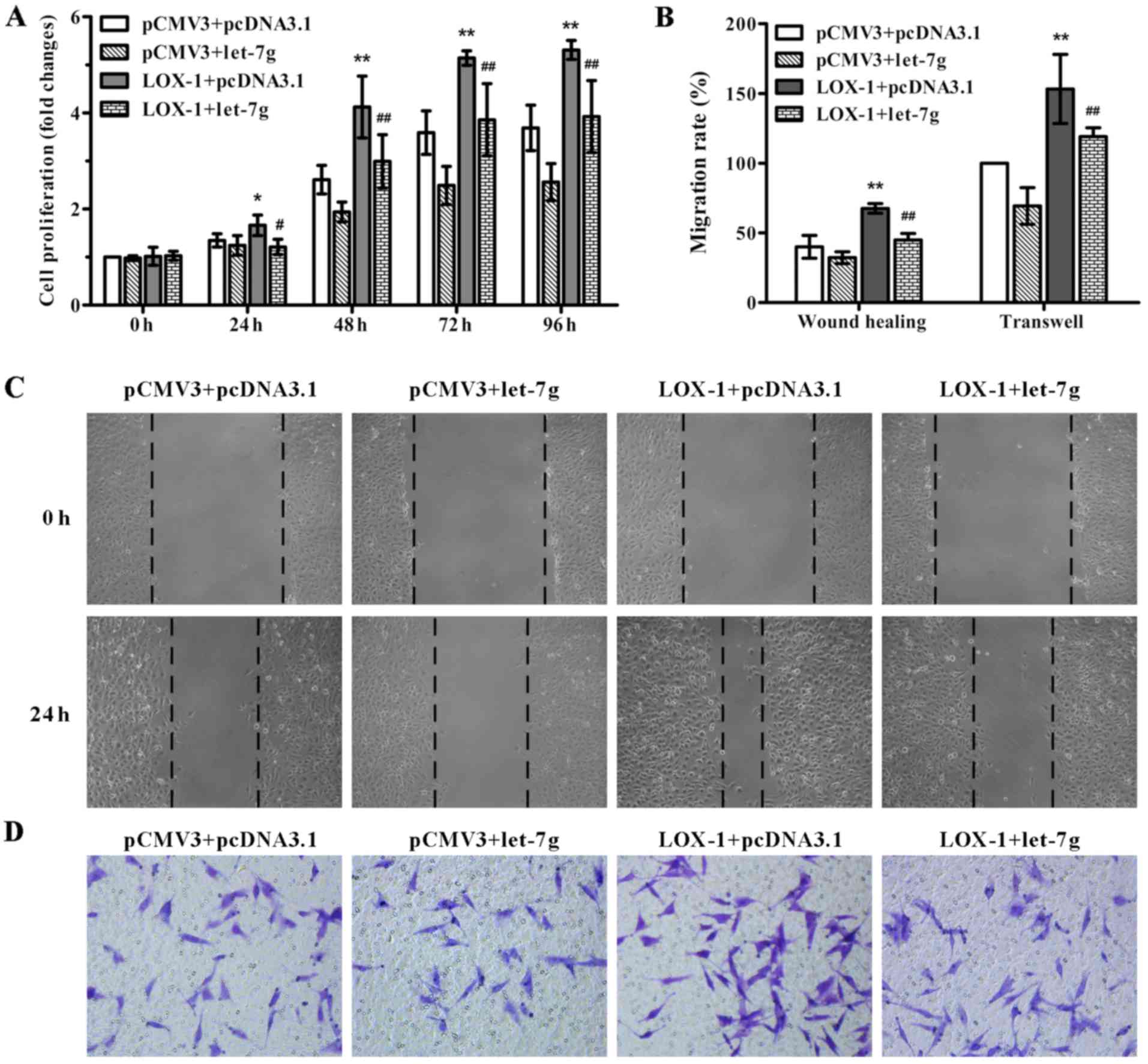
In order to test the effect of miR-let-7g on
LOX-1-induced ASMC proliferation and migration, pCMV3-LOX-1 plasmid
and pcDNA3.1-let-7g plasmid were co-transfected into the ASMCs. As
shown in Fig. 2A, miR-let-7g
overexpression markedly suppressed the LOX-1-stimulated
proliferation of ASMCs compared to the cells co-transfected with
the pCMV3-LOX-1 and scramble pcDNA3.1 vectors as determined by MTT
assay. To confirm the inhibitory effect of miR-let-7g on ASMC
migration, wound healing and Transwell migration assays were
performed. Compared with the ASMCs co-transfected with pCMV3-LOX-1
and scramble pcDNA3.1 plasmids, the number of migratory cells
harboring the pCMV3-LOX-1 plasmid and pcDNA3.1-let-7g plasmid were
obviously decreased (Fig. 2B–D).
These results indicated that miR-let-7g reversed the ASMC
proliferation and migration mediated by LOX-1 overexpression.
miR-let-7g inhibits LOX-1 expression in
ASMCs by binding to its 3′UTR
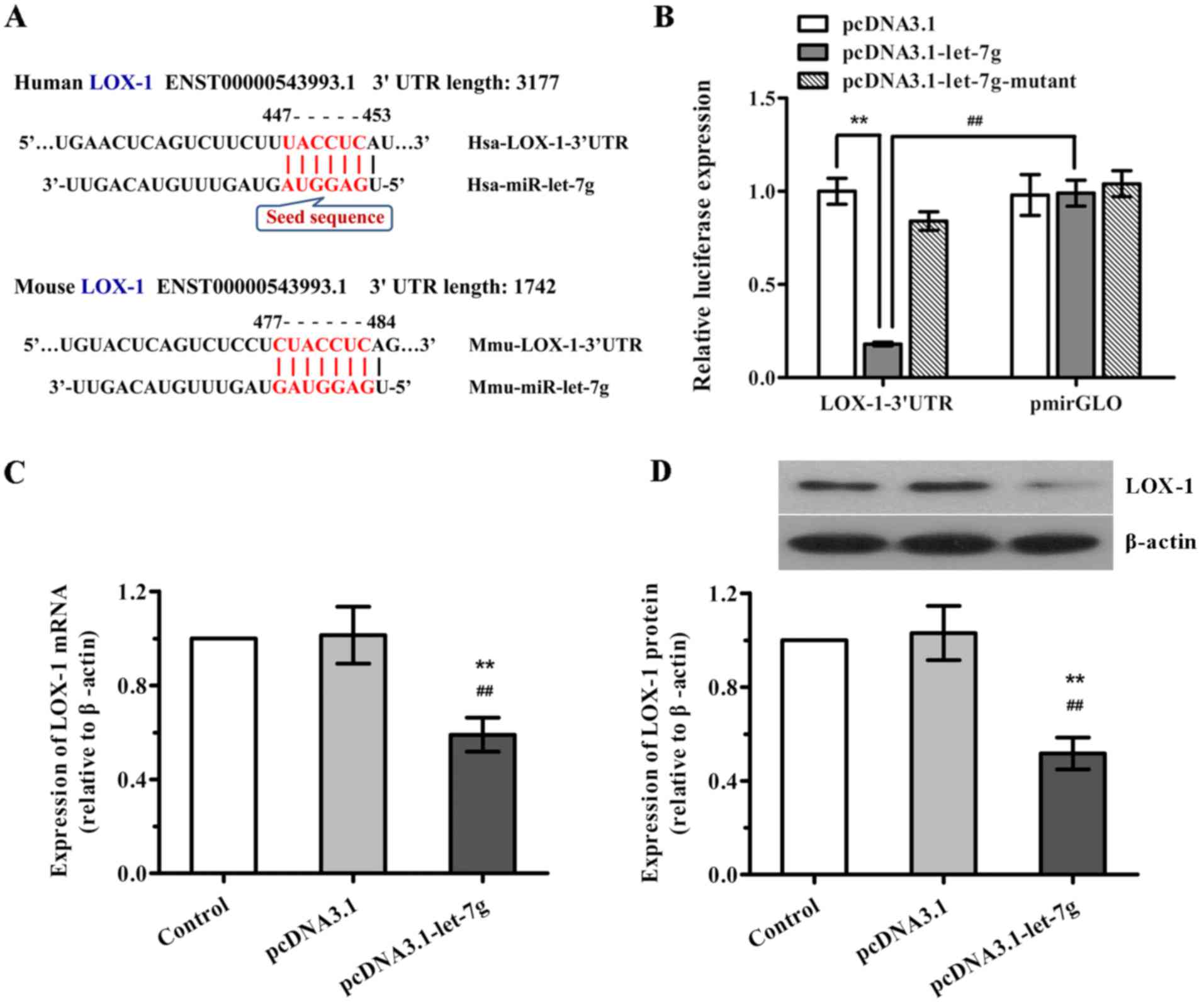
Given that miR-let-7g is predicted to bind to the
3′UTR of LOX mRNA in the human and mouse according to TargetScan
program and miRNA target prediction database (Fig. 3A), we determined whether
miR-let-7g directly binds to the 3′UTR sequence of LOX mRNA and
affects its expression in human ASMCs. Firstly, a luciferase
reporter vector was constructed with the 3′UTR sequence of LOX-1
containing the putative binding site for miR-let-7g, and luciferase
report assay was performed. As shown in Fig. 3B, when LOX-1 3′UTR and
pcDNA3.1-let-7g were co-tranfected into the ASMCs, the luciferase
activity was significantly decreased compared with the scramble
pcDNA3.1. Nevertheless, co-transfection of LOX-1 3′UTR and
miR-let-7g-mutant as well as co-transfection of scramble pmirGLO
and pcDNA3.1-let-7g both had no effects on the luciferase
activities. Subsequently, to further confirm the functional
interaction of miR-let-7g with LOX-1, the effects of miR-let-7g on
the expression levels of LOX-1 mRNA and protein in human ASMCs were
determined by qPCR and western blot analysis, respectively. As
expected, overexpression of miR-let-7g led to a marked reduction in
both LOX-1 mRNA and protein expression (Fig. 3C and D), which was consistent with
the results of the luciferase reporter activity. Taken together,
our findings suggest that miR-let-7g directly targets the 3′UTR of
LOX-1 to suppress LOX-1 expression.
miR-let-7g alleviates atherosclerotic
lesions in ApoE−/− mice
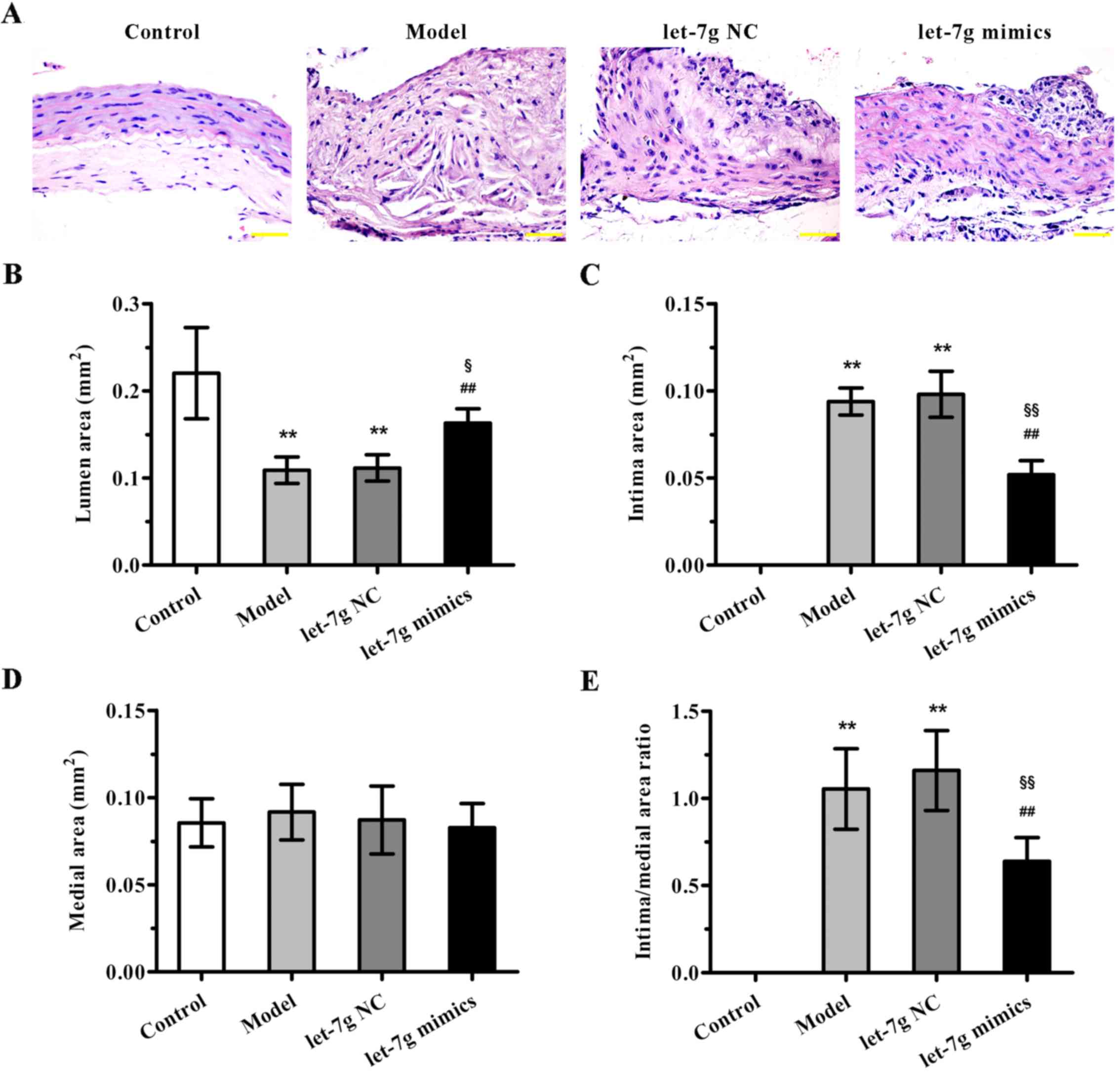
To further evaluate the potential therapeutic
benefit of miR-let-7g in atherosclerosis, high-fat diet fed
ApoE−/− mice were treated with miR-let-7g mimics. As
indicated in Fig. 4A, compared to
the mice fed a normal diet, ApoE−/− mice fed a high-fat
diet for 12 weeks showed significant neointimal hyperplasia.
Importantly, miR-let-7g mimics obviously reduced the neointimal
hyperplasia compared with that noted in the scramble-treated
aortas. Moreover, the quantification results of morphometric
analyses revealed that the parameters for severity of
atherosclerosis, such as lumen area, intima area (Fig. 4B and C), and intima to media area
ratio (Fig. 4E), were markedly
improved by miR-let-7g mimic delivery. However, there was no
significant difference in medial area (Fig. 4D). These data suggest that
miR-let-7g exterts potent anti-atherosclerotic effects in
vivo.
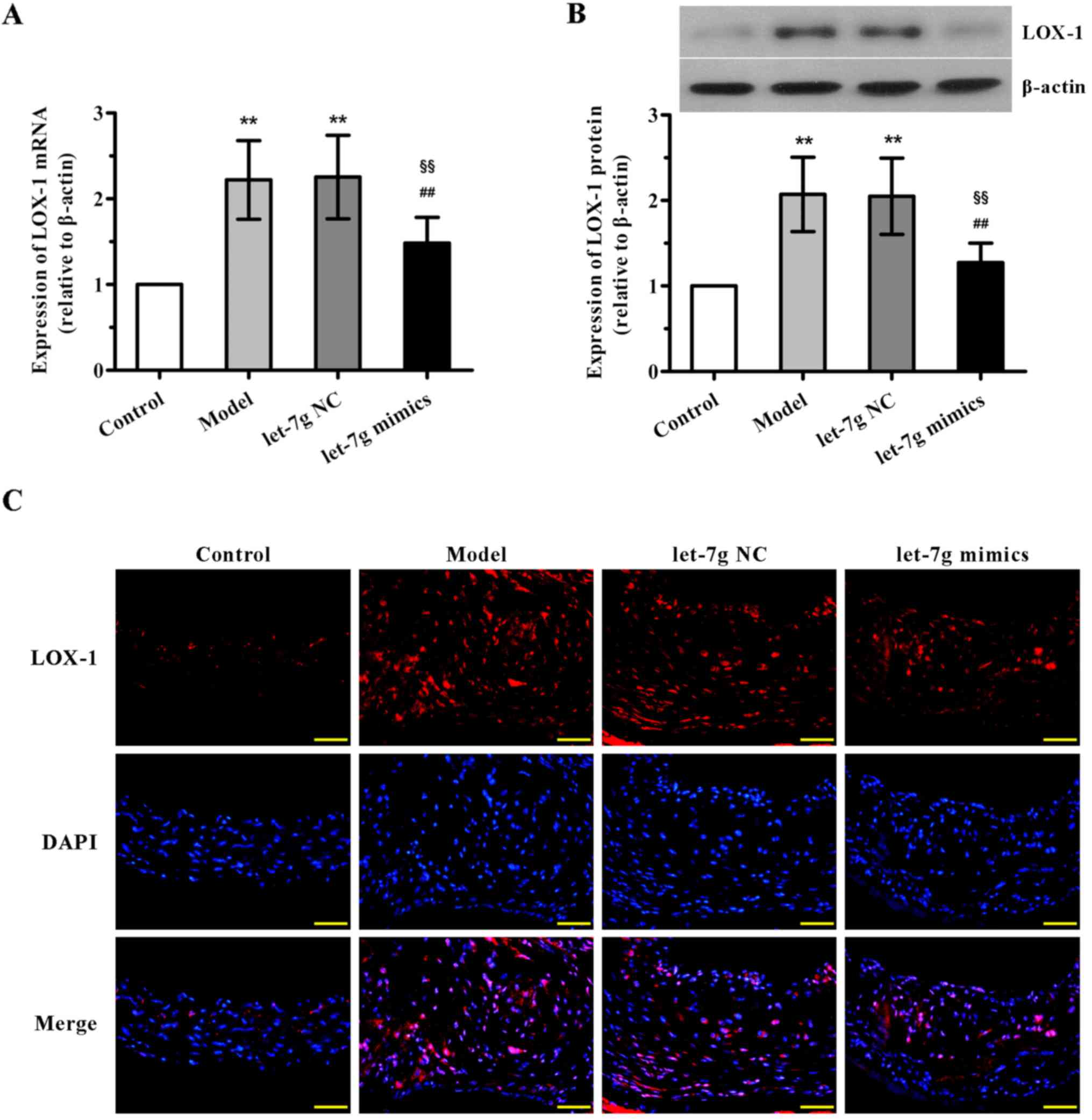
miR-let-7g downregulates the expression
of LOX-1 in the aortas of ApoE−/− mice
Further experiments were undertaken to demonstrate
the underlying mechanisms of interaction between miR-let-7g and
LOX-1 in vivo. The results of qPCR and western blot analysis
showed that the mRNA and protein expression levels of LOX-1 in the
mouse aortas were both notably upregulated in the high-fat fed and
NC-treated groups compared with the control animals, with no
significant difference observed between the model and NC-treated
groups (Fig. 5A and B). By
contrast, a marked reduction in LOX-1 expression in the aortas was
found after the administration of miR-let-7g mimics. Additionally,
concordant with these results, immunofluorescence analysis also
confirmed that treatment with miR-let-7g mimics obviously reversed
the upregulation of LOX-1 expression in the aortas of the high-fat
diet fed ApoE−/− mice. These in vivo observations
were consistent with the effects of miR-let-7g on ASMCs, suggesting
that miR-let-7g may alleviate atherosclerosis via inhibition of
LOX-1 expression.
Discussion
Currently, the incidence of atherosclerosis is
rapidly increasing in developing and developed countries (23). Increasing interest in the
treatment of atherosclerosis is currently being focused on miRNAs
due to their essential regulatory roles in the development of
atherosclerosis (24). In the
present study, we investigated the anti-atherosclerotic effect of
miR-let-7g both in vitro and in vivo. The data
presented here showed that exogenous LOX-1 overexpression had a
promoting effects on ASMC proliferation and migration, whereas
co-transfection with miR-let-7g into ASMCs reversed these effects.
The underlying mechanisms of miR-let-7g likely involve repression
of LOX-1 by directly targeting its 3′UTR. Furthermore, the in
vivo studies showed that systematic delivery of miR-let-7g
attenuated neointima formation in high-fat diet fed
ApoE−/− mice, a well-established animal model for
studying atherosclerosis. The anti-atherosclerotic effects of
miR-let-7g in mice were accompanied by a significant downregulation
of LOX-1, consistent with its effects on ASMCs.
During the atherosclerotic process, VSMCs undergo a
variety of pathological changes. The balance between
differentiation and proliferation of VSMCs plays a critical role in
maintaining healthy blood vessels. However, some stimulation such
as mitogenic substances, growth factors, or loss of vascular ECs
can induce VSMC migration from the media into the intima.
Subsequently, VSMC proliferation can occur in the intima, leading
to neointimal hyperplasia as well as plaque formation (25). Hence, the migration and
proliferation of VSMC is the most common pathological alteration in
the development of atherosclerosis. Ox-LDL plays a key role in the
genesis and progression of atherosclerosis by upregulating its own
receptor, LOX-1, which appears to be the dominant receptor on VSMCs
and ECs (26). Furthermore, the
activation of LOX-1 has also been reported to be involved in many
pathological events of atherosclerosis, such as vascular cell
apoptosis, proliferation as well as vascular remodeling (27,28). In the present study, we
constructed LOX-1-overexpressing ASMCs by transfecting a plasmid
containing the full length cDNA of LOX-1 into human ASMCs, and
confirmed that exogenous LOX-1 overexpression could significantly
induce cell migration and proliferation, suggesting an in
vitro model for the development of atherosclerosis.
The miR-let-7 family, consisting of nine members,
was identified in humans and has been shown to play a pivotal role
in developmental processes (29).
More recently, the functions of let-7 in cardiovascular biology and
disease have drawn increasing attention (30). It has been found that levels of
let-7 are closely associated with coronary artery disease including
atherosclerosis. Among let-7 members, miR-let-7g has been
demonstrated to improve multiple endothelial functions including an
increase in angiogenesis as well as a decrease in monocyte
adhesion, inflammation and senescence by targeting TGF-β and SIRT-1
signaling pathway (22).
Moreover, it has been reported that ox-LDL at lower concentrations
causes VSMC proliferation (<20 μg/ml) while it inducs
cell apoptosis at higher concentrations (>60 μg/ml),
which could be suppressed by miR-let-7g mimic and exacerbated by
its inhibitor (20). In addition,
a previous study also revealed that miR-let-7g could function as an
important modulator of autophagy and apoptosis in ox-LDL-treated
VSMCs (27). In this study, we
demonstrated that when miR-let-7g was co-transfected with LOX-1
into ASMCs, cell migration and proliferation stimulated by LOX-1
overexpression alone were obviously inhibited, which was
accompanied by a decrease in the mRNA and protein levels of LOX-1.
The reporter assay further confirmed the direct binding of
miR-let-7g to the 3′UTR of LOX-1. These observations suggest that
miR-let-7g can interfere with ASMC proliferation and migration by
targeting LOX-1.
Next, we studied the anti-atherosclerotic effect of
miR-let-7g in ApoE−/− mice fed a high-fat diet. We
demonstrated that the LOX-1 expression in aortas of the high-fat
diet fed ApoE−/− mice was markedly increased, consistent
with previous studies supporting the notion that LOX-1 accumulates
in atherosclerotic lesions of experimental animals and humans
(5,31). It has been reported that deletion
of LOX-1 attenuates atherosclerosis in LOX-1-knockout mice fed a
high cholesterol diet (12),
therefore highlighting the key role of LOX-1 in atherosclerosis. Of
note, here, our data showed that administration of miR-let-7g
mimics markedly downregulated the mRNA and protein levels of LOX-1
as well as ameliorated neointima formation and atherosclerotic
lesions, in line with the in vitro findings.
In conclusion, our results demonstrate that
miR-let-7g can suppress ASMC proliferation and migration in
vitro and alleviate atherosclerosis in ApoE−/− mice,
at least partly by directly inhibiting LOX-1 activation. These
observations suggest that miR-let-7g may serve as a potential
therapeutic strategy for treating atherosclerosis.
Acknowledgments
This study was supported by a grant from the
National Undergraduate Innovative Training Program (no.
201610160005).
References
|
1
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stocker R and Keaney JF Jr: Role of
oxidative modifications in atherosclerosis. Physiol Rev.
84:1381–1478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Obikane H, Abiko Y, Ueno H, Kusumi Y,
Esumi M and Mitsumata M: Effect of endothelial cell proliferation
on atherogenesis: a role of p21(Sdi/Cip/Waf1) in monocyte adhesion
to endothelial cells. Atherosclerosis. 212:116–122. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng Y, Gardner SE and Clarke MC: Cell
death, damage-associated molecular patterns, and sterile
inflammation in cardiovascular disease. Arterioscler Thromb Vasc
Biol. 31:2781–2786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Xu S, Huang X, Wang J, Gao S, Li H,
Zhou C, Ye J, Chen S, Jin ZG, et al: Cryptotanshinone, an orally
bioactive herbal compound from Danshen, attenuates atherosclerosis
in apolipoprotein E-deficient mice: role of lectin-like oxidized
LDL receptor-1 (LOX-1). Br J Pharmacol. 172:5661–5675. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White SJ, Sala-Newby GB and Newby AC:
Overexpression of scavenger receptor LOX-1 in endothelial cells
promotes atherogenesis in the ApoE(−/−) mouse model. Cardiovasc
Pathol. 20:369–373. 2011. View Article : Google Scholar :
|
|
7
|
Ulrich-Merzenich G and Zeitler H: The
lectin-like oxidized low-density lipoprotein receptor-1 as
therapeutic target for atherosclerosis, inflammatory conditions and
longevity. Expert Opin Ther Targets. 17:905–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li D, Chen H, Romeo F, Sawamura T, Saldeen
T and Mehta JL: Statins modulate oxidized low-density
lipoprotein-mediated adhesion molecule expression in human coronary
artery endothelial cells: role of LOX-1. J Pharmacol Exp Ther.
302:601–605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitra S, Goyal T and Mehta JL: Oxidized
LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 25:419–429.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Draude G, Hrboticky N and Lorenz RL: The
expression of the lectin-like oxidized low-density lipoprotein
receptor (LOX-1) on human vascular smooth muscle cells and
monocytes and its down-regulation by lovastatin. Biochem Pharmacol.
57:383–386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Li D, Sawamura T, Inoue K and
Mehta JL: Upregulation of LOX-1 expression in aorta of
hypercholesterolemic rabbits: modulation by losartan. Biochem
Biophys Res Commun. 276:1100–1104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mehta JL, Sanada N, Hu CP, Chen J,
Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, et
al: Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice
fed high cholesterol diet. Circ Res. 100:1634–1642. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McDonald RA, Hata A, MacLean MR, Morrell
NW and Baker AH: MicroRNA and vascular remodelling in acute
vascular injury and pulmonary vascular remodelling. Cardiovasc Res.
93:594–604. 2012. View Article : Google Scholar :
|
|
15
|
Madrigal-Matute J, Rotllan N, Aranda JF
and Fernández-Hernando C: MicroRNAs and atherosclerosis. Curr
Atheroscler Rep. 15:3222013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu
Q, Deitch EA, Huo Y, Delphin ES and Zhang C: MicroRNA-145, a novel
smooth muscle cell phenotypic marker and modulator, controls
vascular neointimal lesion formation. Circ Res. 105:158–166. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lovren F, Pan Y, Quan A, Singh KK, Shukla
PC, Gupta N, Steer BM, Ingram AJ, Gupta M, Al-Omran M, et al:
MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation.
126:S81–S90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elia L, Quintavalle M, Zhang J, Contu R,
Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J,
et al: The knockout of miR-143 and -145 alters smooth muscle cell
maintenance and vascular homeostasis in mice: correlates with human
disease. Cell Death Differ. 16:1590–1598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Chen N, Zhang J and Tong Y:
Hsa-let-7g miRNA targets caspase-3 and inhibits the apoptosis
induced by ox-LDL in endothelial cells. Int J Mol Sci.
14:22708–22720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding Z, Wang X, Khaidakov M, Liu S and
Mehta JL: MicroRNA hsa-let-7g targets lectin-like oxidized
low-density lipoprotein receptor-1 expression and inhibits
apoptosis in human smooth muscle cells. Exp Biol Med (Maywood).
237:1093–1100. 2012. View Article : Google Scholar
|
|
21
|
Chen KC, Hsieh IC, Hsi E, Wang YS, Dai CY,
Chou WW and Juo SH: Negative feedback regulation between microRNA
let-7g and the oxLDL receptor LOX-1. J Cell Sci. 124:4115–4124.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao YC, Wang YS, Guo YC, Lin WL, Chang MH
and Juo SH: Let-7g improves multiple endothelial functions through
targeting transforming growth factor-beta and SIRT-1 signaling. J
Am Coll Cardiol. 63:1685–1694. 2014. View Article : Google Scholar
|
|
23
|
Bild DE, McClelland R, Kaufman JD,
Blumenthal R, Burke GL, Carr JJ, Post WS, Register TC, Shea S and
Szklo M: Ten-year trends in coronary calcification in individuals
without clinical cardiovascular disease in the multi-ethnic study
of atherosclerosis. PLoS One. 9:e949162014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Menghini R, Stöhr R and Federici M:
MicroRNAs in vascular aging and atherosclerosis. Ageing Res Rev.
17:68–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu J and Mehta JL: LOX-1: a critical
player in the genesis and progression of myocardial ischemia.
Cardiovasc Drugs Ther. 25:431–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding Z, Wang X, Schnackenberg L, Khaidakov
M, Liu S, Singla S, Dai Y and Mehta JL: Regulation of autophagy and
apoptosis in response to ox-LDL in vascular smooth muscle cells,
and the modulatory effects of the microRNA hsa-let-7g. Int J
Cardiol. 168:1378–1385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding Z, Liu S, Yang B, Fan Y and Deng X:
Effect of oxidized low-density lipoprotein concentration
polarization on human smooth muscle cells' proliferation, cycle,
apoptosis and oxidized low-density lipoprotein uptake. J R Soc
Interface. 9:1233–1240. 2012. View Article : Google Scholar :
|
|
29
|
Boyerinas B, Park SM, Hau A, Murmann AE
and Peter ME: The role of let-7 in cell differentiation and cancer.
Endocr Relat Cancer. 17:F19–F36. 2010. View Article : Google Scholar
|
|
30
|
Bao MH, Feng X, Zhang YW, Lou XY, Cheng Y
and Zhou HH: Let-7 in cardiovascular diseases, heart development
and cardiovascular differentiation from stem cells. Int J Mol Sci.
14:23086–23102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sankaralingam S, Xu Y, Sawamura T and
Davidge ST: Increased lectin-like oxidized low-density lipoprotein
receptor-1 expression in the maternal vasculature of women with
preeclampsia: role for peroxynitrite. Hypertension. 53:270–277.
2009. View Article : Google Scholar
|