Introduction
Gastric cancer (GC) is a multifactorial disease with
a specific pathogenicity mechanism that remains unclear, but which
may be related to diet, lifestyle, or Helicobacter pylori
infection. Mutations in susceptibility genes and epigenetic changes
also play important roles in GC (1–3).
Tumour invasion and metastasis are important factors affecting
prognosis, which is the most difficult aspect of treating malignant
tumours.
Cyclooxygenase (COX) has been considered a potential
target for the treatment and prevention of tumours (4). COX-2 promotes the malignancy of
cells by upregulating the production of prostaglandins, primarily
prostaglandin E2 (PGE2) (5).
COX-2 is associated with the tumourigenesis, histological subtype,
tumour size and developmental stage of GC (6–10)
through multiple pathways, such as angiogenesis, tumour growth,
invasion and immune evasion (11–14).
The Notch family consists of four important
signalling receptors, and the binding of the corresponding ligand
releases the intracellular domain, which enters the nucleus and
regulates gene expression by binding to downstream target gene loci
(15). The Notch family is
involved in the initiation and development of various tumours
(16–20). The binding of Notch1 to the
Jagged1 ligand can activate the STAT3/Twist signalling pathway and
regulate the growth of GC cells (21). Notch2 promotes the growth and
metastasis of bladder tumours through cellular processes, such as
epithelial-to-mesenchymal transition (EMT), the cell cycle and
pluripotency (22). A high
expression of Notch3 in non-small cell lung cancer is related to
drug resistance and poor prognosis in patients, as well as the
expression of the cancer stem cell markers, CD44 and aldehyde
dehydrogenase 1 family, member A1 (ALDH1A1); thus, the inhibition
of Notch3 expression can reduce the clonogenic ability and
ʻstem-likeʼ property of lung cancer cells (23). The Snail family is a superfamily
of zinc-finger transcription factors that mainly binds to promoters
of various effector proteins to regulate transcription and protein
expression (24).
In this study, the expression of Notch family
members and COX-2 in 51 pairs of GC and paracancerous tissues was
examined to determine their correlation with the
clinicopathological features of GC. The changes in Notch and COX-2
expression in GC cell lines treated with celecoxib, PGE2 and small
interfering RNA (siRNA) clarified that the Notch family and COX-2
are involved in GC tumourigenesis, providing a novel and useful
target for the treatment of GC.
Materials and methods
Tissue samples
GC cells and paracancerous tissues (>5 cm from
the tumour foci and with the absence of cancerous cells confirmed
by hematoxylin and eosin staining) were collected for experimental
research from 51 patients with GC who received surgical treatment
at Wuwei Cancer Hospital of Gansu province (China) from October
2009 to April 2010. This study was approved by the Ethical Board of
Wuwei Cancer Hospital and the Ethical Board of the First Hospital
of Lanzhou University. No patient had received radiotherapy or
chemotherapy prior to surgery. A total of 51 pairs of cancerous and
paracancerous tissues were sampled intraoperatively, placed
immediately in liquid nitrogen, and then transferred to a −80°C
freezer for long-term storage. All patients provided informed
consent. All cases were diagnosed by two experienced
pathologists.
Antibodies and reagents
Horseradish peroxidase-conjugated goat anti-rabbit
IgG (ZB-2301) was purchased from Beijing Zhongshan Golden Bridge
Biological Technology Co., Ltd. (Beijing, China). Antibodies
against the Notch1 intracellular domain (N1IC; ab83232), Snail
(ab82846), the Notch2 intracellular domain (N2IC; ab8927) and COX-2
(ab15191) were purchased from Abcam (Cambridge, UK). The anti-GAPDH
antibody (AB-P-R 001) was purchased from Hangzhou Goodhere
Biotechnology Co., Ltd. (Hangzhou, China). Pure celecoxib was
provided by Professor Joe Leung from the the University of Sydney.
PGE2 was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA),
and the siRNA was designed and synthesised by Shanghai Invitrogen
Biotechnology Co., Ltd. (Shanghai, China).
RNA extraction and fluorescence-based
quantitative PCR (qPCR)
RNA was extracted using RNAiso Plus (Takara Bio,
Inc., Kusatsu, Japan) according to the manufacturer's instructions.
Total RNA was reverse-transcribed using a PrimeScript®
RT Master Mix (Perfect Real-Time) kit (Takara Bio, Inc.) according
to the manufacturer's instructions. PCR was performed on a
LightCycler® 480 System (Roche, Basel, Switzerland). The
PCR results were analysed u7sing the 2−ΔΔCT method, and
β-actin was selected as the reference gene.
Protein extraction and western blot
analysis
Total protein was extracted using RIPA lysis buffer
(Beyotime Institute of Biotechnology, Jiangsu, China). The total
protein (30–40 µg) was then subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes. After blocking in 5% skim milk
at room temperature for 2 h, the membranes were incubated overnight
with specific antibodies at 4°C, followed by 1–2 h of incubation
with a goat anti-rabbit secondary antibody (ZB-2301; Beijing
Zhongshan Golden Bridge Biological Technology Co., Ltd.) at room
temperature. Finally, the Super ECL Plus Detection Reagent
(Applygen Technologies, Inc., Beijing, China) was used to detect
specific protein bands.
Cell culture
The GES-1 (immortalized human gastric epithelial
mucosa cells) and AGS (GC cells) cell lines were purchased from the
Beijing Institute of Tumour Cells; the SGC-7901, BGC-823, and
MGC-803 GC cell lines were purchased from the Cell Bank of
Committee on Type Culture Collection of Chinese Academy of
Sciences. All cell lines were routinely cultured in DMEM
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 U/ml streptomycin in an incubator maintained at 37°C and 5%
CO2.
Transfection
The siRNA used in this study was designed and
synthesised by Shanghai Invitrogen Biotechnology Co., Ltd.
si-Notch1 (sense, 5′-CAGGGAGCAUGUGUAACAUTT-3′ and antisense,
5′-AUGUUACACAUGCUCCCUGTT-3′) was used to target Notch1; si-COX-2
(sense, 5′-GCAGCUUCCUGAUUCAAAUTT-3′ and antisense,
5′-AUUUGAAUCAGGAAGCUGCTT-3′) was used to target COX-2; scramble
siRNA (sense, 5′-UUCUCCGAACGUGUCACGUdTdT-3′ and anti-sense,
5′-ACGMGACACGUUCGGAGAAdTdT-3′) was used as a negative control. The
mock group was untransfected cells. The concentration of all siRNAs
was 33 nM. All siRNAs were chemically synthesized. Cells were
inoculated and grown for 24 h prior to transfection, and the siRNA
was transfected into the cells using Lipofectamine™ 2000
(Invitrogen Co., Ltd., Carlsbad, CA, USA) according to the
manufacturer's instructions.
Drug experiments
The gastric cancer cell lines were treated with
celecoxib and PGE2. The concentration gradient of celecoxib
treatment was 0, 25, 50, 75, 100 µmol/l and the intervention
time of celecoxib was 0, 24, 48, 72 h. The concentration gradient
of the PGE2 treatment was 0, 1, 5, 10 µmol/l, the
intervention time of PGE2 was 0, 6, 12, 24 h.
In vitro cell proliferation assay
For growth curve experiments, the cells were plated
in 96-well plates at a density of 2×103 cells/well.
Viable cells were assayed at 1, 2, 3, 4, 5 and 6 days. Cell
proliferation assays were performed using a Cell Counting kit-8
(Beijing Zoman Biotechnology Co., Ltd., Beijing, China). Each
experiment was performed in triplicate and repeated 3 times.
Statistical analysis
SPSS 16.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for data processing, and the measurement data are
represented as the means ± standard deviation. The mean values of
paired samples were compared using a paired-samples t-test.
Comparisons between 2 groups were analysed by an independent
two-sample t-test, and comparisons among multiple groups were
analysed by a one-way analysis of variance. The statistical
significance threshold was 0.05.
Results
Expression of Notch1, Notch2, Notch3,
Jagged1 and COX-2 in GC, and their association with the
clinicopathological characteristics of GC
qPCR and western blot analysis were performed to
determine the expression levels of the aforementioned genes in 51
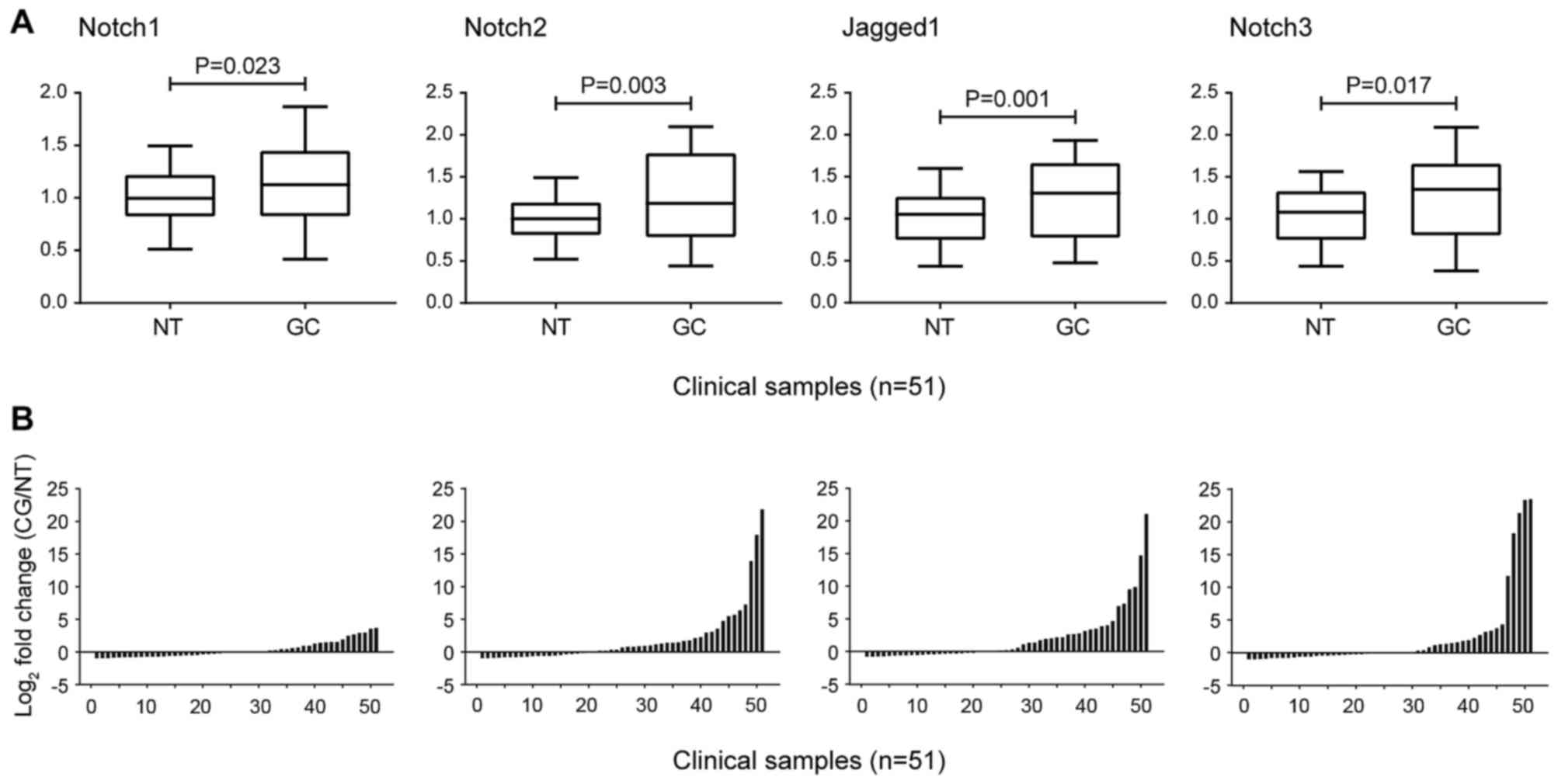
pairs of GC and paracancerous tissues. The Notch1 mRNA expression
level in GC was 1.54±0.22-fold higher (P=0.023) than that in
paracancerous tissues (Fig. 1);
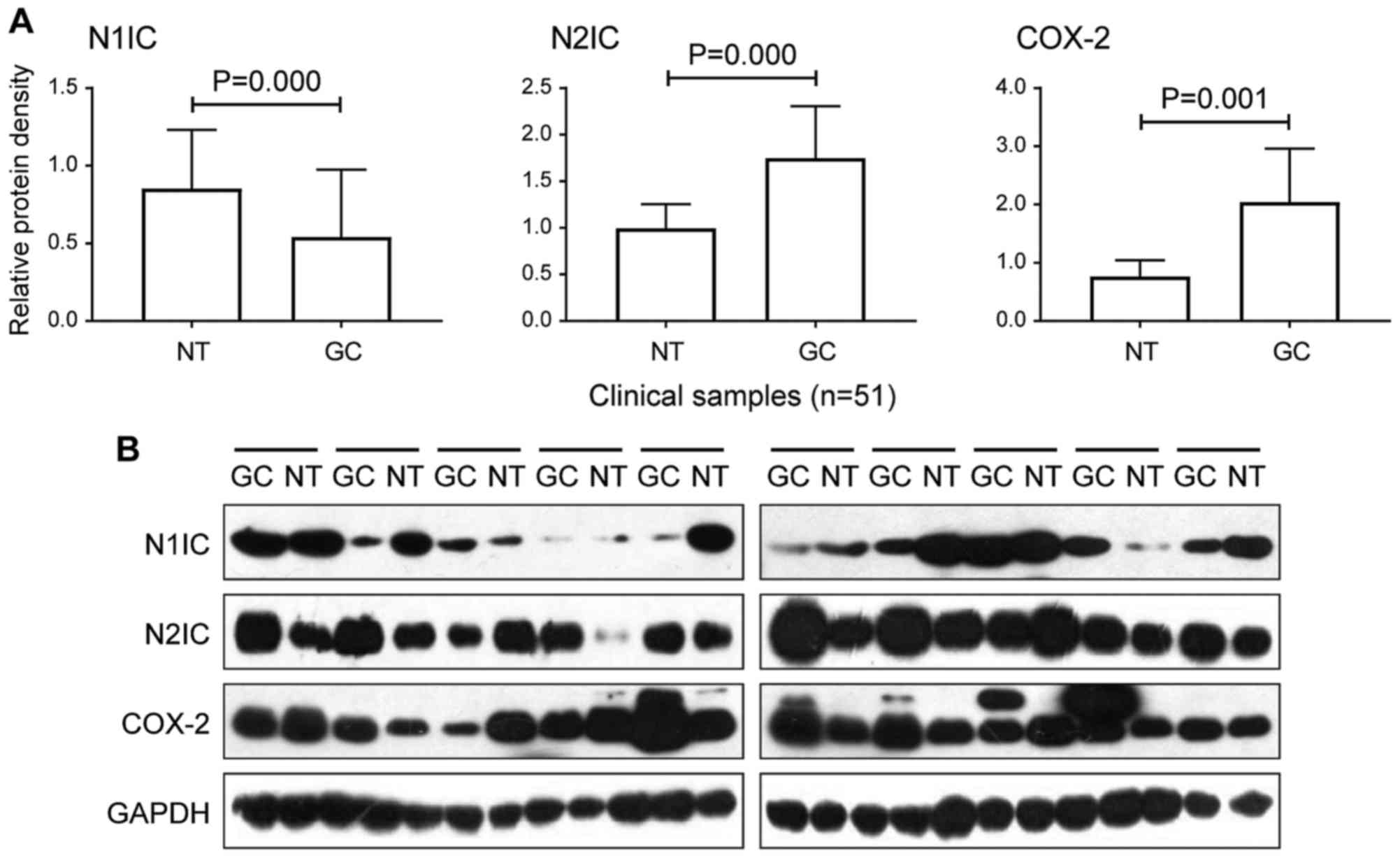
however, the expression level of N1IC in GC (0.29±0.30) was
significantly decreased compared to the corresponding paracancerous
tissues (0.81±1.10; P=0.000; Fig.
2A). The expression of Notch2 mRNA and N2IC in GC was
significantly increased (P=0.003 and P=0.000, respectively;
Figs. 1 and 2A). Similarly, Notch3 mRNA, Jagged1 mRNA
and COX-2 protein expression were at high levels in GC (P=0.017,
P=0.001, and P=0.001, respectively; Figs. 1 and 2A). Clinicopathological analysis
revealed that the expression levels of Notch1 mRNA and N1IC
positively correlated with the depth of invasion and TNM stage
(P=0.003, 0.002; and P=0.027, 0.014, respectively; Tables I and II). The expression of COX-2 in the
undifferentiated and poorly differentiated GC groups was
significantly higher than that in the moderately and highly
differentiated GC groups (P=0.012), and the expression levels
increased with the depth of invasion (P=0.026; Table III). The results of Notch3 mRNA
expression are shown in Table
IV. The mRNA expression of Notch3 was increased in GC and was
significantly increased in the ≤50-year-old GC group (Table IV);
 | Table ICorrelation of Notch1 mRNA expression
with various clinicopathological features of GC. |
Table I
Correlation of Notch1 mRNA expression
with various clinicopathological features of GC.
| Variable | No. of cases | Notch1 mRNA
expression in GC | P-value |
|---|
| Sex |
| Male | 34 | 1.3800±1.26 | 0.641 |
| Female | 17 | 1.2048±1.24 | |
| Age (years) |
| ≤50 | 17 | 1.5302±1.42 | 0.404 |
| >50 | 34 | 1.2173±1.15 | |
| Degree of
differentiation |
| Well and
moderately differentiated | 21 | 1.1103±0.91 | 0.280 |
| Poorly and
undifferentiated | 30 | 1.4695±1.43 | |
| Depth of tumour
invasion |
| Mucosa, submucosa,
muscularis propria | 9 | 0.5429±0.63 | 0.003a |
| Subserosa, serosa,
adjacent structures | 42 | 1.4885±1.28 | |
| TNM stage |
| Stage I/II | 11 | 0.6357±0.64 | 0.002a |
| Stage III/IV | 40 | 1.6548±1.31 | |
| Distant
metastasis |
| Yes | 5 | 1.4048±1.25 | 0.882 |
| No | 46 | 1.3126±1.26 | |
 | Table IICorrelation of N1IC expression with
various clinico-pathological features of GC. |
Table II
Correlation of N1IC expression with
various clinico-pathological features of GC.
| Variable | No. of cases | N1IC expression in
GC | P-value |
|---|
| Sex |
| Male | 34 | 0.2380±0.25 | 0.072 |
| Female | 17 | 0.3997±0.38 | |
| Age (years) |
| ≤50 | 17 | 0.2919±0.39 | 0.911 |
| >50 | 34 | 0.2930±0.25 | |
| Degree of
differentiation |
| Well and
moderately differentiated | 21 | 0.1989±0.26 | 0.058 |
| Poorly and
undifferentiated | 30 | 0.3570±0.32 | |
| Depth of tumour
invasion |
| Mucosa, submucosa,
muscularis propria | 9 | 0.1542±0.15 | 0.027a |
| Subserosa, serosa,
adjacent structures | 42 | 0.3214±0.32 | |
| TNM stage |
| Stage I/II | 11 | 0.1765±0.18 | 0.014a |
| Stage III/IV | 40 | 0.3678±0.34 | |
| Distant
metastasis |
| Yes | 5 | 0.4466±0.46 | 0.233 |
| No | 46 | 0.2751±0.28 | |
 | Table IIICorrelation of COX-2 protein
expression with various clinicopathological features of GC. |
Table III
Correlation of COX-2 protein
expression with various clinicopathological features of GC.
| Variable | No. of cases | COX-2 protein
expression in GC | P-value |
|---|
| Sex |
| Male | 34 | 2.0712±2.65 | 0.899 |
| Female | 17 | 1.9790±1.91 | |
| Age (years) |
| ≤50 | 17 | 1.7217±2.39 | 0.509 |
| >50 | 34 | 2.1999±2.44 | |
| Degree of
differentiation |
| Well and
moderately differentiated | 21 | 1.0402±0.86 | 0.012a |
| Poorly and
undifferentiated | 30 | 2.7407±1.27 | |
| Depth of tumour
invasion |
| Mucosa, submucosa,
muscularis propria | 9 | 1.3183±2.30 | 0.026a |
| Subserosa, serosa,
adjacent structures | 42 | 3.1982±2.43 | |
| TNM stage |
| Stage I/II | 11 | 1.4399±1.32 | 0.750 |
| Stage III/IV | 40 | 2.0756±1.98 | |
| Distant
metastasis |
| Yes | 5 | 1.3733±1.08 | 0.251 |
| No | 46 | 2.1130±2.51 | |
 | Table IVCorrelation of Notch3 expression with
various clinico-pathological features of GC. |
Table IV
Correlation of Notch3 expression with
various clinico-pathological features of GC.
| Variable | No. of cases | Notch3 mRNA
expression in GC | P-value |
|---|
| Sex |
| Male | 34 | 3.4135±1.07 | 0.768 |
| Female | 17 | 4.2245±2.49 | |
| Age (years) |
| ≤50 | 17 | 6.7857±2.88 | 0.042a |
| >50 | 43 | 2.1329±3.84 | |
| Degree of
differentiation |
| Well and
moderately differentiated | 21 | 4.1792±1.42 | 0.69 |
| Poorly and
undifferentiated | 30 | 3.3371±1.56 | |
| Depth of tumour
invasion |
| Mucosa, submucosa,
muscularis propria | 9 | 2.5124±2.91 | 0.65 |
| Subserosa, serosa,
adjacent structures | 42 | 3.7634±2.34 | |
| TNM stage |
| Stage I/II | 11 | 2.6076±1.30 | 0.482 |
| Stage III/IV | 40 | 3.9145±1.29 | |
| Distant
metastasis |
| Yes | 5 | 9.7968±11.16 | 0.062 |
| No | 46 | 3.0194±7.12 | |
COX-2 and Notch1 exhibit an inverse
expression pattern in the normal human gastric mucosal cell line,
GES-1, and different GC cell lines
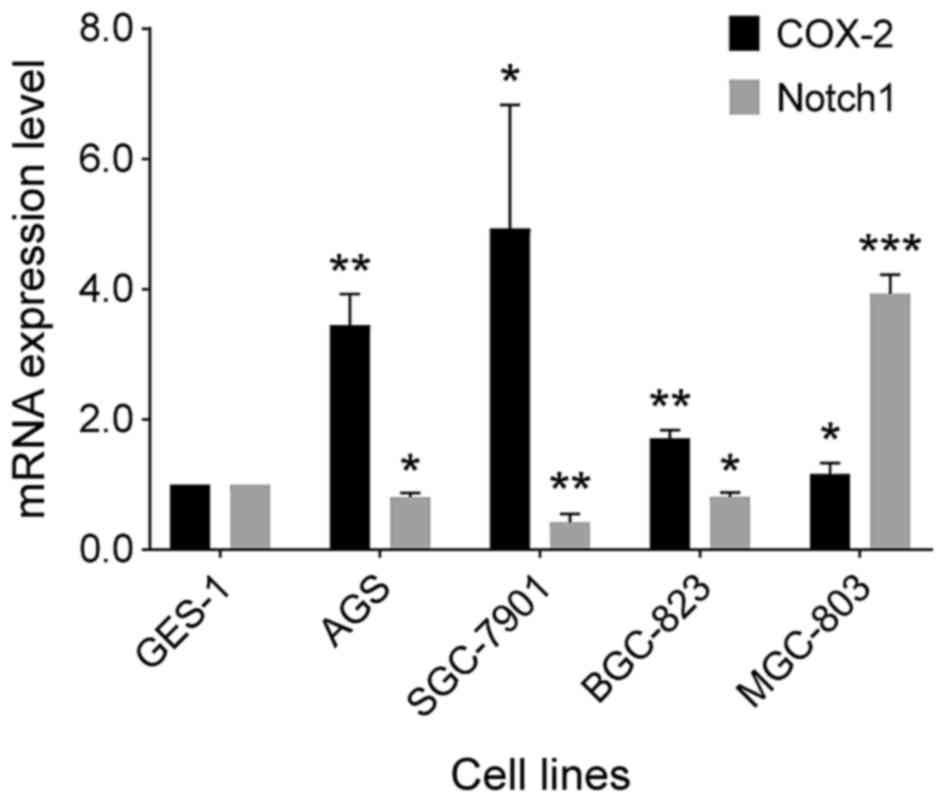
In the previous experiment, the expression of Notch
family members and COX-2 in GC was found to be abnormal;
specifically, there was a significant decrease in the intracellular
domain of Notch1 in GC, whereas COX-2 expression was significantly
increased. To explore the expression trend of Notch1 and COX-2 in
GC cells, the expression of both genes was examined in the GES-1,
AGS, SGC-7901, BGC-823, and MGC-803 cell lines. As shown in
Fig. 3, compared with the normal
gastric epithelial cell line, GES-1, Notch1 mRNA expression was
decreased in the AGS, SGC-7901, and BGC-823 cells, but increased in
the MGC-803 cells (P<0.05). The expression of COX-2 was
increased in the AGS, SGC-7901, BGC-823 and MGC-803 cells compared
with GES-1 cells (P<0.05). Fig.
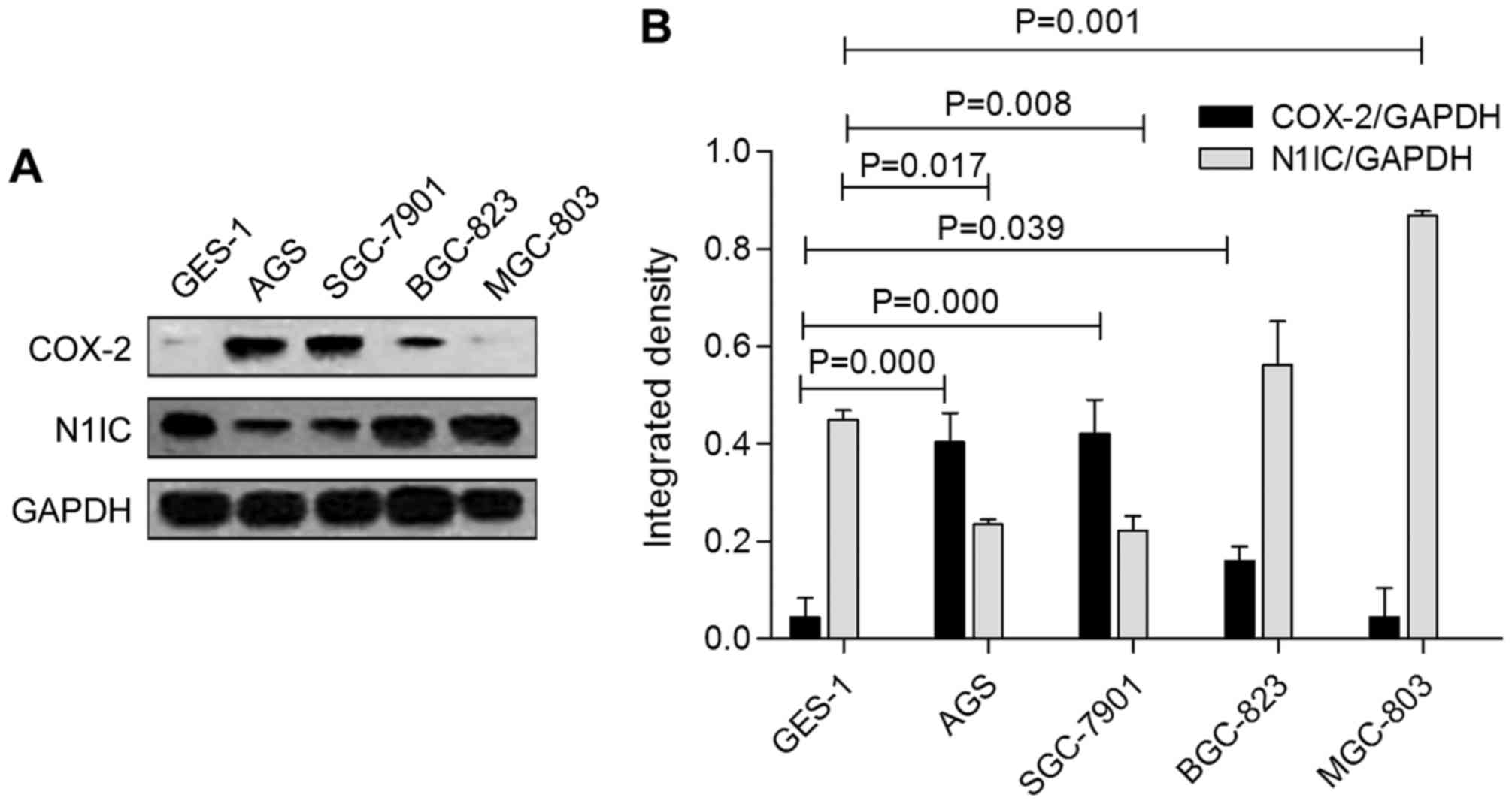
4 shows that N1IC expression was significantly decreased in the
AGS and SGC-7901 cells (P=0.017 and 0.008, respectively), but it
was generally increased in the BGC-823 and MGC-803 cells. Unlike
Notch1, COX-2 was more highly expressed in the AGS, SGC-7901 and
BGC-823 cells (P=0.000, 0.000, and 0.039, respectively), but the
expression was weak in MGC-803 cells. COX-2 expression therefore
inversely correlated with Notch1 expression in GC cells, suggesting
that there is an inverse regulatory relationship between COX-2 and
Notch1 expression in GC cells.
Celecoxib regulates the expression of
Notch1 in GC cells, and PGE2 inhibits the expression of Notch1 in
GC cells
We used pure celecoxib to inhibit COX-2 activity,
while the administration of exogenous PGE2 was used to simulate the
in vitro environment to induce a high COX-2 expression to
further explore the regulatory relationship between Notch1 and
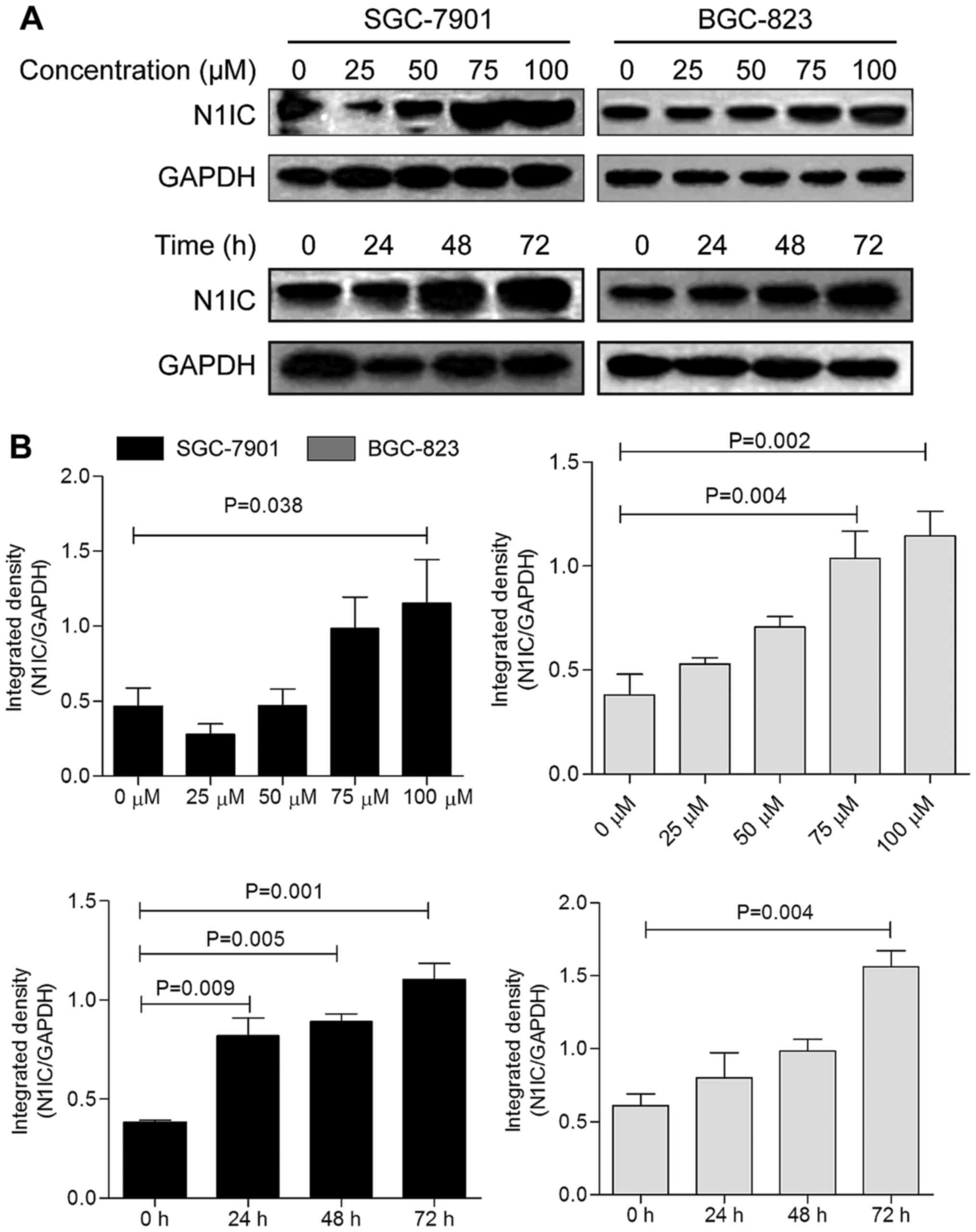
COX-2. As shown in Figs. 5 and
6, celecoxib increased Notch1
mRNA and N1IC expression in the GC cells; Notch1 expression was
increased in a dose-dependent manner over 48 h and exhibited an
increasing trend with the treatment duration. Thus, celecoxib may
upregu-late the expression and activation of Notch1 in SGC-7901 and
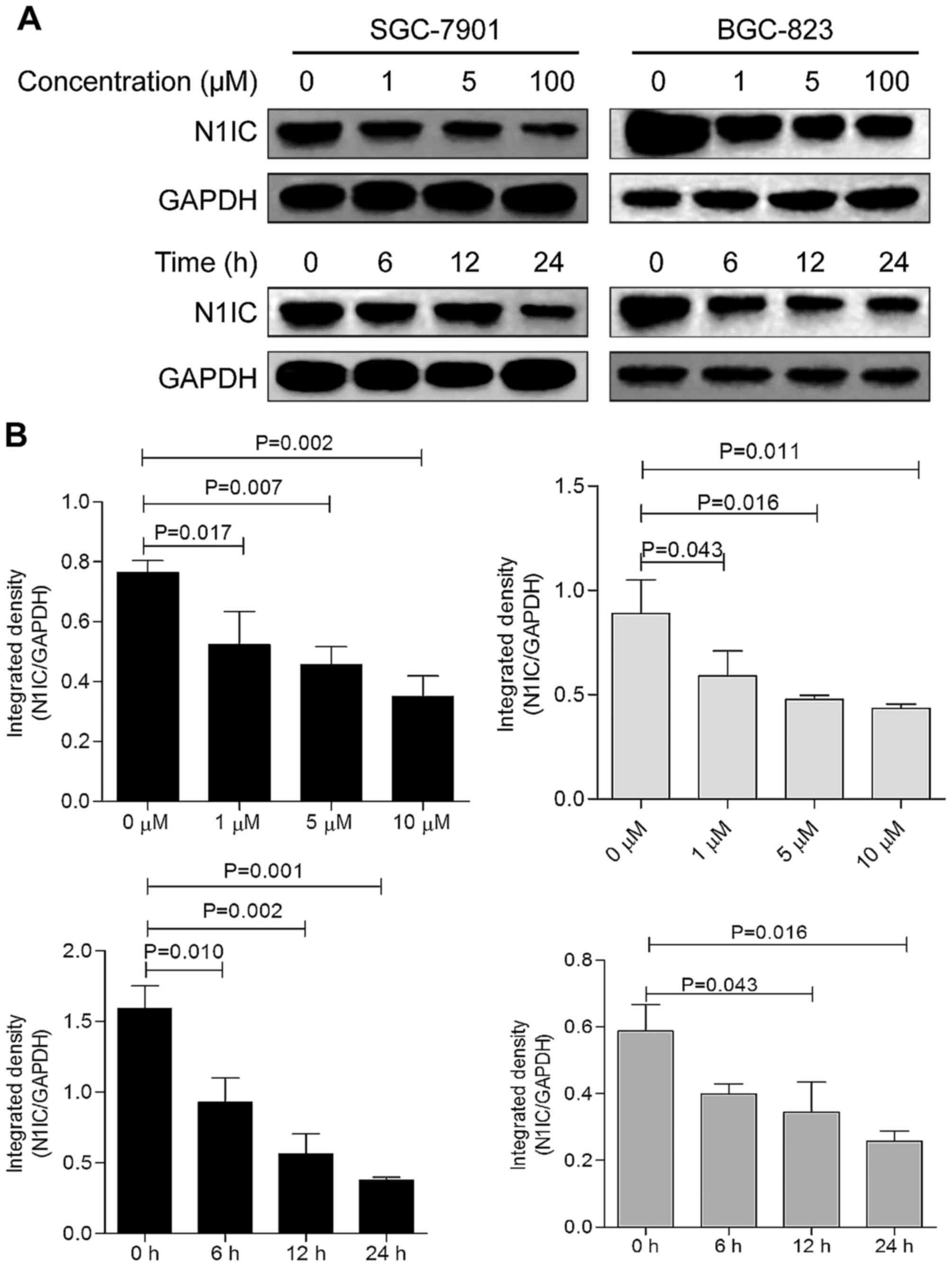
BGC-823 GC cells by inhibiting COX-2 activity. As shown in Figs. 7 and 8, following treatment of the SGC-7901
and BGC-823 cells with various concentrations of PGE2 for 24 h,
Notch1 expression was significantly decreased, and a decreasing
trend in the expression of Notch1 was observed with the increasing
PGE2 concentrations within a certain range. Notch1 expression in
the two cell lines treated with 5 µM PGE2 for 6, 12 and 24 h
decreased in relatively a time-dependent manner.
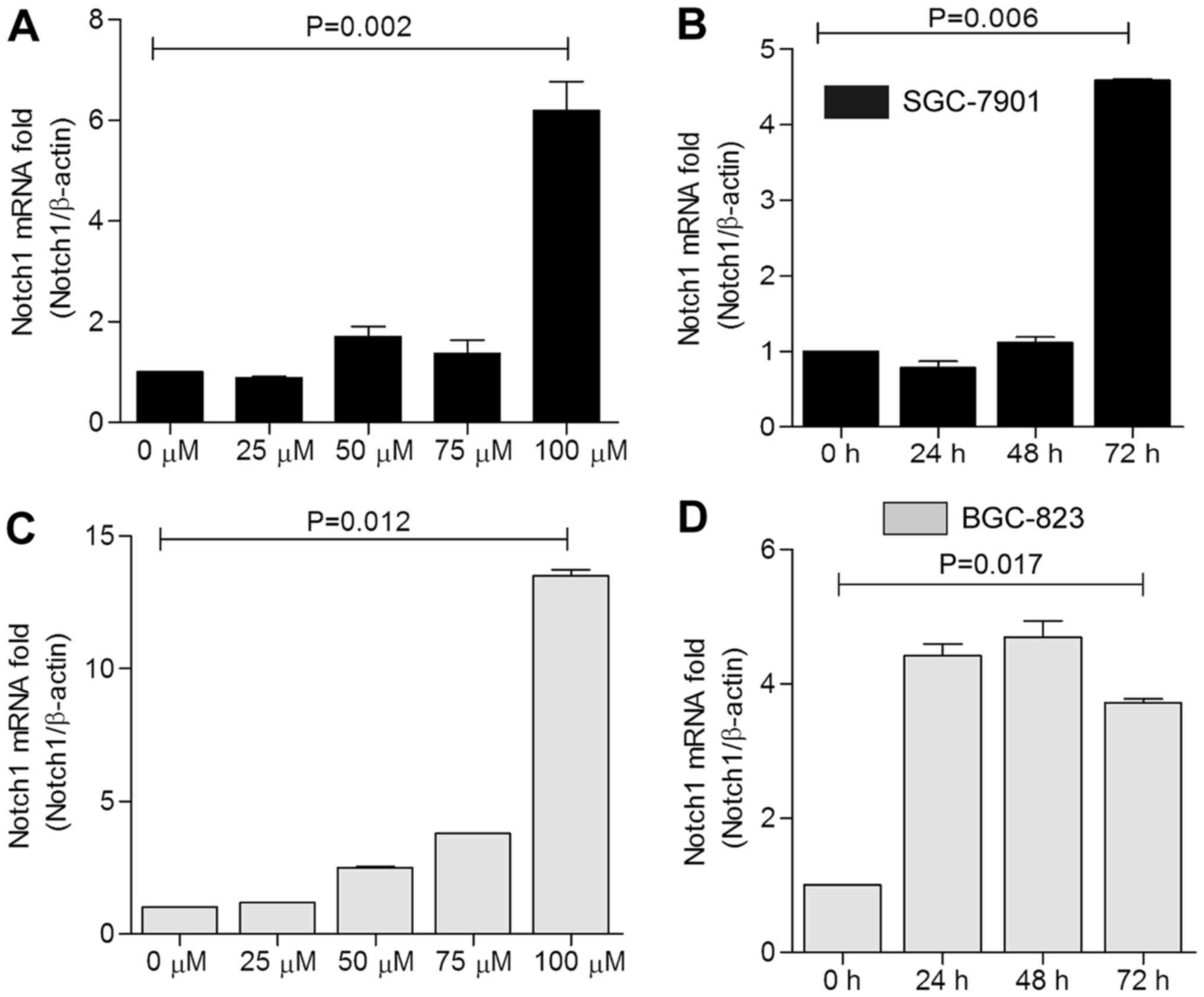 | Figure 5Notch1 mRNA expression trend in
SGC-7901 and BGC-823 cells with celecoxib treatment concentration
and time. Cells treated with 0, 25, 50, 75, and 100 µM
celecoxib for 48 h were used as the dose-dependent group, in which
the 0 µM group of (A) SGC-7901 and (C) BGC-823 cells was
used as the control. Cells treated with 75 µM celecoxib for
0, 24, 48, and 72 h were considered the time-dependent group, in
which the 0 h group of (B) SGC-7901 and (D) BGC-823 cells was used
as the control. In both cell lines, treatment with 100 µM
celecoxib led to the most significant promoting effect on Notch1
mRNA expression (P=0.002, P=0.012). In SGC-7901 cells, 72 h of
treatment resulted in the most significant increase in the Notch1
mRNA level (P=0.006). |
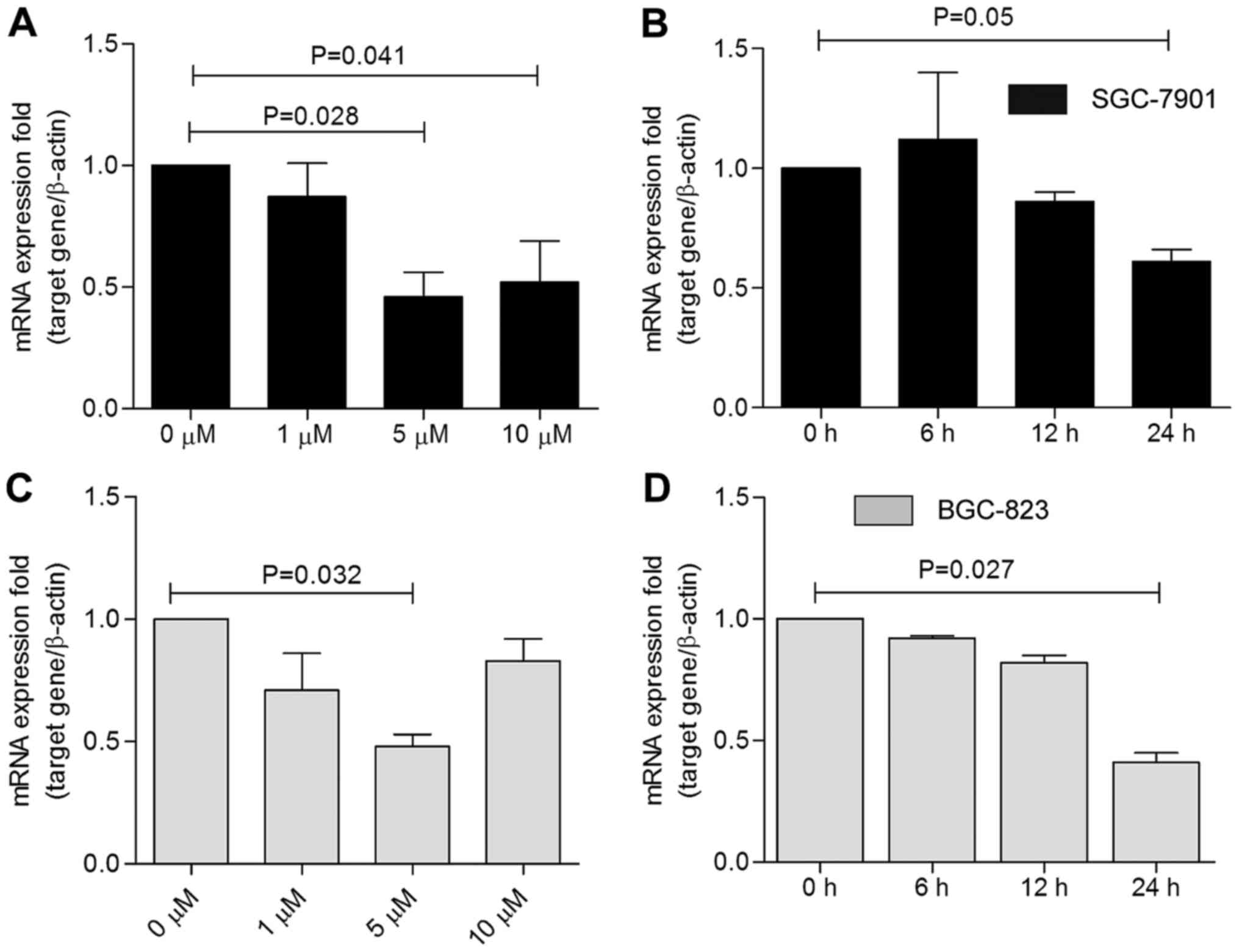 | Figure 7mRNA expression level of Notch1 in
PGE2-treated SGC-7901 and BGC-823 cells. Cells treated with 0, 1,
5, and 10 µM PGE2 for 24 h were used as the dose-dependent
group, in which the 0 µM group of (A) SGC-7901 and (C)
BGC-823 cells was used as the control. Cells treated with 5
µM PGE2 for 0, 6, 12, and 24 h were included in the
time-dependent group, in which the 0 h group of (B) SGC-7901 and
(D) BGC-823 cells was used as the control. In SGC-7901 cells, the 5
and 10 µM groups showed the most significant Notch1
elevation compared with the control group (P=0.028, P=0.041). In
BGC-823 cells, treatment with 5 µM PGE2 for 24 h resulted in
the most significant reduction in Notch1 expression (P=0.032), and
treatment for 24 h resulted in the most significant reduction in
Notch1 mRNA (P=0.027). PGE2, prostaglandin E2. |
Effects of silencing of COX-2 and Notch1
on the expression of Notch1, COX-2 and Snail in GC cells
The drug treatment results revealed a regulatory
association between COX-2 and Notch1. To verify these results,
siRNAs were synthesised and transfected into the SGC-7901 and
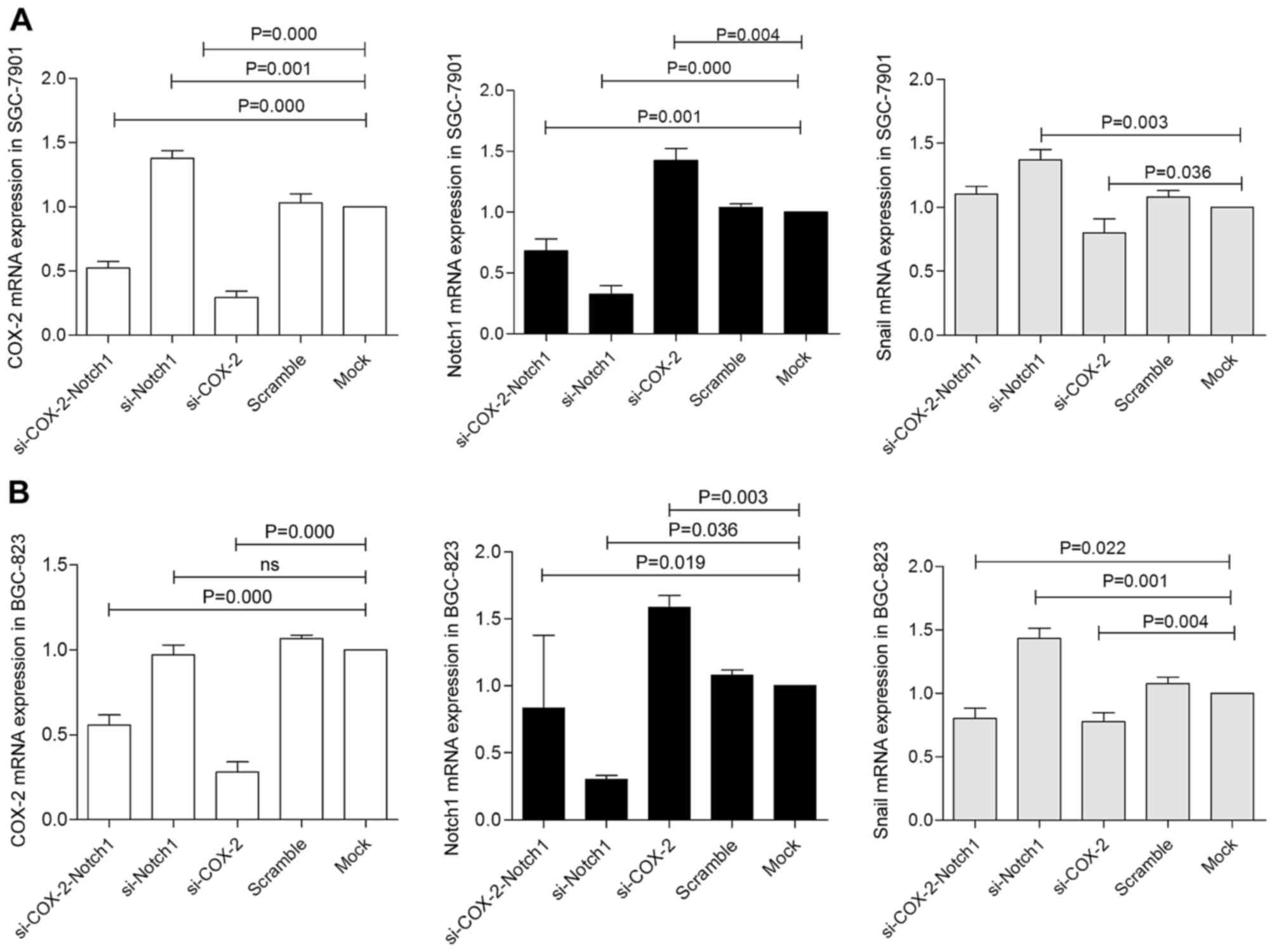
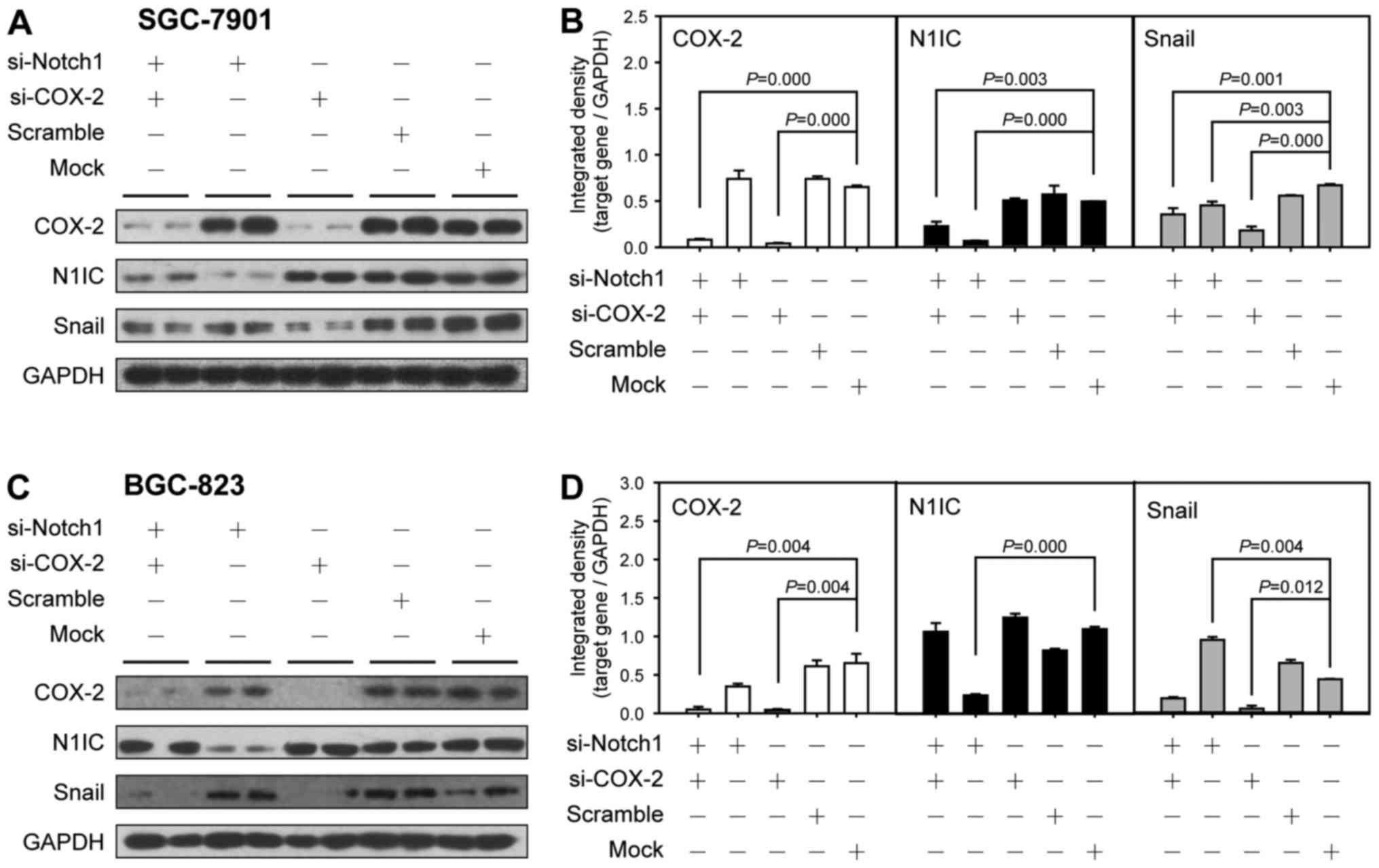
BGC-823 cells. The results are shown in Figs. 9 and 10. In the SGC-7901 cells, the COX-2
mRNA level in the cells transfected with siRNA against Notch1
(si-Notch1 group) was significantly increased (P=0.001). Compared
with the cells transfected with siRNA against COX-2 (si-COX-2
group), the COX-2 protein level in the mock group was slightly
increased, and the expression level of COX-2 in the co-transfection
group was increased. In BGC-823 cells, the mRNA level of COX-2 in
the co-transfection group was higher than that in the si-COX-2
group. In both cell lines, Notch1 mRNA expression in the si-COX-2
group was significantly increased (P=0.001, P=0.019), while N1IC
expression was mildly increased, and N1IC expression in the
co-transfection group of SGC-7901 cells was increased compared to
that in the si-Notch1 group (P=0.024). The N1IC level in the
co-transfected group of BGC-823 cells exhibited no significant
changes compared with the control group. The mRNA and protein
expression of Snail in the si-COX-2 group in both cell lines was
significantly downregulated (P=0.036, 0.000, P=0.004, 0.012). The
mRNA expression of Snail in the si-Notch1 group in both cell lines
was significantly increased (P=0.003, P=0.001). The protein
expression of Snail was upregulated in the si-Notch1 group of
BGC-823 cells (P=0.004) and downregulated in the si-Notch1 group of
SGC-7901 cells (P=0.003); however, the expression of Snail in the
co-transfection group was higher than in the si-COX-2 group.
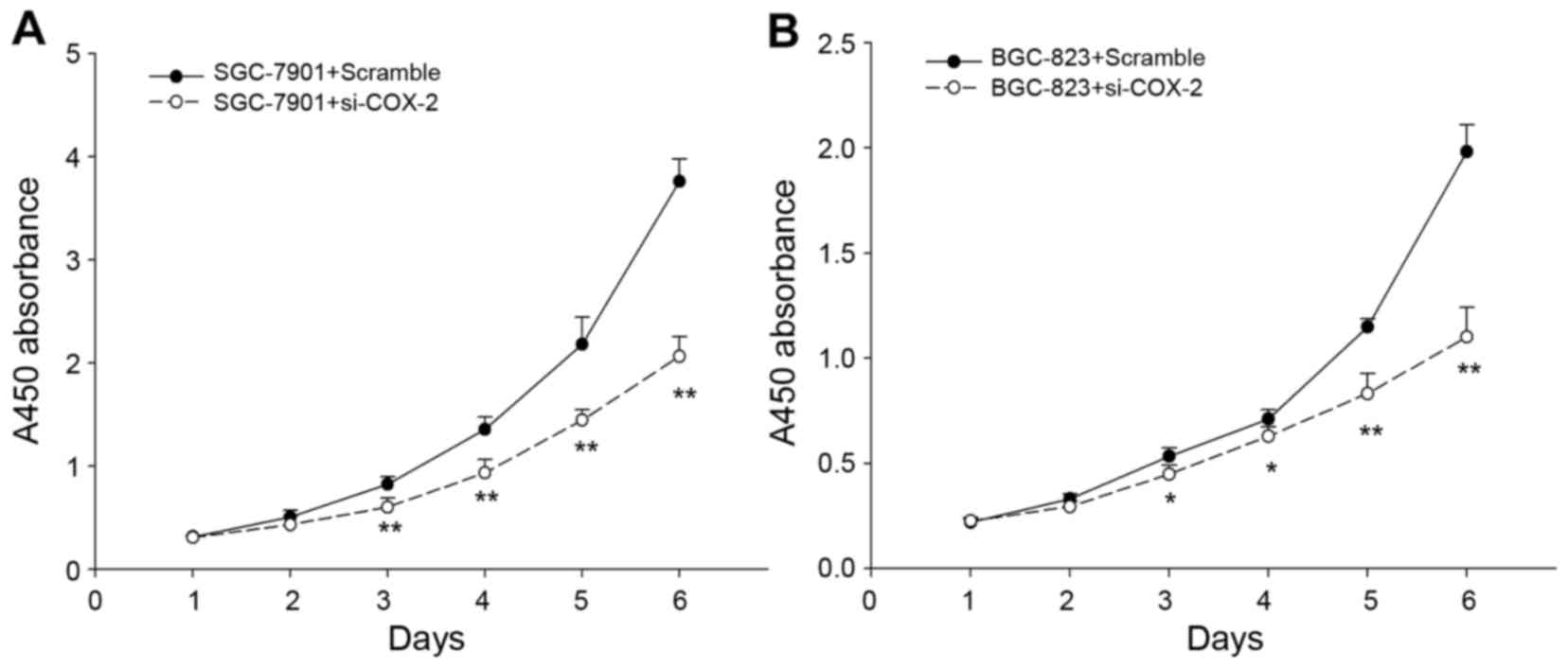
Downregulation of COX-2 inhibits GC
proliferation in vitro
To investigate the role of COX-2 in GC growth,
si-COX-2 or a control siRNA were transiently transfected into the
SGC-7901 and BGC-823 cells, and the effects of the knockdown of
COX-2 were determined by CCK-8 proliferation assays. The
downregulation of COX-2 expression significantly inhibited the
proliferation of GC cells (Fig.
11).
Discussion
The Notch signalling pathway is a highly
evolutionarily conserved signal transduction system (25). The Notch receptor transmits
intracellular signals by interacting with ligands and plays
important regulatory roles in cell proliferation, differentiation
and apoptosis (26–28). Mammals possess four Notch
receptors (Notch1-4), and Notch ligands include Jagged1, Jagged2,
DLL1, DLL3 and DLL4 (29). Notch
acts as an oncogene. Two large-scale whole-exome studies on chronic
lymphocytic leukaemia (CLL) cases in Europe (30,31) confirmed the presence of
gain-of-function mutations in Notch1 in CLL. The abnormal increase
in Notch1 activation due to mutations may be related to the poor
prognosis of CLL. In breast cancer, Jagged1 can promote tumour
metastasis by stimulating the secretion of interleukin-6 from
osteoblasts (32), whereas the
activation of Notch2 by benzyl isothiocyanate in cultured cells and
xenografted tumours can promote the migration of breast cancer
cells (33). Notch3 activation
can induce EMT and suppress the carboplatin-induced apoptosis of
the ovarian cancer cell line, OVCA429 (34). However, some studies have also
suggested that the Notch signalling pathway can inhibit
tumourigenesis and development. Notch1 deficiency in the skin and
in primary keratinocytes can inhibit the β-catenin signalling
pathway and inhibit differentiation and maturation in epidermal
cells, leading to malignant transformation (35). In a K-ras allele-activated and
Notch1-depleted mouse model, Notch1-depleted mice showed a
significantly increased tumour incidence, suggesting that Notch1
acts as a tumour suppressor in pancreatic ductal adenocarcinoma
(36). Notch activation has also
been shown to be reduced following the administration of
γ-secretase inhibitors, which can accelerate the growth of liver
cancer cells, whereas the overexpression of Notch1 in the primary
liver cancer cells of RB-p107-p130-deficient mice led to cell-cycle
arrest and induced apoptosis. Similar experimental results were
observed in the in vitro culture of two hepatocellular
carcinoma cell lines (37).
In this study, the mRNA expression level of Notch1
was higher in GC tissues than in paracancerous tissues, while the
overall expression level of N1IC in GC was significantly reduced
compared with paracancerous normal gastric mucosa, indicating
differences between the mRNA level and the activation level of
Notch1 in GC. The inhibition of Notch1 in GC may have a
tumour-suppressing effect. Jagged1 mRNA, Notch2 mRNA and N2IC
levels were upregulated in GC. As the activation of Notch proteins
is influenced by the corresponding ligand, the abnormal mRNA
expression o f Jagged1 in GC may be one factor causing the abnormal
activation of Notch proteins. However, reports on the function of
enhanced Notch1 and Notch2 activation in paracancerous tissues are
relatively rare; hence, additional studies are required. The mRNA
expression of Notch3 was increased in GC (Fig. 1) and significantly increased in
the ≤50-year-old GC group (Table
IV); however, a larger sample size is needed to confirm these
results.
Consistent with many previous studies (38–41), COX-2 expression was significantly
increased in GC, and its expression was significantly higher in
undifferentiated and poorly differentiated GC than in moderately
and highly differentiated GC; additionally, COX-2 expression
increased with the depth of invasion. Abnormal alterations in cell
adhesion molecules and resulting adhesive behaviours occur during
tumour cell invasion and metastasis (42–44). E-cadherin is a critical adhesion
molecule, and Snail can directly inhibit E-cadherin transcription
and expression by binding to the E-box of the E-cadherin promoter
(45). The preliminary results of
this study demonstrated that COX-2 had a regulatory effect on Snail
(46); however, the mechanisms
through which COX-2 regulates Snail expression remain unclear.
Notch1 expression differed between different GC cell lines and was
decreased in all cell lines that exhibited a high COX-2 expression.
By contrast, COX-2 expression was decreased in cell lines with a
high Notch1 expression. Thus, there was an inverse correlation
between the expression of the two genes.
Following the inhibition of COX-2 activity with pure
celecoxib, the expression of Notch1 was upregulated in a time- and
dose-dependent manner, while it was downregulated in a time- and
dose-dependent manner following the simulation of COX-2 expression
in vitro by exogenous PGE2 administration. Thus, the ability
of celecoxib and PGE2 to regulate the expression of Notch1 through
COX-2 was evaluated. These observations provide new information
regarding the association between COX-2 and Notch1. However, the
antitumour effect of celecoxib also involves non-COX-2-dependent
pathways, while PGE2 is only one of many prostaglandins produced by
COX-2 catalysis. To verify the observations from the drug-based
experiments, siRNAs were used to silence COX-2 and Notch1. The
silencing of COX-2 expression significantly downregulated the
expression of Snail in two GC cell lines, while the transcription
and activation of Notch1 increased to varying degrees. These
results indicate that COX-2 had a regulatory effect on Notch1,
whereas the silencing of Notch1 expression had no significant
effect on the expression of COX-2 in this experiment. There were
differences in the effect of si-Notch1 treatment on Snail between
the two GC cell lines; the silencing of Notch1 caused the
downregulation of Snail expression in SGC-7901 cells and led to the
upregulation of Snail expression in BGC-823 cells, which may be
related to the different roles played by Notch1. The basal
expression level of Notch1 varies in different cellular
environments, and different downstream signal transduction pathways
are activated in different cells. However, its specific mechanism
must be confirmed in future experiments. Cell proliferation assays
revealed that COX-2 downregulation significantly inhibited the
proliferation of GC cells, suggesting that COX-2 could promote the
angiogenesis of GC cells in vitro and that Notch1 may play a
tumour suppressor role in GCs.
The regulation of COX-2 expression by Notch1 has
been reported in previous studies (47,48). In this study, we report that COX-2
knockdown led to the upregulation of the expression of Notch1.
However, we believe that the regulatory relationship is likely to
be indirect or a type of negative feedback regulation. Further
studies are required to define the mode of regulation.
In conclusion, Notch family members were found to be
involved in the tumourigenesis and development of GC and were
associated with the clinicopathological features of GC. Moreover,
different Notch family members may play different roles in GC.
COX-2 can inversely regulate the expression and activation of
Notch1 and is partially dependent on the Notch1 pathway in altering
the expression of Snail in GC. The results of this study revealed
that COX-2 may be involved in the proliferation, invasion and
migration of GC via different signal transduction pathways,
providing a theoretical basis for the targeted molecular therapy of
GC.
Acknowledgments
This study was supported by China's National Science
and Technology Program for Public Wellbeing (grant no.
2012GS620101),National Natural Science Foundation of China (grant
no. 81372145),Gansu Natural Science Foundation of China (grant no.
1606RJZA140 and 1606RJZA141), Gansu Science and Technology Support
Plan (grant no. 1604FKCA105), Lanzhou Chengguan District Science
and Technology Plan Project (grant no. 2014-4-3) and The First
Hospital of Lanzhou University Foundation (grant no.
ldyyyn2015-15).
References
|
1
|
Guggenheim DE and Shah MA: Gastric cancer
epidemiology and risk factors. J Surg Oncol. 107:230–236. 2013.
View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang F, Meng W, Wang B and Qiao L:
Helicobacter pylori-induced gastric inflammation and gastric
cancer. Cancer Lett. 345:196–202. 2014. View Article : Google Scholar
|
|
4
|
Wang ZL, Fan ZQ, Jiang HD and Qu JM:
Selective Cox-2 inhibitor celecoxib induces epithelial-mesenchymal
transition in human lung cancer cells via activating MEK-ERK
signaling. Carcinogenesis. 34:638–646. 2013. View Article : Google Scholar
|
|
5
|
Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H,
Fan Z, Cai J and Li Q: Berberine inhibits invasion and metastasis
of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3
signaling pathway. PLoS One. 10:e01234782015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schildberg C, Abbas M, Merkel S, Agaimy A,
Dimmler A, Schlabrakowski A, Croner R, Leupolt J, Hohenberger W and
Allgayer H: COX-2, TFF1, and Src define better prognosis in young
patients with gastric cancer. J Surg Oncol. 108:409–413. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shin WG, Kim HJ, Cho SJ, Kim HS, Kim KH,
Jang MK, Lee JH and Kim HY: The COX-2-1195AA genotype is associated
with diffuse-type gastric cancer in Korea. Gut Liver. 6:321–327.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su HJ, Zhang Y, Zhang L, Ma JL, Li JY, Pan
KF and You WC: Methylation status of COX-2 in blood leukocyte DNA
and risk of gastric cancer in a high-risk Chinese population. BMC
Cancer. 15:9792015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu XL, Cheng B, Li PY, Huang HJ, Zhao Q,
Dan ZL, Tian DA and Zhang P: MicroRNA-143 suppresses gastric cancer
cell growth and induces apoptosis by targeting COX-2. World J
Gastroenterol. 19:7758–7765. 2013. View Article : Google Scholar
|
|
10
|
Yuan XL, Chen L, Li MX, Dong P, Xue J,
Wang J, Zhang TT, Wang XA, Zhang FM, Ge HL, et al: Elevated
expression of Foxp3 in tumor-infiltrating Treg cells suppresses
T-cell proliferation and contributes to gastric cancer progression
in a COX-2-dependent manner. Clin Immunol. 134:277–288. 2010.
View Article : Google Scholar
|
|
11
|
Cheng J and Fan XM: Role of
cyclooxygenase-2 in gastric cancer development and progression.
World J Gastroenterol. 19:7361–7368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oshima H and Oshima M: The role of
PGE2-associated inflammatory responses in gastric cancer
development. Semin Immunopathol. 35:139–150. 2013. View Article : Google Scholar
|
|
13
|
Yao L, Liu F, Hong L, Sun L, Liang S, Wu K
and Fan D: The function and mechanism of COX-2 in angiogenesis of
gastric cancer cells. J Exp Clin Cancer Res. 30:132011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang P, Luo HS, Li M and Tan SY:
Artesunate inhibits the growth and induces apoptosis of human
gastric cancer cells by downregulating COX-2. Onco Targets Ther.
8:845–854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guruharsha KG, Kankel MW and
Artavanis-Tsakonas S: The Notch signalling system: Recent insights
into the complexity of a conserved pathway. Nat Rev Genet.
13:654–666. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bertrand FE, Angus CW, Partis WJ and
Sigounas G: Developmental pathways in colon cancer: Crosstalk
between WNT, BMP, Hedgehog and Notch. Cell Cycle. 11:4344–4351.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Domingo-Domenech J, Vidal SJ,
Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco
R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J,
et al: Suppression of acquired docetaxel resistance in prostate
cancer through depletion of notch- and hedgehog-dependent
tumor-initiating cells. Cancer Cell. 22:373–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garner JM, Fan M, Yang CH, Du Z, Sims M,
Davidoff AM and Pfeffer LM: Constitutive activation of signal
transducer and activator of transcription 3 (STAT3) and nuclear
factor κB signaling in glioblastoma cancer stem cells regulates the
Notch pathway. J Biol Chem. 288:26167–26176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hassan KA, Wang L, Korkaya H, Chen G,
Maillard I, Beer DG, Kalemkerian GP and Wicha MS: Notch pathway
activity identifies cells with cancer stem cell-like properties and
correlates with worse survival in lung adenocarcinoma. Clin Cancer
Res. 19:1972–1980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McAuliffe SM, Morgan SL, Wyant GA, Tran
LT, Muto KW, Chen YS, Chin KT, Partridge JC, Poole BB, Cheng KH, et
al: Targeting Notch, a key pathway for ovarian cancer stem cells,
sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA.
109:E2939–E2948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu KW, Hsieh RH, Huang KH, Fen-Yau Li A,
Chi CW, Wang TY, Tseng MJ, Wu KJ and Yeh TS: Activation of the
Notch1/STAT3/Twist signaling axis promotes gastric cancer
progression. Carcinogenesis. 33:1459–1467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayashi T, Gust KM, Wyatt AW, Goriki A,
Jäger W, Awrey S, Li N, Oo HZ, Altamirano-Dimas M, Buttyan R, et
al: Not all NOTCH is created equal: The oncogenic role of NOTCH2 in
bladder cancer and its implications for targeted therapy. Clin
Cancer Res. 22:2981–2992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma Y, Li M, Si J, Xiong Y, Lu F, Zhang J,
Zhang L, Zhang P and Yang Y: Blockade of Notch3 inhibits the
stem-like property and is associated with ALDH1A1 and CD44 via
autophagy in non-small lung cancer. Int J Oncol. 48:2349–2358.
2016.PubMed/NCBI
|
|
24
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of Snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hori K, Sen A and Artavanis-Tsakonas S:
Notch signaling at a glance. J Cell Sci. 126:2135–2140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Acar A, Simões BM, Clarke RB and Brennan
K: A role for Notch signalling in breast cancer and endocrine
resistance. Stem Cells Int. 2016:24987642016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Braune EB and Lendahl U: Notch - a
goldilocks signaling pathway in disease and cancer therapy. Discov
Med. 21:189–196. 2016.PubMed/NCBI
|
|
28
|
Kumar R, Juillerat-Jeanneret L and
Golshayan D: Notch antagonists: Potential modulators of cancer and
inflammatory diseases. J Med Chem. 59:7719–7737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Penton AL, Leonard LD and Spinner NB:
Notch signaling in human development and disease. Semin Cell Dev
Biol. 23:450–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fabbri G, Rasi S, Rossi D, Trifonov V,
Khiabanian H, Ma J, Grunn A, Fangazio M, Capello D, Monti S, et al:
Analysis of the chronic lymphocytic leukemia coding genome: Role of
NOTCH1 mutational activation. J Exp Med. 208:1389–1401. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Puente XS, Pinyol M, Quesada V, Conde L,
Ordóñez GR, Villamor N, Escaramis G, Jares P, Beà S, González-Díaz
M, et al: Whole-genome sequencing identifies recurrent mutations in
chronic lymphocytic leukaemia. Nature. 475:101–105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sethi N, Dai X, Winter CG and Kang Y:
Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast
cancer by engaging notch signaling in bone cells. Cancer Cell.
19:192–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SH, Sehrawat A and Singh SV: Notch2
activation by benzyl isothiocyanate impedes its inhibitory effect
on breast cancer cell migration. Breast Cancer Res Treat.
134:1067–1079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gupta N, Xu Z, El-Sehemy A, Steed H and Fu
Y: Notch3 induces epithelial-mesenchymal transition and attenuates
carboplatin-induced apoptosis in ovarian cancer cells. Gynecol
Oncol. 130:200–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nicolas M, Wolfer A, Raj K, Kummer JA,
Mill P, van Noort M, Hui CC, Clevers H, Dotto GP and Radtke F:
Notch1 functions as a tumor suppressor in mouse skin. Nat Genet.
33:416–421. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hanlon L, Avila JL, Demarest RM, Troutman
S, Allen M, Ratti F, Rustgi AK, Stanger BZ, Radtke F, Adsay V, et
al: Notch1 functions as a tumor suppressor in a model of
K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res.
70:4280–4286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Viatour P, Ehmer U, Saddic LA, Dorrell C,
Andersen JB, Lin C, Zmoos AF, Mazur PK, Schaffer BE, Ostermeier A,
et al: Notch signaling inhibits hepatocellular carcinoma following
inactivation of the RB pathway. J Exp Med. 208:1963–1976. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fanelli MFLTDC, Chinen LT, Begnami MD,
Costa WL Jr, Fregnami JH, Soares FA and Montagnini AL: The
influence of transforming growth factor-α, cyclooxygenase-2, matrix
metalloproteinase (MMP)-7, MMP-9 and CXCR4 proteins involved in
epithelial-mesenchymal transition on overall survival of patients
with gastric cancer. Histopathology. 61:153–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lazăr D, Tăban S, Ardeleanu C, Simionescu
C, Sporea I, Cornianu M and Vernic C: Immuno-histochemical
expression of the cyclooxygenase-2 (COX-2) in gastric cancer. The
correlations with the tumor angiogenesis and patients' survival.
Rom J Morphol Embryol. 49:371–379. 2008.
|
|
40
|
Song J, Bai Z and Zhang Z: MicroRNAs are
implicated in the initiation and progression of gastric cancer.
Chin Med J (Engl). 127:554–559. 2014.
|
|
41
|
Yu CH, Li L, Li YM, Zhang BF, Fang J, Zhou
Q, Hu Y and Gao HJ: Expression of tissue microarray p53, p16 and
cyclooxy-genase-2 in gastric cancer. Zhonghua Nei Ke Za Zhi.
45:658–660. 2006.In Chinese. PubMed/NCBI
|
|
42
|
Patriarca C, Macchi RM, Marschner AK and
Mellstedt H: Epithelial cell adhesion molecule expression (CD326)
in cancer: A short review. Cancer Treat Rev. 38:68–75. 2012.
View Article : Google Scholar
|
|
43
|
Valiente M, Obenauf AC, Jin X, Chen Q,
Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, et al:
Serpins promote cancer cell survival and vascular co-option in
brain metastasis. Cell. 156:1002–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Yue P, Page BD, Li T, Zhao W,
Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT, et al: Orally
bioavailable small-molecule inhibitor of transcription factor Stat3
regresses human breast and lung cancer xenografts. Proc Natl Acad
Sci USA. 109:9623–9628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Spaderna S, Schmalhofer O, Wahlbuhl M,
Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A,
et al: The transcriptional repressor ZEB1 promotes metastasis and
loss of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Z, Liu M, Liu X, Huang S, Li L, Song
B, Li H, Ren Q, Hu Z, Zhou Y, et al: COX-2 regulates E-cadherin
expression through the NF-κB/Snail signaling pathway in gastric
cancer. Int J Mol Med. 32:93–100. 2013.PubMed/NCBI
|
|
47
|
Yu LX, Zhou L, Li M, Li ZW, Wang DS and
Zhang SG: The Notch1/cyclooxygenase-2/Snail/E-cadherin pathway is
associated with hypoxia-induced hepatocellular carcinoma cell
invasion and migration. Oncol Rep. 29:362–370. 2013.
|
|
48
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: The down-regulation of Notch1 inhibits the invasion and
migration of hepatocellular carcinoma cells by inactivating the
cyclo-oxygenase-2/Snail/E-cadherin pathway in vitro. Dig Dis Sci.
58:1016–1025. 2013. View Article : Google Scholar
|