Introduction
Acute pancreatitis (AP) is a trypsin-activated
sudden inflammatory response in the pancreas and autodigestion of
the pancreas caused by various etiological factors such as
gallstones, craputence, hyperlipemia, virus, sepsis and shock
(1,2). Currently, AP is divided the mild AP,
moderately severe AP (MSAP) and severe AP (SAP) in the clinic
according to disease severity and prognosis (3). SAP is also called acute necrotizing
pancreatitis (ANP) and generally manifests as diffuse pancreatic
necrosis and hemorrhage, adiponecrosis, neutrophil and monocyte
infiltration, necrosis and apoptosis of pancreatic acinar cells. It
can rapidly develop into systemic inflammation and even multiple
organ dysfunction syndrome (MODS), accompanied by an unacceptably
high mortality rate. However, the therapy of ANP is now limited to
symptomatic and supportive treatments such as analgesia,
spasmolysis, improvement in microcirculation, antishock,
anti-inflammation and antiemetic. Yet up to 30% of ANP patients die
from various complications (4).
Therefore, searching for effective therapeutic targets and drugs
are of great importance.
Today the pathogenesis of ANP has not been fully
elucidated. It has been confirmed that the autodigestion of the
pancreas by intrapancreatic trypsin, excessive inflammation,
necrosis and apoptosis of pancreatic acinar cells may all be
involved in this process (5–7).
However, the use of trypsin inhibitors and anti-inflammatory
therapy directed at a certain inflammatory factor are usually
noneffective in the treatment of ANP. The occurrence and
development of ANP can be described as progression from an initial
injury of pancreatic acinar cells to local and systemic
inflammation (5). Uncontrolled
release of a large amount of various inflammatory factors including
tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β and
IL-10, are not only the initial factors of ANP, but also promote
the necrosis and apoptosis of pancreatic acinar cells and
eventually aggravate the impairment of the pancreas in ANP
(6). Hence, the decrease in the
uncontrolled release of various inflammatory factors may be helpful
to improve the prognosis of AP.
Resveratrol, a polyphenol compound derived from
various plants such as grape skin, peanut, berry and veratrum has
multiple biological activities, including potent anti-inflammatory
activity, antioxidant, anti-aging, insulin sensitization and
cardiac protection. It has been shown that resveratrol has certain
protective effect on cerulein-induced acute edema pancreatitis and
4% sodium taurocholate-induced SAP as well as related complications
in rats, but its effect on L-arginine-induced ANP remains unknown
to date (8,9). Resveratrol has been found to be an
activator of sirtuin 1 (SIRT1). SIRT1 is one of the seven mammalian
orthologs of the yeast protein silent information regulator 2
(Sir2), a conserved NAD-dependent protein deacetylase that plays an
important role in the cell proliferation, differentiation,
apoptosis, metabolism and inflammatory response through
deacetylating certain lysine residues in a variety of downstream
substrates including histone H3, p53, heat shock factor 1 (HSF1),
and further enhancing their transcription activity (10–12). However, the role of SIRT1 in the
development of ANP is largely unknown.
In this study, for the first time, to the best of
our knowledge, the effect of resveratrol on L-arginine-induce ANP
in mice was investigated, and whether the activation of SIRT1 and
its deacetylation of downstream substrates are involved in this
process was determined.
Materials and methods
Animals
Male Balb/c mice weighed 20–25 g were purchased from
the Center of Experimental Animals in Central South University of
China. All mice were kept on a 12-h light/dark cycle at a room
temperature of 23±2°C and a relative humidity of 50±5% and housed
individually with free access to food and water throughout the
experiment. Mice were randomly assigned to the experimental
procedures described as follows. Animal use procedures were
approved by the Animal Welfare Ethics Committee of Central South
University.
Establishment of a mouse model of acute
pancreatitis and treatment
A mouse model of acute pancreatitis was established
by 2 hourly intraperitoneal injections of 8% L-arginine
hydrochloride (pH 7.0, 4 g/kg body weight). The mice were randomly
divided into three groups: control group (Con), L-arginine exposure
group (AP), L-arginine and resveratrol treatment group (AP+RSV).
Mice in the AP+RSV group were treated with intragastric
administration of 80 mg/kg resveratrol every 12 h immediately after
the second injection of L-arginine. Mice in the Con group and AP
group were treated with the same volume of vehicle. Resveratrol was
dissolved in 0.5% sodium carboxymethyl cellulose away from light
and was used immediately after it was ready. Before administration
of L-arginine, the mice were deprived of food and received only
water for 10–12 h. After the first injection of L-arginine, the
mice were given food and water ad libitum immediately and
then sacrificed at 48 and 72 h after the second injection of
L-arginine.
Reagents
The antibodies to HSF1 (1:2,000 dilution; 12972S),
acetylated-lysine (1:1,000 dilution; 9441S), SIRT1 (1:2,000
dilution; 9475S) and p53 (1:1,000 dilution; 2524S) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). The
antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
1:3,000 dilution; G9545), resveratrol, L-arginine hydrochloride,
sodium carboxymethyl cellulose, protease inhibitor cocktail were
purchased from Sigma-Adrich (St. Louis, MO, USA). Mouse TNF-α, IL-6
and IL-10 enzyme-linked immunosorbent assay (ELISA) kits and IgG
horseradish peroxidase-conjugated secondary antibody (1:10,000
dilution; BA1050, BA1054) were purchased from Boster Biological
Technology, Ltd. (Wuhan, China). Lactate dehydrogenase and
myeloperoxidase (MPO) assay kit were purchased from Nanjing
Jiancheng Biology Engineering Institute. In situ cell death
detection kit was purchased from Roche Applied Sciences (Mannheim,
Germany). PureProteome™ Protein A and Protein G magnetic beads were
purchased from Merck Millipore (Billerica, MA, USA). Other reagents
were analytically pure.
Histological examination
Pancreatic tissues were rinsed and fixed in 4%
paraformaldehyde (PFA) at 4°C overnight. The PFA-treated pancreatic
tissues were then processed with sequential clearing and
dehydrating steps, and embedded in paraffin blocks. Samples were
sectioned into 5-μm slices and subjected to standard
hematoxylin and eosin (H&E) staining for the evaluation of
pancreatic tissue injury. Images were taken under an optical
microscope (Olympus, Tokyo, Japan). The severity of pancreatic
tissue lesions was evaluated and scored by two experienced
pathologists in a random and double-blinded manner according to a
modified Grewal method as follows (13): edema was scored from 0–4 points
(0, none; 1, patchy interlobular septum; 2, diffuse inter-lobular
septum; 3, diffuse interlobular and intraacinar septum; 4, diffuse
intercellular space); leukocytic infiltration was scored from 0–4
points (0.5, per 5 leukocytes, 4, >30 leukocytes); the
percentage of acinar cell necrosis was evaluated by ImageJ software
and scored from 0–4 points (0, none; 1, 1–10% necrosis area; 2,
11–20% necrosis area; 3, 21–30% necrosis area; 4, >30% necrosis
area) and hemorrhage was scored from 0–1 points (0, none; 1,
hemorrhage).
Measurement of serological index
Blood was obtained by direct cardiac puncture under
deep anesthesia at 48 h and 72 h after the second injection of
L-arginine and clotted at room temperature for 4 h. To obtain serum
samples, the blood was centrifuged at 1,500 × g for 10 min. The
serum amylase level was further detected at the clinical laboratory
of Xiangya Hospital. ELISA assays were performed to detect the
TNF-α, IL-1β, IL-6 and IL-10 contents in the serum according to the
manufacturer's protocol. Serum lactate dehydrogenase (LDH) activity
was measured by a colorimetric method according to the
manufacturer's protocol.
MPO activity assay
The pancreatic MPO activity was assessed by an MPO
colorimetric activity assay kit according to the manufacturer's
protocol and presented as units per milligram of pancreatic
tissue.
Terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
assay
TUNEL staining was performed to detect the apoptosis
of pancreatic acinar cells through in situ cell apoptosis
detection kits according to the manufacturer's instructions.
Pancreatic tissues were rinsed and fixed in 4% PFA at 4°C overnight
followed by paraffin embedding. Paraffin-embedded sections of the
samples were deparaffinated and hydrated using graded ethanol, and
then incubated in 20 g/ml protease K at 37°C for 30 min. After
being washed with phosphate-buffered saline (PBS) for 5 min three
times, the samples were incubated with 0.1% Triton X-100 for 20 min
at room temperature and then washed with PBS for 5 min three times.
Terminal deoxynucleotidyl transformerase (TDT) and dUTP were mixed
at a 2:29 ratio and then added to the sample and incubated at 37°C
for 2 h. After washing with PBS for 5 min three times, the sections
were immersed into 3% H2O2 prepared by
methanol for 15 min away from light, and then covered with
converter-POD at 37°C for 30 min in a humidified box. After
rinsing, the samples were incubated with DAB substrate kit under a
light microscope. The slides were lightly counterstained with
haematoxylin and then dehydrated and mounted. For each pancreas
specimen, tissue sections were examined under a light microscope at
×100 and ×200 magnifications. Ten random fields per section were
counted and analyzed by Image Pro-Plus 7.0 software and the
percentage of apoptotic cells was finally calculated as the
apoptotic index.
Quantitative (real-time) polymerase chain
reaction
Total RNA of the pancreatic tissues was extracted by
TRIzol and reverse transcribed to cDNA with Primescript™ RT reagent
kit with gDNA Eraser according to the manufacturer's instructions
(Takara Shuzo Co., Kyoto, Japan). The concentration and purity of
total RNA were determined by measuring the OD260 and OD260/OD280
ratio, respectively. The mRNAs of TNF-α, IL-6 and IL-10 were
measured by SYBR Premix Ex Taq™ (Takara Shuzo Co.) through an ABI
7500 real-time PCR system (Life Technology Corp., Carsbad, CA,
USA). Each cDNA sample was analyzed in triplicate. The relative
quantitation of mRNA was analyzed using the equation:
ratio=2−ΔΔCt and normalized to GAPDH. The following
primers synthesized by Sangon Biotech Co., Ltd. (Shanghai, China)
were used: TNF-α forward, 5′-ACCCTCACACTCACAAA CCA-3′ and reverse,
5′-ACAAGGTACAACCCATCGGC-3′; IL-6 forward,
5′-CCCCAATTTCCAATGCTCTCC-3′ and reverse,
5′-CGCACTAGGTTTGCCGAGTA-3′; IL-10 forward,
5′-GCTCTTGCACTACCAAAGCC-3′ and reverse, 5′-CTGCTGATCCTCATGCCAGT-3′;
GAPDH forward, 5′-GGGTCCCAGCTTAGGTTCAT-3′ and reverse,
5′-TACGGCCAAATCCGTTCACA-3′.
Western blotting
Mouse pancreatic tissue (100 mg) was homogenized in
0.5 ml lysate buffer containing 20 mM Tris-HCl (pH 7.4), 5 mM EDTA,
100 mM Na4P2O7, 2 mM
Na3VO4, 100 mM NaF and 1% Nonidet P-40 and
protease inhibitors were added at a fixed volume ratio [lysate
buffer:phenylmethylsulfonyl fluoride (PMSF):protease inhibitor
cocktail, 100:1:1]. Homogenized samples were centrifuged at 4°C, at
14,000 rpm for 15 min. The concentrations of protein from the
supernatant were determined using BCA protein assay reagent.
Protein (25 μg) was loaded for 10% sodium dodecyl
sulfate-polyacrylamide-gel electrophoresis (SDS-PAGE) and then
transferred to polyvinylidene difluoride (PVDF) membranes (Merck
Millipore). After blocking with 5% bovine serum albumin,
immunoblotting was carried out with various primary antibodies at
4°C overnight or at 25°C for 2 h (anti-GAPDH antibody was used as
an internal control). After incubation with IgG horseradish
peroxidase-conjugated secondary antibody at room temperature for 1
h, the membranes were washed three times successively. Then the
specific proteins were detected by enhanced chemiluminescence
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
instructions. The relative band intensity was quantified by
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Co-immunoprecipitation
Fresh mouse pancreatic tissue was rapidly
homogenized in lysate buffer as mentioned above. The protein
concentration was then adjusted to 3 μg/μl and 800
μg total protein and 4 μg capture antibody (SIRT1,
HSF1 or p53) were incubated at 2–8°C with continuous mixing
overnight. Fifty microliters of protein A and protein G magnetic
beads was resuspended and washed with 500 μl PBS containing
0.1% Tween-20. The reaction containing the pre-formed
antibody-antigen complex mentioned above was subsequently added to
the magnetic beads and incubated for 8 h at 2–8°C with continuous
mixing to capture the immune complex. The magnetic beads were
collected by putting the tube into the magnetic stand, allowing the
beads to migrate to the magnet, and then removing the sample with a
pipette. After washing the beads with 500 μl PBS containing
0.1% Tween-20 for 3 times, 60 μl of 5X sample loading buffer
suitable for electrophoresis was added and mixed to resuspend the
beads. The beads were heated at 70–90°C for 10 min and stored at
−80°C until being used for western blotting.
Acetylation assay
Fresh mouse pancreatic tissue was subjected to
immunoprecipitation with mouse HSF1 and p53 antibodies, while
acetylated HSF1 and p53 were detected by western blotting with an
acetylated lysine antibody. Then the acetylation of HSF1 and p53
was evaluated by calculating the ratio of acetylated HSF1 and p53
to total HSF1 and p53, respectively.
Statistical analysis
All data were analyzed by SPSS 18.0 software.
Measurement data are shown as mean ± SEM of three different
experiments and analyzed by unpaired two-tailed Student's t-tests.
Kaplan-Meier analysis was performed to compare the differences in
survival rate between different groups. P<0.05 was considered
statistically significant.
Results
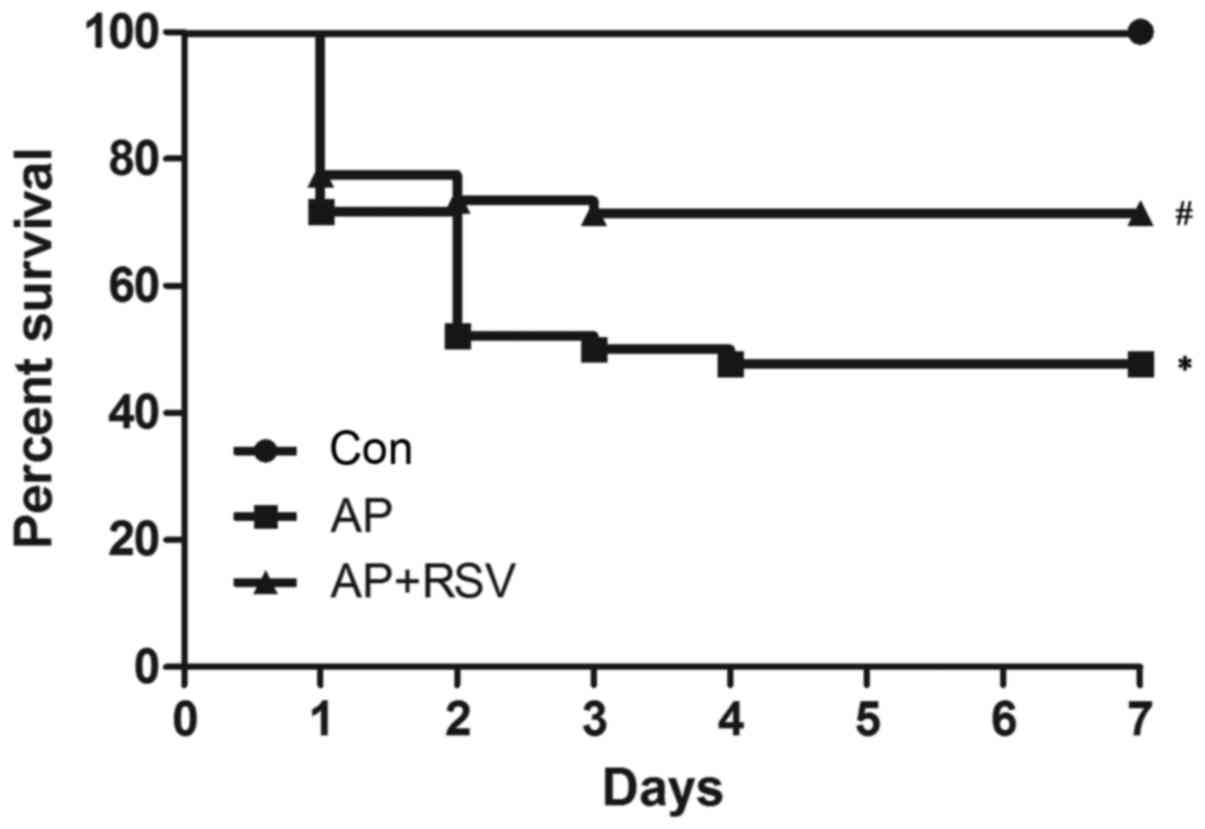
Effect of resveratrol on the survival of
mice with L-argi nine-induced ANP
To assess the effect of resveratrol on the prognosis
of ANP, a mouse model of ANP was established by intraperitoneal
injection of L-arginine as reported previously (14,15). No mouse died in the control group;
the 7-day survival rate was 100%, while the 7-day survival rate of
mice with ANP was obviously decreased to 47.8%, which was
significantly lower than that of the control group (p<0.05). In
contrast, resveratrol enhanced the 7-day survival rate of mice with
ANP to 71.4% (p<0.05) (Fig.
1).
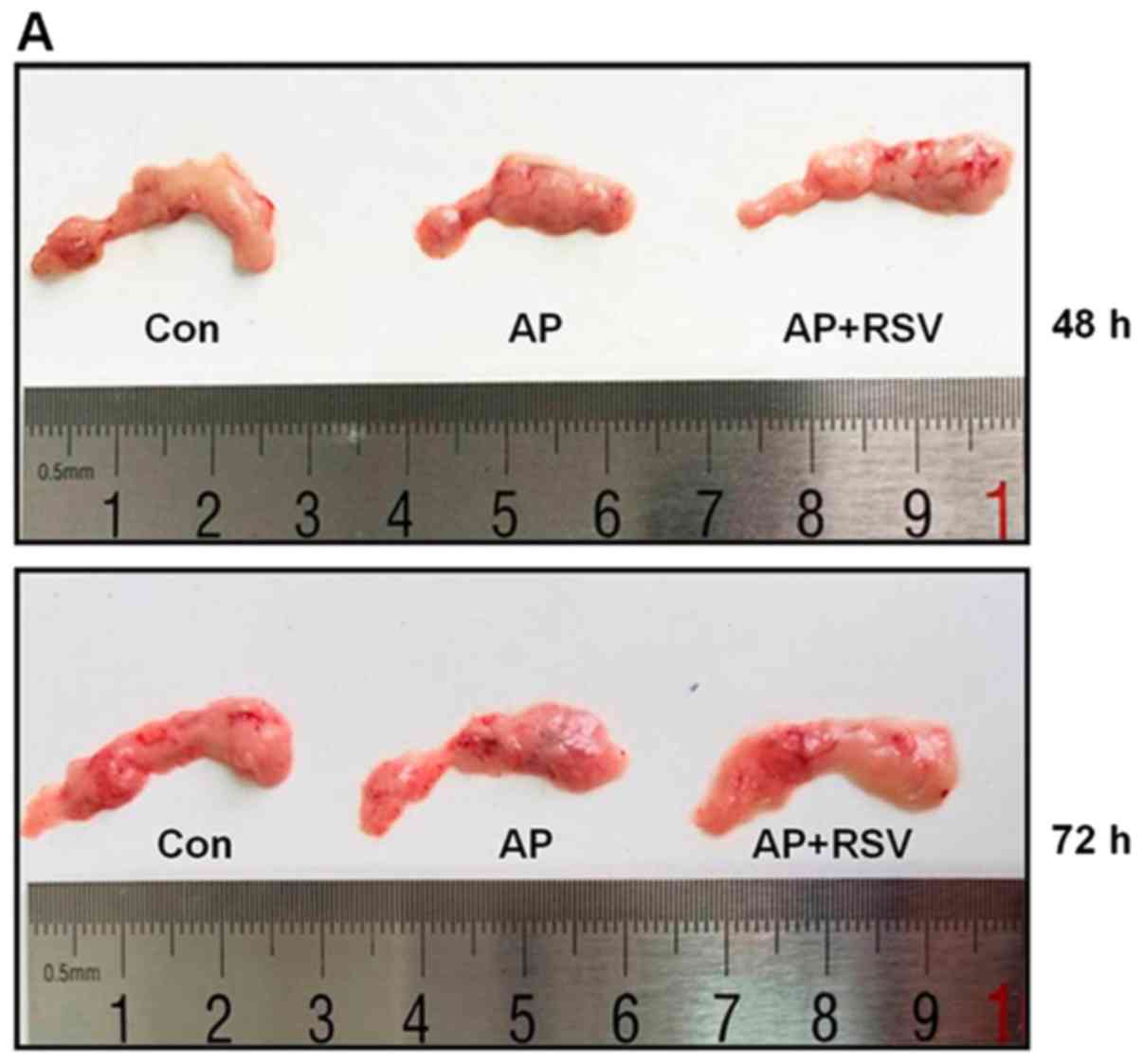
Effect of resveratrol on the pancreatic
morphology of mice with L-arginine-induced ANP
The mice treated with 2 hourly intraperitoneal
injections of 8% L-arginine hydrochloride developed ANP at 48 h
after the second injection of L-arginine. As shown in Fig. 2A, the pancreas of the mice in the
Con group was colored grey-white with normal structure, while the
pancreas of the mice with ANP was colored gray-red with obvious
roughness, necrosis and diffuse hemorrhage at 48 h and 72 h after
the second injection of L-arginine. The pancreas in the
resveratrol-treated mice with ANP showed a lighter color than that
of the mice with ANP and minimal necrosis. The structure of the
pancreas was close to that of the mice in the Con group.
H&E staining showed wider pancreatic
interlobular and acini septum, leukocyte infiltration, and up to a
20% necrotic area as well as hemorrhage in the mice with ANP at 48
h after the second injection of L-arginine, while pancreatic
interlobular and acini septum, and intercellular space were all
widened. A large amount of leukocyte infiltration and a >30%
necrotic area as well as hemorrhage were observed in the mice with
ANP at 72 h after the second injection of L-arginine. However, the
resveratrol-treated mice with ANP showed narrower pancreatic
interlobular and acini septum, less leukocyte infiltration and a
<10% necrotic area (Fig. 2B).
According to the modified Grewal method, the pancreatic damage was
scored. The pathological scores of AP mice at 48 h and 72 h after
the second injection of L-arginine were both significantly higher
than those of the control group, while resveratrol obviously
decreased the pathological scores of the mice with ANP (p<0.05)
(Fig. 2C).
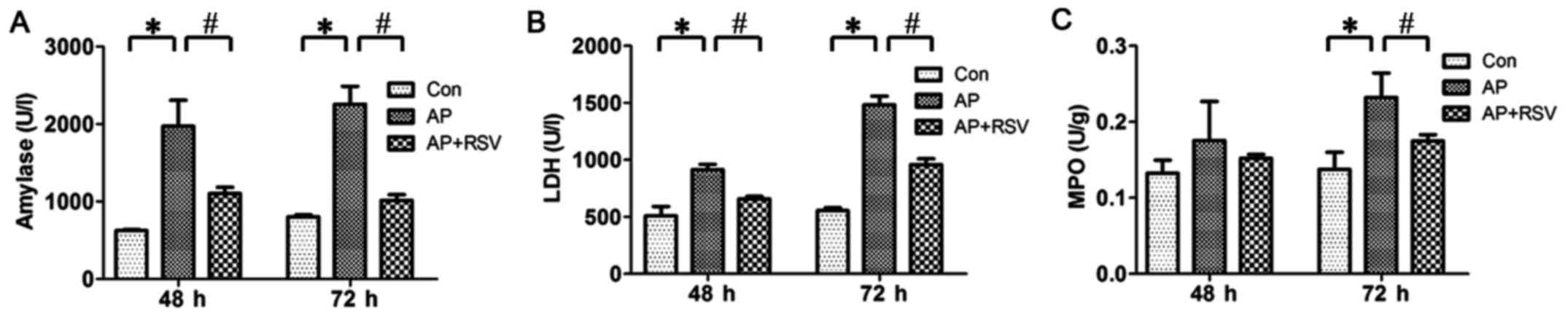
Effect of resveratrol on the serum
amylase level, LDH activity and pancreatic MPO activity of mice
with L-arginine-induced ANP
As the serum amylase level is the most commonly used
biochemical marker of AP, the serum LDH activity could reflect the
degree of necrosis and MPO activity is related to neutrophil
recruitment. Thus, in this study, the serum amylase level, LDH
activity and pancreatic MPO activity were detected at 48 and 72 h
after the second injection of L-arginine. The results showed that
the serum amylase level, LDH activity and pancreatic MPO activity
were significantly higher in the mice with ANP compared with these
parameters of the mice in the Con group (p<0.05). Resveratrol
obviously decreased the serum amylase level, LDH activity and
pancreatic MPO activity of the mice with ANP (p<0.05) (Fig. 3).
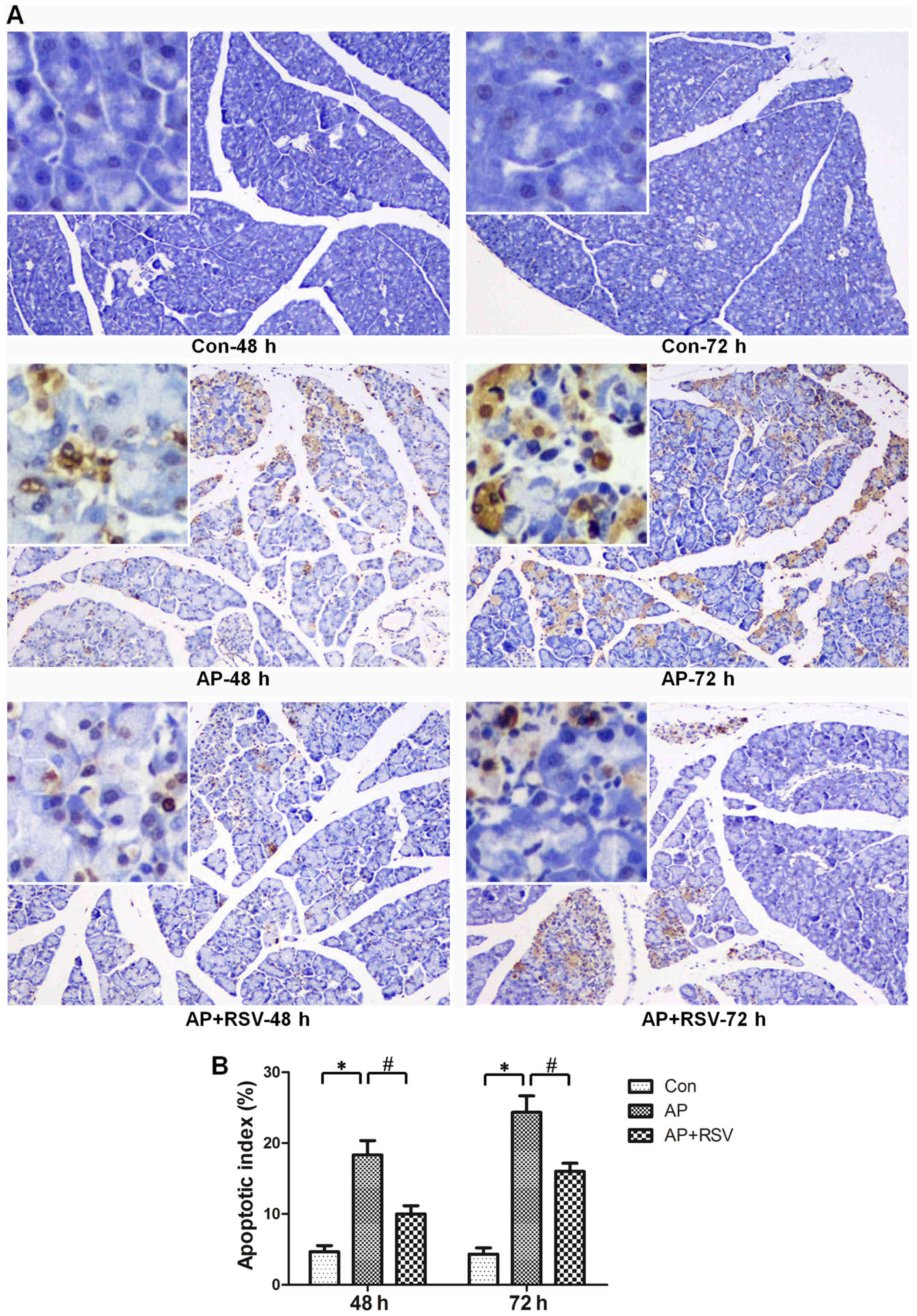
Effect of resveratrol on the apoptosis of
pancreatic acinar cells in mice with L-arginine-induced ANP
The apoptosis of pancreatic acinar cells is also one
of the typical characteristics of ANP. TUNEL staining assay was
used to detect the apoptosis of pancreatic acinar cells.
Significant increases in the apoptotic index were observed in the
mice with ANP (18.33±3.51 vs. 4.67±1.53, 24.33±4.04 vs. 4.33±1.53,
p<0.05) at 48 and 72 h after the second injection of L-arginine,
while resveratrol obviously decreased the apoptotic index of the
mice with ANP (10±2 vs. 18.33±3.51, 16±2 vs. 24.33±4.04, p<0.05)
(Fig. 4).
Effect of resveratrol on pancreatic IL-6,
TNF-α, IL-10 mRNA expression and serum IL-6, TNF-α, IL-10 levels of
the mice with L-arginine-induced ANP
The development of ANP is considered as progression
from the initial injury of exocrine pancreas to local and systemic
inflammatory response (5).
Therefore, the pancreatic IL-6, TNF-α, IL-10 mRNA expression and
serum IL-6, TNF-α, IL-10 levels were detected. It was found that
the pancreatic IL-6, TNF-α mRNA expression and serum IL-6, TNF-α
levels of mice with ANP were significantly increased compared with
the mice in the Con group (p<0.05), while the pancreatic IL-10
mRNA expression and serum IL-10 level were obviously decreased
(p<0.05). Resveratrol did not only markedly decrease the
pancreatic IL-6, TNF-α mRNA expression and serum IL-6, TNF-α levels
of mice with ANP but also enhanced the pancreatic IL-10 mRNA
expression and serum IL-10 level (p<0.05) (Fig. 5).
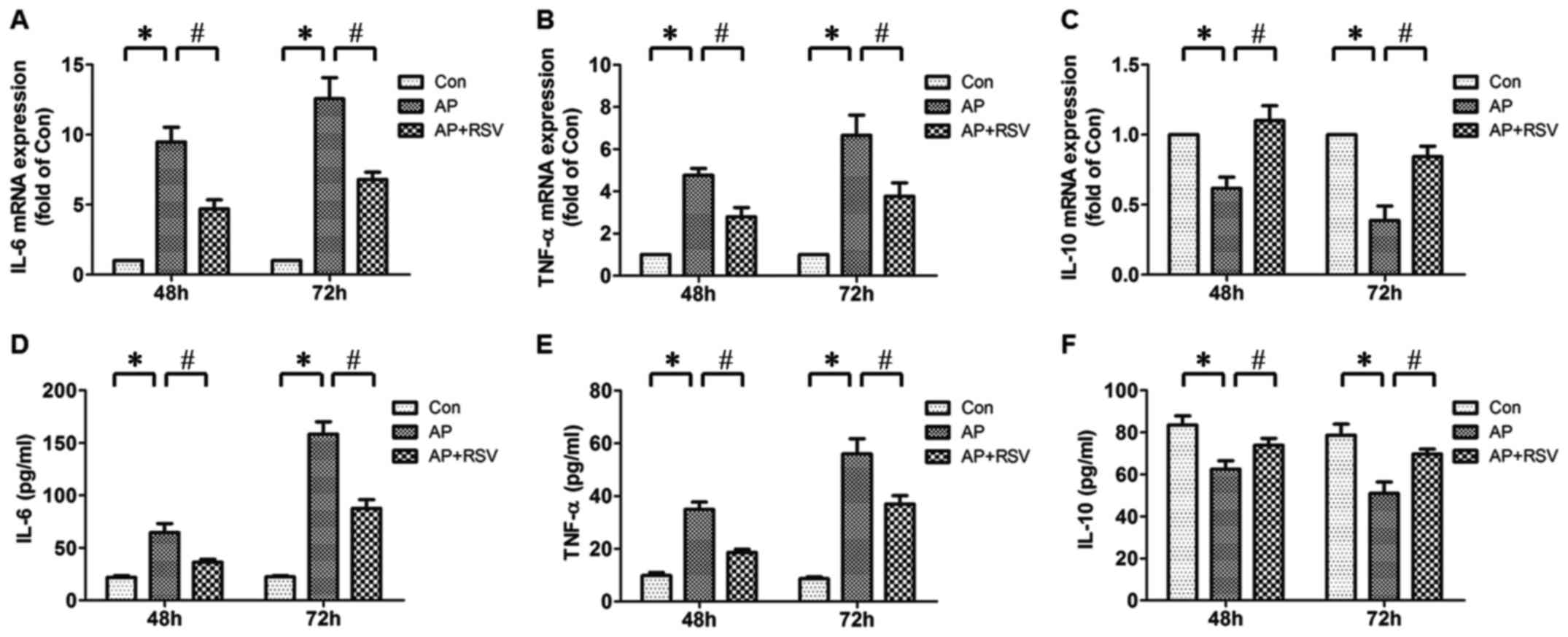 | Figure 5Effect of resveratrol on the
pancreatic interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α),
IL-10 mRNA expression levels and serum IL-6, TNF-α, IL-10 levels in
mice with L-arginine-induced acute necrotizing pancreatitis (ANP).
Pancreatic IL-6 (A), TNF-α (B), IL-10 (C) mRNA expression levels
were detected at 48 h and 72 h after the second injection of
L-arginine by real-time PCR. Serum IL-6 (D), TNF-α (E) and IL-10
(F) levels were detected at 48 h and 72 h after the second
injection of L-arginine by ELISA. The values are shown as means ±
SD. *p<0.05 vs. Con; #p<0.05 vs. acute
pancreatitis (AP) (n=5). Con, control group; AP, L-arginine
exposure group; AP+RSV, L-arginine and resveratrol treatment
group. |
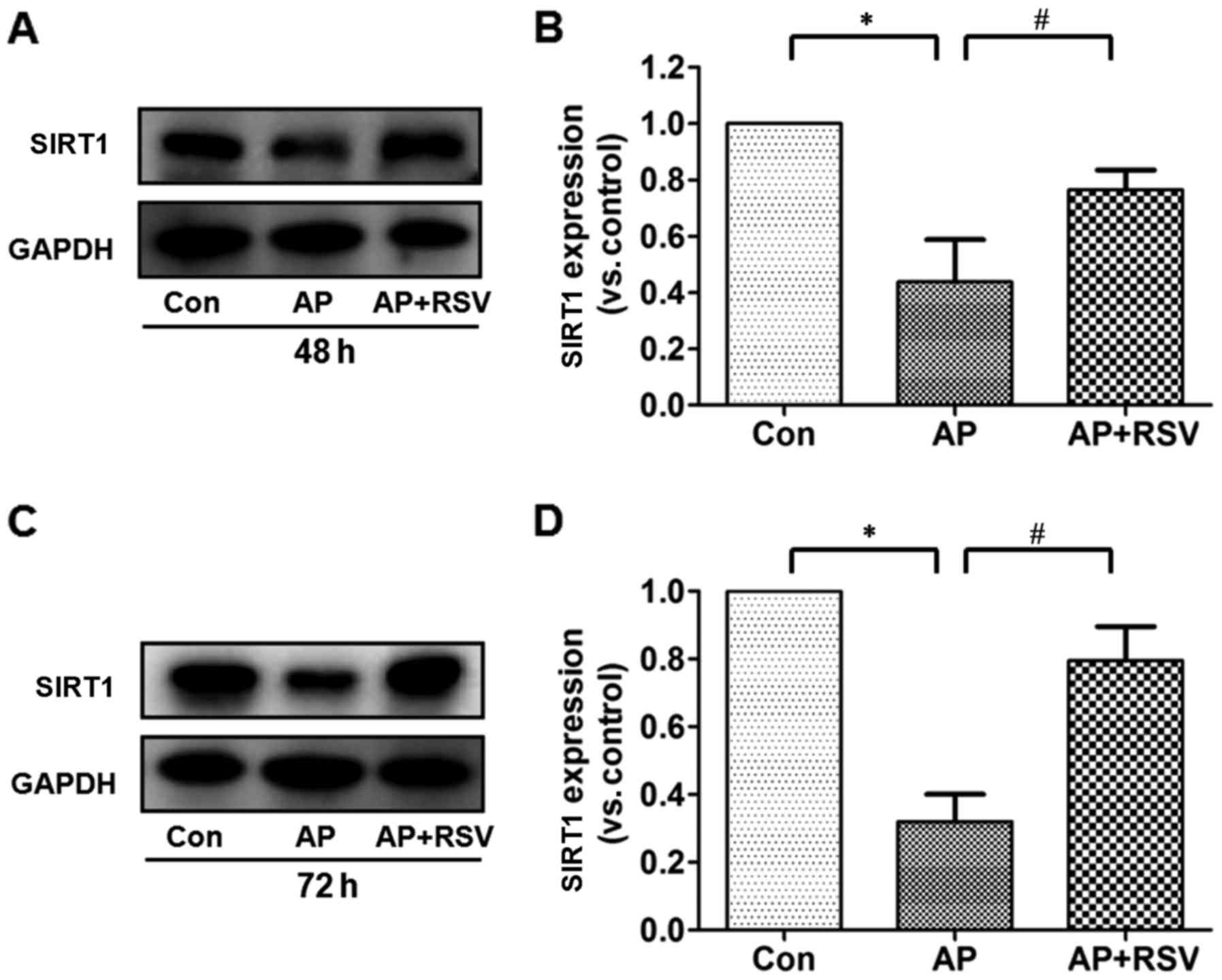
Effect of resveratrol on the SIRT1
protein expression and activity in the pancreas of mice with
L-arginine-induced ANP
Resveratrol has been confirmed as a classic SITR1
activator, but whether SIRT1 is involved in the pathogenesis of
L-arginine-induced acute pancreatitis is still unknown. The
expression and activity were further detected. It was shown that
the expression of SIRT1 in the pancreas of mice with ANP was
significantly decreased to ~40% of that in the mice in the control
group at 48 and 72 h after the second injection of L-arginine
(p<0.05), but obviously increased SIRT1 expression was detected
in the resveratrol-treated mice with ANP (p<0.05) (Fig. 6).
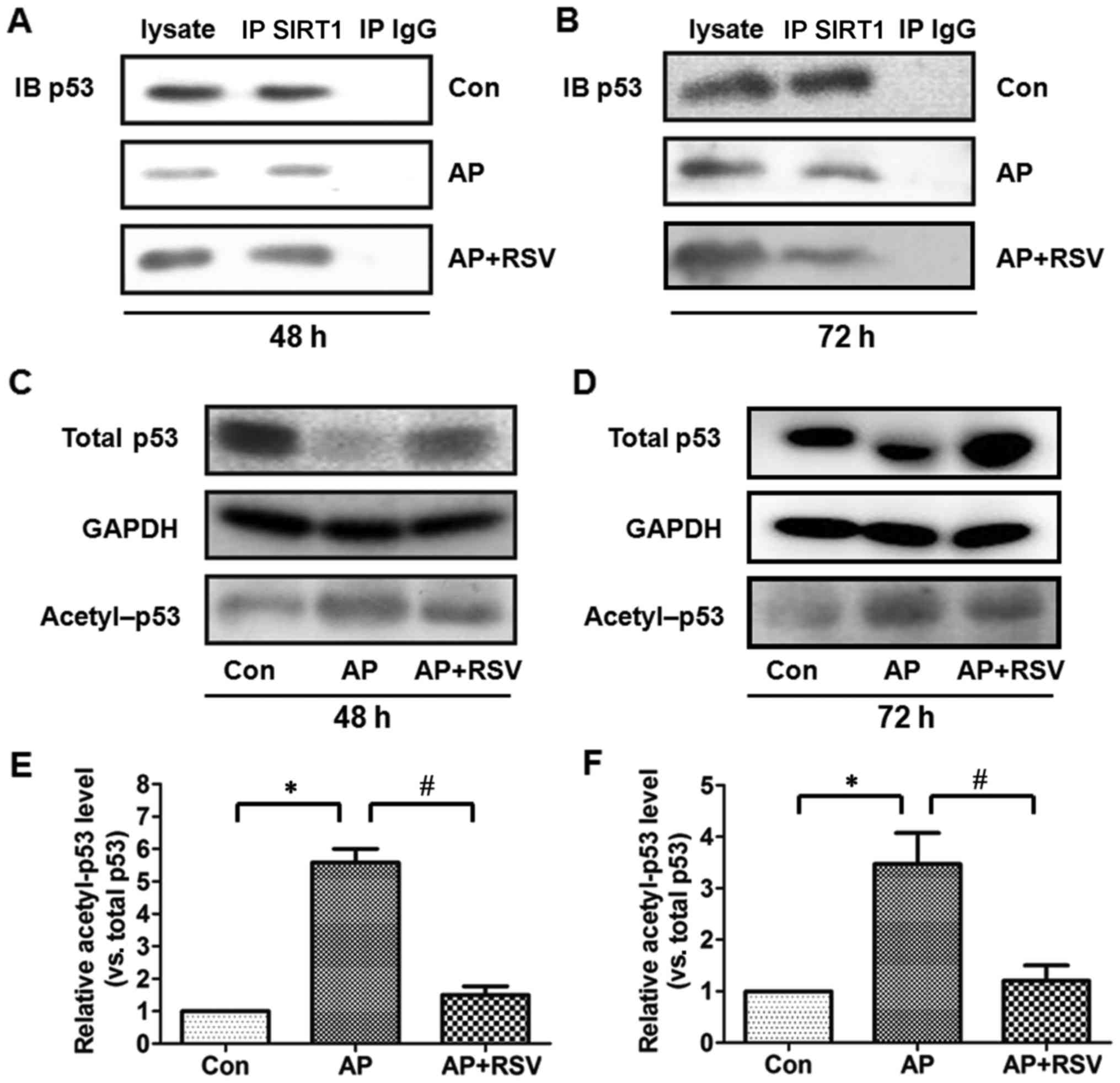
p53 is a pro-apoptotic protein, the deacetylation of
which indirectly reflects the activity of SIRT1 (5,16,17). Firstly, the expression of p53 was
detected. p53 protein expression was significantly downregulated in
the pancreas of the mice with ANP at 48 h and 72 h after the second
injection of L-arginine (p<0.05), while resveratrol obviously
upregulated the p53 protein expression of the mice with ANP
(p<0.05). Co-immunoprecipitation assay showed that p53 was
precipitated by the SIRT1 antibody and further acetylation assay
showed that the ratio of acetylated p53 was markedly higher in the
pancreas of the mice with ANP at 48 h and 72 h after the second
injection of L-arginine (p<0.05), but resveratrol markedly
attenuated the acetylation of p53 in the mice with ANP (p<0.05)
(Fig. 7).
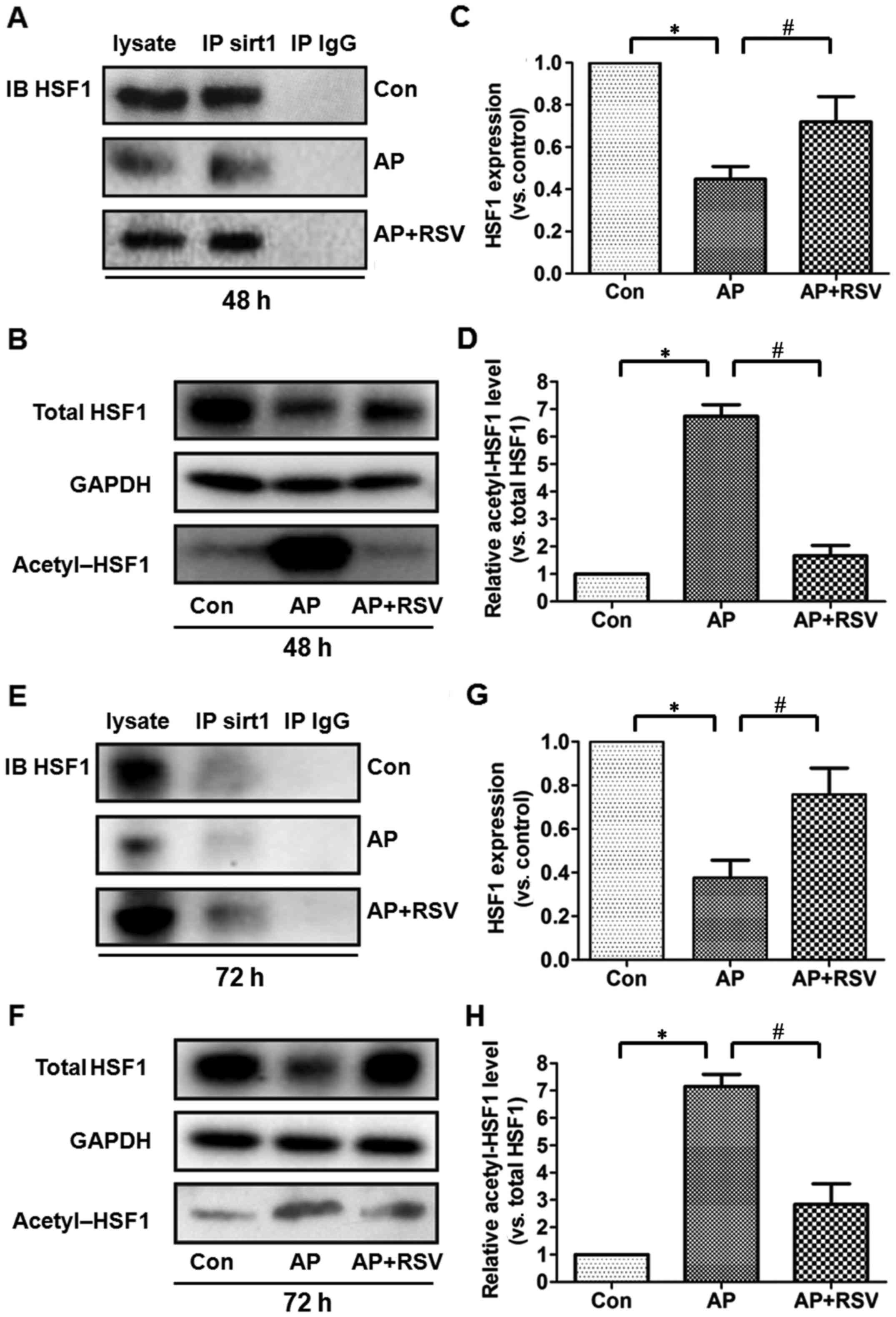
Effect of resveratrol on the expression
and acetylation of HSF1 in the pancreas of mice with
L-arginine-induced ANP
HSF1 is a crucial transcription factor in heat shock
response and also plays an important role in the inflammatory
response and cell apoptosis. The activity of HSF1 is closely
related to its acetylation. To investigate the role of HSF1 in the
L-arginine-induced ANP, co-immunoprecipitation assay was firstly
performed to detect the interation between HSF1 and SIRT1. HSF1 was
precipitated by the SIRT1 antibody in the different groups,
although the total expression of HSF1 was significantly decreased
in the pancreas of the mice with ANP at 48 and 72 h after the
second injection of L-arginine (p<0.05). Acetylation assay
showed that the ratio of acetylated HSF1 was significantly
upregulated (p<0.05). However, resve-ratrol obviously enhanced
the total expression of HSF1 but decreased the acetylation of HSF1
in the pancreas of the mice with ANP (p<0.05) (Fig. 8).
Discussion
AP, a common inflammatory disorder of the pancreas,
has become the leading cause of admission to intensive care units
(ICUs) worldwide (18). It has
been confirmed that AP is initiated by the activation of various
enzymes in pancreatic acinar cells, which leads to the injury of
acinar cells, followed by the release of pro-inflammatory factors
such as TNF-α, IL-1β and IL-6 (19). The chemokines further released
from the injured acinar cells then promote inflammatory cells
including neutrophils and macrophages to accumulate in the
pancreas, which results in exacerbating the local inflammatory
response in the pancreas and progressing rapidly into a systemic
inflammatory response (5). Under
this condition, the excessive release of various inflammatory
factors increases the microvessel density, induces thrombosis and
hemorrhage and finally increases the apoptosis and necrosis of
acinar cells. In addition, the gut barrier dysfunction is usually
found in AP, which can result in bacterial translocation, further
activate the macrophages to release a large quantity of
inflammatory factors and induce a secondary attack to the pancreas
(20,21). Hence, controlling the rapidly
progressive inflammatory response and inhibiting the injury of
pancreatic acinar cells may be beneficial to improve the prognosis
of ANP.
Resveratrol is a natural polyphenol compound mainly
found in various plants and red wine. It is well known for its
anti-inflammatory and antioxidant effects. It has been confirmed
that resveratrol has protective effects on sodium
taurocholate-induced ANP and cerulean-induced acute edema
pancreatitis. L-arginine-induced experimental model of ANP was
firstly reported by Tani et al (22), since L-arginine selectively
destroys pancreatic acinar cells by inducing amino acid imbalance,
decreasing the synthesis of polyamine, nucleic acid and proteinase
and resulting in excessive activation of zymogen. In addition,
L-arginine could induce the expression of different types of
inflammatory factors and apoptosis-related genes and proteins in
the pancreatic acinar cells. Compared with other invasive
experimental models of ANP, the L-arginine-induced experimental
model of ANP has the advantages of non-invasive, easier operation,
higher success rate and lower cost, although the death rate is
high. It is commonly used as an investigative tool for screening
and developing effective therapies for ANP. In this study, the
7-day survival rate of the L-arginine-induced mice with ANP was
47.8%, while administration of 20 and 40 mg/kg resveratrol every 12
h immediately after the second injection of L-arginine had no
significant effect on the survival of the mice with ANP (data not
shown). However, administration of 80 mg/kg resveratrol
significantly enhanced the 7-day survival rate of the mice with ANP
to 71.4%. Further studies showed that the L-arginine-induced ANP
model was successfully established at 48 h after the second
injection of L-arginine, which was mainly manifested as pancreatic
and systemic inflammation, apoptosis and necrosis of pancreatic
acinar cells. Administration of 80 mg/kg resveratrol every 12 h
immediately after the second injection of L-arginine markedly
ameliorated L-arginine-induced pancreatic inflammation and injury
as well as systemic inflammation. These results proved that
resveratrol could protect L-arginine-induced ANP through
alleviating the pancreatic inflammatory response.
In recent years, accumulating evidence suggests that
the biological activities of resveratrol are closely related to the
activation of SIRT1. SIRT1 has been proven to be involved in a
variety of cellular functions, such as proliferation,
differentiation, apoptosis, aging and metabolism. It also
participates in the regulation of inflammation, but its role in ANP
is poorly understood to date. Our data showed that the pancreatic
expression of SIRT1 in L-arginine-induced mice with ANP was
significantly decreased compared with that in the normal mice,
which was reversed by resveratrol treatment. The activation of
SIRT1 enhances the transcriptional activities of its downstream
targets mainly through deacetylating the lysine residues at certain
sites. p53 has been identified as a downstream target of SIRT1 and
the deacetylation level of p53 was proven to be able to indirectly
reflect the activity of SIRT1. In this study, the results showed
that p53 interacted with SIRT1 in the different experimental
groups, the p53 expression was downregulated while the ratio of
acetylated p53 was upregulated in the L-arginine-induced mice with
ANP, suggesting that the SIRT1 activity was significantly decreased
in the mice with ANP, while resveratrol could enhance the SIRT1
activity in the mice with ANP. SIRT1 deacetylates p53 and activates
itself to trigger cell apoptosis (16,17) while p53 has been proven to play an
important role in the apoptosis of pancreatic acinar cells
(23). It seemed to be
contradictory that resveratrol activiated SIRT1 and subse quently
promoted p53-mediated apoptosis of pancreatic acinar cells by
increasing the deacetylation of p53. Actually, studies increasingly
suggested that the promotion of apoptosis of pancreatic acinar
cells was beneficial to ameliorate ANP because of the decreasing
necrosis and severity of the pancreatitis (24–26). Therefore, we speculated that the
protective effect of resveratrol on ANP might also be related to
the enhancement of SIRT1 activation and subsequent promotion of
pancreatic acinar cell apoptosis mediated by p53 deacetylation,
while resveratrol eventually decreased the apoptosis of pancreatic
acinar cells through other anti-apoptotic mechanisms (27,28).
Inflammatory response persists in the occurrence and
development of ANP which is described as progression from an
initial injury of pancreatic acinar cells to local and systemic
inflammation. HSF1, an important heat shock transcription factor,
is widely expressed in various types of cells and plays an
important role in inflammation by regulating the transcription of
inflammatory factors (29–31).
The acetylation of HSF1 decreases its DNA binding activity, and
thus promotes the transcription of pro-inflammatory factors and
inhibits the transcription of anti-inflammatory factors. In our
previous study, HSF1-knockout mice developed more severe acute
edema pancreatitis induced by cerulein than the wild-type mice.
However, the role of HSF1 in ANP is poorly understood. Our results
showed that HSF1 expression was significantly decreased in the
pancreatic tissues of mice with ANP, indicating that HSF1 is
involved in the pathogenesis of ANP. It is particularly noteworthy
that HSF1 is a deacetylase substrate for SIRT1 (10,32). Ghemrawi et al found that a
decrease in the cellular availability of B12 led to ER stress
activation mediated by decreased SIRT1 expression, which in turn
led to both lower HSF1 expression and HSF1 hyper-acetylation
(10). Similarly, in our study,
although the total HSF1 expression was decreased in the pancreatic
tissue of the L-arginine-induced mice with ANP, the ratio of
acetylated HSF1 was sharply increased up to 6- to 8-fold of the
normal mice, which may consequently increase the transcription of
pro-inflammatory factors (TNF-α and IL-6) but decrease the
transcription of anti-inflammatory factors (IL-10). However,
resveratrol did not only markedly enhance the total expression of
HSF1, but also diminished acetylated HSF1. These results suggest
that resveratrol enhances the deacetylation of HSF1 in the pancreas
of ANP, and subsequently exhibits its potent anti-inflammatory
effect and eventually ameliorates ANP by mediating the
transcription of inflammatory factors.
In addition to the deacetylation of p53 and HSF1,
other intracellular target proteins and signaling pathways may also
be involved in the protective effect of SIRT1 activation on ANP.
For instance, nuclear factor-κB (NF-κB), forkhead box protein
(Fox)O1, FoxO3 and FoxO4 are also the deacetylation targets of
SIRT1 (33,34). Sirt1 inhibits apoptosis and
inflammation via deacetylating these proteins and thereby
modulating their activities, which may also contribute to the
protective effect of resveratrol on ANP. Mammalian target of
rapamycin (mTOR), an important target of SIRT1, inhibits autophagy
and has been implicated in the development of pancreatitis
(35,36). SIRT1 influences different
pathophysiological processes including metabolism, cell apoptosis
and aging by downregulating mTOR (37–39). Interestingly, resveratrol
exhibited a protective effect on various diseases via the
upregulation of mTOR and autophagy. Therefore, the protective
effect of resveratrol on ANP may also be attributed to the SIRT1
mTOR axis-enhanced autophagy in the pancreatic acinar cells.
Furthermore, the Toll-like receptor 4 (TLR4)/NF-κB pathway mediates
the production of various pro-inflammatory factors and has been
found to play important roles in the development of panceatitis,
whereas resveratrol was found to relieve the inflammatory injury by
inhibiting the TLR4/NF-κB pathway (45,46). Hence, the inhibition of the
TLR4/NF-κB pathway may also contribute to the protective effect of
resveratrol on ANP.
In conclusion, to the best of our knowledege, our
data demonstrated for the first time that resveratrol effectively
improved the survival, relieved the inflammatory response and
decreased the acinar necrosis and apoptosis in L-arginine-induced
ANP in mice, which may be related to the enhancement of
SIRT1-mediated deacetylation of p53 and HSF1. Notably, the increase
in SIRT1-mediated deacetylation of p53 can promote the apoptosis of
pancreatic acinar cells, which might further decrease the necrosis
of pancreatic acinar cells and relieve ANP. This may be another
important protective effect of resveratrol besides its
anti-inflammatory activity.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 81000846, 81270201 and 81470408)
and the Natural Science Foundation of Hunan Province of China (no.
12JJ4084).
References
|
1
|
Weitz G, Woitalla J, Wellhöner P, Schmidt
K, Büning J and Fellermann K: Does etiology of acute pancreatitis
matter? A review of 391 consecutive episodes. JOP. 16:171–175.
2015.PubMed/NCBI
|
|
2
|
Agarwal S, George J, Padhan RK, Vadiraja
PK, Behera S, Hasan A, Dhingra R, Shalimar and Garg PK: Reduction
in mortality in severe acute pancreatitis: A time trend analysis
over 16 years. Pancreatology. 16:194–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dellinger EP, Forsmark CE, Layer P, Lévy
P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G,
Whitcomb DC, et al: Pancreatitis Across Nations Clinical Research
and Education Alliance (PANCREA): Determinant-based classification
of acute pancreatitis severity: An international multidisciplinary
consultation. Ann Surg. 256:875–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sandakov PIa, Samartsev VA and Mineev DA:
Surgical and therapeutic treatment of acute pancreatitis.
Khirurgiia (Mosk). 10:56–63. 2014.In Russian.
|
|
5
|
Hegyi P, Pandol S, Venglovecz V and
Rakonczay Z Jr: The acinar-ductal tango in the pathogenesis of
acute pancreatitis. Gut. 60:544–552. 2011. View Article : Google Scholar
|
|
6
|
Bhatia M: Inflammatory response on the
pancreatic acinar cell injury. Scand J Surg. 94:97–102.
2005.PubMed/NCBI
|
|
7
|
Lv JC, Wang G, Pan SH, Bai XW and Sun B:
Lycopene protects pancreatic acinar cells against severe acute
pancreatitis by abating the oxidative stress through JNK pathway.
Free Radic Res. 49:151–163. 2015. View Article : Google Scholar
|
|
8
|
Carrasco C, Holguín-Arévalo MS,
Martín-Partido G, Rodríguez AB and Pariente JA: Chemopreventive
effects of resveratrol in a rat model of cerulein-induced acute
pancreatitis. Mol Cell Biochem. 387:217–225. 2014. View Article : Google Scholar
|
|
9
|
Sha H, Ma Q, Jha RK, Wu Z, Qingyuan Z,
Wang Z, Ma Z, Luo X and Liu C: Resveratrol suppresses
microcirculatory disturbance in a rat model of severe acute
pancreatitis. Cell Biochem Biophys. 67:1059–1065. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghemrawi R, Pooya S, Lorentz S, Gauchotte
G, Arnold C, Gueant JL and Battaglia-Hsu SF: Decreased vitamin B12
availability induces ER stress through impaired SIRT1-deacetylation
of HSF1. Cell Death Dis. 4:e5532013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JT and Gu W: SIRT1: Regulator of p53
Deacetylation. Genes Cancer. 4:112–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X
and Zhai Q: SIRT1 improves insulin sensitivity under
insulin-resistant conditions by repressing PTP1B. Cell Metab.
6:307–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grewal HP, Mohey EDA, Gaber L, Kotb M and
Gaber AO: Amelioration of the physiologic and biochemical changes
of acute pancreatitis using an anti-TNF-alpha polyclonal antibody.
Am J Surg. 167:214–219. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dawra R, Sharif R, Phillips P, Dudeja V,
Dhaulakhandi D and Saluja AK: Development of a new mouse model of
acute pancreatitis induced by administration of L-arginine. Am J
Physiol Gastrointest Liver Physiol. 292:G1009–G1018. 2007.
View Article : Google Scholar
|
|
15
|
Kui B, Balla Z, Vasas B, Végh ET, Pallagi
P, Kormányos ES, Venglovecz V, Iványi B, Takács T, Hegyi P, et al:
New insights into the methodology of L-arginine-induced acute
pancreatitis. PLoS One. 10:e01175882015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong W, Tatsuo S, Shou-Dong W, Qian Z,
Jian-Feng H, Jue W, Chen J, Hai-Yan Q and Yue-Jin Y: Resveratrol
Upregulates Cardiac SDF-1 in mice with acute myocardial infarction
through the deacetylation of cardiac p53. PLoS One.
10:e01289782015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kume S, Haneda M, Kanasaki K, Sugimoto T,
Araki S, Isono M, Isshiki K, Uzu T, Kashiwagi A and Koya D: Silent
information regulator 2 (SIRT1) attenuates oxidative stress-induced
mesangial cell apoptosis via p53 deacetylation. Free Radic Biol
Med. 40:2175–2182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lankisch PG, Apte M and Banks PA: Acute
pancreatitis. Lancet. 386:85–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayer J, Rau B, Gansauge F and Beger HG:
Inflammatory mediators in human acute pancreatitis: Clinical and
pathophysiological implications. Gut. 47:546–552. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vandenabeele P, Galluzzi L, Vanden Berghe
T and Kroemer G: Molecular mechanisms of necroptosis: An ordered
cellular explosion. Nat Rev Mol Cell Biol. 11:700–714. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Capurso G, Zerboni G, Signoretti M,
Valente R, Stigliano S, Piciucchi M and Delle Fave G: Role of the
gut barrier in acute pancreatitis. J Clin Gastroenterol. 46(Suppl):
S46–S51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tani S, Itoh H, Okabayashi Y, Nakamura T,
Fujii M, Fujisawa T, Koide M and Otsuki M: New model of acute
necrotizing pancreatitis induced by excessive doses of arginine in
rats. Dig Dis Sci. 35:367–374. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Chen J, Wang X, Wang C, Cao W,
Zhao Y, Zhang B, Cui M, Shi Q and Zhang G: Ligustrazine alleviates
acute pancreatitis by accelerating acinar cell apoptosis at early
phase via the suppression of p38 and Erk MAPK pathways. Biomed
Pharmacother. 82:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura Y, Do JH, Yuan J, Odinokova IV,
Mareninova O, Gukovskaya AS and Pandol SJ: Inflammatory cells
regulate p53 and caspases in acute pancreatitis. Am J Physiol
Gastrointest Liver Physiol. 298:G92–G100. 2010. View Article : Google Scholar :
|
|
25
|
Mareninova OA, Sung KF, Hong P, Lugea A,
Pandol SJ, Gukovsky I and Gukovskaya AS: Cell death in
pancreatitis: Caspases protect from necrotizing pancreatitis. J
Biol Chem. 281:3370–3381. 2006. View Article : Google Scholar
|
|
26
|
Liu Y, Yuan J, Tan T, Jia W, Lugea A,
Mareninova O, Waldron RT and Pandol SJ: Genetic inhibition of
protein kinase Cε attenuates necrosis in experimental pancreatitis.
Am J Physiol Gastrointest Liver Physiol. 307:G550–G563. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu J, Hu W and Zhang DD: Resveratrol, a
polyphenol phytoalexin, protects against doxorubicin-induced
cardiotoxicity. J Cell Mol Med. 19:2324–2328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han G, Xia J, Gao J, Inagaki Y, Tang W and
Kokudo N: Anti-tumor effects and cellular mechanisms of
resveratrol. Drug Discov Ther. 9:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Zhang L, Yu F, Liu Y, Liang Q,
Deng G, Chen G, Liu M and Xiao X: HSF1 is a transcriptional
activator of IL-10 gene expression in RAW264.7 macrophages.
Inflammation. 35:1558–1566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen S, Zuo X, Yang M, Lu H, Wang N, Wang
K, Tu Z, Chen G, Liu M, Liu K, et al: Severe multiple organ injury
in HSF1 knockout mice induced by lipopolysaccharide is associated
with an increase in neutrophil infiltration and surface expression
of adhesion molecules. J Leukoc Biol. 92:851–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tong Z, Jiang B, Zhang L, Liu Y, Gao M,
Jiang Y, Li Y, Lu Q, Yao Y and Xiao X: HSF-1 is involved in
attenuating the release of inflammatory cytokines induced by LPS
through regulating autophagy. Shock. 41:449–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raynes R, Pombier KM, Nguyen K, Brunquell
J, Mendez JE and Westerheide SD: The SIRT1 modulators AROS and DBC1
regulate HSF1 activity and the heat shock response. PLoS One.
8:e543642013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim HN, Han L, Iyer S, de Cabo R, Zhao H,
O'Brien CA, Manolagas SC and Almeida M: Sirtuin1 suppresses
osteoclastogenesis by deacetylating FoxOs. Mol Endocrinol.
29:1498–1509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shinozaki S, Chang K, Sakai M, Shimizu N,
Yamada M, Tanaka T, Nakazawa H, Ichinose F, Yamada Y, Ishigami A,
et al: Inflammatory stimuli induce inhibitory S-nitrosylation of
the deacetylase SIRT1 to increase acetylation and activation of p53
and p65. Sci Signal. 7:ra1062014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu YY, Zhou CH, Dou WH, Tang W, Hu CY, Hu
DM, Feng H, Wang JZ, Qian MJ, Cheng GL, et al: Improved autophagic
flux is correlated with mTOR activation in the later recovery stage
of experimental acute pancreatitis. Pancreatology. 15:470–477.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji L, Li L, Qu F, Zhang G, Wang Y, Bai X,
Pan S, Xue D, Wang G and Sun B: Hydrogen sulphide exacerbates acute
pancreatitis by over-activating autophagy via AMPK/mTOR pathway. J
Cell Mol Med. 20:2349–2361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kwon HS and Ott M: The ups and downs of
SIRT1. Trends Biochem Sci. 33:517–525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ghosh HS, McBurney M and Robbins PD: SIRT1
negatively regulates the mammalian target of rapamycin. PLoS One.
5:e91992010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou XL, Xu JJ, Ni YH, Chen XC, Zhang HX,
Zhang XM, Liu WJ, Luo LL and Fu YC: SIRT1 activator (SRT1720)
improves the follicle reserve and prolongs the ovarian lifespan of
diet-induced obesity in female mice via activating SIRT1 and
suppressing mTOR signaling. J Ovarian Res. 7:972014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Diaz-Gerevini GT, Repossi G, Dain A,
Tarres MC, Das UN and Eynard AR: Beneficial action of resveratrol:
How and why? Nutrition. 32:174–178. 2016. View Article : Google Scholar
|
|
41
|
Jung MJ, Lee J, Shin NR, Kim MS, Hyun DW,
Yun JH, Kim PS, Whon TW and Bae JW: Chronic repression of mTOR
complex 2 induces changes in the gut microbiota of diet-induced
obese mice. Sci Rep. 6:308872016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alayev A, Berger SM and Holz MK:
Resveratrol as a novel treatment for diseases with mTOR pathway
hyperactivation. Ann NY Acad Sci. 1348:116–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gu J, Hu W, Song ZP, Chen YG, Zhang DD and
Wang CQ: Resveratrol-induced autophagy promotes survival and
attenuates doxorubicin-induced cardiotoxicity. Int Immunopharmacol.
32:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park D, Jeong H, Lee MN, Koh A, Kwon O,
Yang YR, Noh J, Suh PG, Park H and Ryu SH: Resveratrol induces
autophagy by directly inhibiting mTOR through ATP competition. Sci
Rep. 6:217722016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Z, Chen N, Liu JB, Wu JB, Zhang J,
Zhang Y and Jiang X: Protective effect of resveratrol against acute
lung injury induced by lipopolysaccharide via inhibiting the
myd88-dependent Toll-like receptor 4 signaling pathway. Mol Med
Rep. 10:101–106. 2014.PubMed/NCBI
|
|
46
|
Zhong K: Curcumin mediates a protective
effect via TLR-4/NF-κB signaling pathway in rat model of severe
acute pancreatitis. Cell Biochem Biophys. 73:175–180. 2015.
View Article : Google Scholar : PubMed/NCBI
|